Abstract
Background:
Ankle sprains may damage both the lateral ligaments of the hindfoot and the osteochondral tissue of the ankle joint. When nonoperative treatment fails, operative approaches are indicated to restore both native motion patterns at the hindfoot and ankle joint contact mechanics. The goal of the present study was to determine the effect of lateral ligament injury, repair, and reconstruction on ankle joint contact mechanics and hindfoot motion patterns.
Methods:
Eight cadaveric specimens were tested with use of robotic technology to apply combined compressive (200-N) and inversion (4.5-Nm) loads to the hindfoot at 0° and 20° of plantar flexion. Contact mechanics at the ankle joint were simultaneously measured. A repeated-measures experiment was designed with use of the intact condition as control, with the other conditions including sectioned anterior talofibular and calcaneofibular ligaments, the Broström and Broström-Gould repairs, and graft reconstruction.
Results:
Ligament sectioning decreased contact area and caused a medial and anterior shift in the center of pressure with inversion loads relative to those with the intact condition. There were no significant differences in inversion or coupled axial rotation with inversion between the Broström repair and the intact condition; however, medial translation of the center of pressure remained elevated after the Broström repair relative to the intact condition. The Gould modification of the Broström procedure provided additional support to the hindfoot relative to the Broström repair, reducing inversion and axial rotation with inversion beyond that of intact ligaments. There were no significant differences in center-of-pressure excursion patterns between the Broström-Gould repair and the intact ligament condition, but this repair increased contact area beyond that with the ligaments intact. Graft reconstruction more closely restored inversion motion than did the Broström-Gould repair at 20° of plantar flexion but limited coupled axial rotation. Graft reconstruction also increased contact areas beyond the lateral ligament-deficient conditions but altered center-of-pressure excursion patterns relative to the intact condition.
Conclusions:
No lateral ankle ligament reconstruction completely restored native contact mechanics of the ankle joint and hindfoot motion patterns.
Clinical Relevance:
Our results provide a rationale for conducting long-term, prospective, comparative, in vivo studies to assess the impact of altered mechanics following lateral ligament injury, and its nonoperative and operative treatment, on the development of ankle osteoarthritis.
Ankle sprains, particularly lateral ankle sprains, are the most common athletic injuries1-3. It is well established that these injuries damage the lateral ankle ligaments and also may damage the osteochondral tissues of the ankle joint4-10. In fact, osteochondral lesions of the ankle are being recognized as an increasingly common injury and may occur in association with as many as 50% of acute ankle sprains and fractures, particularly among patients with sports injuries11-13.
Lateral ankle sprains involve sudden, forceful inversion of the hindfoot. This is associated with aberrant hindfoot kinematics and impaction of the articulating surfaces of the ankle joint, possibly altering the load-transfer patterns across the joint surfaces. Abnormal contact mechanics could damage the osteochondral tissues and predispose the patient to premature ankle osteoarthritis10. However, studies have only explored contact behavior during simulated stance14 in the presence of compromised lateral ligaments, not under inversion loading conditions. A connection between altered contact mechanics and inversion loading has not yet been established, despite the potential role of this association in the development of osteochondral injury to the articular surfaces of the ankle joint during the inversion injury.
When nonoperative treatment fails, ankle instability frequently requires operative treatment. The Broström procedure, a direct repair of the anterior talofibular and calcaneofibular ligaments, can restore hindfoot kinematics if viable ligamentous tissue allows this repair15. The Gould modification of the Broström procedure involves the same direct repair of the ruptured lateral ligaments with reinforcement of the repair with use of the inferior extensor retinaculum16, providing additional support against inversion loads17.
More recently, reconstruction of the lateral ligaments with free tendon grafts has been advocated15,18-20. Surgeons may resort to this option when the quality of the torn ligament tissue is insufficient for direct repair21. These techniques have been associated with improved stability of the hindfoot as shown by in vitro biomechanical15 and clinical studies22,23. Previous studies15,17-20,22,24,25, however, have focused on the effect of ligament reconstruction on primary motions in the direction of the applied load. Coupled motions, or motions in directions other than the applied load, are seldom explored26, although they form an important component of normal hindfoot motion27,28.
In addition to quantifying the degree to which lateral ligament repairs and reconstructions improve hindfoot stability, it is important to characterize their ability to restore native load-transfer properties at the ankle joint because altered contact mechanics may be an important factor in the development of premature ankle osteoarthritis14. For example, following tenodesis procedures, there is an onset of radiographic findings consistent with osteoarthritis29,30 over a five to fifteen-year period after surgery31-36.
Ideally, lateral ankle ligament repair or reconstruction techniques should restore contact mechanics at the tibiotalar joint and should remove joint laxity without restricting ankle motion15. To examine the ability of these surgical techniques to achieve these goals, we defined four objectives for this study: (1) to determine whether inversion loading in the presence of ruptured lateral ligaments causes abnormal ankle contact mechanics, (2) to examine whether the Broström repair could restore normal hindfoot kinematics and ankle contact mechanics, (3) to assess whether the Gould modification of the Broström procedure provided additional hindfoot support without altering coupled hindfoot motion patterns and ankle contact mechanics, and (4) to determine whether graft reconstruction may be an alternative to direct repair by comparing hindfoot motion patterns and contact mechanics resulting from a graft reconstruction with those obtained with the Broström-Gould procedure.
Materials and Methods
Eight cadaveric lower limbs were utilized for the present study. To justify this sample size, we used previously reported data on one of our primary outcome measures: medial-lateral shift of center of pressure with inversion37. In that study, an inversion moment (3 Nm) with an axial load (600 N) caused 8.7 ± 1.1 mm of medial translation of the center of pressure in the native ankle joint with the foot placed at 0° of plantar flexion. Six specimens were required to detect differences of 25% with 80% power. This estimate included adjustment of our alpha value by the number of conditions in our study (α = 0.05/5 = 0.01).
Fresh-frozen specimens were thawed at room temperature for twelve hours prior to testing. Data on age and sex were not available for these specimens. Anterior arthrotomies were performed, and the articulating surfaces of the ankle joint were carefully inspected for any signs of cartilage degeneration. Specimens were excluded if any gross joint abnormalities, instability, or cartilage degeneration was observed. Skin and soft tissues were removed, with care being taken to avoid damage to the ligamentous and retinacular structures. The peroneal tendons were left intact in their sheath. Specimens were sectioned at the proximal part of the tibia and fibula, leaving approximately 25 cm of the shaft of each bone. Two syndesmotic screws were placed across the tibia and fibula proximally to stabilize the tibiofibular joint. The calcaneus was potted in bonding cement (Bondo/3M, Atlanta, Georgia) with use of three axial 7.3-mm hip screws. The proximal part of the tibia was also potted with use of the same material.
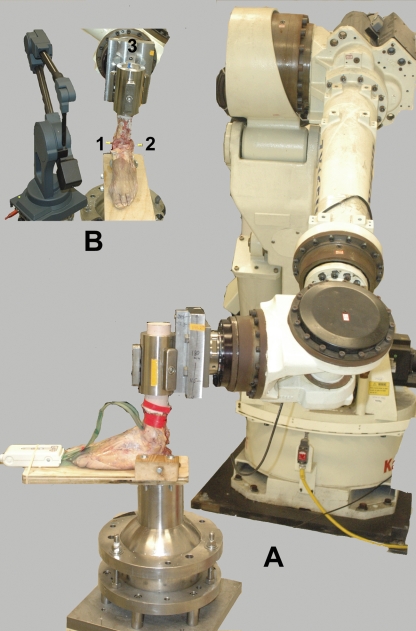
The limbs were loaded using a six-degrees-of-freedom robotic arm (ZX165U; Kawasaki Robotics, Wixom, Michigan) with 0.3-mm accuracy and ±0.3-mm repeatability38 (Fig. 1). A universal force-moment sensor (Delta; ATI, Apex, North Carolina) was mounted to the end of the robotic arm to enable measurement of the forces acting across the hindfoot complex. The potted calcaneus was secured to a pedestal that was fixed to the floor. The remainder of the foot rested on a platform that was attached to the pedestal. One additional screw was placed transversely in the calcaneal tuberosity and was fixed to the pedestal for additional stability. The tibia-fibula complex was attached to the robotic arm with use of a custom fixture. The specimen was aligned in neutral flexion, and reference frames were defined to describe motion of the hindfoot (motion of the calcaneus relative to the tibia-fibula complex) with use of a digitizer (MicroScribe; Immersion, San Jose, California) (Fig. 1) according to the standard description of hindfoot kinematics provided by the International Society of Biomechanics39. This is a commonly employed standard for describing hindfoot motion25,27,40 because axes correspond to anatomical directions (medial-lateral, anterior-posterior, superior-inferior); therefore, reported motions lend themselves well to clinical interpretation. Briefly, the intermalleolar axis defined dorsiflexion-plantar flexion and was directed laterally and medially for right and left specimens, respectively. The long axis of the tibia was directed superiorly and defined internal-external rotation. The common perpendicular of these two axes faced anteriorly and defined inversion-eversion.
Fig. 1.
A: The robotic system was used to load the hindfoot with use of a pressure-measurement sensor that was inserted into the ankle joint. B: The three-dimensional digitizer was used to define the coordinate system of the hindfoot. A line defined by Points 1 and 2 (medial and lateral malleoli) identifies the flexion axis, and a line defined by the midpoint of this axis and Point 3 (located at the center of the proximal part of the tibia) identifies the long axis of the tibia.
The loading conditions were 4.5 Nm of inversion with a 200-N axial compressive load at both 0° and 20° of plantar flexion. Inversion of the hindfoot at 0° of plantar flexion engages the calcaneofibular ligament, whereas inversion at 20° of plantar flexion loads the anterior talofibular ligament26. The robot control algorithm employs force feedback, allowing the tibia to move along the kinematic path that satisfies the desired loading conditions41. The control algorithm minimizes the difference between the current and desired loading conditions within prescribed tolerances (5 N and 0.5 Nm) with use of a Newton-Raphson numerical technique. The robot does not constrain hindfoot motion other than maintaining a fixed flexion angle. We did not constrain the motion of the talus relative to the fixed calcaneus at the subtalar joint in any direction. Thus, subtalar joint motion could occur in its native axis of rotation as governed by the articulating surface shapes and the ligamentous constraints. Outcome measures for kinematics included motion in the direction of the applied inversion moment and coupled axial rotation, defined as the rotation about the long axis of the tibia while loading the foot in inversion.
The motion path with all ligaments intact was obtained initially. The anterior talofibular ligament and the calcaneofibular ligament were then cut at their fibular insertions. Next, the repairs were performed, starting with the Broström procedure, followed by the Broström procedure with the Gould modification, and, finally, the free graft reconstruction procedure. Ligament sectioning, repair, and reconstruction were performed by one surgeon (V.R.P.) with the specimen in an unloaded neutral position.
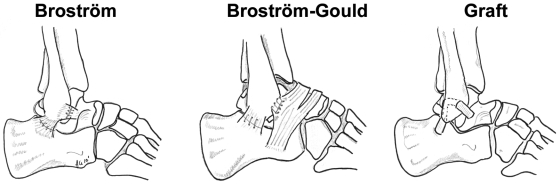
The Broström procedure was performed with use of a #1 braided nonabsorbable suture to reattach the ligaments to the fibula with use of transosseous horizontal mattress sutures. The sutures were placed about 5 mm from the cut edge of the ligament (Fig. 2). The Gould modification was performed by suturing the inferior extensor retinaculum to the fibular periosteum by means of two horizontal mattress #1 braided nonabsorbable sutures over the Broström repair for the anterior talofibular and calcaneofibular ligaments (Fig. 2). The retinaculum was sutured about 5 mm from its proximal edge.
Fig. 2.
Sketches illustrating the Broström (left), Broström-Gould (center), and graft reconstruction (right) surgical techniques.
After testing of the specimens after the Broström-Gould repair, the retinaculum and the ligaments were dissected off the fibula. The graft reconstruction was performed with use of a 5-mm-thick strip of the peroneus brevis to replace the anterior talofibular and calcaneofibular ligaments (Fig. 2). A 5-mm tunnel was drilled in the talus at the insertion of the native anterior talofibular ligament. The graft was secured to the talus with use of a 4.75-mm Bio-Tenodesis screw (Arthrex, Naples, Florida). Next, the graft was passed through a 5-mm fibular tunnel placed at the origin of the anterior talofibular ligament. This tunnel was curved posteriorly and inferiorly with a burr in an attempt to match the tunnel exit to the origin of the calcaneofibular ligament. With traction on the graft consistent with that used clinically, a second 4.75-mm biotenodesis screw was placed in the fibular tunnel. Another 5-mm tunnel was placed in the calcaneus, and the graft was secured in this tunnel with a third 4.75-mm Bio-Tenodesis screw.
Initially, the previously-recorded motion paths were repeated for the intact, sectioned, repaired, and reconstructed conditions for two cycles to precondition the soft tissues. Similar to the findings in a previous study26, however, the sutures began to pull through the tissues after multiple preconditioning cycles for the Broström and Broström-Gould repairs; therefore, hindfoot kinematics were measured from the first loading cycle.
After determination of the motion path for each condition, a pressure sensor that was specially designed to accommodate the size and shape of the ankle joint (5033; Tekscan, South Boston, Massachusetts) was inserted and was secured to the tibia with masking tape. Portions of the posterior capsule were removed to slide the pressure sensor through the joint space, although this did not affect ankle stability in inversion42. The sensor measures only the axial component of the loads acting on it. It was lubricated with petroleum jelly at the start of the experiment to minimize any artifact from shear forces37.
After insertion of the sensor, the motion path was repeated for each condition while pressure measurements were recorded. The pressure measurements were used to determine (1) translation of the center of pressure from the neutral to the loaded position in the anterior-posterior and medial-lateral directions, and (2) contact area, contact force, peak pressure, and mean pressure at the position corresponding to the target axial and inversion loads.
The pressure sensor was calibrated prior to testing by loading the sensor to 20% and 80% of the maximum expected load and then fitting the data with a two-parameter power function. This calibration technique yielded <3% root mean square error in pressure readings across the full sensing range43. The calibration accuracy was tested by loading the sensor in an MTS loading system (MTS Systems, Eden Prairie, Minnesota) with an Instron controller (8500; Instron, Norwood, Massachusetts) and a 444.8-N (100-lb) load cell (Interface, Scottsdale, Arizona) both after calibration and at least twice over the course of testing. If load measurements of the sensor diverged by >10% from that applied with use of the MTS loading system, the sensor was recalibrated.
Statistical Methods
All data sets were checked for normality with use of the Kolmogorov-Smirnov test and for equal variance. A one-way repeated-measures analysis of variance with the Student-Newman-Keuls post hoc test was used to determine if significant differences (p ≤ 0.05) emerged among conditions. In instances in which p values did not reach significance, 95% confidence intervals were examined to consider potentially important effects. Data that were not normally distributed were transformed to the logarithmic scale. These data were again checked to confirm that they achieved a normal distribution. Subsequently, the one-way repeated-measures analysis of variance and post hoc test described above were performed. The mean and 95% confidence interval of the transformed data were calculated. The inverse logarithm of these values was then determined to express the mean and 95% confidence interval on a linear scale.
Source of Funding
Salary support and funding for materials were partially supported by the National Institutes of Health-sponsored Clinical and Translational Science Center at Weill Cornell Medical College (KL2RR024997) and by the Clark and Kirby Foundations. Donation of surgical equipment by Arthrex is gratefully acknowledged.
Results
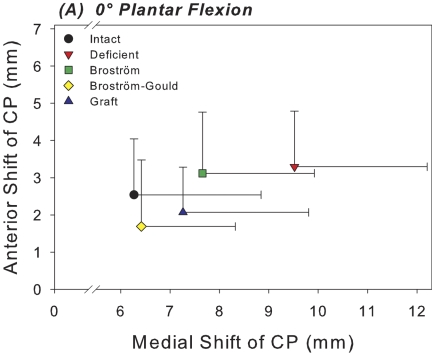
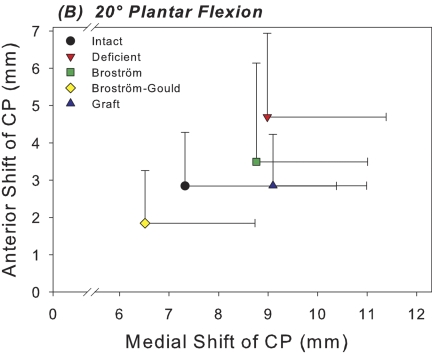
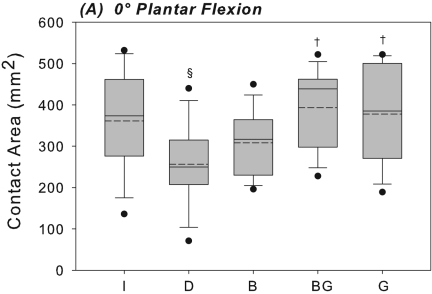
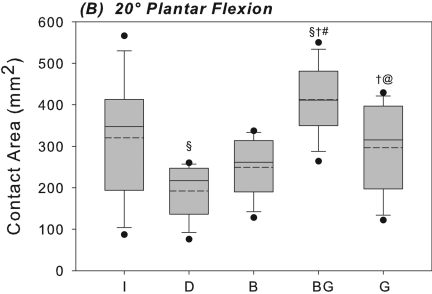
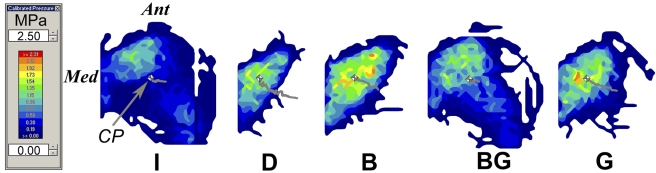
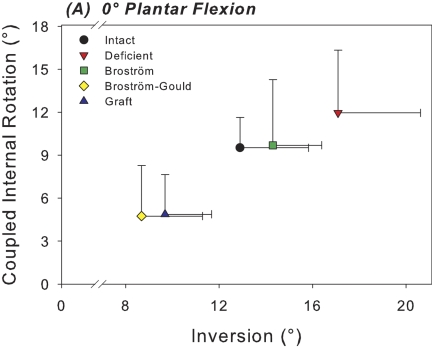
At 0° of plantar flexion, sectioning of the anterior talofibular and calcaneofibular ligaments caused a 52% increase in the medial shift of the center of tibiotalar pressure under inversion loading in comparison with the intact condition; this difference was significant (p = 0.003) (Fig. 3-A). This coincided with a 29% decrease in the tibiotalar contact area with the applied inversion moment at 0° of plantar flexion; this difference was also significant (p = 0.018) (Fig. 4-A). At 20° of plantar flexion, deficiency of the anterior talofibular and calcaneofibular ligaments caused a significant medial and anterior shift in the center of tibiotalar pressure, with increases of 23% (p = 0.039) and 65% (p = 0.035), respectively (Figs. 3-B and 5). This coincided with a 40% decrease in tibiotalar contact area; this difference was also significant (p = 0.008) (Figs. 4-B and 5). Contact forces on the talar dome decreased by 22% (p = 0.017) and 25% (p = 0.034) at 0° and 20° of plantar flexion, respectively (Table I). Ligament sectioning also increased hindfoot inversion at 0° and 20° of plantar flexion by 33% (p = 0.002) and 37% (p < 0.001), respectively (Figs. 6-A and 6-B).
Fig. 3-A Fig. 3-B.
Figs. 3-A and 3-B Scatter plots of the mean medial and anterior shift in the center of pressure (CP) for each of the five test conditions at 0° (Fig. 3-A) and 20° (Fig. 3-B) of plantar flexion. Horizontal and vertical whiskers indicate the standard deviations for the mean medial and anterior shift of the center of pressure, respectively.
Fig. 4-A Fig. 4-B.
Figs. 4-A and 4-B Box plots of the contact area at the ankle joint with the hindfoot inverted at 0° (Fig. 4-A) and at 20° (Fig. 4-B) of plantar flexion. The solid and dashed lines indicate the median and mean, respectively. The boxes indicate the quartiles, and the whiskers indicate the fifth and ninety-fifth percentiles. The dots indicate outliers outside of the fifth and ninety-fifth percentiles. The level of significance was set at p < 0.05. I = intact, D = deficient anterior talofibular and calcaneofibular ligaments, B = Broström, BG = Broström-Gould, and G = graft. §Significantly different from intact condition. †Significantly different from deficient condition. #Significantly different from Broström repair. @Significantly different from Broström-Gould repair.
Fig. 5.
Representative two-dimensional contour plot of contact pressure on the talar dome with the hindfoot in the inverted and axially loaded position at 20° of plantar flexion. The gray line in each image indicates the path of the center of pressure (CP) from the axially loaded position with no inversion moment to the axially loaded position with inversion moment. The gray and white box in each image indicates the location of the center of pressure under both axial and inversion loads. Ant = anterior, Med = medial, I = intact, D = deficient anterior talofibular and calcaneofibular ligaments, B = Broström, BG = Broström-Gould, and G = graft.
TABLE I.
Force on Talar Dome Under Inversion and Axial Loads*
| Force (N) |
|||||
| Intact | Deficient | Broström | Broström-Gould | Graft | |
| Neutral | 166.4 ± 29.8 | 130.5 ± 27.5† | 135.6 ± 40.3† | 158.5 ± 23.1‡§ | 164.1 ± 30.1‡§ |
| 20° plantar flexion | 149.6 ± 41.2 | 112.9 ± 15.8† | 133.2 ± 40.7 | 167.9 ± 38.9‡§ | 138.9 ± 35.5 |
The values are given as the mean and the standard deviation.
Significantly different from intact condition (p < 0.05).
Significantly different from deficient condition (p < 0.05).
Significantly different from Broström repair (p < 0.05).
Fig. 6-A Fig. 6-B.
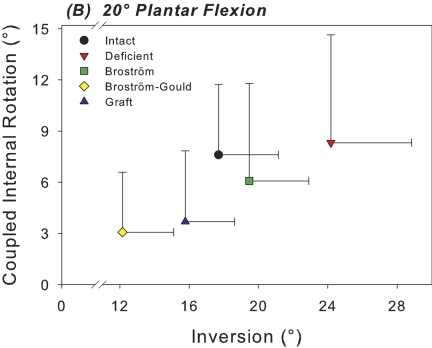
Figs. 6-A and 6-B Scatter plots of the mean inversion of the calcaneus relative to the tibia versus mean coupled internal rotation of the calcaneus with inversion for each test condition at 0° (Fig. 6-A) and 20° (Fig. 6-B) of plantar flexion. The horizontal and vertical whiskers indicate the standard deviations for mean inversion and coupled internal rotation, respectively.
The Broström repair significantly reduced medial shift in the center of tibiotalar pressure at 0° of plantar flexion by 20% in comparison with the ligament-deficient condition (p = 0.027) (Fig. 3-A). However, at 20° of plantar flexion, medial shift in the center of pressure was still 20% higher than the intact level (p = 0.033) (Fig. 3-B). There were no significant differences in contact area between the intact condition and the Broström repair condition at either flexion angle (Figs. 4-A and 4-B). The mean differences in tibiotalar contact area between the intact and Broström repair specimens were 52.9 mm2 (95% confidence interval, –7.6 to 113.4 mm2) and 70.9 mm2 (95% confidence interval, –19.1 to 160.8 mm2) at neutral and 20° of plantar flexion, respectively. A negative value for the mean difference and 95% confidence interval indicates a contact area greater than that of the intact condition. The Broström repair significantly decreased contact force on the talar dome with inversion by 19% relative to the intact condition at 0° (p = 0.034) (Table I).
The Broström repair significantly reduced hindfoot inversion relative to the ligament-deficient condition at both 0° (p = 0.017) and 20° of plantar flexion (p < 0.001) by 16% and 20%, respectively (Figs. 6-A and 6-B). There were no significant differences in hindfoot inversion with the Broström repair in comparison with the intact condition. The mean differences were –1.4° (95% confidence interval, –3.3° to 0.5°) and –1.8° (95% confidence interval, –5.0° to 1.4°) at 0° and 20° of plantar flexion, respectively. The mean differences in coupled axial rotation with inversion were –0.2° (95% confidence interval, –3.0° to 2.6°) and 1.5° (95% confidence interval, –1.7° to 4.7°) at 0° and 20° of plantar flexion, respectively. A negative value for the mean difference and confidence interval indicates hindfoot inversion and coupled axial rotation greater than the intact condition.
The Gould modification of the Broström repair showed no significant differences compared with the intact condition in terms of medial shift of the center of pressure at either flexion angle (Figs. 3-A, 3-B, and 5). The mean difference was –0.2 mm (95% confidence interval,–3.3 to 3.6 mm) and 0.8 mm (95% confidence interval, –2.7 to 5.5 mm) at 0° and 20° of plantar flexion, respectively (Figs. 3-A, 3-B, and 5). The Broström-Gould repair also showed no significant difference in terms of contact area under inversion loads in comparison with the intact condition at 0°, with a mean difference of –32.2 mm2 (95% confidence interval, –97.5 to 33.0 mm2) (Fig. 4-A). At 20° of plantar flexion, however, contact area increased significantly by 29% in comparison with the intact condition (p = 0.018) (Fig. 4-B).
The Gould modification also significantly reduced primary hindfoot inversion beyond that of the intact condition at both 0° and 20° of plantar flexion by 33% (p = 0.007) and 31% (p < 0.001), respectively (Figs. 6-A and 6-B). The Gould modification also further decreased inversion relative to the Broström repair at 0° and 20° by 39% (p < 0.001) and 38% (p < 0.001), respectively. The Gould modification of the Broström procedure also limited coupled axial rotation relative to the intact condition at both 0° and 20° of plantar flexion by 50% (p < 0.001) and 60% (p = 0.003), respectively (Figs. 6-A and 6-B). It also decreased coupled axial rotation relative to the Broström repair alone at 0° and 20° by 51% (p < 0.001) and 50% (p = 0.022), respectively.
Graft reconstruction did not significantly alter contact area from its intact value under inversion loading at either flexion angle. The mean differences were –16.9 mm2 (95% confidence interval, –136.6 to 102.9 mm2) and 24.1 mm2 (95% confidence interval, –83.0 to 131.3 mm2) at 0° and 20° of plantar flexion, respectively (Figs. 4-A and 4-B). Contact area was significantly less (28%) than that associated with the Broström-Gould repair at 20° (p = 0.01). Graft reconstruction did not restore medial shift of the center of pressure to intact levels at 20°, where medial shift was significantly greater (by 24%) relative to the intact condition (p = 0.046). Anterior shift of the center of pressure was not different from the intact condition at either flexion angle under inversion load, with mean differences of 0.5 mm (95% confidence interval, –0.8 to 1.7 mm) and –0.01 mm (95% confidence interval, –1.2 to 1.1 mm) at 0° and 20° of plantar flexion, respectively. No significant changes in peak or mean pressures were detected when comparing across any conditions (Table II).
TABLE II.
Peak and Mean Pressure on the Talar Dome Under Inversion and Axial Loads*
| Intact | Deficient | Broström | Broström-Gould | Graft | |
| Peak pressure (MPa) | |||||
| Neutral | 1.5 (1.1 to 2.1) | 1.6 (1.2 to 2.2) | 1.4 (1.1 to 1.7) | 1.3 (1.1 to 1.6) | 1.5 (1.1 to 2.0) |
| 20° plantar flexion | 1.5 (1.1 to 2.1) | 1.7 (1.4 to 2.2) | 1.5 (1.3 to 1.8) | 1.3 (1.1 to 1.4) | 1.5 (1.2 to 1.9) |
| Mean pressure (MPa) | |||||
| Neutral | 0.5 (0.3 to 0.7) | 0.5 (0.4 to 0.8) | 0.4 (0.4 to 0.5) | 0.4 (0.3 to 0.5) | 0.4 (0.4 to 0.5) |
| 20° plantar flexion | 0.5 (0.3 to 0.8) | 0.6 (0.4 to 0.9) | 0.5 (0.4 to 0.7) | 0.4 (0.4 to 0.5) | 0.5 (0.4 to 0.6) |
The values are given as the mean, with the 95% confidence interval in parentheses. No significant differences were detected.
Graft reconstruction significantly reduced hindfoot inversion relative to the intact condition by 25% at 0° of plantar flexion (p = 0.007) (Fig. 6-A). At 20°, there were no significant differences in range of motion of the hindfoot in inversion between the intact condition and after graft reconstruction, with a mean difference of 1.9° (95% confidence interval, –0.4° to 4.3°). At 20°, the graft reconstruction provided significantly greater hindfoot inversion (30%) than allowed by the Broström-Gould repair (p = 0.006) (Fig. 6-B). Graft reconstruction significantly limited coupled axial rotation relative to the intact condition at 0° and 20° by 49% (p < 0.001) and 52% (p = 0.003), respectively.
Discussion
In the present study, we assessed the effect of lateral ligament injury, direct surgical repair, and reconstruction techniques on contact mechanics at the ankle joint and on inversion and coupled axial rotation of the hindfoot under axial compression and inversion loading. None of the surgical techniques fully restored normal contact behavior and motion patterns in our in vitro model. As altered contact mechanics and abnormal motion patterns might contribute to the premature onset and progression of osteoarthritis44-48, these results provide a basis for long-term prospective and comparative in vivo studies that assess the role of these mechanical factors on the development of osteoarthritis after ligament injury, repair, and reconstruction.
The medial shift in the center of pressure at the tibiotalar joint under inversion loading is consistent with the findings of previous in vitro studies of contact mechanics at the ankle joint of the intact hindfoot37,49. The present study also demonstrated further medial shift of the center of pressure and decreased contact area with a lateral ligament deficit, a finding that may correlate with the development of medial osteochondral lesions of the talar dome4-10.
Similar to previous studies26,50, we showed that direct repair of the ankle ligaments may restore motion patterns of the hindfoot. However, this repair did not completely restore contact mechanics. The possible restoration of motion patterns might suggest more aggressive early operative treatment of grade-3 ligament injuries50, although this is still a topic of debate51.
Additional support of the hindfoot in inversion when the Gould modification is added to the Broström repair is consistent with previously reported data17. Although the Broström-Gould repair limited primary and coupled motions beyond that of the intact condition, the additional stability that it provides might be responsible for the improved subjective satisfaction and feelings of stability reported by patients managed with this repair16.
Other previous studies have presented conflicting results with regard to the success of the Broström-Gould procedure. An in vitro biomechanical study24 demonstrated that the Gould modification restored native range of motion in inversion. In contrast, an in vivo study52 demonstrated that the Gould modification limited inversion by 4.5° compared with that in the uninjured, contralateral limb. We also found that the Gould modification limited primary motion of the hindfoot in inversion at 0° of plantar flexion to a similar extent (4.2°). Differences between the in vitro study and the current study may be attributable to more aggressive recruitment of the retinacular tissues when performing the Broström-Gould repair. Furthermore, we did not precondition the repair constructs to avoid damaging them, as has been previously reported26. In contrast, Fujii et al.24 tested their specimens with two trials of both inversion and internal rotation, which may have compromised the repair. Overall, direct comparison between previous in vitro studies15,24 and the current study is difficult as an axial load was not applied in the other studies.
The Broström-Gould repair and graft reconstruction limited primary motions, compared with the native hindfoot complex, at neutral ankle flexion. In contrast, the graft reconstruction may restore the native level of inversion at 20° of plantar flexion, whereas the Gould modification did not. A previous study of graft reconstruction15 also demonstrated that this technique could restore range of motion of the hindfoot in inversion.
The tendency of the graft reconstruction to limit coupled axial rotation with inversion may be caused by greater stiffness of the graft tissue compared with the native anterior talofibular ligament and calcaneofibular ligament53. This may indicate the importance of matching graft stiffness to that of native tissues. Limited hindfoot coupling also suggests altered motion patterns at the ankle joint or the subtalar joint; however, this was not the focus of the present study. Furthermore, the graft reconstruction may restore the native contact mechanics of the ankle joint more closely than the Broström procedure alone and avoids the propensity of the Gould modification to increase contact areas beyond that of the intact status. This finding lends support to the use of free tendon graft reconstructions for repairing lateral ankle ligaments.
In the presence of chronic instability, the remaining lateral ligaments may be degraded or insufficient; thus, primary repairs may not be possible. In this situation, a graft reconstruction to approximate the anatomy of the anterior talofibular ligament and calcaneofibular ligament may be an alternative as we showed that graft reconstruction increased hindfoot stability in inversion after sectioning of the lateral ligaments. However, graft reconstruction limited coupled axial rotation of the hindfoot and increased medial shift in the center of pressure at 20° of plantar flexion relative to the intact condition. Therefore, additional research must be done to determine appropriate graft tissue source, size, positioning, and tensioning to most closely restore hindfoot motion and contact patterns.
The tandem decrease in both contact area and load across the dome of the talus likely explains why pressure measurements were not significantly different after sectioning of the lateral ligaments, even in the presence of significantly decreased contact areas. The decrease in load with ligament sectioning under an inversion moment is not surprising as load is shared by the talar dome and the medial plafond of the talus in the native ankle joint37,49. Ligament injury increased inversion, which decreased load on the talar dome and likely increased load on the medial plafond. In contrast, the surgical procedures reduced inversion, causing the opposite effect.
Despite wide population variability in articular surface and ligament morphology, our repeated-measures study design controlled for this variability as a potential confounding factor as each specimen acted as its own control. Similarly, the repeated-measures study design controlled for variability in bone and soft-tissue quality across specimens. Overall, the use of a cadaveric model limits clinical interpretation of our results. Time and load-dependent biological phenomena such as scarring and healing may confer more or less stability to the repairs over time and cannot be studied in a cadaveric model. In vivo studies of joint function, similar to previous studies25,40,54,55, are needed to overcome this limitation.
In conclusion, lateral ligament deficiency altered contact behavior at the ankle joint and motion patterns of the hindfoot, whereas none of the surgical techniques fully restored normal contact and motion in our in vitro model. As altered contact mechanics and motion patterns might contribute to the onset and progression of premature ankle osteoarthritis, our results provide a rationale for conducting long-term prospective and comparative in vivo studies to assess the impact of lateral ligament injury, and its nonoperative and operative treatment, on hindfoot mechanics as well as on the development of ankle osteoarthritis.
Acknowledgments
Note: This research was partially supported by the National Institutes of Health-sponsored Clinical and Translational Science Center at Weill Cornell Medical College (KL2RR024997) and by the Clark and Kirby Foundations. The authors gratefully acknowledge the donation of test specimens by Dr. Jonathan Deland and manuscript editing by Dr. Timothy Wright. The current investigators would also like to thank Arthrex for donating a biotenodesis set for the study (Bio-Tenodesis Screw System; Arthrex, Naples, Florida).
Footnotes
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the Clinical and Translational Science Center at Weill Cornell Medical College (#KL2RR024997) and of less than $10,000 from the Clark and Kirby Foundations. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Broström L. Sprained ankles. 3. Clinical observations in recent ligament ruptures. Acta Chir Scand. 1965;130:560-9 [PubMed] [Google Scholar]
- 2.Broström L. Sprained ankles. V. Treatment and prognosis in recent ligament ruptures. Acta Chir Scand. 1966;132:537-50 [PubMed] [Google Scholar]
- 3.Garrick JG, Requa RK. Role of external support in the prevention of ankle sprains. Med Sci Sports. 1973;5:200-3 [PubMed] [Google Scholar]
- 4.Elias I, Zoga AC, Morrison WB, Besser MP, Schweitzer ME, Raikin SM. Osteochondral lesions of the talus: localization and morphologic data from 424 patients using a novel anatomical grid scheme. Foot Ankle Int. 2007;28:154-61 [DOI] [PubMed] [Google Scholar]
- 5.Harrington KD. Degenerative arthritis of the ankle secondary to long-standing lateral ligament instability. J Bone Joint Surg Am. 1979;61:354-61 [PubMed] [Google Scholar]
- 6.Hintermann B, Boss A, Schäfer D. Arthroscopic findings in patients with chronic ankle instability. Am J Sports Med. 2002;30:402-9 [DOI] [PubMed] [Google Scholar]
- 7.Taga I, Shino K, Inoue M, Nakata K, Maeda A. Articular cartilage lesions in ankles with lateral ligament injury. An arthroscopic study. Am J Sports Med. 1993;21:120-7 [DOI] [PubMed] [Google Scholar]
- 8.Takao M, Ochi M, Uchio Y, Naito K, Kono T, Oae K. Osteochondral lesions of the talar dome associated with trauma. Arthroscopy. 2003;19:1061-7 [DOI] [PubMed] [Google Scholar]
- 9.Valderrabano V, Hintermann B, Horisberger M, Fung TS. Ligamentous posttraumatic ankle osteoarthritis. Am J Sports Med. 2006;34:612-20 [DOI] [PubMed] [Google Scholar]
- 10.van Dijk CN, Bossuyt PM, Marti RK. Medial ankle pain after lateral ligament rupture. J Bone Joint Surg Br. 1996;78:562-7 [PubMed] [Google Scholar]
- 11.Tol JL, Struijs PA, Bossuyt PM, Verhagen RA, van Dijk CN. Treatment strategies in osteochondral defects of the talar dome: a systematic review. Foot Ankle Int. 2000;21:119-26 [DOI] [PubMed] [Google Scholar]
- 12.Schenck RC, Jr, Goodnight JM. Osteochondritis dissecans. J Bone Joint Surg Am. 1996;78:439-56 [PubMed] [Google Scholar]
- 13.Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35:1680-7 [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum D, Bertsch C, Claes LE. NOVEL Award 1996: 2nd prize. Tenodeses do not fully restore ankle joint loading characteristics: a biomechanical in vitro investigation in the hind foot. Clin Biomech (Bristol, Avon). 1997;12:202-9 [DOI] [PubMed] [Google Scholar]
- 15.Schmidt R, Cordier E, Bertsch C, Eils E, Neller S, Benesch S, Herbst A, Rosenbaum D, Claes L. Reconstruction of the lateral ligaments: do the anatomical procedures restore physiologic ankle kinematics? Foot Ankle Int. 2004;25:31-6 [DOI] [PubMed] [Google Scholar]
- 16.Hamilton WG, Thompson FM, Snow SW. The modified Brostrom procedure for lateral ankle instability. Foot Ankle. 1993;14:1-7 [DOI] [PubMed] [Google Scholar]
- 17.Aydogan U, Glisson RR, Nunley JA. Extensor retinaculum augmentation reinforces anterior talofibular ligament repair. Clin Orthop Relat Res. 2006;442:210-5 [DOI] [PubMed] [Google Scholar]
- 18.Coughlin MJ, Schenck RC, Jr, Grebing BR, Treme G. Comprehensive reconstruction of the lateral ankle for chronic instability using a free gracilis graft. Foot Ankle Int. 2004;25:231-41 [DOI] [PubMed] [Google Scholar]
- 19.Sugimoto K, Takakura Y, Kumai T, Iwai M, Tanaka Y. Reconstruction of the lateral ankle ligaments with bone-patellar tendon graft in patients with chronic ankle instability: a preliminary report. Am J Sports Med. 2002;30:340-6 [DOI] [PubMed] [Google Scholar]
- 20.Takao M, Komatsu F, Naito K, Uchio Y, Ochi M. Reconstruction of lateral ligament with arthroscopic drilling for treatment of early-stage osteoarthritis in unstable ankles. Arthroscopy. 2006;22:1119-25 [DOI] [PubMed] [Google Scholar]
- 21.Karlsson J, Bergsten T, Lansinger O, Peterson L. Reconstruction of the lateral ligaments of the ankle for chronic lateral instability. J Bone Joint Surg Am. 1988;70:581-8 [PubMed] [Google Scholar]
- 22.Colville MR, Grondel RJ. Anatomic reconstruction of the lateral ankle ligaments using a split peroneus brevis tendon graft. Am J Sports Med. 1995;23:210-3 [DOI] [PubMed] [Google Scholar]
- 23.Takao M, Oae K, Uchio Y, Ochi M, Yamamoto H. Anatomical reconstruction of the lateral ligaments of the ankle with a gracilis autograft: a new technique using an interference fit anchoring system. Am J Sports Med. 2005;33:814-23 [DOI] [PubMed] [Google Scholar]
- 24.Fujii T, Kitaoka HB, Watanabe K, Luo ZP, An KN. Comparison of modified Broström and Evans procedures in simulated lateral ankle injury. Med Sci Sports Exerc. 2006;38:1025-31 [DOI] [PubMed] [Google Scholar]
- 25.Ringleb SI, Udupa JK, Siegler S, Imhauser CW, Hirsch BE, Liu J, Odhner D, Okereke E, Roach N. The effect of ankle ligament damage and surgical reconstructions on the mechanics of the ankle and subtalar joints revealed by three-dimensional stress MRI. J Orthop Res. 2005;23:743-9 [DOI] [PubMed] [Google Scholar]
- 26.Bahr R, Pena F, Shine J, Lew WD, Tyrdal S, Engebretsen L. Biomechanics of ankle ligament reconstruction. An in vitro comparison of the Broström repair, Watson-Jones reconstruction, and a new anatomic reconstruction technique. Am J Sports Med. 1997;25:424-32 [DOI] [PubMed] [Google Scholar]
- 27.Lapointe SJ, Siegler S, Hillstrom H, Nobilini RR, Mlodzienski A, Techner L. Changes in the flexibility characteristics of the ankle complex due to damage to the lateral collateral ligaments: an in vitro and in vivo study. J Orthop Res. 1997;15:331-41 [DOI] [PubMed] [Google Scholar]
- 28.Reinschmidt C, van den Bogert AJ, Lundberg A, Nigg BM, Murphy N, Stacoff A, Stano A. Tibiofemoral and tibiocalcaneal motion during walking: external vs. skeletal markers. Gait Posture. 1997;6:98-109 [Google Scholar]
- 29.Karlsson J, Bergsten T, Lansinger O, Peterson L. Lateral instability of the ankle treated by the Evans procedure. A long-term clinical and radiological follow-up. J Bone Joint Surg Br. 1988;70:476-80 [DOI] [PubMed] [Google Scholar]
- 30.Labs K, Perka C, Lang T. Clinical and gait-analytical results of the modified Evans tenodesis in chronic fibulotalar ligament instability. Knee Surg Sports Traumatol Arthrosc. 2001;9:116-22 [DOI] [PubMed] [Google Scholar]
- 31.Kaikkonen A, Lehtonen H, Kannus P, Järvinen M. Long-term functional outcome after surgery of chronic ankle instability. A 5-year follow-up study of the modified Evans procedure. Scand J Med Sci Sports. 1999;9:239-44 [DOI] [PubMed] [Google Scholar]
- 32.Krips R, van Dijk CN, Halasi PT, Lehtonen H, Corradini C, Moyen B, Karlsson J. Long-term outcome of anatomical reconstruction versus tenodesis for the treatment of chronic anterolateral instability of the ankle joint: a multicenter study. Foot Ankle Int. 2001;22:415-21 [DOI] [PubMed] [Google Scholar]
- 33.Krips R, van Dijk CN, Halasi T, Lehtonen H, Moyen B, Lanzetta A, Farkas T, Karlsson J. Anatomical reconstruction versus tenodesis for the treatment of chronic anterolateral instability of the ankle joint: a 2- to 10-year follow-up, multicenter study. Knee Surg Sports Traumatol Arthrosc. 2000;8:173-9 [DOI] [PubMed] [Google Scholar]
- 34.Krips R, van Dijk CN, Lehtonen H, Halasi T, Moyen B, Karlsson J. Sports activity level after surgical treatment for chronic anterolateral ankle instability. A multicenter study. Am J Sports Med. 2002;30:13-9 [DOI] [PubMed] [Google Scholar]
- 35.Rosenbaum D, Becker HP, Sterk J, Gerngross H, Claes L. Functional evaluation of the 10-year outcome after modified Evans repair for chronic ankle instability. Foot Ankle Int. 1997;18:765-71 [DOI] [PubMed] [Google Scholar]
- 36.Sugimoto K, Takakura Y, Akiyama K, Kamei S, Kitada C, Kumai T. Long-term results of Watson-Jones tenodesis of the ankle. Clinical and radiographic findings after ten to eighteen years of follow-up. J Bone Joint Surg Am. 1998;80:1587-96 [DOI] [PubMed] [Google Scholar]
- 37.Tochigi Y, Rudert MJ, Saltzman CL, Amendola A, Brown TD. Contribution of articular surface geometry to ankle stabilization. J Bone Joint Surg Am. 2006;88:2704-13 [DOI] [PubMed] [Google Scholar]
- 38.C series controller operations and programming manual. Wixom: Kawasaki Robotics (USA), Inc, Training and Documentation Department; 1999. p I9-11 [Google Scholar]
- 39.Wu G, Siegler S, Allard P, Kirtley C, Leardini A, Rosenbaum D, Whittle M, D'Lima DD, Cristofolini L, Witte H, Schmid O, Stokes I; Standardization and Terminology Committee of the International Society of Biomechanics ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion—part I: ankle, hip, and spine. International Society of Biomechanics. J Biomech. 2002;35:543-8 [DOI] [PubMed] [Google Scholar]
- 40.Siegler S, Lapointe S, Nobilini R, Berman AT. A six-degrees-of-freedom instrumented linkage for measuring the flexibility characteristics of the ankle joint complex. J Biomech. 1996;29:943-7 [DOI] [PubMed] [Google Scholar]
- 41.Fujie H, Mabuchi K, Woo SL, Livesay GA, Arai S, Tsukamoto Y. The use of robotics technology to study human joint kinematics: a new methodology. J Biomech Eng. 1993;115:211-7 [DOI] [PubMed] [Google Scholar]
- 42.Fitzpatrick DC, Otto JK, McKinley TO, Marsh JL, Brown TD. Kinematic and contact stress analysis of posterior malleolus fractures of the ankle. J Orthop Trauma. 2004;18:271-8 [DOI] [PubMed] [Google Scholar]
- 43.Brimacombe JM, Wilson DR, Hodgson AJ, Ho KC, Anglin C. Effect of calibration method on Tekscan sensor accuracy. J Biomech Eng. 2009;131:034503. [DOI] [PubMed] [Google Scholar]
- 44.Chen CT, Bhargava M, Lin PM, Torzilli PA. Time, stress, and location dependent chondrocyte death and collagen damage in cyclically loaded articular cartilage. J Orthop Res. 2003;21:888-98 [DOI] [PubMed] [Google Scholar]
- 45.Chen CT, Burton-Wurster N, Lust G, Bank RA, Tekoppele JM. Compositional and metabolic changes in damaged cartilage are peak-stress, stress-rate, and loading-duration dependent. J Orthop Res. 1999;17:870-9 [DOI] [PubMed] [Google Scholar]
- 46.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619-36 [DOI] [PubMed] [Google Scholar]
- 47.Torzilli PA, Deng XH, Ramcharan M. Effect of compressive strain on cell viability in statically loaded articular cartilage. Biomech Model Mechanobiol. 2006;5:123-32 [DOI] [PubMed] [Google Scholar]
- 48.Torzilli PA, Grigiene R, Borrelli J, Jr, Helfet DL. Effect of impact load on articular cartilage: cell metabolism and viability, and matrix water content. J Biomech Eng. 1999;121:433-41 [DOI] [PubMed] [Google Scholar]
- 49.Calhoun JH, Li F, Ledbetter BR, Viegas SF. A comprehensive study of pressure distribution in the ankle joint with inversion and eversion. Foot Ankle Int. 1994;15:125-33 [DOI] [PubMed] [Google Scholar]
- 50.Cass JR, Morrey BF, Katoh Y, Chao EY. Ankle instability: comparison of primary repair and delayed reconstruction after long-term follow-up study. Clin Orthop Relat Res. 1985;198:110-7 [PubMed] [Google Scholar]
- 51.Kerkhoffs GM, Handoll HH, de Bie R, Rowe BH, Struijs PA. Surgical versus conservative treatment for acute injuries of the lateral ligament complex of the ankle in adults. Cochrane Database Syst Rev. 2007;2:CD000380. [DOI] [PubMed] [Google Scholar]
- 52.Messer TM, Cummins CA, Ahn J, Kelikian AS. Outcome of the modified Broström procedure for chronic lateral ankle instability using suture anchors. Foot Ankle Int. 2000;21:996-1003 [DOI] [PubMed] [Google Scholar]
- 53.Attarian DE, McCrackin HJ, Devito DP, McElhaney JH, Garrett WE., Jr A biomechanical study of human lateral ankle ligaments and autogenous reconstructive grafts. Am J Sports Med. 1985;13:377-81 [DOI] [PubMed] [Google Scholar]
- 54.Caputo AM, Lee JY, Spritzer CE, Easley ME, DeOrio JK, Nunley JA, 2nd, DeFrate LE. In vivo kinematics of the tibiotalar joint after lateral ankle instability. Am J Sports Med. 2009;37:2241-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Asla RJ, Wan L, Rubash HE, Li G. Six DOF in vivo kinematics of the ankle joint complex: application of a combined dual-orthogonal fluoroscopic and magnetic resonance imaging technique. J Orthop Res. 2006;24:1019-27 [DOI] [PubMed] [Google Scholar]