Abstract
Background
A low level of response (LR) to alcohol has been shown to relate to a higher risk for alcohol use disorders (AUDs). However, no previous research has examined the association between LR and the development of AUDs in the context of additional robust risk factors for AUDs. This study evaluated whether LR and other related characteristics predicted the occurrence of AUDs across adulthood using discrete-time survival analysis (DTSA).
Methods
297 probands from the San Diego Prospective Study reported on the LR to alcohol, a family history (FH) of AUDs, the typical drinking quantity, the age of drinking onset, the body mass index and the age at the baseline (T1) assessment. Alcohol use disorders (AUDs) were evaluated at the 10-year (T10), T15, T20, and T25 follow-ups.
Results
A low LR to alcohol predicted AUD occurrence over the course of adulthood even after controlling for the effects of other robust risk factors. Interaction effects revealed that the impact of FH on AUDs was only observed for subjects with high T1 drinking levels, and probands with high T1 drinking were at high risk for AUDs regardless of their age of onset.
Conclusion
The findings illustrate that LR is a unique risk factor for AUDs across adulthood, and not simply a reflection of a broader range of risk factors. The continued investigation of how LR is related to AUD onset later in life will help inform treatment providers about this high-risk population, and future longitudinal evaluations will utilize DTSA to assess rates of AUD remission as well as the onset of drinking outcomes in adolescent samples.
Keywords: Level of Response, Alcoholism, Survival Analysis, Longitudinal, Family History, Drinking Onset
I. Introduction
A low level of response (LR) to alcohol is one of several endophenotypes related to a higher risk for the alcohol use disorders (AUDs) of abuse and dependence (Schuckit, 2009). A low LR is indicated by either a low intensity of reaction to alcohol at a given blood alcohol concentration (BAC) on an alcohol challenge (Schuckit and Gold, 1988; Schuckit & Smith, 2000), or as a retrospective report of the need for more drinks for effects earlier in life (Schuckit et al., 1997a, 1997b). This lower intensity of response relates to heavier alcohol intake even among relatively light and infrequent drinkers as young as age 12 (Schuckit et al., 2008a; 2008b). In all studies to date, a lower LR earlier in life predicted later heavier drinking and alcohol problems even after controlling for alcohol intake and pattern of problems at the time the response to alcohol was measured (Schuckit, 2009; Schuckit et al., 2008a; 2008b; Schuckit et al., in press; Volavka et al., 1996). The ability to control for baseline quantity is an important step to minimize the possibility that a low LR reflects tolerance acquired through a prior pattern of heavier drinking. The heritability of LR as established through family or twin studies is between 40% and 60% (Schuckit et al., 2001; Schuckit et al., 2005; Schuckit et al., 2006).
Within a structural equation framework, a low LR to alcohol was related to the family history (FH) of AUDs, and was associated with heavier drinking and alcohol problems both directly and as partially mediated by heavier peer drinking, alcohol expectancies, and the use of alcohol to cope with stress (Schuckit et al., 2008a; 2008c; Schuckit et al., in press). Therefore, in light of the 40% or more contribution of environment to LR, the relationship between a low LR and later alcohol problems is best considered in the context of additional characteristics that relate to future AUDs. These include the FH of AUDs with studies demonstrating that the link between LR and alcohol outcomes tends to be stronger in family history positives (Schuckit & Smith, 1996), perhaps reflecting other risk factors in addition to LR (Schuckit, 2009), LR partially mediates the relationship between FH and adverse alcohol outcomes (Cotton, 1979; Jacob et al., 2003; Jennison & Johnson, 1998; Ohannassian et al., 2004; Schuckit et al., 2005; Schuckit & Smith, 2000). The familial pattern of AUDs is also associated with an earlier onset of drinking, which itself predicts a higher risk for future alcohol-related difficulties (Grant & Dawson, 1997; McGue et al., 2001; Obot et al., 2001; Prescott & Kendler, 1999; Seljamo et al., 2006; Warner et al., 2007; York et al., 2004). Earlier onset of alcohol use also relates to a range of additional problems including physical fights, driving arrests, drug use, and other antisocial behaviors (Hingson et al., 2001; King & Chassin, 2007; Kuperman et al., 2001; McGue et al., 2001). Studies have reported that once drug use and antisocial characteristics are considered, the relationship between an early onset of drinking and alcohol outcomes is likely to diminish (Dawson et al., 2008; King & Chassin, 2007; Kuperman et al., 2001; Labouvie & White, 2002).
Another characteristic associated with both LR and alcohol outcomes is heavier drinking and drug use earlier in life (Schulenberg & Maggs, 2008; Trim et al, 2008). The intake of alcohol can decrease the LR to alcohol, perhaps through the development of tolerance, and prior substance use patterns have been used as covariates in evaluations of LR (Eng et al., 2005; Schuckit & Gold, 1988). A person’s age is also important because the risk for heavy drinking and AUDs increases during adolescence and subsequently diminishes after age 30 (Hingson et al., 2008; Nelson et al., 1998), and the average age of onset of alcohol dependence is in the mid-20’s, although earlier problems are likely to be seen for men with preexisting antisocial problems (Liu et al., 2004; Ohannessian & Hesselbrock, 1993; Schuckit et al., 1998; Schuckit & Smith, 2001; Slutsky et al., 1998). Increasing age can also relate to a higher LR through higher blood alcohol concentrations (BACs) per drink due to higher body fat (with lower proportions of body water), slightly slower metabolism of alcohol, and increased brain sensitivity to depressant drugs with advancing age (Kalant, 1998; Lynsky et al., 2003; Wang et al., 2001).
Reflecting these data, LR has been compared across higher and lower AUD risk groups (e.g., children of alcoholics vs. FH negative subjects) while controlling for age, the number of years of drinking, percent body water, and the prior pattern of the use of alcohol and other drugs (Schuckit & Gold, 1988; Schuckit & Smith, 2000). However, while alcohol outcomes associated with a low LR have been documented in recent longitudinal evaluations (Trim et al., 2008), the associations between LR and the development of AUDs over time has not yet been fully examined. Also, potential interactions among predictors of AUDs in the context of a low LR have rarely been evaluated.
Therefore, this paper evaluated the relations between LR and other related risk factors (FH of AUDs, the age of drinking onset, prior drinking quantities, age, and body mass index) on the occurrence of AUDs across adulthood using discrete-time survival analysis (DTSA) with probands from the ongoing San Diego Prospective Study. Our primary hypothesis was that a low LR to alcohol would relate to the occurrence of AUDs across adulthood even after controlling for other robust risk factors for AUDs. We also expected that a positive FH of AUDs, early drinking onset, and higher levels of baseline drinking would all predict AUD occurrence. Furthermore, we proposed several interactions among the risk factors for AUDs including: an enhanced association for all risk factors on AUDs within FHP subjects, reflecting the fact that risk for AUDs is heterogeneous and FHP subjects with additional risk factors would be at increased risk for heavier drinking and AUDs in adulthood (Schuckit, 2009); higher levels of baseline typical quantity of alcohol use would facilitate and amplify the impact of other risk factors on AUDs by creating an environment in which those predictors would be likely to have a greater impact on problematic drinking; and an earlier onset of drinking would amplify the impact of additional risk factors through the lack of experience and less intense executive control likely to be observed in earlier adolescence (Brown et al., 2008).
II. Materials and Methods
Participants
These probands were originally recruited as 18–25 year old (mean age ~20) Caucasian drinking but not alcohol dependence men through questionnaires mailed to random students and nonacademic staff at the University of California, San Diego between 1978 and 1988 (Schuckit & Gold, 1988; Schuckit & Smith, 2000). Potential participants were subsequently evaluated in person with a semi-structured interview based on the Renard Diagnostic Interview and the Structured Clinical Diagnostic Interview (SCID) of the Diagnostic and Statistical Manual of Mental Disorders (DSM; American Psychiatric Association, 1987). All probands participated in an alcohol challenge with 0.75ml/kg (0.61gm/kg) of ethanol, consumed over 8–10 minutes, to evaluate their LR over three hours through self reports of subjective feelings of intoxication, alterations in body sway/standing steadiness, and alcohol-related changes in cortisol (Schuckit & Gold, 1988; Schuckit & Smith, 2000).
All 453 men were located approximately 10 years (T10) after the initial testing when 99% completed semi-structured interviews about their interval alcohol and drug use, and corroborating data was gathered from an additional informant, usually a spouse. At the 15 year followup (T15), 98% of the original subjects and resource persons were evaluated using an interview expanded to include material from the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) instrument and to gather information about additional areas of functioning including peer drinking, alcohol expectancies, and drinking to cope (Bucholz et al., 1994). The same data collection methods were used to gather information on these variables during the 20-year follow-up (T20, approximately 96% retention), and as part of the ongoing 25 year followup (T25) protocol with an estimated 94% data retention over the 25 years.
Measures
Level of Response to Alcohol
The LR value used here was obtained during a traditional alcohol challenge at T1 (~age 20; Schuckit & Gold, 1988; Schuckit & Smith, 2000). Changes in self-report of subjective feelings of intoxication, body sway/standing steadiness, and cortisol levels from before the drink to 60 minutes after consuming the alcohol (the usual time of peak blood alcohol concentration) were converted to z-scores relative to other individuals tested in the laboratory. The overall LR z-score was log-transformed for these analyses due to the right skewness of this variable.
Family History of Alcoholism
Probands reported on the presence of DSM-III-R alcohol abuse and dependence in their parents at T1 using an instrument similar to the Family History Module (FHAM) from the Collaborative Study on the Genetics of Alcoholism (COGA) protocol. The FHAM has a specificity of 98% and a positive predictive value of about 50%, with a positive indication associated with a 13.6 odds ratio of a diagnosis for the relative at interview (Rice et al., 1995).
Typical Alcohol Quantity
This continuous variable is the baseline self-reported typical number of standard (10–12g of ethanol) drinks per drinking occasion over the 6-months prior to the T1 interview. This variable is included to help ensure that the LR result is not a consequence of prior heavy drinking and acquired tolerance.
Age of Drinking Onset
At T1, probands self-reported the age at which they first consumed at least one standard drink.
Body Mass Index (BMI)
BMI at baseline was calculated from proband’s height (in inches) and weight (in pounds) using the formula: (Weight × 703)/(Height2).
Age
The proband’s chronological age at T1.
Alcohol Use Disorders (AUDs) at T10/T15/T20/T25
Probands were assessed for lifetime DSM-III-R alcohol abuse and dependence at each follow-up using an interview similar to the SSAGA instrument. If positive for AUD, the instrument probed the earliest age at which diagnostic criteria were met for AUD. That interview has Cronbach’s alpha >.85, 1-wk reliabilities ~.8 for substance disorders, and correlations with diagnoses from the SCAN average ~.7 (Bucholz et al., 1994). Through the T25 assessment, 35.0% of this sample had been diagnosed with an AUD at some point in their lifetime.
Data Structure
In order to estimate the DTSA models in a latent variable framework, the raw data were prepared as outlined by Muthen & Masyn (2005). Since there was considerable age heterogeneity at each measurement wave, we modeled the hazard/survival curves as a function of age rather than of measurement occasion. A total of 37 binary time-specific event indicators were constructed to reflect yearly intervals between the youngest proband age at baseline interview (20 years) to the oldest proband age at the most recent interview (55 years) for this subsample (note that those probands aged 18–19 at baseline had not yet been interviewed at the current T25 assessment). For reasons of model parsimony and interpretation, these yearly intervals were then collapsed into five-year intervals resulting in seven age categories (referred to as w1–w7): 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–55 years (this also helped ensure convergence during model estimation). The proband was considered to have experienced the event of interest (AUD occurrence) at the earliest age they met criteria for AUD, designated by a “1” score on the binary time-specific event indicator for the corresponding age interval; prior intervals were scored “0” to reflect no event, and subsequent intervals were scored “99” to reflect missing due to having experienced the event. For example, the event history for a proband with AUD onset at age 31 would be [0, 0, 1, 99, 99, 99, 99]. Right-censoring occurred when a proband did not report an AUD onset by the T25 assessment (mean age 47 years). Such probands would have “0” scores for all intervals that included up to their age at most recent interview, with any subsequent intervals being considered missing with a score of “99”. For example, the event history for a proband with no AUD onset by age 43 would be [0, 0, 0, 0, 0, 99, 99]. The frequency of right-censored probands across the age intervals was: 0 for w1–w4, 1 for w5, 42 for w6, and 152 for w7. This illustrates a relative sparseness of data at the last age interval (50+ years) due to the fact that the average proband age during the most recent assessment (T25) was 47 years. It should be noted that since no probands met criteria for AUD at the baseline interview (per screening requirements), no left-censoring was needed for the current analyses.
Data Analytic Approach
The relations between baseline covariates and the outcome of AUDs were evaluated with a series of discrete-time survival analyses (DTSA) conducted using a latent hazard function representing the event time distribution. The discrete-time hazard is the conditional probability that an individual will be diagnosed with an AUD in a time period, given that he was not diagnosed in any earlier time period (Singer & Willett, 2003). The DTSA model has been described as “a number of logistic regressions fitting the incremental probability of survival” (page 185; Asparouhov et al., 2006), which informs both whether and when an event of interest (here, an AUD diagnosis) occurs. A related indicator of event occurrence is the survival function, which is the sample’s cumulative probability of not being diagnosed with an AUD over time, and which is expressed as a function of the hazard function (Muthen & Masyn, 2005). This survival function has been shown to approximate the Cox regression model used in traditional continuous time survival models, and is preferred when the data are categorical and the number of categories is less than 20 (Asparouhov et al., 2006).
The modeling approach followed procedures outlined by Muthen & Masyn (2005) wherein conventional DTSA is considered a special case within the general latent variable framework which corresponds to a single-class latent class analysis with binary time-specific event indicators. The first step was to fit an unconditional survival model that included only the seven binary time-specific event indicators for the occurrence of AUDs across adulthood. The constant hazard assumption was then evaluated by comparing the unconditional survival model, which allowed the hazard rate to vary across time, to a model that constrained the hazard rate to equality across intervals using a likelihood-ratio test (LRT) based on the model deviance statistics.
The next step evaluated the proportionality assumption for each of the covariates to determine whether the covariate effects were identical across all time points. Proportionality was assessed separately for each covariate by comparing a model with time-varying covariate effects to a model that constrained the covariate effects to equality across time. If the unconstrained covariate model fit better than the constrained model (based on LRTs), the covariate violated the proportionality assumption. In subsequent analyses, proportionality was relaxed for such covariates to allow for time-varying effects on the occurrence of AUD across adulthood.
A multivariate model including all the covariates was then estimated such that the latent hazard function with its seven binary time-specific event indicators was regressed on the set of time-invariant covariates (all factor loadings fixed to 1.0 to reflect proportional covariate effects across time) and any covariate that violated the proportionality assumption had freely estimated regression paths on the binary time-specific event indicators to allow for time-varying effects. The results of this model allowed interpretation for each individual covariate effect on AUD occurrence after adjusting for all other covariates.
Follow-up analyses evaluated a series of statistical interactions in the DTSA model to determine whether the linear additivity assumption had been violated. This assumption postulates that differences in the value of a predictor correspond to fixed differences in the hazard rate, such that a predictor’s effect does not depend upon the values of other predictors in the model. Following the procedure of Singer & Willett (2003), these analyses were restricted to examine interactions among the robust risk factors of AUDs (LR to alcohol, family history, baseline drinking, age of onset) as described previously. Each of the six possible interactions was tested by creating a cross-product term and adding it to a model that included both main effect covariates. This model was compared to a model excluding the cross-product using LRT to determine whether the inclusion of each interaction significantly improved model fit.
III. Results
These data were generated from the first 297 SDPS probands evaluated in the ongoing 25-year (T25) follow-up of this longitudinal study. The current group is similar to those men not yet eligible for the T25 assessment on education, income, religion, and drinks per occasion at the prior followup (T20). Probands in the current sample were different from those not yet evaluated on T20 measures of age (41.9 vs. 40.9 years, t(425)=3.32, p=.001), ethnicity (2% vs. 6% white Hispanic, χ2(1)=4.87, p=.03), and marital status (75% married/10% divorced vs. 82% married/3% divorced, χ2(2)=6.22, p=.04). At T25, the subjects reported on here had a mean age 41.9 years; all were Caucasian (including 2% white Hispanic subjects); they had completed an average of 17.5 years of education; 75% were married or living as married, 10% were separated or divorced, 15% had never been married; and regarding religion, 46% reported no preference, 30% were Protestant, 20% were Catholic, and 4% reported another religion.
Table 1 reports descriptive statistics for all covariate predictors measured at the baseline assessment (~age 20). In addition to the demographics described above, this subsample had a mean LR score of −.47 which reflects a lower average LR than would be expected since LR was converted to z-score at baseline for the full sample. 40% of the probands had a parent meet criteria for AUD at T1. These subjects had an average of less than 3 drinks per drinking occasion at T1, and an average onset of drinking at ~15 years. The mean BMI was 22 (in the “normal weight” range), and mean age ~23 years at T1. A third of the sample met criteria for AUD by the T25 followup assessment.
Table 1.
Descriptive Data for Covariates Measured at Baseline (n=297)
| Mean | Std Dev | Range | |
|---|---|---|---|
| LR | −.47 | .80 | −3.14 – +2.05 |
| FH | .40 | .49 | 0–1 |
| Quant | 2.71 | 1.41 | 1–8 |
| Onset | 14.80 | 2.33 | 7–21 |
| BMI | 22.40 | 2.16 | 18–37 |
| Age | 22.68 | 1.75 | 20–28 |
Note. LR (Level of response to alcohol) at baseline (~age 20) is the z-score generated by the sum of up to three variables representing change from baseline to 60 minutes after the drink of three variables during an alcohol challenge; FH (family history of AUDs) is the presence of alcohol abuse or dependence in either biological parent at T1; Quant is the typical number of standard drinks consumed per drinking occasion over the past 6 months reported at T1; Onset is the age at which probands reported first having one standard drink; BMI is the body mass index calculated from height and weight at T1; Age is the age of probands at T1
Prior to analyses, all continuous variables were first centered (average score subtracted from each raw score) to reduce confounds due to multicollinearity, and the binary variable of family history was set to values of 0 and 1. All models were run in Mplus v5.1 (Muthen & Muthen, 1998–2007) with full information maximum likelihood (FIML) estimation under the assumption of data missing at random (MAR) with robust standard errors. In order to increase confidence in the final maximum likelihood values, automatically generated starting values with random perturbations (100 random sets of starting values with 10 full optimizations) were used for all models described below.
Unconditional Survival Model
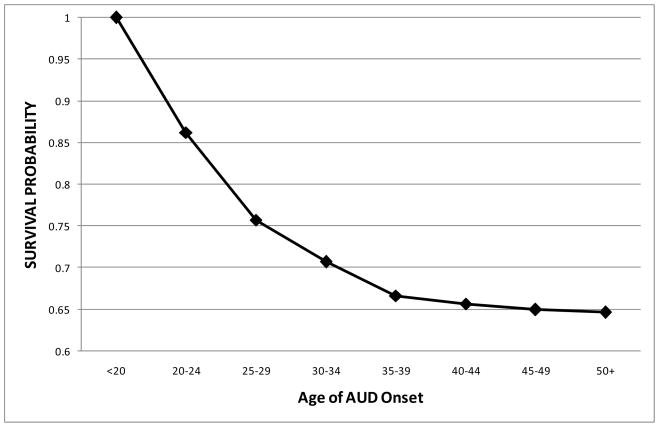
We first fitted an unconditional discrete-time survival model for the occurrence of AUDs across adulthood in the probands using only the binary time-specific event indicators. The estimated hazard function captured the conditional probability that an individual would be diagnosed with an AUD in a given age interval given that he was not diagnosed in an earlier interval, and this was used to calculate the survival function over time. Figure 1 illustrates a steep decrease in survival curve of AUD occurrence in early adulthood that gradually leveled out in the 30’s and 40’s. The probability of not having an AUD through age 55 in this sample was .65. The constant baseline hazard assumption was next evaluated to assess whether the hazard rate varied significantly across adulthood. The unconditional hazard model (with time-varying hazard rates) was found to significantly improve fit compared to a model that constrained the hazard rate to equality (χ2 = 51.166, df=6, p<.001). Thus, the constant baseline hazard assumption was rejected and the hazard function was allowed to vary across age intervals in all models.
Figure 1.
Fitted survival probabilities for onset of AUD across adulthood (n=297).
Univariate Covariate Effects
The next step evaluated the proportionality assumption for each of the covariates by comparing a model with time-varying covariate effects (non-proportional) to a model that constrained the covariate effects to equality (proportional). The only variable that resulted in a significantly better model fit when allowed to vary across time was Time 1 age (χ2 = 33.108, df=6, p=.01). The parameter estimates and odds ratios for the univariate effects of age (allowed to vary across time) and the other five covariates (assumed to be proportional) are listed in Table 2 under the Univariate Effects heading. All covariate effects, except for BMI, were significant in the expected direction: LR to alcohol and age of alcohol onset were negatively related to the hazard function such that a low LR and an earlier age of alcohol onset were associated with AUD occurrence; family history of AUD and T1 typical quantity were positively related to the hazard function such that having an alcoholic parent and higher levels of baseline drinking were associated with AUDs across adulthood; and there was a negative relation between T1 age and AUD occurrence in the 20’s (significant in the 20–24 year range) such that an older age at the baseline interview was associated with a lower occurrence of AUD; this trend reversed in subsequent years with an older age at baseline interview becoming associated with a higher rate of AUD occurrence (significant in the 45–49 year range). The parameter estimates listed for the age intervals represent the negative logits of the hazard probability for each age interval and the corresponding percentages listed under odds ratios are the probability of AUD onset for those probands not considered missing at each age interval.
Table 2.
Predictors of the AUD Hazard Function (n=297)
| Univariate Effects |
Full Model |
|||||
|---|---|---|---|---|---|---|
| Est (SE) | t | OR (95% CI) | Est (SE) | t | OR (95% CI) | |
| Age 20–24 (w1) | 1.83 (.17)*** | 10.89 | 13.8% | 2.60 (.26)*** | 10.07 | 6.9% |
| Age 25–29 (w2) | 1.98 (.19)*** | 10.35 | 12.1% | 2.23 (.22)*** | 10.08 | 9.7% |
| Age 30–34 (w3) | 2.64 (.27)*** | 9.87 | 6.7% | 3.00 (.36)*** | 8.28 | 4.8% |
| Age 35–39 (w4) | 2.80 (.30)*** | 9.43 | 5.7% | 3.04 (.33)*** | 9.21 | 4.6% |
| Age 40–44 (w5) | 4.17 (.58)*** | 7.17 | 1.5% | 4.88 (.90)*** | 5.43 | 0.7% |
| Age 45–49 (w6) | 4.32 (.71)*** | 6.08 | 1.3% | 5.69 (.70)*** | 8.11 | 0.3% |
| Age 50+ (w7) | 3.69 (1.01)*** | 3.64 | 2.4% | 4.76 (1.08)*** | 4.41 | 0.8% |
| LR | −1.46 (.46)** | −3.15 | .23 (.09–.57) | −1.42 (.44)** | −3.27 | .24 (.10–.57) |
| FH | .48 (.21)* | 2.29 | 1.61 (1.07–2.43) | .39 (.22)† | 1.78 | 1.48 (.96–2.27) |
| Quant | .37 (.07)*** | 5.47 | 1.44 (1.26–1.66) | .35 (.07)*** | 5.17 | 1.41 (1.23–1.63) |
| Onset | −.11 (.04)* | −2.59 | .89 (.83–.97) | −.09 (.05)† | −1.90 | .92 (.83–1.01) |
| BMI | −.00 (.04) | −.03 | .99 (.93–1.08) | −.01 (.05) | −.28 | .99 (.90–1.09) |
| w1 on Age | −.67 (.13)*** | −4.98 | .51 (.40–.66) | −.61 (.13)*** | −4.51 | .55 (.42–.70) |
| w2 on Age | −.10 (.11) | −.98 | .90 (.73–1.12) | −.04 (.12) | −.36 | .96 (.76–1.22) |
| w3 on Age | .25 (.18) | 1.36 | 1.28 (.90–1.82) | .32 (.19) | 1.63 | 1.37 (.95–2.00) |
| w4 on Age | .08 (.16) | .48 | 1.08 (.79–1.48) | .14 (.17) | .81 | 1.15 (.82–1.61) |
| w5 on Age | .49 (.35) | 1.42 | 1.63 (.82–3.24) | .61 (.40) | 1.50 | 1.83 (.84–4.03) |
| w6 on Age | .69 (.13)*** | 5.46 | 1.99 (1.54–2.57) | .88 (.12)*** | 7.49 | 2.41 (1.91–3.05) |
| w7 on Age | .19 (.13) | 1.46 | 1.21 (.93–1.56) | .45 (.14)** | 3.16 | 1.58 (1.19–2.06) |
p<.10.
p< .05.
p<.01.
p<.001.
Note. Refer to Table 1 for explanation of abbreviations. The OR values listed for the age intervals under Univariate Effects represent the probability of AUD onset for non-missing probands at each interval. The OR values listed for the age intervals under Full Model represent the probabilities of AUD onset for probands with zero scores on the covariates not missing at each interval.
Full Multivariate Survival Model
Finally, we estimated all covariates simultaneously to determine each individual covariate effect on AUD occurrence across adulthood after adjusting for all other covariates. Figure 2 illustrates the final multivariate model estimated in Mplus, with the binary time-specific event indicators each regressed on T1 age to allow for time-varying effects, and the latent hazard function (estimated from the seven binary time-specific event indicators) regressed on the set of covariates assumed to be proportional across time. Note that the discrete-time survival model is specified as a single-class latent class analyses (represented by the constant latent class “c” variable) in order to obtain the logistic estimates of interest within the SEM framework (Muthen & Masyn, 2005). The results of this model are found in Table 2 under the Full Model heading. After adjusting for all other covariates, the LR to alcohol and Time 1 typical quantity were still significant predictors of the AUD occurrence in adulthood, while the effects of family history (b=.39, p=.076) and age of onset (b=−.09, p=.058) were reduced to marginal significance. Body mass index remained non-significant and the effects of T1 age on the AUD hazard function remained significant in 20–24 and 45–49 age range, and became significant in the 50+ age range. The parameter estimates listed for the age intervals represent the negative logits of the hazard probability for each age interval and the corresponding percentages listed under odds ratios are the probability of AUD onset for those probands with zero scores on all covariates (i.e. FHN probands with an average score on all centered continuous covariates) not considered missing at each age interval.
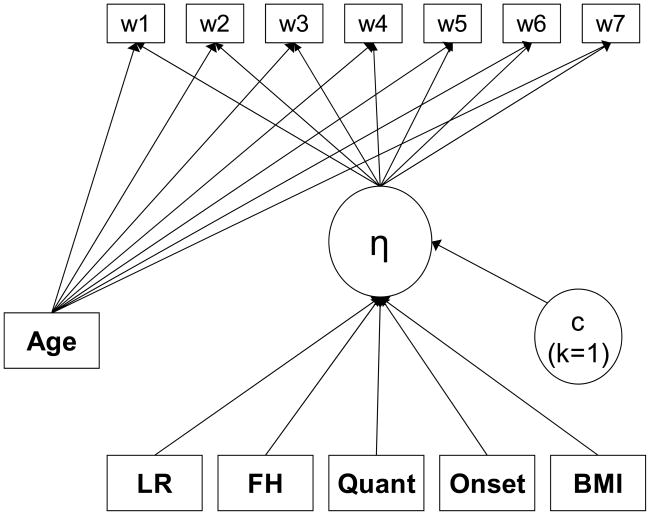
Figure 2.
Full DTSA model for AUD occurrence as estimated in Mplus. The hazard function (η) is defined by the absence or presence of an AUD at each of the seven age intervals. The effects of T1 age were allowed to vary across time; all other predictors were proportional and had time-invariant effects on the hazard function. LR= Level of response to alcohol; FH= Family history of AUDs at T1; Quant= T1 typical drinking quantity; Onset= Age of drinking onset; BMI= Body Mass Index at T1; AGE= Age at T1 interview. Note that the latent variable “c” reflects that a latent class analysis with one class (k=1) was used for estimation purposes.
Expressed in terms of odd ratios, the AUD occurrence among FHP subjects was 48% larger than among FHN subjects (HR=1.48); probands with a 1-drink increase in T1 drinking quantity were diagnosed with an AUD at a 41% higher rate than those with the lower T1 drinking quantity (HR=1.41); and those with a 1-year increase in age of drinking onset were diagnosed with an AUD at a 8% lower rate than those with the earlier age of drinking onset (HR=0.92). The hazard ratio for the continuous LR score was difficult to place into perspective due to prior standardization and log-transformation. Therefore, the full model from Table 2 was re-run using a categorical measure of LR based on a median split of the continuous LR score used in these analyses, revealing that the AUD rate among high LR subjects was 53% smaller than among low LR subjects (HR=0.47).
Interaction Effects among Risk Factors
As described earlier, a set of follow-up analyses were conducted to determine whether the linear additivity assumption could be confirmed regarding the robust risk factors examined in these models. A total of six 2-way interactions were created from the alcohol-related covariates of LR, family history, T1 typical quantity, and age of alcohol onset. Two interactions yielded a significant LRT when included in the full model from Table 2: family history by T1 quantity (χ2 = 4.146, df=1, p=.04; b=.30, p=.03) and age of onset × T1 quantity (χ2 = 4.464, df=1, p=.03; b=.07, p=.01). Thus, allowing for these interactions improved overall model fit and there was evidence to reject the linear additivity assumption for these covariate effects.
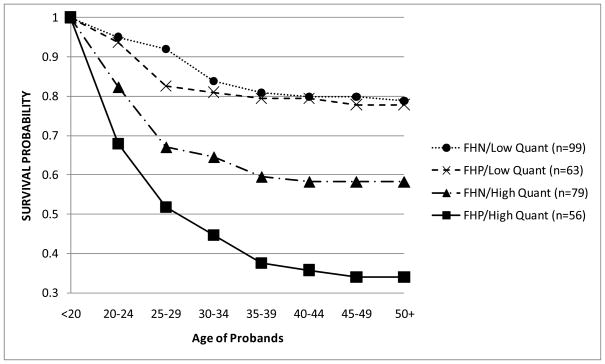
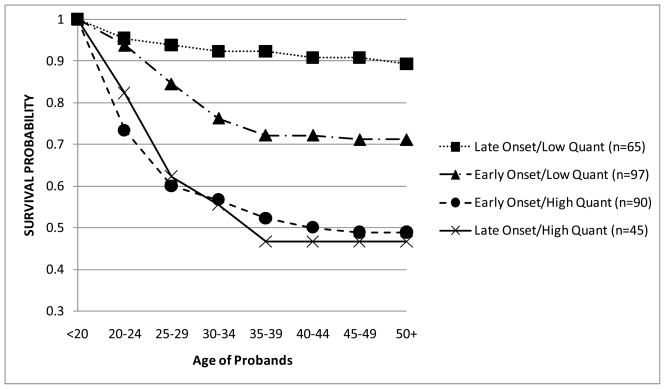
In order to better visualize these two significant interactions, a series of tables (not shown) for the occurrence of AUD across adulthood was created using dichotomized subgroups of the component covariates of the interactions. For these analyses, FH was still treated as a binary category (FHN/FHP), while the age of drinking onset (<age 15 vs. 15+) and the T1 drinking quantity (< 3 vs. 3+) were dichotomized using a median split on each variable. For each significant interaction, four subgroups were created from the variables described above, with the survival function then calculated for each subgroup individually and graphed over time. Figure 3 describes the development of AUDs over time by four combinations of the FH and T1 drinking quantity groupings. If alcohol intake at ~age 20 was low, the survival rate (i.e. those not diagnosed with an AUD by T25) was ~78% regardless of the FH. The impact of FH, however, was apparent among the high T1 drinking quantity group where the survival rate was lower for FHP (34%) compared to FHN (58%). Figure 4 illustrates the survival curves of AUD for the combinations of T1 drinking quantity and the age of drinking onset. Here, probands with high T1 drinking quantity, regardless of the age of drinking onset, had the lowest survival rates of being diagnosed with an AUD at ~47%. Those with an early onset and a low T1 drinking quantity had a survival rate of 71%, and the probands with neither risk factor (i.e. late onset and low T1 quantity) had the highest survival rate at 89%.
Figure 3.
Survival probabilities of AUDs by family history/T1 drinking quantity subgroups. Groups were created by median split of T1 quantity (<3 drinks and 3+ drinks per occasion) and FHP/FHN categories.
Figure 4.
Survival probabilities of AUDs by age of drinking onset/T1 drinking quantity subgroups. Groups were created by median split of age of drinking onset (<15 years and 15+ years) and T1 quantity (<3 drinks and 3+ drinks per occasion) categories.
IV. Discussion
A low LR to alcohol has been related to a higher risk for alcohol use disorders in a wide range of studies (Schuckit, 2009). This paper extends these findings by applying a discrete-time survival analysis to more precisely identify the relation between a low LR measured early in life and the occurrence of AUDs across adulthood.
The current results offer support for our primary hypotheses that a low LR to alcohol predicts a higher rate of AUD onset over time in adult probands from the ongoing San Diego Prospective Study. The effect of LR was present even after controlling for other robust risk factors for AUDs, such as the FH of AUDs, the age of drinking onset, and baseline drinking levels. The inclusion of baseline quantity as a covariate helps ensure that LR was not just a consequence of earlier heavier drinking or tolerance. These data provide prospective evidence that the LR to alcohol is a unique risk factor for alcohol-related problems, and not simply a reflection or “marker” for a broader range of risk factors which influence adulthood AUD (King et al., 2006; Trim et al., 2007).
The use of discrete-time survival analyses offers a more clear analytic and graphical depiction than previous studies regarding the patterns of onset of AUDs with increasing age in this sample. Figure 2 illustrates that the greatest drop in the survival curve for the entire sample occurs between ages 20 and 30, which is consistent with previous literature identifying this period of early adulthood to be associated with the highest risk for alcohol problems and AUD onset (Hasin et al., 2007; Kessler et al., 2005). While 69% of probands who become diagnosed with an AUD in this sample did so before age 30, the number of subjects with an onset of AUD after age 30 was still notable. Even though the rates of new AUD diagnosis decreased later in life, 6% of probands in this subsample became newly diagnosed after age 40. The continued investigation of the clinical profile and risk factors associated with AUD onset later in life is important to the current ongoing study, and these unique individuals may represent a relatively understudied high-risk population deserving of further clinical attention (Jacob et al, 2005).
It should be noted that an early drinking onset, the baseline drinking quantity, and FH all had significant univariate effects on AUD occurrence across adulthood; these relations became marginal for FH and early drinking onset after adjusting for all other covariates. This finding is consistent with a wide range of previous studies that have shown each of these risk factors to be related to alcohol use and problems over time (Grant & Dawson, 1997; King & Chassin, 2007; Schuckit et al., 2008a). A younger baseline age predicted AUDs in the early 20’s, which may identify a high-risk subgroup with a rapid onset of alcohol problems even after indirectly controlling for the duration of the drinking career with the age of onset covariate, while an older baseline age predicted AUDs in the late 40’s and 50’s, which may reflect a smaller subgroup at risk for AUDs due to late-life consequences associated with longer drinking careers. Body mass index (reflecting a standardized weight to height ratio) was not associated with the AUD onset, which may reflect the fact that original alcohol dose was based on weight and the two major risk groups (based on FH) were matched on height to weight ratio.
The secondary hypotheses related to the prediction of interactions among the more robust predictors of developing an AUD. Indeed, the FH of AUDs and baseline drinking did interact such that the impact of FH was only observed for subjects with higher T1 drinking levels. This may indicate that efforts to help children of alcoholics to keep their drinking quantities low in the late teens and early adulthood may help decrease the AUD risk associated with their FH. While it is also possible that additional characteristics such as lower socioeconomic status (SES) and higher levels of antisocial behavior might have been more prevalent in those with higher early drinking, these mechanisms may be less likely to operate in the higher educated, higher income, more functional SDPS probands studied here.
Another interaction effect occurred wherein the presence of either early onset or high baseline drinking (absent the other risk factor) independently increased AUD rates over time, but the combined effects were no greater than those for high baseline drinking only. In this regard, high baseline drinking appears to have subsumed any unique effects attributable to early onset drinking, and the effects of early onset drinking on AUD occurrence in adulthood may at least be partially mediated by drinking levels in early adulthood. It is also interesting to note that there were no interactions with LR. Thus, at least within the SDPS sample, LR appeared to function as a robust predictor of AUDs regardless of the presence or absence of other risk factors.
While this longitudinal evaluation provides greater understanding of the unique impact of a low LR to alcohol on the development of AUDs across adulthood, several caveats should be noted. First, once probands were diagnosed at any given assessment they were considered to have experienced that event even if their diagnosis remitted at subsequent assessments. Future survival-based studies will examine both the onset and offset of AUDs, possibly using the “multiple spells” methodology which captures repeatable outcomes over time (Lavori et al., 1996; Singer & Willett, 2003). The SDPS is in a unique position to follow-through on such analyses with additional future data collection, as well as examine specific characteristics that predict subsequent remission for probands diagnosed with AUD at the first followup (T10). Second, the predictors were all taken from the baseline assessment to ensure temporal precedence. While time-varying effects of these predictors were examined (and allowed for in the case of T1 age) in the tests for proportionality, future studies will expand on these findings by evaluating the effects of time-varying covariates measured at the followups (i.e. proband drinking, peer drinking, drinking to cope) on AUD occurrence using DTSA techniques. Third, the outcome of interest was based on retrospective reports of the age of first meeting criteria for AUD, and reliability could be a concern due to the timing gap between assessments (10 years for the first followup, 5 years for each subsequent followup). However, there is recent evidence that retrospective report of similar drinking measures is fairly stable and reliable well into the 50’s (Koenig et al., in press). Finally, these results were drawn for a modest sized sample of high-functioning Caucasian males, and future replications using women, non-Caucasians, and lower SES and more antisocial groups are needed to establish the generalizability of these findings to more diverse populations. The application of these DTSA models will also be critical in determining whether LR measured in adolescence operates in a similar fashion for the onset of a range of alcohol outcomes (i.e. onset of drinking, onset of negative consequences) using future assessments of SDPS offspring.
Acknowledgments
This work was supported by NIAAA 5R01 AA005526; NIAAA T32 AA013525-05; the Veterans Affairs Research Service; and by funds provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R) 3. American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- Asparouhov T, Masyn K, Muthen B. Continuous time survival in latent variable models. ASA section on Biometrics; Proceedings of the Joint Statistical Meeting; Seattle. August 2006; 2006. pp. 180–187. [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Martin C, Chung T, Tapert SF, Sher K, Winters KC, Lowman C, Murphy S. Developmental perspective on alcohol and youths. Pediatrics. 2008;121:s290–s310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new semistructured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3. Erlbaum; Mahwah, NJ: 2003. [Google Scholar]
- Cotton NS. The familial incidence of alcoholism: a review. J Stud Alcohol. 1979;40:89–116. doi: 10.15288/jsa.1979.40.89. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng MY, Schuckit MA, Smith TL. The level of response to alcohol in daughters of alcoholics and controls. Drug Alcohol Depend. 2005;79:83–93. doi: 10.1016/j.drugalcdep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States. Arch Gen Psychiat. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Edwards EM. Age at drinking onset, alcohol dependence, and their relation to drug use and dependence, driving under the influence of drugs, and motor-vehicle crash involvement because of drugs. J Stud alcohol Drugs. 2008;69:192–201. doi: 10.15288/jsad.2008.69.192. [DOI] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Zakocs R. Age of drinking onset and involvement in physical fights after drinking. Pediatrics. 2001;108:872–877. doi: 10.1542/peds.108.4.872. [DOI] [PubMed] [Google Scholar]
- Jacob T, Waterman B, Heath C, True W, Bucholz KK, Haber R, Scherrer J, Fu Q. Genetic and environmental effects on offspring alcoholism. Arch Gen Psychiatry. 2003;60:1265–1272. doi: 10.1001/archpsyc.60.12.1265. [DOI] [PubMed] [Google Scholar]
- Jacob T, Bucholz KK, Sartor CE, Howell DN, Wood PK. Drinking trajectories from adolescence to the mid-forties among alcohol dependent males. J Stud Alcohol. 2005;66:745–755. doi: 10.15288/jsa.2005.66.745. [DOI] [PubMed] [Google Scholar]
- Jennison K, Johnson K. Alcohol dependence in adult children of alcoholics: longitudinal evidence of early risk. J Drug Educ. 1998;28:19–33. doi: 10.2190/BRRQ-W96E-UGJN-GA9R. [DOI] [PubMed] [Google Scholar]
- Kalant H. Pharmacological interactions of ageing and alcohol, in Alcohol Problems and Ageing. In: Gomberg ESL, Hegedus AM, Zucker RA, editors. Research Monograph No. 33. Bethesda: US Department of Health and Human Services; 1998. pp. 99–116. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey replication. Arch Gen Psychiat. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- King KM, Chassin L. A prospective study of the effects of age of initiation of alcohol and drug use on young adult substance dependence. J Stud Alcohol Drugs. 2007;68:256–265. doi: 10.15288/jsad.2007.68.256. [DOI] [PubMed] [Google Scholar]
- King KM, Meehan BT, Trim RS, Chassin L. Marker or mediator? The effects of adolescent substance use on young adult educational attainment. Addiction. 2006;101:1730–1740. doi: 10.1111/j.1360-0443.2006.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig LB, Jacob T, Haber JR. Validity of the lifetime drinking history: A comparison of retrospective and prospective quantity-frequency measures. J Stud Alcohol Drugs. doi: 10.15288/jsad.2009.70.296. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman S, Schlosser SS, Kramer JR, Bucholz K, Hesselbrock V, Reich T, Reich W. Risk domains associated with an adolescent alcohol dependence diagnosis. Addiction. 2001;96:629–636. doi: 10.1046/j.1360-0443.2001.96462911.x. [DOI] [PubMed] [Google Scholar]
- Labouvie E, White HR. Drug sequences, age of onset, and use trajectories as predictors of abuse/dependence in young adulthood. In: Kandel DB, editor. States and Pathways of Involvement in Drug Use: Examining the Gateway Hypothesis. New York: Cambridge Univ. Press; 2002. pp. 19–41. [Google Scholar]
- Lavori PW, Dawson R, Mueller TI, Warshaw M, Swartz A, Leon A. Analysis of the course of psychopathology: transitions among states of health and illness. Int J Method Psych. 1996;6:321–334. [Google Scholar]
- Liu I-C, Blacker DL, Xu R, Fitzmaurice G, Lyons MJ, Tsuang MT. Genetic and environmental contributions to the development of alcohol dependence in male twins. Arch Gen Psychiatry. 2004;61:897–903. doi: 10.1001/archpsyc.61.9.897. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Day C, Hall W. Alcohol and other drug use disorders among older-aged people. Drug Alcohol Rev. 2003;22:125–133. doi: 10.1080/09595230100100552. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II. Familial risk and heritability. Alcohol Clin Exp Res. 2001;25:1166–1173. [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. Associations with substance use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25:1156–1165. [PubMed] [Google Scholar]
- Muthen BO, Masyn K. Discrete-time survival mixture analysis. J Educ Behav Stat. 2005;30:27–58. [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide. 4. Muthen & Muthen; Los Angeles, CA: 1998–2007. [Google Scholar]
- Nelson CB, Heath AC, Kessler RC. Temporal progression of alcohol dependence symptoms in the U.S. Household Population: Results from the National Comorbidity Survey. J Consulting Clin Psychology. 1998;66:474–483. doi: 10.1037//0022-006x.66.3.474. [DOI] [PubMed] [Google Scholar]
- Obot I, Wagner F, Anthony J. Early onset and recent drug use among children of parents with alcohol problems: data from a national epidemiologic survey. Drug Alcohol Depend. 2001;65:1–8. doi: 10.1016/s0376-8716(00)00239-8. [DOI] [PubMed] [Google Scholar]
- Ohannessian CM, Hesselbrock VM. The influence of perceived social support on the relationship between family history of alcoholism and drinking behaviors. Addiction. 1993;88:1651–1658. doi: 10.1111/j.1360-0443.1993.tb02040.x. [DOI] [PubMed] [Google Scholar]
- Ohannessian CM, Hesselbrock VM, Kramer J, Kuperman S, Bucholz KK, Schuckit MA, Nurnberger JI., Jr The relationship between parental alcoholism and adolescent psychopathology: A systematic examination of parental comorbid psychopathology. J Abnorm Child Psychol. 2004;32:519–533. doi: 10.1023/b:jacp.0000037781.49155.a6. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: A noncausal association. Alcohol Clin Exp Res. 1999;23:101–107. [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–23. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treatment. 2009;36:s5–s14. [PubMed] [Google Scholar]
- Schuckit MA, Daeppen J-B, Tipp JE, Hesselbrock M, Bucholz KK. The clinical course of alcohol-related problems in alcohol dependent and nonalcohol dependent drinking women and men. J Stud Alcohol. 1998;59:581–590. doi: 10.15288/jsa.1998.59.581. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, Bierut L, Goate A, Foroud T. A genome-wide search for genes that relate to a low level of response to alcohol. Alcohol Clin Exp Res. 2001;25:323–329. [PubMed] [Google Scholar]
- Schuckit MA, Gold EO. A simultaneous evaluation of multiple markers of ethanol/placebo challenges in sons of alcoholics and controls. Arch Gen Psychiatry. 1988;45:211–216. doi: 10.1001/archpsyc.1988.01800270019002. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. J Stud Alcohol. 2000;61:827–835. doi: 10.15288/jsa.2000.61.827. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The clinical course of alcohol dependence associated with a low level of response to alcohol. Addiction. 2001;96:903–910. doi: 10.1046/j.1360-0443.2001.96690311.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Anderson KG, Brown SA, Kuperman S, Kramer J, Hesselbrock V, Bucholz K. Evaluation of a level of response to alcohol-based structural equation model in adolescents. J Stud Alcohol. 2005;66:174–184. doi: 10.15288/jsa.2005.66.174. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Trim RS, Bucholz KK, Edenberg HJ, Hesselbrock V, Kramer J, Dick DM. An evaluation of the full level of response to alcohol model of heavy drinking in COGA offspring. J Stud Alcohol Drugs. doi: 10.15288/jsad.2009.70.436. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Pierson J, Danko GP, Beltran IA. Relationships among the level of response to alcohol and the number of alcoholic relatives in predicting alcohol-related outcomes. Alcohol Clin Exp Res. 2006;30:1308–1314. doi: 10.1111/j.1530-0277.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Tipp JE. The Self-Rating of the Effects of Alcohol (SRE) form as a retrospective measure of the risk for alcoholism. Addiction. 1997a;92:979–988. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim RS, Heron J, Horwood J, Davis JM, Hibbeln JR the ALSPAC Study Team. The performance of elements of a “level of response to alcohol”-based model of drinking behaviors in 13-year-olds. Addiction. 2008a;103:1786–1792. doi: 10.1111/j.1360-0443.2008.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim RS, Heron J, Horwood J, Davis JM, Hibbeln JR the ALSPAC Study Team. The self-rating of the effects of alcohol questionnaire as a predictor of alcohol-related outcomes in 12-year-old subjects. Alcohol Alcohol. 2008b;43:638–643. doi: 10.1093/alcalc/agn077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim R, Kreikebaum S, Hinga B, Allen R. Testing the level of response to alcohol-based model of heavy drinking and alcohol problems in the offspring from the San Diego Prospective Study. J Stud Alcohol Drugs. 2008c;69:571–579. doi: 10.15288/jsad.2008.69.571. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Wiesbeck GA, Kalmijn J. The relationship between self-rating of the effects of alcohol and alcohol challenge results in ninety-eight young men. J Stud Alcohol. 1997b;58:397–404. doi: 10.15288/jsa.1997.58.397. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Wilhelmsen K, Smith TL, Feiler H, Lind P, Lange L, Kalmijn J. Autosomal linkage analysis for the level of response to alcohol. Alcohol Clin Exp Res. 2005;29:1976–1982. doi: 10.1097/01.alc.0000187598.82921.27. [DOI] [PubMed] [Google Scholar]
- Schulenberg JE, Maggs JL. Destiny matters: distal developmental influences on adult alcohol use and abuse. Addiction. 2008;103:1–6. doi: 10.1111/j.1360-0443.2008.02172.x. [DOI] [PubMed] [Google Scholar]
- Seljamo S, Aromaa M, Koivusilta L, Rautava P, Sourander A, Helenius H, Sillanpää M. Alcohol use in families: a 15-year prospective follow-up study. Addiction. 2006;101:984–992. doi: 10.1111/j.1360-0443.2006.01443.x. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. It’s about time: using discrete-time survival analysis to study duration and the timing of events. J Educ Stat. 1993;18:155–195. [Google Scholar]
- Slutske WE, Heath AC, Dinwiddie SH, Madden PAF, Bucholz KK, Dunne MP, Statham DJ, Martin NG. Common genetic risk factors for conduct disorder and alcohol dependence. J Abnorm Psychology. 1998;107:363–374. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- Trim RS, Meehan BT, King KM, Chassin L. The relation between adolescent substance use and young adult internalizing symptoms: findings from a high-risk longitudinal sample. Psychol Addict Behav. 2007;21:97–107. doi: 10.1037/0893-164X.21.1.97. [DOI] [PubMed] [Google Scholar]
- Trim RS, Schuckit MA, Smith TL. Level of response to alcohol within the context of alcohol-related domains: An examination of longitudinal approaches assessing changes over time. Alcohol Clin Exp Res. 2008;32:472–480. doi: 10.1111/j.1530-0277.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Goodwin DW, Gabrielli WF, Jr, Penick EC, Mednick SA, Jensen P, Knop J, Schulsinger F. The electroencephalogram after alcohol administration in high-risk men and the development of alcohol use disorders 10 years later. Preliminary findings. Arch Gen Psychiatry. 1996;53:258–263. doi: 10.1001/archpsyc.1996.01830030080012. [DOI] [PubMed] [Google Scholar]
- Wang PS, Bohn RL, Glynn RJ, Morgun H, Avorn J. Hazardous benzodiazepine regimens in the elderly: effects of half-life, dosage, and duration on risk of hip fracture. Am J Psychiatry. 2001;158:892–898. doi: 10.1176/appi.ajp.158.6.892. [DOI] [PubMed] [Google Scholar]
- Warner LA, White HR, Johnson V. Alcohol initiation experiences and family history of alcoholism as predictors of problem-drinking trajectories. J Studies Alcohol Drugs. 2007;68:56–65. doi: 10.15288/jsad.2007.68.56. [DOI] [PubMed] [Google Scholar]
- York JL, Welte J, Hirsch J, Hoffman JH, Barnes G. Association of age at first drink with current alcohol drinking variables in a national general population sample. Alcohol Clin Exp Res. 2004;28:1379–1387. doi: 10.1097/01.alc.0000139812.98173.a4. [DOI] [PubMed] [Google Scholar]