Abstract
Peripheral neuropathy in primary (AL) amyloidosis is usually lower limb predominant, length-dependent, symmetrical, and affects small (pain and autonomic) fibers, as much or more than large fibers. We report a patient with step-wise progressive, multiple upper limb mononeuropathies that were due to nerve biopsy-proven primary amyloidosis (lambda light chain), with no systemic or autonomic features. Recognition that light chain amyloidosis may be the cause of a multiple mononeuropathy pattern adds to the differential diagnosis of this clinical phenotype.
Keywords: amyloidosis, amyloid neuropathy, mononeuritis multiplex, necrotizing vasculitis, nerve biopsy
Primary (AL) amyloidosis is an acquired disorder characterized by abnormal proliferation and deposition of monoclonal immunoglobulin light chains. Peripheral nerves are affected in approximately 15% of patients with AL amyloidosis.1 Peripheral neuropathy may be the initial manifestation, although other organ involvement is usually found - a fact that is helpful in evaluating these patients. The typical pattern of amyloid neuropathy is diffuse, symmetrical, length-dependent, lower-limb predominant, with prominent involvement of small (pain and autonomic features) greater than large fibers. We describe an unusual neuropathic presentation of primary amyloidosis – our patient had large fiber predominant multiple mononeuropathies in the absence of a more diffuse peripheral neuropathy.
CASE REPORT
A 76 year old woman, originally from Mexico, but residing for twenty years in the United States, first developed symptoms two years prior to our evaluation. She experienced gradual worsening of tingling and prickling pain of the palmar surface of the 1st through 3rd fingers and over the thenar eminence of her left hand. She developed numbness (both difficulty feeling objects and loss of pain sensation) in the same area and weakness in the left thenar hand muscles. Evaluation elsewhere included nerve conduction studies which showed bilateral median neuropathies. She had carpal tunnel release on the left followed by two cortisone injections into the carpal tunnel, without symptomatic improvement. Her symptoms continued to worsen. One year after symptom onset, she developed symptoms on the other side in the form of right hand weakness (with difficulty pouring coffee), and prickling, tingling sensations of the right lateral dorsal hand and dorsal 1st and 2nd fingers. Based on electrophysiologic studies, an axonal right radial neuropathy was reported.
Aside from her hand symptoms, she felt well. She had no fevers, chills, weight loss, rash, red swollen joints, sicca symptoms, lightheadedness, change in sweating pattern, facial numbness, change in speech or swallowing, other bulbar symptoms, change in bowel or bladder function, shortness of breath, or lower extremity symptoms. Her past medical history was remarkable for hypothyroidism, hypertension and hypercholesterolemia. She was on thyroid replacement, anti-hypertensive and anti-hyperlipidemic agents and pain medications for her hand discomfort. She had never been a smoker, and was a social drinker (4 drinks per week). She was a homemaker with no chemical or heavy metal exposures, and no known tick or insect bites prior to the onset of her symptoms.
Because of concern for an “inflammatory mononeuritis multiplex” (necrotizing vasculitis), she was treated with 8 infusions of rituximab over 8 weeks without benefit. She was subsequently treated with intravenous immunoglobulin without improvement or stabilization.
At our initial evaluation, neurologic examination was remarkable for multiple mononeuropathies (bilateral radial and left median neuropathies). Strength examination using the Mayo Clinic muscle strength grading system (−1=25% weak; −2=50% weak; −3=75% weak, −4=100% weak) revealed −1 weakness of the right triceps and −4 weakness of the right wrist and digit extensors; −1 left wrist extensors, −2 left digit extensors, −1 left median-innervated deep finger flexors, −2 left thenar strength. Sensory examination demonstrated reduced sensation in left median and bilateral superficial radial (left more so than right) distributions of both large and small fiber modalities. Lower extremity strength and sensory examinations were completely normal. There were no deficits in mentation, cranial nerve function, cerebellar function or gait.
Laboratory evaluation included an elevated sedimentation rate (73 mm/hour; normal 0-29 mm/hour), elevated angiotensin-converting enzyme (ACE) (67 U/L; normal 7-46 U/L), mildly elevated calcium (10.3 mg/dL; normal 8.9-10.1 mg/dL), elevated fasting plasma glucose (117 mg/dL; normal 70-100 mg/dL), decreased hemoglobin (11.9 g/dL; normal 12-15.5 g/dL), and a monoclonal IgM lambda (1 g/dL) on serum protein electrophoresis/immunofixation. The following studies were normal or negative: antinuclear antibodies, antibodies to extractible nuclear antigens, circulating anti-neutrophil cytoplasmic antibodies, perinuclear anti-neutrophil cytoplasmic antibodies, rheumatoid factor, myeloperoxidase antibodies, proteinase 3 antibodies, cyclic citrullinated peptide antibodies, paraneoplastic panel, human immunodeficiency virus, syphilis, Lyme serology, cryoglobulins, urine heavy metals, and genetic testing for hereditary neuropathy with liability to pressure palsies. Cerebrospinal fluid analysis revealed 4 nucleated cells, an elevated protein of 60 mg/dL, glucose of 61 mg/dL, normal IgG index, no oligoclonal bands, and negative cytology.
Lip biopsy was negative for lymphoid infiltrate. Quantitative sensory testing (using CASE IV)2 was performed in the left hand; the study demonstrated predominantly large but also some small fiber sensory dysfunction – monofilament testing was abnormal, vibration detection threshold was abnormal (99.6%), cooling detection threshold was within the normal range (67.0%), and heat pain detection threshold was markedly low (0.01%), suggestive of hyperalgesia. An autonomic reflex screen showed normal sudomotor function. Chest CT showed a calcified granuloma in the right upper lobe with calcified right hilar and mediastinal nodes, with no evidence for sarcoidosis or lymphoma.
Nerve conduction studies and electromyography were performed and showed absent left median compound muscle action potential (CMAP) and sensory nerve action potential (SNAP), absent right radial CMAP and SNAP, decreased left radial SNAP, and decreased right peroneal CMAP (Table). No conduction blocks were observed. The ulnar and sural SNAPs were preserved. Needle examination showed severe changes, with fibrillation potentials and long duration motor unit potentials in radial innervated muscles bilaterally (right brachioradialis, extensor digitorum communis, triceps, and left extensor digitorum communis), moderate changes of active and chronic denervation in median-innervated muscles bilaterally (right pronator teres and abductor pollicis brevis, left abductor pollicis brevis), with normal ulnar-innervated muscles (right flexor carpi ulnaris and first dorsal interosseus, left first dorsal interosseus). There were long duration motor unit potentials in some non-radial and non-median innervated upper limb muscles and right lower extremity muscles, mostly without abnormal spontaneous activity (except for rare fibrillation potentials in the gastrocnemius) (Table). The electrophysiologic findings were most consistent with multiple asymmetric mononeuropathies that involved motor and sensory fibers in the upper limbs (bilateral radial and median).
Table.
| Nerve Conduction Studies | ||||
|---|---|---|---|---|
| Stimulate (Record) | A (mV/μV) | CV (m/s) | DL (ms) | F-wave latency (ms) |
| R Median motor (APB) | 2.8 (>4) | 55 (>48) | 3.3 (<4.5) | 25.7 |
| R Median sensory (Index) | 18 (>15) | 55 (>56) | 3.2 (<3.6) | |
| R Ulnar motor (ADM) | 8.8 (>6) | 61 (>51) | 2.1 (<3.6) | 25.8 |
| R Ulnar sensory (Fifth) | 31 (>10) | 60 (>54) | 2.2 (<3.1) | |
| R Radial motor (EDC) | 0 | NR (>67) | NR (<3.1) | |
| R Radial sensory | 0 (>20) | NR (>49) | NR (<2.9) | |
| L Median motor (APB) | 0.0 (>4) | NR (>48) | NR (<4.5) | |
| L Median sensory (Index) | 0 (>15) | NR (>56) | NR (<3.6) | |
| L Radial motor (EDC) | 4.4 | 58 (>67) | 3 (<3.1) | |
| L Radial sensory | 7 (>20) | – (>49) | 2.6 (<2.9) | |
| R Peroneal motor (EDB) | 0.7 (>2) | 43 (>41) | 4.5 (<6.6) | |
| L Peroneal motor (EDB) | 2.4 (>2) | 42 (>41) | 3.2 (<6.6) | |
| R Tibial motor (AH) | 8.1 (>4) | 44 (>40) | 3.7 (<6.1) | |
| R Sural sensory (Ankle) | 11 (>0) | 49 (>40) | 3.6 (<4.5) | |
| EMG | |||||
|---|---|---|---|---|---|
| Muscle | Insertional Activity |
Fibrillation Potentials |
Motor Unit Potentials | ||
| Recruitment | Long Duration |
High Amplitude |
|||
| R APB | Increased | 1+ | 2− | 2+ | 3+ |
| R biceps brachii | Normal | 0 | 0 | 1+ | 2+ |
| R brachioradialis | Increased | 4+ | No units | No units | No units |
| R deltoid | Normal | 0 | 1− | 2+ | 1+ |
| R EDC | Increased | 4+ | No units | No units | No units |
| R FDI | Normal | 0 | 0 | 0 | 0 |
| R FCU | Normal | 0 | 0 | 0 | 0 |
| R FPL | Normal | 0 | 1− | 2+ | 1+ |
| R pronator teres | Increased | 2+ | 2− | 2+ | 2+ |
| R triceps brachii | Increased | 2+ | 2− | 2+ | 2+ |
| L APB | Increased | 2+ | 2− | 2+ | 2+ |
| L biceps brachii | Normal | 0 | 0 | 0 | 0 |
| L deltoid | Normal | 0 | 0 | 1+ | 1+ |
| L EDC | Increased | 3+ | 3− | 3+ | 2+ |
| L FDI | Normal | 0 | 0 | 0 | 0 |
| R medial gastrocnemius | Increased | 1+ | 1− | 1+ | 1+ |
| R TFL | Normal | 0 | 0 | 0 | 0 |
| R Tibialis anterior | Normal | 0 | 1− | 2+ | 2+ |
| R vastus medialis | Normal | 0 | 1− | 1+ | 1+ |
| R cervical paraspinals | Normal | 0 | 0 | 0 | 0 |
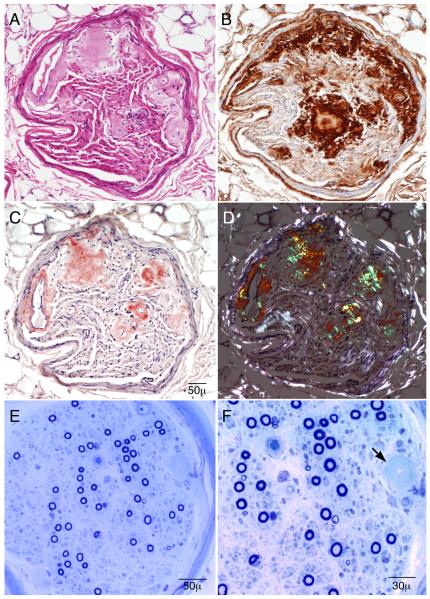
A left superficial radial nerve biopsy was performed. The left side was chosen due to greater clinical sensory loss compared to the right. On teased fiber preparation, there was an increased rate of axonal degeneration (24%) and an increased number of empty nerve strands (39 [out of 106]). The myelinated nerve fiber density was moderately decreased in a multifocal pattern, with greater involvement of small fibers (Fig.). There was no evidence of necrotizing vasculitis. There was deposition of amorphous material within fascicles and around endoneurial microvessels which stained positively with Congo red and methyl violet preparations, and reacted positively with serum amyloid protein and lambda free light chain immunopreparations (Fig.). These findings were diagnostic of primary AL type amyloidosis.
Figure.
Superficial radial nerve biopsy (A-D, paraffin sections and E-F, epoxy sections). Serial paraffin cross sections show (A, hematoxylin and eosin stain) areas of eosinophilic amorphous deposits in the endoneurium and in the walls of endoneurial microvessels. The deposits react to lambda light chain preparations (B), stain salmon-pink on Congo red stain (C), and, when viewed under polarized light (D), show apple-green birefringence in the areas of amorphous material. The semithin epoxy sections (E and F) show a moderately reduced density of myelinated fibers. Unlike the clinical symptoms and findings (that are large fiber predominant), the biopsy shows relatively more severe reduction of small myelinated fibers in comparison to large myelinated fibers. The arrow shows a region of amyloid infiltration of an endoneurial microvessel. These findings are diagnostic of primary amyloidosis from a lambda light chain.
Bone marrow biopsy showed a plasma cell proliferative disorder with 5% lambda light chain restricted plasma cells and minimal amyloid deposition within periosteal vessels. Fat aspirate was positive for amyloid. Transthoracic echocardiogram with strain study showed no evidence of cardiac involvement by amyloid. Troponin T was less than 0.01 ng/mL (normal <=0.03 ng/mL), B-type natriuretic peptide was elevated (150 pg/mL; normal <=140 pg/mL), and beta2-microglobulin was elevated (2.31 μg/mL; normal 0.70-1.80 μg/mL). Familial amyloidosis transthyretin DNA sequencing was negative. The patient’s case was reviewed with by a hematologist; melphalan and prednisone were recommended for treatment, but the patient declined.
DISCUSSION
Amyloidosis was first described in the 1700s,3 but the advent of methyl violet stains in the 1800s and Congo red stains in the 1900s made its identification easier. There are several types of amyloidosis, including primary (AL), secondary, familial, and dialysis-related. Primary amyloidosis, as diagnosed in our patient, results from the overproduction of monoclonal immunoglobulin light chains (lambda in approximately two-thirds of cases) and subsequent deposition of light chains (or their variable components) into vulnerable tissue (frequently kidney, heart, liver, and nerve). Systemic complaints, such as fatigue and weight loss, are common. Other symptoms/signs reflect dysfunction in the tissues where amyloid has been deposited and can include nephrotic syndrome, hepatomegaly, and neuropathy.4
Amyloid neuropathy occurs in approximately 15% of patients with primary amyloidosis, and there is a median lag time of approximately 29 months between onset of symptoms and diagnosis.1 Peripheral neuropathy in primary amyloidosis patients is usually lower-extremity predominant, primarily axonal, with prominent small fiber features, producing autonomic dysfunction (lightheadedness, reduced or abnormal sweating, early satiety, GI dysmotility) and pain (sharp, stabbing, burning, and aching).5 Typically, amyloid neuropathy is diffuse and progressive, and ultimately, over time, both motor and sensory, and both large and small fibers, become involved.6 Generally, the peripheral neuropathic manifestations of amyloidosis do not show improvement, even with treatment.7 Nerve conduction studies and electromyography typically show changes of a primarily axonal sensorimotor neuropathy, with decreased CMAP amplitudes, decreased to absent SNAPs, and normal or mild decreases in conduction velocities, and changes of denervation on EMG.1
As early as the 1960s, Dyck, Lambert, and colleagues8 described the electrophysiological and pathological changes in amyloid neuropathy by performing in-vitro nerve conduction studies and nerve histology on the same nerve specimens, and showed that the same populations of nerve fibers were involved using both techniques. The nerve pathology showed marked alteration in fiber size distribution in sural nerves, with greater preservation of large myelinated fibers than of small myelinated and unmyelinated fibers. The in-vitro nerve conduction studies showed a relatively preserved Aα potential, a reduced delta potential, and an absent C potential (unmyelinated fibers). These findings correlate well with small fiber symptoms (pain, loss of pain perception, and autonomic symptoms) experienced by the patients studied.
A study of nerve biopsies in patients with peripheral nerve amyloidosis revealed the location of amyloid deposition in the endoneurium (12/13 biopsies) and epineurium (9/13 biopsies) in the majority, and in the perineurium (2/13 biopsies) in the minority. Most had moderate degrees (25-75%) of myelinated fiber loss, and in most (8 of 10), the pathology showed primarily findings of axonal degeneration rather than of demyelination.9 In another study, all patients (15/15) with amyloidotic neuropathy who had a thermoregulatory sweat test performed demonstrated an abnormal sweating pattern.1 Morphometric analysis of interomediolateral column neuron cell bodies in patients with amyloid neuropathy has revealed reduction in cell counts.10 The contribution of ischemia as the primary mechanism of amyloidotic neuropathy was postulated in the 1940s,11 but the actual mechanism of this neuropathy is still in question, and may be multifactorial. There is ongoing debate about the relative importance of ischemic, compressive, and toxic or metabolic mechanisms.
Our patient presented with a multifocal process, involving several individual upper limb nerves in a stepwise progression, rather than the usual symmetric, slowly progressive, lower-limb and distal predominant symptoms and signs. These features were misleading for the diagnosis of amyloid neuropathy. The elevated sedimentation rate (which was likely due to the presence of the monoclonal protein) and the pattern of multiple mononeuropathies led to further suspicion of necrotizing vasculitis. The likelihood of vasculitis was considered so high that our patient was initially treated with immunotherapy without a nerve biopsy. Similarly, the elevated ACE and calcium levels and calcified granulomas on chest CT raised the possibility of sarcoidosis. In contrast to most amyloid neuropathies which are small fiber predominant, our case clinically had large fiber (weakness and vibratory loss) greater than small fiber involvement (hyperalgesia), and the autonomic reflex screen showed normal sudomotor function. We believe the cause of the upper limb multiple mononeuropathies was extensive amyloid infiltration of nerve. The nerve biopsy did not show necrotizing vasculitis (the common finding in patients with this clinical presentation), nor did it show non-caseating granulomas typical of sarcoidosis (a possibility because of the elevated ACE level). There were no systemic symptoms, although there was evidence of amyloid infiltration of both bone marrow and fat.
Multiple mononeuropathy, especially of the upper limbs, is an unusual presentation of primary amyloidosis and, to our knowledge, it has not been previously confirmed through nerve pathology. A case of multiple mononeuropathies due to inherited (transthyretin) amyloidosis has been reported.12 There is a case of a patient with systemic amyloidosis (with amyloid found pathologically on lymph node biopsy) who developed primarily upper extremity neuropathic signs, but sural nerve biopsy failed to demonstrate amyloid.13 Isolated amyloidomas (localized amyloid) have been described in different body tissues, including lungs and skin. They have also been found in the central nervous system14 and in the peripheral nervous system as lumbosacral radiculoplexus neuropathies,15 or in vertebrae with subsequent compromise of neural tissue,16,17 but they have not presented as multiple mononeuropathies.
This case outlines several important points. The first is that amyloidosis can produce focal rather than generalized peripheral nerve dysfunction; this is not only true for cases of plasmacytoma with localized amyloid infiltrating or from amyloidoma arising from nerve, but also for systemic amyloidosis. In fact, AL amyloid neuropathy is probably usually a multifocal process that causes a symmetric length-dependent sensorimotor polyneuropathy, because the lesions are widely and probably evenly distributed. In contrast, this patient’s particular neuropathic presentation was that of multifocal nerve involvement, and it persisted over two years from the first neurologic symptom, without clinical generalization of her neuropathy. Secondly, autonomic symptoms or signs are not always prominent in amyloid neuropathy. Our patient presented with large fiber predominant symptoms and findings (although the nerve biopsy did show a relative loss of small myelinated fibers). Finally, this case shows the importance of nerve biopsy in clarifying the etiology of difficult cases of multiple mononeuropathies. It is important not to assume that multiple mononeuropathies are due to vasculitis without careful evaluation of other potential etiologies. Immunomodulatory therapies can have significant side effects, and in a non-responding patient, there is a risk of escalating to more toxic treatments. It is important to get pathological confirmation of the cause of neuropathy, particularly when the presentation is atypical or the patient is not responding as expected to treatment. Recognition of the diversity of patterns of presentation of amyloidosis may assist in earlier diagnosis and appropriate treatment.
Supplementary Material
Acknowledgments
We would like to thank Dr. Robert Spinner for assistance in the management of this patient.
Supported in part by a grant obtained from the National Institutes of Neurological Disorders and Stroke (NS36797).
Abbreviations
- A
amplitude
- ADM
abductor digiti minimi
- AH
abductor hallucis
- APB
abductor pollicis brevis
- CMAP
compound muscle action potential
- CV
conduction velocity
- DL
distal latency
- EDB
extensor digitorum brevis
- EDC
extensor digitorum communis
- EMG
electromyography
- FCU
flexor carpi ulnaris
- FDI
first dorsal interosseus
- FPL
flexor pollicis longus
- SNAP
sensory nerve action potential
- TFL
tensor fascia latae
References
- 1.Rajkumar SV, Gertz MA, Kyle RA. Prognosis of patients with primary systemic amyloidosis who present with dominant neuropathy. Am J Med. 1998;104(3):232–237. doi: 10.1016/s0002-9343(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 2.Dyck PJ, Zimmerman I, Gillen DA, Johnson D, Karnes JL, O’Brien PC. Cool, warm, and heat-pain detection of receptors: testing methods and inferences about anatomic distribution of receptors. Neurology. 1993;43:1500–1508. doi: 10.1212/wnl.43.8.1500. [DOI] [PubMed] [Google Scholar]
- 3.Wainewright J. In: An anatomical treatise of the liver, with the diseases incident to it. Lucy J, Clarke J, editors. London: 1722. pp. 29–30. [Google Scholar]
- 4.Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR. Amyloidosis. Best Pract Res Clin Haematol. 2005;18:709–727. doi: 10.1016/j.beha.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Kelly JJ, Jr., Kyle RA, O’Brien PC, Dyck PJ. The natural history of peripheral neuropathy in primary systemic amyloidosis. Ann Neurol. 1979;6:1–7. doi: 10.1002/ana.410060102. [DOI] [PubMed] [Google Scholar]
- 6.Kim D. Quantitative sensation and autonomic test abnormalities in transthyretin amyloidosis polyneuropathy. Muscle Nerve. 2009 doi: 10.1002/mus.21332. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gertz MA, Kyle RA, Greipp PR. Response rates and survival in primary systemic amyloidosis. Blood. 1991;77(2):257–262. [PubMed] [Google Scholar]
- 8.Dyck PJ, Lambert EH. Dissociated sensation in amyloidosis: Compound action potential, quantitative histologic and teased fiber, and electron microscopic studies of sural nerve biopsies. ArchNeurol. 1969;20:490–507. doi: 10.1001/archneur.1969.00480110054005. [DOI] [PubMed] [Google Scholar]
- 9.Rajani B, Rajani V, Prayson RA. Peripheral nerve amyloidosis in sural nerve biopsies: a clinicopathologic analysis of 13 cases. Arch Pathol Lab Med. 2000;124(1):114–118. doi: 10.5858/2000-124-0114-PNAISN. [DOI] [PubMed] [Google Scholar]
- 10.Low PA, Dyck PJ, Okazaki H, Kyle R, Fealey RD. The splanchnic autonomic outflow in amyloid neuropathy and Tangier disease. Neurology. 1981;31(4):461–463. doi: 10.1212/wnl.31.4.461. [DOI] [PubMed] [Google Scholar]
- 11.Kernohan JW, Woltman HW. Amyloid Neuritis. Archives of Neurology and Psychiatry. 1942;47:132–140. [Google Scholar]
- 12.Briemberg HR, Amato AA. Transthyretin amyloidosis presenting with multifocal demyelinating mononeuropathies. Muscle Nerve. 2004;29(2):318–322. doi: 10.1002/mus.10614. [DOI] [PubMed] [Google Scholar]
- 13.Gorson KC, Hedley-White ET, Skinner M. Case 9-2001-A 64-yeaar-old woman with peripheral neuropathy, paraproteinemia, and lymphadenopathy. N Engl J Med. 2001;344:917–923. doi: 10.1056/NEJM200103223441208. [DOI] [PubMed] [Google Scholar]
- 14.Laeng RH, Altermatt HJ, Scheithauer BW, Zimmermann DR. Amyloidomas of the nervous system: a monoclonal B-cell disorder with monotypic amyloid light chain lambda amyloid production. Cancer. 1998;82:362–374. doi: 10.1002/(sici)1097-0142(19980115)82:2<375::aid-cncr18>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Ladha SS, Dyck PJ, Spinner RJ, Perez DG, Zeldenrust SR, Amrami KK, et al. Isolated amyloidosis presenting with lumbosacral radiculoplexopathy: description of two cases and pathogenic review. J Peripher Nerv Syst. 2006;11:346–352. doi: 10.1111/j.1529-8027.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- 16.Porchet F, Sonntag VK, Vrodos N. Cervical amyloidoma of C2. Case report and review of the literature. Spine. 1998;23(1):133–138. doi: 10.1097/00007632-199801010-00027. [DOI] [PubMed] [Google Scholar]
- 17.Unal A, Sutlap PN, Kyyyk M. Primary solitary amyloidoma of thoracic spine: a case report and review of the literature. Clinical Neurology & Neurosurgery. 2003;105(3):167–169. doi: 10.1016/s0303-8467(02)00141-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.