Abstract
Background:
Modulation of the mechanical environment may profoundly affect the healing tendon graft-bone interface. The purpose of this study was to determine how controlled axial loading after anterior cruciate ligament reconstruction affects tendon-to-bone healing. Our hypothesis was that controlled cyclic axial loading after a period of immobilization would improve tendon-to-bone healing compared with that associated with immediate axial loading or prolonged immobilization.
Methods:
One hundred and fifty-six male Sprague-Dawley rats underwent anterior cruciate ligament reconstruction with use of a flexor digitorum longus autograft. A custom-designed fixture was used to apply an external fixator across the knee parallel to the anterior cruciate ligament graft. Animals were randomly assigned to be treated with immobilization (n = 36) or controlled knee distraction along the long axis of the graft to achieve approximately 2% axial strain beginning (1) immediately postoperatively (n = 36), (2) on postoperative day 4 (“early delayed loading,” n = 42), or (3) on postoperative day 10 (“late delayed loading,” n = 42). The animals were killed at fourteen or twenty-eight days postoperatively for biomechanical testing, micro-computed tomography, and histomorphometric analysis of the bone-tendon-bone complex. Data were analyzed with use of a two-way analysis of variance followed by a post hoc Tukey test with p < 0.05 defined as significant.
Results:
Delayed initiation of cyclic axial loading on postoperative day 10 resulted in a load to failure of the femur-anterior cruciate ligament-tibia complex at two weeks that was significantly greater than that resulting from immediate loading or prolonged immobilization of the knee (mean and standard deviation, 9.6 ± 3.3 N versus 4.4 ± 2.3 N and 4.4 ± 1.5 N, respectively; p < 0.01). The new-bone formation observed in the tibial tunnels of the delayed-loading groups was significantly increased compared with that in the immediate-loading and immobilization groups at both two and four weeks postoperatively (1.47 ± 0.11 mm3 [postoperative-day-10 group] versus 0.89 ± 0.30 mm3 and 0.85 ± 0.19 mm3, respectively, at two weeks; p < 0.003). There were significantly fewer ED1+ inflammatory macrophages and significantly more ED2+ resident macrophages at the healing tendon-bone interface in both delayed-loading groups compared with the counts in the immediate-loading and immobilization groups at two and four weeks (2.97 ± 0.7 [postoperative day 10] versus 1.14 ± 0.47 and 1.71 ± 1.5 ED2+ cells, respectively, per high-power field at two weeks; p < 0.02). The numbers of osteoclasts in the delayed-loading groups were significantly lower than those in the immediate-loading and immobilization groups at two and four weeks postoperatively (0.35 ± 0.15 [postoperative-day-10 group] versus 1.02 ± 0.08 and 1.44 ± 0.2 cells, respectively, per high-power field at two weeks; p < 0.01), and the delayed-loading groups also had significantly reduced interface tissue vascularity compared with the other groups (p < 0.003).
Conclusions:
Delayed application of cyclic axial load after anterior cruciate ligament reconstruction resulted in improved mechanical and biological parameters of tendon-to-bone healing compared with those associated with immediate loading or prolonged postoperative immobilization of the knee.
Clinical Relevance:
This study of anterior cruciate ligament reconstruction may have important implications for rehabilitation after soft-tissue reconstructive procedures in the knee. Controlled mechanical loads after a delay to allow resolution of acute postoperative inflammation may be most favorable to the healing enthesis.
The healing of tendon to bone is a basic requirement for the long-term survival of an anterior cruciate ligament reconstruction. The weakest link following reconstruction is not the graft itself, but rather the fixation between the graft and the bone until graft osseointegration occurs1-4. Current techniques for anterior cruciate ligament reconstruction all require tendon-to-bone healing in a surgically created bone tunnel. Nonetheless, the biological and mechanical influences in this unique healing environment remain incompletely understood1-4, despite the importance of understanding these relationships when establishing optimal rehabilitation protocols.
Previous studies have demonstrated that mechanical loading plays a critical role in maintaining the homeostasis of native musculoskeletal tissues5-12. Uninjured ligaments and tendons as well as their entheses are sensitive to their mechanical environment and generally demonstrate an increase in their tensile modulus in response to increasing loads8. In contrast, stress deprivation is associated with a decline in mechanical properties5-14. The influence of mechanical loads on a healing enthesis, however, remains unclear, and studies have been limited by an inability to quantify or control the loads applied to the tendon-bone interface11-14. Therefore, the biological response to known amounts and types of applied mechanical stimulus is unknown, and comparison across studies in the literature is difficult.
We developed a novel rat model of anterior cruciate ligament reconstruction using an external fixator that allows controlled cyclic axial loading of the healing tendon-bone interface15. Unlike previous models, this approach allows control of the onset, frequency, duration, and magnitude of the external loading environment on the healing bone-graft-bone construct. Our long-term goal is to determine the effect of mechanical load on healing between soft tissue and bone. The purpose of this study was to ascertain how controlled axial loading after anterior cruciate ligament reconstruction affects tendon-to-bone healing. Our overall hypothesis was that controlled cyclic axial loading after a period of immobilization would improve tendon-to-bone healing after anterior cruciate ligament reconstruction compared with that resulting from immediate axial loading or prolonged immobilization. Our specific hypotheses were that delayed loading would lead to (1) less inflammatory cell accumulation at the healing tendon-bone interface, (2) less angiogenesis, (3) increased new-bone formation with less osteoclast accumulation, and (4) increased load to failure and stiffness at the healing enthesis.
Materials and Methods
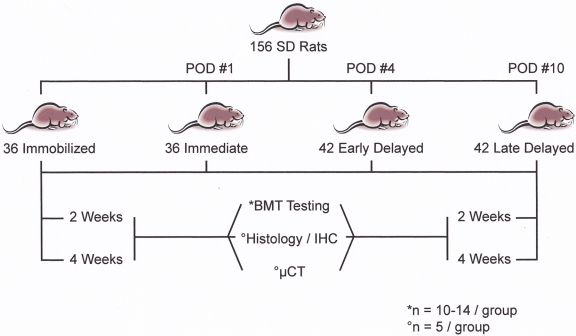
After refining the surgical technique by performing it in cadaveric animals, we carried out an anterior cruciate ligament reconstruction with use of a flexor digitorum longus autograft in 156 adult, male Sprague-Dawley rats (weight, 250 to 350 g; age, less than three months). The rats were randomly assigned to one of four postoperative regimens: immobilization (n = 36) or loading beginning (1) immediately postoperatively (n = 36), (2) on postoperative day 4 (“early delayed loading,” n = 42), or (3) on postoperative day 10 (“late delayed loading,” n = 42). The loading regimen was cycled under displacement control of the knee joint distraction along the long axis of the tendon graft to achieve approximately 2% axial strain. The animals were killed at fourteen or twenty-eight days postoperatively for biomechanical testing (n = 10 to 14 per group), micro-computed tomography (n = 5 per group), and histomorphometric analysis (n = 5 per group) of the bone-tendon-bone complex (Fig. 1). All animal procedures were approved by the animal care and use committee at the Hospital for Special Surgery.
Fig. 1.
Study design. POD = postoperative day, SD = Sprague-Dawley, BMT = biomechanical testing, IHC = immunohistochemistry, and μCT = micro-computed tomography.
Surgical Technique
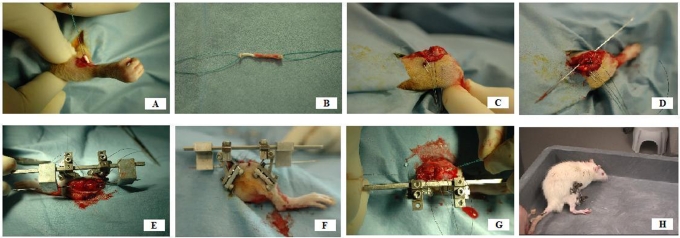
The surgical procedure was done on the right lower limb of the rat after induction of general anesthesia and surgical preparation. A flexor digitorum longus autograft was harvested from the ipsilateral limb through a small posteromedial ankle incision and midline plantar foot incision. The tendon was stripped of attached muscle, and a 4-0 Ethibond locking Krackow suture (Ethicon, Somerville, New Jersey) was placed in each end of the tendon (Fig. 2). A midline incision was made over the knee, and a medial parapatellar arthrotomy was performed. The patellar tendon, collateral ligaments, anterior and posterior cruciate ligaments, and anterior aspect of the capsule were all sectioned, and the ligament stumps were resected from the joint. The posterior aspect of the capsule was bluntly elevated and was detached from the posterior aspect of the tibia but not divided. After the tibiofemoral joint was confirmed to be completely free of restraint (except for the posterior musculature and neurovascular bundle), the tibiofemoral joint was flexed to approximately 60°. A Keith needle (1.2 mm in diameter) was used to drill the tibial and femoral tunnels with use of a transtibial technique, with care taken to capture the tibial and femoral anterior cruciate ligament footprints. The Keith needle was left in place across the knee and used to guide parallel alignment of the external fixator. The external fixator was aligned with use of custom-fabricated jigs that were positioned on the Keith needle, allowing the external fixator bar to be secured parallel to the bone tunnels and anterior cruciate ligament graft (Fig. 2). Custom-designed connectors were fixed to the threaded pins and the fixation bar and could be interfaced to the loading device for daily cyclic loading (described below). To place the external fixator, two parallel 0.9-mm threaded pins (1600-635T Kirschner wire; MicroAire Surgical Instruments, Charlottesville, Virginia) were placed centrally into the distal part of the femur from the lateral side through separate stab incisions spaced approximately 5 mm apart. The external fixator was then secured to the femoral pins and used to guide the insertion of two 0.9-mm threaded pins in the central-proximal part of the tibia from the lateral side. The entire external fixator was then tightly secured to maintain the fixator bar in a position parallel to the Keith needle and eventually to the anterior cruciate ligament graft (Fig. 2). The Keith needle was then used to pull the flexor digitorum longus autograft tendon through the bone tunnels, replacing the native anterior cruciate ligament. The ends of the tendon graft were manually tensioned and were secured to the periosteum and surrounding fascia of the distal part of the femur and proximal part of the tibia with 3-0 Ethibond suture. The length of the graft was measured with use of calipers from the extra-articular entrance of the tibial tunnel to the exit of the femoral tunnel, and the wound was closed in a standard layered fashion.
Fig. 2.
Surgical technique. The flexor digitorum longus tendon is percutaneously harvested from the ipsilateral rat limb (A) and is prepared with locking Krackow stitches at each end (B). A medial parapatellar arthrotomy is performed, and all capsular and ligamentous restraints are circumferentially released (C). The tibial and femoral anterior cruciate ligament footprints are identified and are captured centrally with a Keith needle via a transtibial technique (D). The Keith needle is used to guide the placement of an external fixator across the knee joint parallel to the drilled tunnels for the graft (E). The fixator is secured across the knee with use of threaded 0.9-mm Kirschner wires (F). With the fixator secure, the graft is pulled into position with the Keith needle (G). The graft is then secured proximally and distally with suspensory fixation to the adjacent periosteum. The animals showed that they tolerated the procedure well during cage activity postoperatively (H).
Description of Loading Device
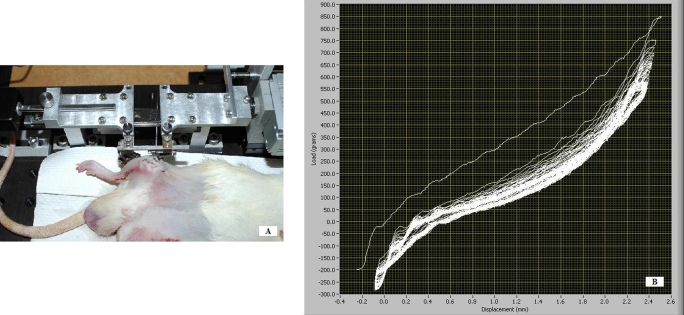
A custom computer-controlled loading device that could be secured to the connectors of the external fixator was used. After the connectors were secured to the loading device, the fixation bar was removed. A computer-controlled stepper motor directed the loading device to apply axial distraction or compression to the tibiofemoral joint, parallel to the graft tunnels, thus enabling the application of axial strain to the graft. The loading device recorded the resulting load-displacement response (Fig. 3).
Fig. 3.
A: A novel computer-controlled loading device was developed to interface with the connectors of the external fixator and apply axial distraction or compression to the tibiofemoral joint, parallel to the graft tunnels, thereby enabling the application of axial strain to the graft. B: The loading protocol was performed daily in the treatment group for fifty cycles, allowing load-displacement curves to be monitored and recorded on a daily basis in the postoperative period until the animals were killed.
Daily Loading Protocol
In a separate pilot study, the applied knee distraction was quantified with use of an optical tracking system (ProReflex MCU; Qualisys, Gothenburg, Sweden) (accuracy = 50 μm) after anterior cruciate ligament reconstruction in nine cadaveric animals. Reflective markers were placed on the graft at the graft tunnel exits of both the femur and the tibia. As the distraction device pulled the knee joint apart at a frequency of 0.05 Hz according to the experimental protocol, the cameras tracked the three-dimensional spatial position of the two markers. The total distraction distance was then calculated as the magnitude of the distance between the markers at maximal load. Subsequently, the slack distance was defined in each animal by sectioning all surrounding tissues except for the graft and determining the distance necessary for the isolated bone-graft-bone construct to achieve 30 g of load. The mean measured initial slack length (and standard deviation) was 0.55 ± 0.23 mm while the mean knee distraction was 0.88 ± 0.33 mm. Therefore, the mean graft elongation was 2.2% when normalized to the average graft length of 15 mm (measured from the femoral end fixation to the tibial end fixation).
The loading protocol was defined on the basis of this information and then was performed daily in the treatment group for fifty cycles. After induction of 2% isoflurane inhalation anesthesia, the external fixator was connected to the loading device. The amount of graft elongation was chosen on the basis of previous studies demonstrating 2% to 5% elongation of both the native anterior cruciate ligament and anterior cruciate ligament grafts in the human knee during light rehabilitation exercises such as pedaling on a stationary bicycle16-18. A compressive force of 1.96 N (200 g) was first applied across the joint to establish a consistent starting position. The joint was then distracted at 0.24 mm/sec until the tibia and femur reached the total distraction distance as defined by the evaluation experiments described above and was then returned to the starting position for a total of fifty cycles at a frequency of 0.05 Hz. The resulting load-displacement response was recorded for each cycle. The entire loading protocol required approximately twenty minutes of anesthesia daily, which was well tolerated by all animals. After completion of the loading protocol, the fixation bar was reattached to the external fixator and the animal was returned to the cage, awakened from anesthesia, and resumed normal cage activity.
Animals assigned to the immobilization group were allowed normal cage activity with the external fixator in place and did not undergo any controlled loading throughout the study period. However, the animals in the immobilization group were connected to the computer-controlled loading device (while they were under anesthesia) immediately before they were killed, to obtain a one-time load-displacement response for comparison with the experimental groups.
Histological Analysis
After the animal was killed, the intra-articular portion of the graft was divided at its midsubstance to provide separate tibial and femoral specimens19. The tissues were trimmed and then were fixed for two days with 10% neutral buffered formalin with 1% cetylpyridinium chloride added to preserve proteoglycans. The plane of sectioning was perpendicular to the bone tunnel, so that cross sections of the tendon in the tunnel were made. The first perpendicular cut was made 1 mm below the joint surface to permit evaluation of healing at the intra-articular tunnel aperture but below the articular surface of the knee joint. The tissues were embedded in paraffin, and 5-μm-thick sections were cut in the coronal plane. Staining was performed with hematoxylin and eosin and safranin O. Healing between the tendon and the bone was assessed by measuring new tissue formation (fibrovascular granulation tissue, fibrous tissue, cartilage, and bone) between the tendon and bone and by assessing narrowing of the tendon-bone interface20.
For immunohistochemical analysis, serial sections were treated with 3% H2O2 to quench endogenous peroxidase activity, and nonspecific antibody binding was blocked with 5% goat serum. One percent bovine serum albumin in phosphate-buffered saline solution was used as a negative secondary reagent control. Each primary antibody was applied to separate serial sections for sixty minutes at 37°C. Bound antibodies were visualized with use of a goat avidin-biotin peroxidase system with 3,3' diaminobenzidine (DAB; DAKO, Carpinteria, California) as a substrate. The following antibodies were used to localize hematopoietic lineage cells: mouse anti-rat ED1-macrophage (ED1 antigen is a lysosomal glycoprotein expressed only by a subpopulation of macrophages and monocytes) and mouse anti-rat ED2-macrophage (ED2 antigen is a membrane glycoprotein found only on mature tissue macrophages) (Serotec, Raleigh, North Carolina)21,22. Osteoclast activity was evaluated by chemical staining for tartrate-resistant acid phosphatase (TRAP; Zymed, San Francisco, California). Proliferating blood vessels were localized with use of a monoclonal antibody to α-smooth muscle actin (α-SMA), a marker of myofibroblasts and vascular pericytes (Sigma, St. Louis, Missouri)20. Negative controls were processed in an identical manner except for incubation with bovine serum albumin rather than the primary antibody.
Analysis of Histological Data
The histological sections were examined with use of light microscopy (Eclipse E800; Nikon, Melville, New York), and digital images of the stained tissue sections were made with use of a SPOT RT camera (Diagnostic Instruments, Sterling Heights, Michigan). The illumination and detection parameters of the microscope were kept constant between specimens to allow for direct comparisons. The number of positively-stained cells was counted in thirty randomly-selected high-power 100 by 100-μm (40× magnification) microscopic fields distributed along the bone tunnel23. These measurements were limited to the tibial tunnel since there was variability in the quality of the femoral tunnel sections due to the small size of the bone. Three observers (D.K., A.B., and A.J.S.F.) who were experienced with immunohistochemical analyses independently counted positively-stained cells on the ED1 and ED2-stained slides23. A review of approximately fifty slides showed >95% agreement among the observers. On the basis of this analysis, all of the sections were scored by one observer. Random data checking was performed for the other specific immunohistochemical analyses to confirm that this level of agreement was maintained throughout the histological analysis. The number of positively-stained cells for all other markers was also counted at the tendon-bone interface.
Micro-Computed Tomography Analysis
Trabecular architecture, bone formation, and bone remodeling along the tendon-bone interface were assessed with use of micro-computed tomography (MS-8 Small Specimen Scanner; Enhanced Vision Systems, London, Ontario, Canada)24,25. Time-zero analysis was also performed on five specimens to provide a baseline reference for comparison with the control and experimental groups. The tibial tunnels were scanned and reconstructed at 22.5-μm isotropic resolution. Each sample was placed in the holder surrounded by saline solution and scanned at 80 V and 80 mA. The scans included a phantom containing air, saline solution, and a bone reference material for calibration of Hounsfield units to tissue mineral density. The images were thresholded to distinguish bone voxels with use of a global threshold for each specimen based on the histogram of computed tomography attenuation values based on the Otsu discriminant26.
After thresholding, six outcome measures were evaluated: new-bone volume (mm3), tissue mineral content (mg), tissue mineral density (mg/mL), trabecular thickness (μm), trabecular number, and trabecular spacing (μm)25,27. The total bone mineral content, bone volume fraction (bone volume divided by total volume), and mineral distribution were calculated for a cylindrical volume of interest centered along the graft tunnel for the entire length of each graft. The bone volume is the total number of thresholded bone voxels within the total volume of interest25. The mineral distribution along the graft was determined from the computed tomography value for each voxel of the micro-computed tomography scan. An SB3 standard (1100 g of hydroxyapatite/cm3) was used to calibrate tissue mineral density on each scan.
Biomechanical Testing
At the two or four-week time point, the femur-graft-tibia construct was carefully harvested and was stored at –80°C. Prior to testing, the specimens were thawed and all soft tissue was resected under the microscope, with preservation of only the graft crossing the knee. The tibia and femur were potted in cement (Bondo; 3M Bondo Corp., Atlanta, Georgia) to ensure secure fixation. Specimens were mounted on a custom-designed tensile testing apparatus with use of a specially designed jig that allowed alignment of the axis of distraction parallel to the long axis of the graft. Saline solution spray was used to maintain specimen hydration throughout testing at room temperature. The specimens were first preconditioned for five cycles between 0 and 0.2 N and then loaded in tension to failure at a rate of 10 mm/min (167 μm/sec). The ultimate load (N) was obtained from the recorded load-deformation curve, and stiffness (N/mm) was calculated from the linear portion of this curve with use of Microsoft Office Excel 2002 (Microsoft, Redmond, Washington). The site of graft failure (the bone attachment site versus a midsubstance rupture) was also recorded.
Statistical Analysis
Statistical analysis of the biomechanical and micro-computed tomography data was performed with use of two-way analysis of variance with appropriate post hoc corrections. Analysis of the histological data was performed with use of two-way analysis of variance between treatment groups as well as repeated-measures analysis of variance for comparison of histomorphometric measures at different locations (the intra-articular aperture, midpart of the tunnel, and extra-articular aperture) within the same bone tunnel. All statistical analyses were performed with use of SigmaStat Software (Ashburn, Virginia), and significance was set at p < 0.05.
Source of Funding
This study was financially supported by National Institutes of Health Grant R01 AR053689-01A1.
Results
The animals tolerated the anterior cruciate ligament reconstruction procedure. However, we did observe an approximately 15% rate of postoperative fracture (n = 25), usually at or near threaded-pin sites in the distal part of the femur or the proximal part of the tibia, during mechanical loading. Nine animals died, from anesthetic complications or postoperative infection, during the study period. These animals were replaced to maintain the sample sizes specified in the Materials and Methods section.
Histological Findings
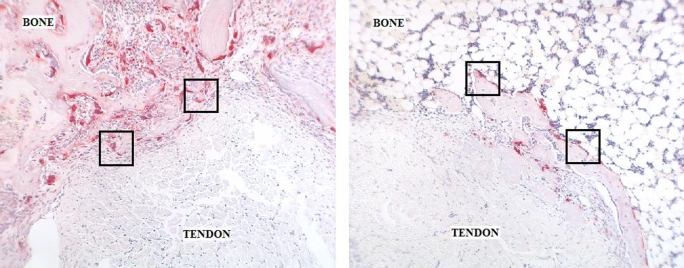
Qualitative analysis demonstrated less interposed fibrovascular scar tissue at the tendon-bone interface in the delayed-loading groups (the animals that underwent loading on postoperative day 4 or 10) than in the immediate-loading or immobilization group at two and four weeks. Safranin-O staining revealed no significant fibrocartilage formation at the healing tendon-bone interface in any treatment group.
Macrophages
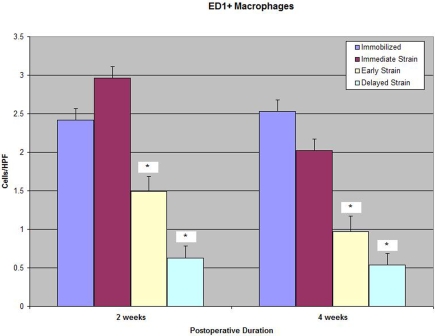
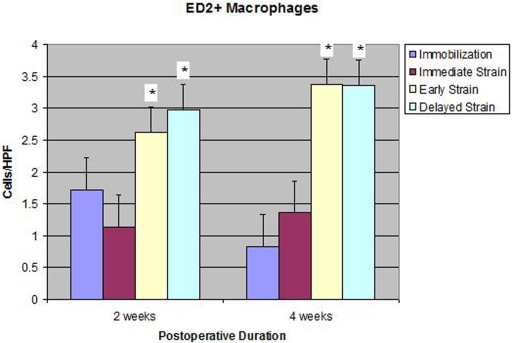
There were significantly fewer ED1+ inflammatory macrophages at the tendon-bone interface in both delayed-loading groups (postoperative day 4 and postoperative day 10) than there were in the immediate-loading or immobilization group at two and four weeks (p < 0.01; Table I and Figs. 4-A, 4-B, and 4-C). ED1+ macrophages are phagocytic cells that remove debris and appear in high concentrations early after injury21,22. Conversely, the quantities of ED2+ resident macrophages seen at the tendon-bone interface in the delayed-loading groups were significantly greater than those in the immediate-loading or immobilization group at both two and four weeks (mean and standard deviation, 2.97 ± 0.7 [postoperative-day-10 group] versus 1.14 ± 0.47 and 1.71 ± 1.5 ED2+ cells, respectively, per high-power field at two weeks; p < 0.02). ED2+ macrophages are derived from local cells, accumulate later in the healing cycle, and appear to have an anabolic role in tendon repair (Fig. 5)21,22. There was no significant difference between the immobilization and immediate-loading groups with regard to the number of ED1+ or ED2+ macrophages observed at the tendon-bone interface at either two or four weeks postoperatively.
TABLE I.
Quantitative Analysis of Cells at the Tendon-Bone Interface of the Tibial Tunnel After Anterior Cruciate Ligament Reconstruction
| Cells per High-Power Field* |
||||
| Group | Immobilization | Immediate Loading | Early Delayed Loading (Postop. Day 4) | Late Delayed Loading (Postop. Day 10) |
| ED1+ macrophages | ||||
| 2 weeks | 2.42 ± 0.12a | 2.96 ± 0.05b | 1.49 ± 0.17a,b | 0.63 ± 0.2a,b |
| 4 weeks | 2.53 ± 0.82c | 2.02 ± 0.91d | 0.97 ± 0.21c,d | 0.54 ± 0.13c,d |
| ED2+ macrophages | ||||
| 2 weeks | 1.71 ± 1.5e | 1.14 ± 0.47f | 2.62 ± 0.54e,f | 2.97 ± 0.7e,f |
| 4 weeks | 0.83 ± 0.6g | 1.36 ± 0.16h | 3.37 ± 0.29g,h | 3.35 ± 0.51g,h |
| Osteoclasts (tartrate-resistant acid phosphatase staining) | ||||
| 2 weeks | 1.44 ± 0.2i | 1.02 ± 0.08j | 0.58 ± 0.37i,j | 0.35 ± 0.15i,j |
| 4 weeks | 0.69 ± 0.08k | 1.22 ± 0.17l | 0.29 ± 0.14k,l | 0.33 ± 0.15k,l |
| Neovascularity (smooth-muscle-actin staining) | ||||
| 2 weeks | 0.78 ± 0.39m | 1.54 ± 0.22n | 0.3 ± 0.17m,n | 0.27 ± 0.15m,n |
| 4 weeks | 1.03 ± 0.62o | 0.83 ± 0.46p | 0.31 ± 0.16o,p | 0.22 ± 0.1o,p |
Data are reported as the mean and standard deviation. Values that share a letter were significantly different from one another, with a, b, c, d, i, j, k, and l indicating p < 0.01; e, f, g, and h indicating p < 0.02; and m, n, o, and p indicating p < 0.003.
Fig. 4-A Fig. 4-B Fig. 4-C.
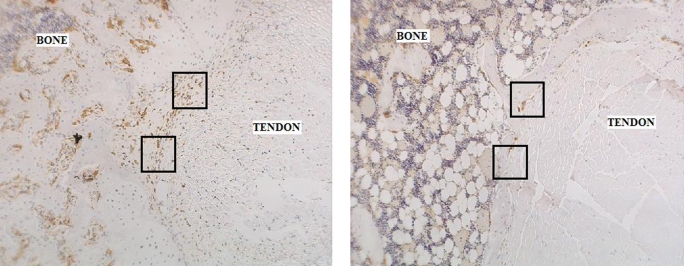
Fig. 4-A Immunohistochemical staining for ED1+ macrophages on an axial section of the tibial tunnel from an animal subjected to immediate loading and killed at four weeks (×40). Fig. 4-B Immunohistochemical staining for ED1+ macrophages on an axial section of the tibial tunnel from an animal treated with delayed loading starting on postoperative day 4 and killed at four weeks (×40). Note the substantially reduced quantity of ED1+ macrophages at the tendon-bone interface in the specimen from the delayed-loading group compared with that from the immediate-loading group. Fig. 4-C Significantly fewer catabolic ED1+ macrophages were observed at the tendon-bone interface of both delayed-loading groups compared with the number in the immediate-loading or immobilization group at two and four weeks postoperatively (p < 0.01). HPF = high-power field.
Fig. 5.
Significantly more ED2+ resident macrophages were observed at the tendon-bone interface of both delayed-loading groups compared with the number in the immediate-loading or immobilization group at two and four weeks postoperatively (p < 0.02). HPF = high-power field.
Angiogenesis
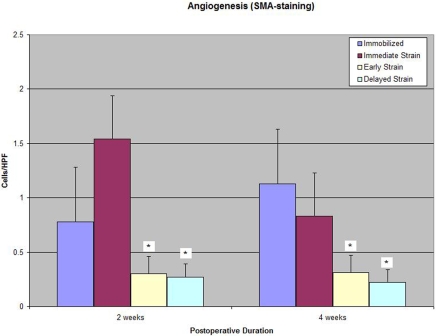
Smooth-muscle-actin staining of myofibroblasts revealed significantly less neovascularity at the tendon-bone interface in the delayed-loading groups as compared with that in the immediate-loading or immobilization group at both two and four weeks postoperatively (0.27 ± 0.15 [postoperative-day-10 group] versus 1.54 ± 0.22 and 0.78 ± 0.39 cells, respectively, per high-power field at two weeks; p < 0.003; Table I and Fig. 6). No significant difference in neoangiogenesis at the tendon-bone interface was observed between the immobilization and immediate-loading groups at two or four weeks postoperatively.
Fig. 6.
Quantitative immunohistochemical analysis with smooth-muscle-actin (SMA) staining revealed reduced interface tissue vascularity in both delayed-loading groups compared with that in the immediate-loading or immobilization group at two and four weeks postoperatively (p < 0.003). HPF = high-power field.
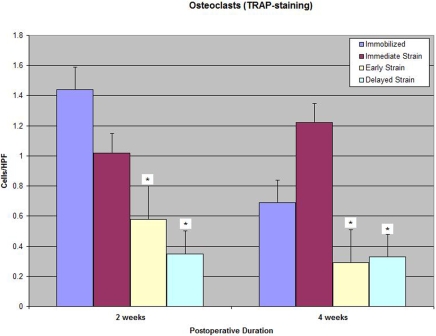
Osteoclasts
Tartrate-resistant acid phosphatase staining of osteoclasts revealed significantly fewer osteoclasts at the tendon-bone interface in both delayed-loading groups compared with the numbers in the immediate-loading and immobilization groups at two and four weeks postoperatively (0.35 ± 0.15 [postoperative-day-10 group] versus 1.02 ± 0.08 and 1.44 ± 0.2 cells, respectively, per high-power field at two weeks; p < 0.01; Table I and Figs. 7-A, 7-B, and 7-C). No significant difference in the osteoclast number at the tendon-bone interface was observed between the immobilization and immediate-loading groups at two or four weeks postoperatively.
Fig. 7-A Fig. 7-B Fig. 7-C.
Fig. 7-A Tartrate-resistant acid phosphatase (TRAP) staining for osteoclasts on an axial section of the tibial tunnel from an animal treated with immediate loading and killed at four weeks (×40). Fig. 7-B Tartrate-resistant acid phosphatase staining for osteoclasts on an axial section of the tibial tunnel from an animal treated with delayed loading starting on postoperative day 4 and killed at four weeks (×40). Note the substantially reduced number of osteoclasts at the tendon-bone interface in the specimen from the delayed-loading group compared with that from the immediate-loading group. Fig. 7-C Significantly fewer osteoclasts were observed at the tendon-bone interface in both delayed-loading groups compared with the number in the immediate-loading or immobilization group at two and four weeks postoperatively (p < 0.01). HPF = high-power field.
Effect of Postoperative Duration
Differences over time in the quantity of ED1+ macrophages, ED2+ macrophages, osteoclasts, and neoangiogenesis at the tendon-bone interface were analyzed within each treatment group. Significantly fewer osteoclasts were noted in the immobilization group at four weeks postoperatively compared with the number at two weeks (p = 0.003). Significantly fewer ED1+ macrophages and less neoangiogenesis were seen at four weeks compared with the findings at two weeks postoperatively in the immediate-loading group (p = 0.03). No significant differences in quantitative histomorphometry findings were observed between the postoperative time points in the delayed-loading groups.
Micro-Computed Tomography
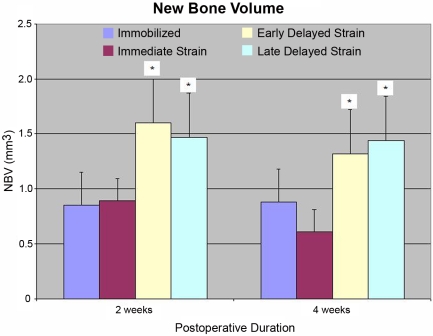
The new-bone volume, tissue mineral content, and tissue mineral density observed in the tibial tunnels of the delayed-loading groups were significantly greater than those in the immediate-loading, immobilization, and time-zero control groups at both two and four weeks postoperatively (new-bone volume, 1.47 ± 0.11 mm3 [postoperative-day-10 group] versus 0.89 ± 0.30 mm3 and 0.85 ± 0.19 mm3 [immediate-loading and immobilization groups, respectively] at two weeks; p < 0.003; Table II and Figs. 8 and 9). No significant differences in new-bone volume, tissue mineral content, or tissue mineral density were observed between the immobilization and immediate-loading groups at any time point. Regional tunnel analysis revealed that the new-bone volume, tissue mineral content, and tissue mineral density at the intra-articular aperture (p < 0.01) and midpart of the tunnel (p < 0.01) were significantly greater in the delayed-loading groups than they were in the immobilization or immediate-loading group, but there were no differences between groups at the extra-articular aperture.
TABLE II.
Results of Micro-Computed Tomography Analysis of the Tibial Tunnels*
| Time Zero | Immobilization | Immediate Loading | Early Delayed Loading (Postop. Day 4) | Late Delayed Loading (Postop. Day 10) | |
| 2 weeks | |||||
| New-bone volume (mm3) | 0.82 ± 0.04 | 0.85 ± 0.19 | 0.89 ± 0.30 | 1.60 ± 0.54† | 1.47 ± 0.11† |
| Tissue mineral content (mg) | 0.45 ± 0.03 | 0.48 ± 0.12 | 0.49 ± 0.15 | 1.05 ± 0.38‡ | 0.97 ± 0.09‡ |
| Tissue mineral density (mg/mL) | 543 ± 25 | 542 ± 32 | 536 ± 40 | 639 ± 16‡ | 643 ± 21‡ |
| 4 weeks | |||||
| New-bone volume (mm3) | 0.82 ± 0.04 | 0.88 ± 0.28 | 0.61 ± 0.09 | 1.32 ± 0.19§ | 1.44 ± 0.45‡ |
| Tissue mineral content (mg) | 0.45 ± 0.03 | 0.49 ± 0.17 | 0.37 ± 0.08 | 0.92 ± 0.13‡ | 1.03 ± 0.34‡ |
| Tissue mineral density (mg/mL) | 543 ± 25 | 534 ± 40 | 575 ± 39 | 684 ± 27† | 706 ± 18† |
The values are given as the mean and standard deviation.
P < 0.003 compared with the immobilization and immediate-loading groups.
P < 0.001 compared with the immobilization and immediate-loading groups.
P < 0.01 compared with the immobilization and immediate-loading groups.
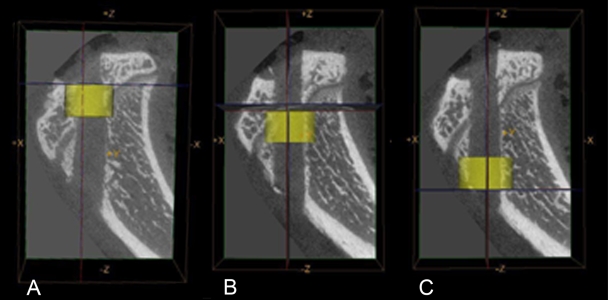
Fig. 8.
Sagittal micro-computed tomography images of the tibial tunnel. New-bone formation, tissue mineral content, and tissue mineral density were quantified for the entire tibial tunnel as well as for the intra-articular aperture (A), midpart of the tunnel (B), and extra-articular aperture (C).
Fig. 9.
Significantly greater new-bone volume (NBV) was observed in the tibial tunnels of both delayed-loading groups compared with that in the immediate-loading or immobilization group at two and four weeks postoperatively (p < 0.003).
Biomechanical Testing
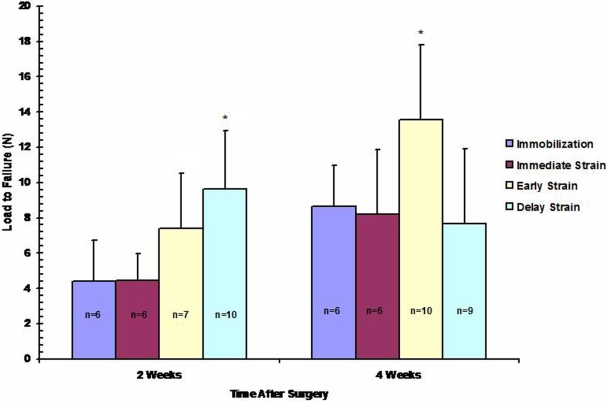
Delayed initiation of cyclic axial loading on postoperative day 10 resulted in a significantly increased load to failure of the bone-graft-bone complex at two weeks compared with that resulting from immediate loading or prolonged immobilization of the knee (9.6 ± 3.3 N versus 4.4 ± 2.3 N and 4.4 ± 1.5 N, respectively; p < 0.01; Fig. 10). In addition, stiffness in the postoperative-day-10 group was significantly greater than that in all other groups at two weeks postoperatively (6.1 ± 2.2 N/mm versus ∼3.8 ± 1.8 N/mm, respectively; p = 0.04). Initiation of cyclic loading on postoperative day 4 also resulted in a greater load to failure at two weeks compared with that in the immediate-loading or immobilization group (7.4 ± 3.1 N versus 4.4 ± 2.3 N and 4.4 ± 1.5 N, respectively), but this difference did not reach significance (p = 0.09; Fig. 10). At four weeks, the group in which delayed loading had been initiated on postoperative day 4 demonstrated a load to failure that was significantly greater than that in all other groups (13.5 ± 4 N versus ∼8 ± 4 N, respectively; p = 0.01). No significant difference in construct stiffness was observed between any groups at four weeks postoperatively.
Fig. 10.
A significantly greater ultimate load to failure of the bone-tendon-bone complex was observed in the late (postoperative-day-10) delayed-loading groups compared with that in the immediate-loading or immobilization group at two weeks postoperatively (p < 0.01). The late delayed-loading group demonstrated a significantly greater load to failure compared with that in the other groups at four weeks postoperatively (p = 0.01).
In forty of forty-eight tested specimens, the mode of failure of the construct was pullout of the graft from either the tibial or the femoral tunnel. The eight remaining specimens failed by midsubstance rupture of the graft. There were no significant differences in the mode of failure between treatment groups.
Effect of Postoperative Duration
Differences in the load to failure and stiffness of the bone-tendon-bone complex over time were analyzed within each treatment group. In the immobilization group and in the group with early delayed loading (on postoperative day 4), the failure and stiffness at four weeks postoperatively were significantly greater than they were at two weeks (p = 0.04 and p = 0.001, respectively). No significant differences in the load to failure or stiffness were observed between the postoperative time points in the immediate-loading or late-delayed-loading group.
Discussion
The purpose of this study was to determine the effect of controlled axial loading on tendon-to-bone healing after anterior cruciate ligament reconstruction. We hypothesized that controlled mechanical stimulation of the healing anterior cruciate ligament reconstruction after a period of postoperative immobilization would provide an improved healing response compared with that resulting from immediate mechanical stimulation or prolonged immobilization. Mechanical stimulation was applied via a displacement-control loading protocol involving a novel cyclic distraction system, which provided a unique opportunity to evaluate the influence of in vivo loads applied at various postoperative times on tendon-to-bone healing. In our study, the delayed application of cyclic axial load after anterior cruciate ligament reconstruction resulted in improved mechanical and biological parameters of tendon-to-bone healing compared with those resulting from immediate loading or prolonged postoperative immobilization of the knee. A more mature tendon-bone interface was achieved, as demonstrated by significantly increased load to failure, new-bone formation in the tunnel, and anabolic ED2+ macrophages as well as reduced interface scar tissue, catabolic ED1+ macrophages, osteoclasts, and vascularity of the interface tissue.
Previous studies have clearly demonstrated that mechanical loading plays a critical role in maintaining the homeostasis of both the biological response and the mechanical behavior of native musculoskeletal tissues. Uninjured ligaments and tendons as well as their entheses are sensitive to their mechanical environment and generally demonstrate an increase in tensile modulus in response to increasing loads, while stress deprivation is correspondingly associated with a decline in mechanical properties5-12,28-31. Similarly, bone is very sensitive to mechanical loads. Increased loading promotes new-bone formation and increased mechanical properties, while stress deprivation can lead to profound decreases in bone mineral density and rapid resorption30,31.
The influence of mechanical loads on a healing enthesis after a surgical repair, however, has been controversial. For example, using a clinically relevant model of flexor tendon injury, Thomopoulos et al. recently found that muscle loading was beneficial to healing at the tendon-bone interface12. Muscle loading significantly improved biomechanical properties, especially in the physiological subfailure range, compared with those of unloaded flexor tendon repairs12. In a prior study by the same group, however, the authors found that early exercise after repair of an acute rotator cuff tear impaired healing compared with that resulting from immobilization in a rat model13. In a rabbit model of anterior cruciate ligament reconstruction, Sakai et al. similarly found healing and graft attachment strength to be better in animals treated with postoperative immobilization than in those allowed normal cage activity postoperatively14. Animals not treated with immobilization demonstrated a maximum load to failure that was lower than that in animals treated with immobilization, and the graft was partly separated from the fibrous tissue covering the osseous wall in the former group14. Similarly, Kamps et al. found increased cyclic stress to be detrimental to healing and biomechanical properties in a rabbit patellar tendon midsubstance injury model11.
Clinical studies have also been performed to address the role of mechanical stimulation after anterior cruciate ligament reconstruction. Ito et al. prospectively examined thirty consecutive patients who had been randomized to be treated with three days or two weeks of immobilization after single-bundle anterior cruciate ligament reconstruction32. No significant differences were noted in anterior laxity, joint proprioception, or thigh muscle strength at one year postoperatively. Henriksson et al. examined fifty patients treated with anterior cruciate ligament reconstruction who were randomized to be managed with an early range of motion or with plaster cast immobilization for four additional weeks, and they found no significant difference in sagittal knee laxity or Lysholm and Tegner scores33. An important limitation of all of these animal and clinical studies, however, is that none were able to quantify or control the loads applied to the tendon-bone interface during healing. Yu and Paessler examined the relationship between tunnel widening after anterior cruciate ligament reconstruction and different postoperative rehabilitation programs in sixty-five patients34. They reported significantly greater tunnel widening at six months in patients who had undergone early, aggressive rehabilitation compared with those who had undergone a conservative rehabilitation program. However, no differences in clinical outcomes and objective measures of knee stability were observed between the groups34.
Our observations of improved biomechanical and histological properties of the healing enthesis after anterior cruciate ligament reconstruction when mechanical loads were applied after a period of immobilization supported our main hypothesis. In contrast, immediate initiation of cyclic axial loading postoperatively conferred no improvement in healing at the tendon-bone interface compared with that following prolonged immobilization. Delayed loading was associated with significant improvements in both ultimate load to failure and stiffness of the bone-tendon-bone complex. In addition, histological evaluation revealed significantly less interface scar tissue and a greater prevalence of anabolic ED2+ macrophages with a corresponding reduction in catabolic ED1+ macrophages associated with the acute inflammatory reaction. Bone homeostasis was also affected, with significant increases in new-bone formation and tissue mineral density in the tunnel on micro-computed tomography scans of the delayed-loading groups and corresponding reductions in osteoclasts at the tendon-bone interface compared with the values in the immediate-loading and immobilization groups.
While the etiology of these differences may be multifactorial, we hypothesize that controlled mechanical loading of the tissues in the physiological range as applied in this study (∼2% strain at time zero) may be most favorable after resolution of the acute inflammatory reaction in the immediate postoperative period. This magnitude of strain was selected on the basis of previous in vivo studies of the anterior cruciate ligament during activities of daily living16-18,35. Fleming et al. measured in vivo anteromedial bundle strain in five subjects during stair-climbing activities and noted strain magnitudes approximating 2.7%35. Consistent with other studies of anterior cruciate ligament biomechanics, these values increased as the knee was moved from a flexed to an extended position16-18,35. Ongoing studies with use of our novel system are in progress to provide further insight into the effects of variable magnitudes of axial load on the healing interface after anterior cruciate ligament reconstruction. Furthermore, quantification and localization of downstream inflammatory markers at the tendon-bone interface will provide insight into whether the results observed in this study reflect the direct effect of mechanotransduction on cells or an indirect effect related to micromotion and modulation of the inflammatory reaction at the healing enthesis.
While these findings may have important implications for postoperative rehabilitation following ligament reconstruction, the clinical relevance of these results must be interpreted with some caution. The applied motion in this study was primarily along the long axis of the graft, which differs from normal knee motion, in which there is likely a complex loading environment of axial, shear, and compressive forces on the tendon-bone interface with daily activities16-18. Our goal was to isolate the loading environment to axial strain, which could then be used as an independent study parameter. This approach provided a unique framework for assessing the role of a controlled mechanical stimulus in the cellular and molecular mechanisms of tendon-to-bone healing. While an experimental model of knee motion in the postoperative period may offer greater clinical relevance, the unknown components of the applied mechanical stimulus may confound the ability to define relationships between this independent experimental variable and biological and mechanical outcomes.
Our study is not without limitations. The threaded pins in the distal part of the femur and proximal part of the tibia are stress risers and can precipitate fracture with daily axial loading. In our series we noted an approximately 15% rate of postoperative fracture that required animals to be killed prematurely. In order to isolate and apply a controlled mechanical stimulus to the healing graft, our model required sectioning of all other soft tissue traversing the knee joint. For this reason, we recognize that the direct translation of our findings to the clinical setting of anterior cruciate ligament reconstruction is somewhat limited. We also recognize that postoperative healing of the anterior cruciate ligament graft is accompanied by concomitant healing of these sectioned periarticular soft tissues, such that axial loads applied with increasing postoperative duration may be shared by both the graft and the healing surrounding soft tissues. Our independent mechanical input was knee distraction, which is imparted even in the presence of healing of the surrounding tissues. However, the percentage of graft elongation may change over time as the graft-bone interface adapts and the effective length of the graft changes as a result of graft-to-bone healing. In addition, future studies should be done to examine the effect of axial loading with use of higher levels of applied strain, longer durations of loading, or more frequent loading sessions. The addition of a positive control group allowing ad libitum cage activity without external fixation may provide useful information for comparison as well. It is also important to note that we had several primary outcomes, so the significance of the magnitude of the differences between the p values without a Bonferroni correction must be interpreted with some caution.
In conclusion, we found that a physiological level of controlled mechanical stimulation after anterior cruciate ligament reconstruction was beneficial to tendon-to-bone healing when it was applied after a period of immobilization in the immediate postoperative period. The applied external mechanical stimulus was also sensitive to the time of application in the postoperative period. It appears that the application of physiological loads may be optimally initiated after resolution of the acute inflammatory reaction, related to surgical trauma, in the immediate postoperative period.
Acknowledgments
Note: The authors acknowledge Dr. Peter Torzilli for his guidance throughout the design and completion of these experiments. They also thank Lilly Ying for her technical expertise and the preparation of all histological specimens.
Footnotes
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the National Institutes of Health (Grant R01 AR053689-01A1). Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795-803 [DOI] [PubMed] [Google Scholar]
- 2.Whiston TB, Walmsley R. Some observations on the reactions of bone and tendon after tunneling of bone and insertion of tendon. J Bone Joint Surg Br. 1960;42:377-86 [DOI] [PubMed] [Google Scholar]
- 3.Grana WA, Egle DM, Mahnken R, Goodhart CW. An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med. 1994;22:344-51 [DOI] [PubMed] [Google Scholar]
- 4.Panni AS, Milano G, Lucania L, Fabbriciani C. Graft healing after anterior cruciate ligament reconstruction in rabbits. Clin Orthop Relat Res. 1997;343:203-12 [PubMed] [Google Scholar]
- 5.Woo SL, Gomez MA, Sites TJ, Newton PO, Orlando CA, Akeson WH. The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. J Bone Joint Surg Am. 1987;69:1200-11 [PubMed] [Google Scholar]
- 6.Gelberman RH, Woo SL, Lothringer K, Akeson WH, Amiel D. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg Am. 1982;7:170-5 [DOI] [PubMed] [Google Scholar]
- 7.Gomez MA, Woo SL, Amiel D, Harwood F, Kitabayashi L, Matyas JR. The effects of increased tension on healing medial collateral ligaments. Am J Sports Med. 1991;19:347-54 [DOI] [PubMed] [Google Scholar]
- 8.Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res. 1995;13:907-14 [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H, Manske PR, Pruitt DL, Larson BJ. Effect of cyclic tension on lacerated flexor tendons in vitro. J Hand Surg Am. 1995;20:467-73 [DOI] [PubMed] [Google Scholar]
- 10.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106-13 [DOI] [PubMed] [Google Scholar]
- 11.Kamps BS, Linder LH, DeCamp CE, Haut RC. The influence of immobilization versus exercise on scar formation in the rabbit patellar tendon after excision of the central third. Am J Sports Med. 1994;22:803-11 [DOI] [PubMed] [Google Scholar]
- 12.Thomopoulos S, Zampiakis E, Das R, Silva MJ, Gelberman RH. The effect of muscle loading on flexor tendon-to-bone healing in a canine model. J Orthop Res. 2008;26:1611-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106-13 [DOI] [PubMed] [Google Scholar]
- 14.Sakai H, Fukui N, Kawakami A, Kurosawa H. Biological fixation of the graft within bone after anterior cruciate ligament reconstruction in rabbits: effects of the duration of postoperative immobilization. J Orthop Sci. 2000;5:43-51 [DOI] [PubMed] [Google Scholar]
- 15.Eng MS, Imhauser C, Packer J, Bedi A, Brophy R, Kovacevic D, Jackson K, Deng XH, Rodeo SA, Torzilli P. A novel in vivo joint loading system to investigate the effect of daily mechanical load on a healing anterior cruciate ligament reconstruction. J Med Device. 2010;4:15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beynnon BD, Johnson RJ, Fleming BC, Renström PA, Nichols CE, Pope MH, Haugh LD. The measurement of elongation of anterior cruciate-ligament grafts in vivo. J Bone Joint Surg Am. 1994;76:520-31 [DOI] [PubMed] [Google Scholar]
- 17.Beynnon BD, Uh BS, Johnson RJ, Fleming BC, Renström PA, Nichols CE. The elongation behavior of the anterior cruciate ligament graft in vivo. A long-term follow-up study. Am J Sports Med. 2001;29:161-6 [DOI] [PubMed] [Google Scholar]
- 18.Heijne A, Fleming BC, Renstrom PA, Peura GD, Beynnon BD, Werner S. Strain on the anterior cruciate ligament during closed kinetic chain exercises. Med Sci Sports Exerc. 2004;36:935-41 [DOI] [PubMed] [Google Scholar]
- 19.Binkley JM, Peat M. The effect of immobilization on the ultrastructure and mechanical properties of the medial collateral ligament in rats. Clin Orthop Relat Res. 1986;203:301-8 [PubMed] [Google Scholar]
- 20.Bedi A, Kawamura S, Ying L, Rodeo SA. Differences in tendon graft healing between the intra-articular and extra-articular ends of a bone tunnel. HSS J. 2009;5:51-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamura S, Ying L, Kim HJ, Dynybil C, Rodeo SA. Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res. 2005;23:1425-32 [DOI] [PubMed] [Google Scholar]
- 22.Honda H, Kimura H, Rostami A. Demonstration and phenotypic characterization of resident macrophages in rat skeletal muscle. Immunology. 1990;70:272-7 [PMC free article] [PubMed] [Google Scholar]
- 23.Hays PL, Kawamura S, Deng XH, Dagher E, Mithoefer K, Ying L, Rodeo SA. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90:565-79 [DOI] [PubMed] [Google Scholar]
- 24.Rodeo SA, Kawamura S, Ma CB, Deng XH, Sussman PS, Hays P, Ying L. The effect of osteoclastic activity on tendon-to-bone healing: an experimental study in rabbits. J Bone Joint Surg Am. 2007;89:2250-9 [DOI] [PubMed] [Google Scholar]
- 25.Rodeo SA, Kawamura S, Kim HJ, Dynybil C, Ying L. Tendon healing in a bone tunnel differs at the tunnel entrance versus the tunnel exit: an effect of graft-tunnel motion? Am J Sports Med. 2006;34:1790-800 [DOI] [PubMed] [Google Scholar]
- 26.Otsu N. A threshold selection method from gray-level histograms. IEEE Trans. 1979;9:62-6 [Google Scholar]
- 27.Gulotta LV, Kovacevic D, Ying L, Ehteshami JR, Montgomery S, Rodeo SA. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am J Sports Med. 2008;36:1290-7 [DOI] [PubMed] [Google Scholar]
- 28.Thornton GM, Shao X, Chung M, Sciore P, Boorman RS, Hart DA, Lo IK. Changes in mechanical loading lead to tendon-specific alterations in MMP and TIMP expression: influence of stress-deprivation and intermittent cyclic hydrostatic compression on rat supraspinatus and Achilles tendons. Br J Sports Med. 2010;44:698-703 [DOI] [PubMed] [Google Scholar]
- 29.Arnoczky SP, Lavagnino M, Egerbacher M, Caballero O, Gardner K. Matrix metalloproteinase inhibitors prevent a decrease in the mechanical properties of stress-deprived tendons: an in vitro experimental study. Am J Sports Med. 2007;35:763-9 [DOI] [PubMed] [Google Scholar]
- 30.Wolff J. Das Gesetz der Transformation der Knochen (Berlin A. Hirchwild). [The law of bone remodeling]. Berlin: Springer-Verlag; 1892 [Google Scholar]
- 31.Bostrom MPG, Boskey A, Kauffman JK, et al. Form and function of bone. Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic basic science. 2nd ed Rosemont, IL: AAOS; 2000. p319-70 [Google Scholar]
- 32.Ito Y, Deie M, Adachi N, Kobayashi K, Kanaya A, Miyamoto A, Nakasa T, Ochi M. A prospective study of 3-day versus 2-week immobilization period after anterior cruciate ligament reconstruction. Knee. 2007;14:34-8 [DOI] [PubMed] [Google Scholar]
- 33.Henriksson M, Rockborn P, Good L. Range of motion training in brace vs. plaster immobilization after anterior cruciate ligament reconstruction: a prospective randomized comparison with a 2-year follow-up. Scand J Med Sci Sports. 2002;12:73-80 [DOI] [PubMed] [Google Scholar]
- 34.Yu JK, Paessler HH. Relationship between tunnel widening and different rehabilitation procedures after anterior cruciate ligament reconstruction with quadrupled hamstring tendons. Chin Med J Engl. 2005;118:320-6 [PubMed] [Google Scholar]
- 35.Fleming BC, Beynnon BD, Renstrom PA, Johnson RJ, Nichols CE, Peura GD, Uh BS. The strain behavior of the anterior cruciate ligament during stair climbing: an in vivo study. Arthroscopy. 1999;15:185-91 [DOI] [PubMed] [Google Scholar]