Abstract
Our intestinal microbiota serve many roles vital to the normal daily function of the human gastrointestinal tract. Many probiotics are derived from our intestinal bacteria, and have been shown to provide clinical benefit in a variety of gastrointestinal conditions. Current evidence indicates that probiotic effects are strain-specific, they do not act through the same mechanisms, and nor are all probiotics indicated for the same health conditions. However, they do share several common features in that they exert anti-inflammatory effects, they employ different strategies to antagonize competing microorganisms, and they induce cytoprotective changes in the host either through enhancement of barrier function, or through the upregulation of cytoprotective host proteins. In this review we focus on a few selected probiotics – a bacterial mixture (VSL#3), a Gram-negative probiotic (E. coli Nissle 1917), two Gram-positive probiotic bacteria (LGG, L. reuteri), and a yeast probiotic (S. boulardii) – for which sound clinical and mechanistic data is available. Safety of probiotic formulations is also discussed.
Keywords: Probiotics, intestinal microbiota, inflammation, colitis
Introduction
Bacteria form an integral component of the normal daily function of the human body. Our bacteria outnumber our human cells by 10 to 1[1], and the number of bacteria in our gastrointestinal tract measures in the range of 1012 in magnitude. These microorganisms play a key role in human metabolism and nutrition. They synthesize compounds such as vitamin K and B vitamins, they break down cholesterol, they produce short chain fatty acids such as butyrate, and digest dietary polysaccharides that would not otherwise be salvageable for energy use [2, 3]. They also contribute to host defense by priming the dendritic cells of the immune system [4], and they inhibit the colonization of pathogenic bacteria through competition for binding sites along the intestinal epithelial cell surface, a phenomenon known as “colonization resistance” [5]. In addition, they produce bactericidal products, such as small molecular weight peptides called bacteriocins, that kill other pathogenic bacteria [6]. Bacteriocins produced by lactobacilli, for example, are able to kill common food-borne pathogens such as Listeria monocytogenes [7], Bacillus cereus, Clostridium botulinum and Staphylococcus aureus [6]. Our commensal bacteria also provide defense by competing with pathogenic bacteria for nutrients. For the most part, we have developed a harmonious, symbiotic relationship with our commensal microbes: we provide them with food and a habitat, and in turn they play a key role in human metabolism and nutrition, and protect us from harmful pathogens (Fig. (1)). Hence, it comes as no surprise that many probiotic bacteria used today were originally isolated from our expansive repertoire of human commensal bacteria.
Fig. (1). Beneficial effects of commensal and probiotic bacteria.
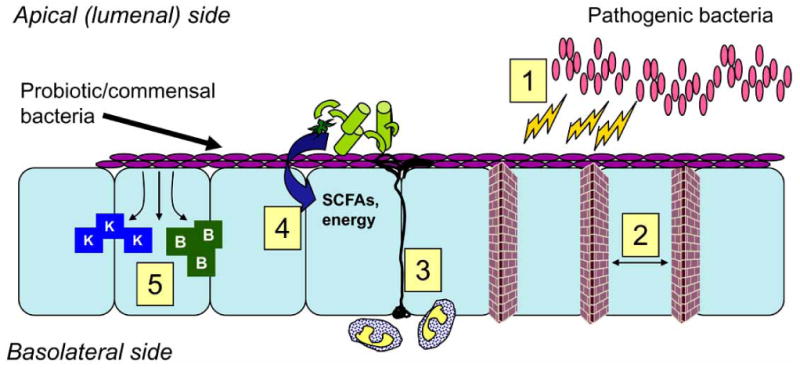
Schematic of the many vital roles of bacteria in the intestine. Commensal/probiotic bacteria are purple, epithelial cells are in light blue, apical and basolateral sides of epithelial cells are also indicated. Our intestinal bacteria serve an important role by 1) Inhibiting pathogen growth through production of antimicrobials; 2) Enhancing tight junction and barrier function; 3) Priming dendritic cells (drawn in black) and the immune system; 4) Assisting in digestion and breakdown of micro-nutrients from otherwise undigestable material (indicated in light green), synthesis of short chain fatty acids (SCFAs); 5) Synthesizing key vitamins such as vitamin K and B vitamins
The United Nations and World Health Organization define probiotics as “live microorganisms which, when administrated in adequate amounts, confer a health benefit on the host”. To the average person, the quantity of probiotic bacteria now available on the market is daunting in number, and this number only continues to rise. Regrettably, many of the bacteria advertised as probiotics have never been evaluated for clinical efficacy. Since they are considered as food and food supplements instead of pharmacologic agents, probiotics are not regulated with the same standards as drugs, even though they are often marketed with the same health claims as many drugs. Several studies of marketed probiotics have found that the viability of organisms and the composition of the probiotic formulation are often not as advertised. For example, a study in Britain tested several probiotic supplements and found that the numbers of viable bacteria, and even the identity of the actual strains of bacteria, were not correctly indicated by the product labels [8]. Lack of consistency in terms of dose, species and strain used, origin of strain, delivery vehicle (pill, liquid, food, etc.), makes interpretation of the available data from clinical trials even more difficult. Indeed, because probiotics are not inert chemical compounds but are live organisms which (like all bacteria) possess the capacity to mutate/change their phenotypes, they present their own unique challenges for regulatory agencies and for researchers performing clinical trials on probiotics [9, 10].
Recognizing this fact, in 2002 the United Nations FAO/WHO Working Group generated new guidelines for the development and evaluation of probiotics found in foods [11]. These guidelines included the following recommendations: (1) appropriate methods to identify genus and species of the probiotic strain, (2) in vitro tests to screen potential probiotic organisms as well as target-specific in vitro tests to correlate with in vivo results, (3) standards to ensure that a probiotic strain is safe and free of contamination, (4) In vivo studies using animals and humans (clinical trials) to test specific health claims of the probiotic in question, and (5) guidelines on how the probiotic should be labeled, including proper storage conditions and minimum viable numbers of organism at the end of the indicated shelf-life.
This review is not meant to be a comprehensive treatise on all probiotics, but rather will attempt to address some basic concepts of probiotics and to illustrate the fact that “not all probiotics are created equal”. Current evidence indicates that probiotic effects are strain-specific, they do not act through the same mechanisms, and nor are all probiotics indicated for the same health conditions. We will focus on a few selected probiotics, of different species, for which clinical and mechanistic data is available. Other probiotic reviews may provide the reader with more information on the subject [12-14].
Examples of Probiotics VSL#3: A Mixture of Different Gram-positive Bacteria
The probiotic VSL#3 is a mixture of 8 different species of bacteria, namely Streptococcus salivarius subsp. thermophilus, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, Bifidobacteria longum, Bifidobacteria infantis, and Bifidobacteria breve. These bacteria are all Gram-positive bacteria and they were originally isolated from the stool of a healthy human volunteer [15]. The bacteria in VSL#3 survive well in the human gastrointestinal tract, being recoverable in stool as viable bacteria after ingestion [15].
Clinical Evidence
VSL#3 has been shown to improve the clinical outcome of chronic intestinal inflammation in clinical trials. In a randomized, double-blinded, placebo-controlled trial of 40 patients suffering from at least 3 relapses per year of recurrent pouchitis, relapse occurred in only 15% (3/20) of the patients assigned to receive twice daily dosing of VSL#3 whereas those patients assigned to receive twice daily placebo all relapsed within four months [16]. Similar results were obtained in a subsequent study of once daily dosing of VSL#3 in patients with recurrent pouchitis. Out of 36 patients enrolled, remission was maintained after 12 months in 17 patients (85%) on VSL#3 and in one patient (6%) on placebo [17]. A standardized questionnaire was used as a scoring tool to determine quality of life assessment, and scores were much higher in the VSL#3 group as compared to placebo [17]. Another study examining the onset of acute pouchitis in the year following ileal pouch-anal anastomosis after colectomy in patients with ulcerative colitis suggested that VSL#3 may be helpful as prophylactic treatment in patients with pouchitis, in addition to its utility in maintenance therapy [18].
In an open label trial to determine whether patients with active UC would benefit from VSL#3, patients with mild to moderate UC (n=34) were given the probiotic mixture for 6 weeks and then reassessed. Using intention to treat analysis, a remission rate of 53% was noted in the VSL#3 group, no response was seen in 9% and worsening of symptoms noted in 9%, with 5% failing to complete the final assessment [19]. An interesting component of this study was the determination of biopsy-associated bacteria using nucleic acid-based sequencing of 16S rRNA to test for the presence of VSL#3 species. The investigators were able to demonstrate that two of the bacteria found in VSL#3, S. salivarius subspecies thermophilus and B. infantis, were detectable in the biopsies of 3 of the patients in remission. Of interest, none of the other VSL#3 bacteria were detected, which led the authors to postulate that these two components of VSL#3 may therefore be the primary active ingredients of the bacterial mixture in vivo and led them to suggest that these strains may be of interest in future studies of probiotic treatment of UC.
Basic Science
In the laboratory, VSL#3 displays many interesting properties both in vitro and in vivo which may account for its clinical activities. VSL#3 attenuates intestinal inflammation in the IL-10 deficient mouse model of enterocolitis, resulting in a decrease in TNF-alpha secretion and an improvement in histologic scores. In addition, less epithelial hyperplasia, less mucosal ulceration, and decreased neutrophilic infiltration was observed in the VSL#3 group as compared to controls [20]. Barrier function was also assessed using mannitol flux assays and after 4 weeks of VSL#3 treatment, barrier function normalized in these mice. Using the T84 human intestinal epithelial cell line it was further shown that VSL#3 and products secreted by these bacteria enhanced intestinal epithelial barrier function in vitro, and pre-exposure of T84 monolayers to VSL#3 provided a dose-dependent decrease in cellular invasion by the pathogenic bacteria Salmonella enterica serovar Dublin. A subsequent study corroborated these findings and further found that VSL#3 upregulated the expression of several mucins which are postulated to play an important cytoprotective role in host defense against pathogens [21].
Further work on VSL#3 showed this probiotic secreted products which inhibited the key pro-inflammatory transcription factor NF-kappaB, and inhibited degradation of IkappaB (an NF-kappaB inhibitor), by blocking proteasome activity in intestinal epithelial cells [22]. In addition, products produced by VSL#3 induced the expression of heat shock proteins which protected cells against oxidant injury. Heat shock proteins were induced through activation of the transcription factor HSF-1 (Heat Shock Factor-1). Both the live VSL#3 bacteria and secreted bacterial factors were able to elicit heat shock protein induction in intestinal epithelial cells, in a dose-dependent manner [22].
Two studies tested DNA isolated from VSL#3 bacteria for anti-inflammatory activity in two different models of experimental colitis [23, 24]. In the first study, Il-10 deficient mice were fed DNA from VSL#3 for 2 weeks and then their colons removed for analysis. The animals receiving VSL#3 DNA showed less histologic disease and a decrease in TNF-alpha as compared to controls [23]. In the second study, a DSS animal model of experimental colitis was used. Animals were pretreated with DNAse-treated, methylated or unmethylated DNA from VSL#3, or E. coli DNA for 10 days prior to DSS exposure and then colitis severity was assessed after 7 days of DSS treatment. It was found that the DSS-treated groups who received VSL#3 probiotic DNA - and the E. coli DNA – displayed less severe colitis than the groups that received DNAase-treated probiotic and methylated probiotic DNA. Further studies using TLR9-deficient mice led the authors to conclude that TLR9 signaling played an essential role in mediating these anti-inflammatory effects [24].
Taken together, the data from all of these studies suggest that VSL#3 may act through several different mechanisms to protect the intestine against inflammatory injury and colitis. Given that VSL#3 is a complex bacterial mixture of multiple different types of bacteria, it is likely that different species within the mixture account for so many of these different mechanisms, and one would expect that many mechanisms of action for VSL#3 have yet to be elucidated.
E. Coli Nissle 1917: A Gram-negative Probiotic
E. coli Nissle 1917 is unusual as a probiotic because it is a Gram-negative bacillus, whereas most bacterial probiotics are Gram positive bacteria. It also has a very long and interesting history. First discovered in 1917, the microbiologist Alfred Nissle isolated this strain from the stool of a soldier in World War I who was relatively unaffected by an outbreak of Shigellosis [25]. Nissle characterized the strain of E. coli, and then marketed it for the treatment of infectious diarrhea long before the availability of conventional antibiotics. This strain has now been on the market for nearly 100 years for the treatment of diarrhea, and in Germany it is used as an acceptable alternative to mesalazine for maintaining remission of ulcerative colitis [26].
Clinical Evidence
Several studies comparing E. coli Nissle to the gold standard mesalazine have shown equivalent efficacy of E. coli Nissle in maintaining remission of ulcerative colitis [26-29]. In 1997, a double-blinded study of 120 patients with inactive ulcerative colitis were given either mesalazine or the probiotic E. coli Nissle for 12 weeks and then relapse rates, relapse-free times and global assessments were compared. Relapse rates of 11.3% were reported for mesalazine and 16.0% for E. coli Nissle, with a relapse-free time of 103 +/- 4 days for mesalazine and 106 +/- 5 days for E. coli Nissle 1917. Global assessments and tolerability were similar for both groups [28]. Another clinical trial in the U.K. showed similar results, with E. coli Nissle being as effective as mesalazine in maintaining remission of ulcerative colitis [29]. A larger double-blind clinical trial followed, with 327 UC patients in disease remission randomized to receive either mesalazine or E. coli Nissle for 12 months, and then assessed using endoscopic and histological activity indices. The relapse rate by “per protocol” analysis was 40/110 (36.4%) in the E. coli Nissle group and 38/112 (33.9%) in the mesalazine group (significant equivalence p = 0.003). Using “Intention to Treat” analysis, including all patients who did not strictly follow protocol but who took at least one dose of the study medication, a relapse rate of 45.1% was calculated for the Nissle group and 37.0% for the mesalazine group (significant equivalence p = 0.013). No serious adverse events were reported [27].
Basic Science
Unlike the harmful strains of E. coli that cause human disease, the probiotic E. coli Nissle 1917 lacks many of the virulence factors normally found in its more pathogenic relatives. In addition, it possesses several “fitness factors” which confer a survival advantage over both pathogenic and non-pathogenic strains of E. coli [26]. Based on clinical trial data, clearly, E. coli Nissle must confer some anti-inflammatory and cytoprotective effects on the bowel mucosa, but the mechanisms by which this probiotic exerts it anti-inflammatory effects remain unclear. Several groups have shown that Nissle confers a protective effect in animal models of inflammatory colitis [30-32]. In an attempt to determine whether the protective effects of Nissle in inflammatory colitis were TLR-mediated, a DSS model of experimental colitis was used. Wild-type, TLR2-deficient and TLR4-deficient mice were fed Nissle or saline control, and then the animals were assessed for disease activity, mucosal damage, and cytokine secretion. E. coli Nissle improved colitis scores and decreased TNF-alpha and MCP-1 secretion in the wild-type mice but there was no improvement in DAI score, microscopic inflammation or in neutrophil recruitment (MPO activity) observed with the TLR-2 or TLR-4 knockout mice [31], suggesting that the anti-inflammatory mechanism of E. coli Nissle 1917 may occur through TLR-2- and TLR-4-dependent pathways, presumably mediated through NF-kappaB. However, another study performed in the human intestinal epithelial cell line HCT15 showed that E. coli Nissle 1917 inhibited TNF-alpha-induced IL-8 production but did not affect NF-kappaB activation, nuclear translocation, or DNA binding [33]. Thus, the mechanism(s) by which Nissle exerts its anti-inflammatory effects still continues to be a subject of intense investigation.
Two other properties also likely contribute to E. coli Nissle's effectiveness as a probiotic organism. Like VSL#3, E. coli Nissle may enhance intestinal barrier function. In experiments measuring transepithelial resistance of infected T84 cells, E. coli Nissle protected T84 monolayers against barrier disruption by enteropathogenic E. coli (EPEC) infection [34]. Based on DNA microarray analysis, the authors concluded that the mechanism involved downregulation of PKCzeta activity (one of the protein kinase C isoforms important in epithelial barrier disruption), and modulation of tight junction protein expression, such as zonula occludin-2 (ZO-2) [34]. However, unlike VSL#3, this protective barrier effect was not seen with E. coli Nissle when monolayers were infected with the invasive pathogen Salmonella enterica serovar Dublin [21], indicating that probiotics may not all possess the same protective capabilities against all intestinal pathogens.
Another group of investigators showed that E. coli Nissle upregulates human beta-defensin expression [35, 36]. Defensins are antimicrobial peptides produced by the gut which, as the name implies, protect and defend the host against bacterial pathogens. There is an association between IBD and low defensin levels [37], therefore it is possible that Nissle provides protection through a defensin mechanism, for example by limiting the adherence of certain harmful populations of bacteria to the intestinal epithelial cell lining of the gut.
LGG and L. Reuteri: Gram-positive Probiotics
The next two probiotics that will be discussed (LGG, L. reuteri) are both Gram- positive bacilli of the Lactobacillus genus. Of all bacteria, Lactobacillus species are probably the most commonly used as probiotics. They are generally regarded as safe because of their long history of use in the food and dairy industry, which probably accounts for their popularity as probiotic choices. Interestingly, however, overall they comprise only a small portion of the natural intestinal microbiota [38]. As the name implies, they all produce lactate and lactic acid.
Lactobacillus Rhamnosus GG
Lactobacillus rhamnosus GG, or LGG was first discovered by Sherwood Gorbach and Barry Goldwin. In a search for good candidate probiotic organisms, bacteria were isolated from the stool specimens of healthy human volunteers and screened for their ability to survive bile and acid exposure; their adherent properties to epithelial cells were also tested [39]. Out of this screening process, LGG was first discovered. Subsequent studies were performed in which 76 volunteers were given LGG either in the form of a frozen concentrate of bacteria or a fermented milk preparation and then feces analyzed for the presence of viable LGG [40]. LGG was recovered from 87% of volunteers after four days of stopping the LGG and in 33% of volunteers one week after terminating the treatment, confirming the ability of LGG to survive in the human GI tract. Further tests on the viability of LGG, ingested in the form of gelatin capsules, have confirmed that Lactobacillus GG also survives well as a capsular formulation and can be recovered from the feces of humans after 3 days of daily ingestion of capsules containing 1.2 × 1010 CFU of bacteria [41].
Clinical Evidence
Several clinical trials have been performed using LGG for the treatment of diarrhea, and the organism has been used successfully in the treatment of acute diarrhea in pediatric populations [42-45]. LGG appears most effective against rotavirus diarrhea, resulting in a decrease both in duration and frequency of diarrhea in this group of patients [44, 46]. It is also effective in the treatment of nosocomial and antibiotic-associated diarrhea [47, 48]. A meta-analysis examining the use of probiotics for antibiotic-associated diarrhea included a total of 6 trials using LGG, which yielded a total combined number of 817 patients. The calculated weighted event rates were 8% for the LGG group and 27% for the control, resulting in an average NNT (number to treat) of six [49, 50]. In other words, to prevent one patient from developing antibiotic-associated diarrhea, one would need to treat 6 patients receiving antibiotics with LGG. Results from another meta-analysis yielded similar results [51]. Therefore, most experts agree that LGG is one of the probiotics for which there is the most evidence of clinical efficacy, particularly for antibiotic-associated diarrhea and for rotavirus diarrhea in children [50, 52-54].
Basic Science
LGG has been shown to produce antimicrobial substances which inhibit other intestinal bacteria such as Clostridium and other anaerobes, Pseudomonas, Salmonella and E. coli, as well as Staphylococcus and Streptococcus species [55]. However, based on the small molecular size and other properties, the authors concluded that rather than resembling a bacteriocin, the antimicrobial substance was more likely to be similar to the microcins produced by E. coli [55]. The antimicrobial from LGG showed no inhibitory effect on other lactobacilli.
LGG also enhances barrier function. In a study of children with mild to moderately active Crohn's disease, daily administration of LGG resulted in improved intestinal permeability as measured by a cellobiosemannitol sugar permeability test, with a maximal effect being seen after 12 weeks of probiotic treatment [56]. In animals, LGG also improves intestinal barrier function [57]. Two week old rats were gavaged with either cow's milk, milk plus LGG, or water and Ussing chambers were then used to assess intestinal permeability. LGG was found to protect against the increased gut permeability induced by cow's milk in suckling rats. In vitro, LGG pre-treatment of epithelial cells results in protection of barrier function against the intestinal pathogen enterohemorrhagic Escherichia coli (EHEC) O157:H7 [58]. In this study, cells were pretreated with LGG, infected with E. coli O157:H7 and then examined using electron microscopy. Although not able to prevent cytoplasmic vacuolization, treatment with LGG protected cellular architecture, particularly tight junction disruption, caused by E. coli O157:H7. It was further noted that LGG pretreatment partially protected against E. coli O157:H7-induced loss of barrier function in human intestinal T84 cells grown in monolayers, as measured by transepithelial resistance (TER). Collectively, these human, in vivo, and in vitro studies provide evidence that LGG protects intestinal barrier function.
Other potential mechanisms that may contribute to the probiotic effects of LGG include the induction of cytoprotective heat shock proteins [59]. Small molecular weight compounds synthesized and released by LGG induce a time and concentration-dependent induction of cytoprotective heat shock proteins Hsp25 and Hsp72 in intestinal epithelial cells. In this study, pretreatment of cells with LGG factors (LGGCM) caused activation of the transcription factor Heat Shock Factor – 1, upregulated Hsp72 expression and Hsp25 expression on microarray analysis and real-time PCR, and protected intestinal epithelial cells against oxidant injury. When siRNA was used to silence the expression of Hsp25 and Hsp72, it was found that Hsp72 (and not Hsp25) played the major role in protecting the intestinal epithelial cells against oxidant injury. This finding is in keeping with other published data, showing that Hsp72 stabilizes and prevents denaturation of cellular proteins, and protects intestinal epithelial cells against oxidant-induced damage [60]. Two proteins secreted by LGG have been described, p40 and p75, which also likely contribute to protecting intestinal epithelial cells against oxidant injury. These LGG-derived proteins were found to attenuate hydrogen-peroxide-induced oxidant injury of intestinal monolayers in vitro [61]. In addition, they play a role in inhibiting cytokine-induced apoptosis in human and mouse epithelial cells [62].
Lactobacillus Reuteri
Lactobacillus reuteri was named after the German microbiologist Gerhard Reuter and was first recognized as a distinct species of the Lactobacillus genus in 1980. Studies indicate that each animal has a species-specific strain of L. reuteri which has presumably evolved to adapt to its particular host and L. reuteri is a ubiquitous colonizer of the intestine, having been isolated from the gastrointestinal tract of humans and of many animal species including pigs, rats, and even poultry [63-65].
Clinical Evidence
Clinical trials indicate that, like LGG, L. reuteri may be useful for the treatment of acute diarrhea, particularly in children [52]. In one study, 40 children aged 6 to 36 months hospitalized with acute diarrhea were randomized to receive either human-origin L. reuteri or placebo for the length of hospitalization or up to 5 days. Analysis of the stool samples showed an increase of roughly 5 log of L. reuteri after 48 h in the L. reuteri group. The total amount of measurable lactobacilli also increased by 2 logs in the L. reuteri group after 48 h. Levels of total lactobacilli in feces were low in the placebo group, and L. reuteri was not detectable. L. reuteri decreased the duration of watery diarrhea as compared to the placebo-treated group, with watery diarrhea persisting in 26% of the L. reuteri group as compared to 81% in the control group by the second day [66]. Results from another study focused primarily on pediatric rotavirus gastroenteritis were similar, with a decrease in the duration of diarrhea noted in the L. reuteri group as compared to placebo [67].
Basic Science
In an IL-10 deficient animal model of experimental colitis, L. reuteri colonization was shown to attenuate the development of colitis [68]. Its anti-inflammatory properties are likely not limited to effects on the intestinal mucosa, as demonstrated by a study using monocyte-derived macrophages from children with Crohn's disease (both with active disease and in remission). It was found that L. reuteri suppressed TNF-alpha and MCP-1 release from activated cells, leading the authors to suggest that L. reuteri may decrease inflammation by suppressing both monocyte and macrophage chemotaxis and cellular activation. Further mechanistic investigations revealed that, unlike other lactobacilli [23, 69], L. reuteri achieved suppression of TNF-alpha release through the suppression of c-Jun phosphorylation and AP-1 activation rather than through inhibition of NF-kappaB – in fact, NF-kappaB activity appeared to be unaffected [70]. It is interesting to note that, in contrast, the same group did report suppression of TNF-alpha-induced NF-kappaB activation by L. reuteri in a different type of cell (human myeloid cells), which in this case led to increased cellular apoptosis; these effects were mediated at least in part through inhibition of ubiquitination of the inhibitor of NF-kappaB (IkappaB-alpha) and through MAPK signaling [71].
Under anaerobic growth conditions, Lactobacillus reuteri produces a potent antimicrobial compound called reuterin, which is a β-hydroxypropionaldehyde derivative of glycerol. This compound exerts broad-spectrum antimicrobial activity against multiple pathogenic intestinal bacteria. Several human-derived strains of L. reuteri tested for their ability to inhibit growth of enterohemorrhagic E. coli (EHEC), enterotoxigenic E. coli (ETEC), Salmonella enterica, Shigella sonnei and Vibrio cholerae were all able to inhibit growth of the intestinal pathogens but to varying degrees, indicating some strains are more effective than others [72]. In addition, the antimicrobial capacity of the L. reuteri strains did not always correlate with their level of reuterin production, indicating that other as-yet-unidentified antimicrobial factors may be synthesized by these bacteria.
Saccharomyces Boulardii: A Probiotic Yeast
A yeast distantly related to Saccharomyces cerevisiae (Brewer's yeast), Saccharomyces boulardii is one of the few microorganisms commonly used as a probiotic which is not of bacterial origin. It was first described in the 1920's by the microbiologist Henri Boulard, who isolated the yeast from the skins of lychee nuts from his travels in Indochina [73]. The yeast, which now bears his name, is a substrain of Saccharomyces cerevisiae, has an optimal growth temperature of 37° C and survives passage through all levels of the GI tract. It does not permanently colonize the colon, however, and kinetic studies indicate that it disappears within 5 days of discontinuing administration [74].
Clinical Evidence
Several clinical trials of S. boulardii have demonstrated its efficacy in the prevention of antibiotic-associated diarrhea and in treating recurrent Clostridium difficile-associated disease [73, 75, 77-82]. Clostridium difficile-associated disease, or CDAD, is caused by Clostridium difficile, a toxin-producing bacteria which usually results in a diarrheal illness. When severe, CDAD can cause colitis which can be life-threatening and even fatal. A risk factor for acquiring CDAD is antimicrobial use, and CDAD accounts for 15-25% of antibiotic-associated diarrhea [83]. Many people receive treatment for CDAD, experience multiple relapses and never completely clear their infection. S. boulardii has been shown in several studies to provide clinical benefit in ameliorating antibiotic-associated diarrhea as well as to improve the eradication of recurrent C. difficile colitis [78-81]. S. boulardii has been shown to decrease recurrent C. difficile disease, especially when administered in combination with high-dose vancomycin [82]. Some suggest that S. boulardii may decrease CDAD recurrences by up to 50% [84].
Although a promising probiotic candidate for the treatment of AAD and recurrent CDAD, experts recommend that caution be used with this probiotic. There have been several reports of fungemia associated with S. boulardii use, and there is even a report of a hospital outbreak of Saccharomyces boulardii bloodstream infections occurring in three patients who did not actually receive the probiotic, but who shared the same hospital ward as other patients who had received S. boulardii [85]. Therefore, concerns have been raised about the safety of administering this live yeast as a probiotic, especially in the elderly, the very ill and in immunocompromised patient populations [86, 87].
Basic Science
Saccharomyces boulardii has some interesting properties: it adheres well to epithelial cells throughout all levels of the gastrointestinal tract, and it produces a protease which can cleave C. difficile toxins A and B [88, 89]. In addition, S. boulardii administration in rodent models stimulates secretory IgA and induces a specific IgA immune response to C. difficile toxin A [90, 91]. This has particularly important implications for the treatment of CDAD, because evidence suggests that IgA titers are protective against the toxin [92].
It has been demonstrated that S. boulardii protects and preserves epithelial barrier function in the setting of EPEC infection and decreases the extent of bacterial invasion and translocation [76]. Our own studies have shown that heat-inactivated media taken from cultures of S. boulardii protects epithelial cells in vitro against the injurious effects of C. difficile toxin A on barrier function. Intestinal epithelial cells were grown on transwell permeable supports and pretreated overnight with heat-inactivated S. boulardii CM prior to addition of 100ng/ml of C. difficile toxin A to the apical compartment. TER measurements were then taken using an EVOM chopstick voltohmmeter one hour prior to addition of toxin and then at intervals after toxin addition as indicated (Fig. (2)). The cells pretreated with S. boulardii CM displayed less loss of barrier function than controls, suggesting that S. boulardii may synthesize and secrete additional, yet-to-be-identified factors which protect intestinal epithelial cells against injury.
Fig. (2). Heat-treated media from S. boulardii protects epithelial barrier function against C. difficile toxin A.
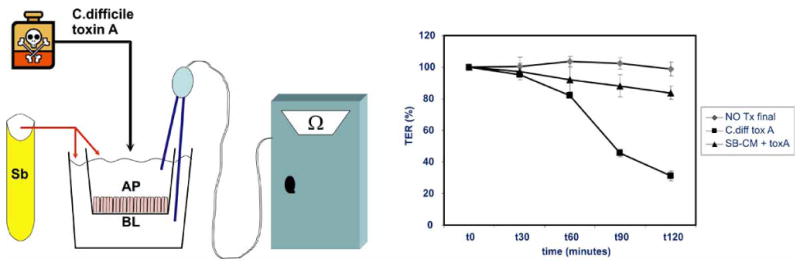
Human intestinal Caco2 Bbe cells were grown to confluence in monolayers on transwell supports. S. boulardii conditioned media (SB-CM) was boiled for 10 minutes and then added to the apical (AP) and basolateral (BL) sides (1:10) and allowed to incubate overnight (boiled media was used as control). C. difficile toxin A (kind gift of Dr. C. Pothoulakis) was then added to the apical side of the cells and the transepithelial resistance of the monolayers was measured every 30 minutes for 120 minutes with a chopstick voltohmmeter. Schematic of the experimental design is shown on the left. SB-CM protected the intestinal epithelial barrier function, with only 17% loss of barrier function recorded after 120 minutes as compared to 69% loss of barrier function noted in the C. difficile toxin A-treated group that received control media only (n=3, error bars calculated as S.E.M.)
Conclusions
Although more clinical trials are needed, what we know to date indicates that probiotics are useful for certain clinical indications (e.g. VSL#3 for pouchitis, E. coli Nissle for some subsets of UC patients, LGG and L. reuteri for rotavirus diarrhea, S. boulardii for certain subsets of C. difficile disease, LGG and S. boulardii for antibiotic-associated diarrhea, etc). It can also be seen that, in addition to differences in clinical use and composition, probiotics vary in their proposed mechanisms of action. Nonetheless, many of them seem to have cytoprotective (induction of heat shock protein, mucin expression) as well as anti-inflammatory effects (often by affecting the same inflammatory pathways but at different steps – see Fig. (3)). There is an urgent need to better define their appropriate clinical use, especially as probiotics are not always benign [87, 93]. There are many reports of probiotics causing infections, and in particular there is an increased risk of invasive infection in patients with indwelling intravenous catheters [86, 94]. Probiotic use can even turn deadly: in one clinical trial examining probiotics for pancreatitis, the trial had to be stopped early because the probiotic group actually fared much worse [95]. There were 24 deaths in the probiotic group and 9 in the control group, and 9 cases of bowel ischemia reported in the probiotic group, whereas none were seen in the control group [95]. The results of this trial provide a good illustration of how we still do not entirely understand the complex mechanisms of action of probiotics, and of the urgent need to better determine the scientific basis for their function. This clinical trial used rigorously-tested probiotics, so it is not difficult to imagine the additional potential dangers lurking in probiotics of inadequately controlled quality [8, 96]. Fortunately, probiotic use is generally safe and it shows much promise for clinical efficacy in many gastrointestinal disorders, but we still have a long way to go. Ongoing research in this area, and a better understanding of host-microbial interactions through ongoing research on the human microbiome [1, 97, 98], will undoubtedly lead to further advances in this important field of Gl research.
Fig. (3). Different probiotics act at different steps along the NF-κB activation pathway.
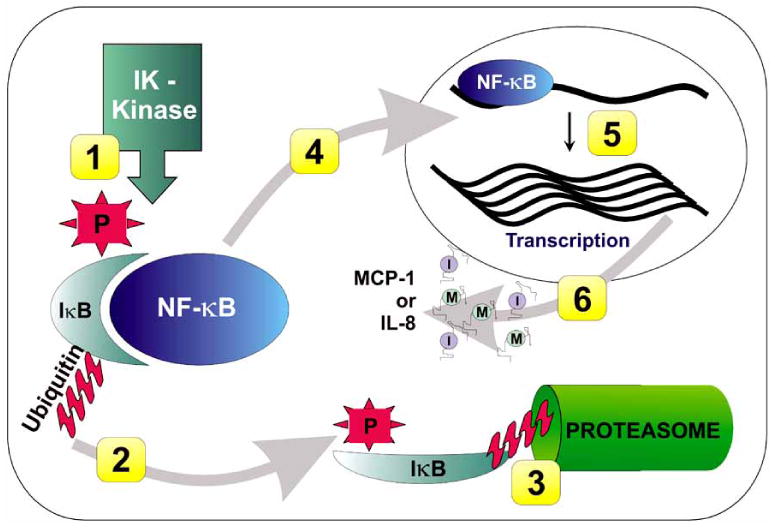
NF-κB activation involves several steps, including: 1) Phosphorylation of the inhibitor IκB molecule; 2) Ubiquitination of the inhibitor IκB molecule, which targets it for degradation (L. reuteri acts here); 3) Degradation of the inhibitor IκB molecule by the proteasome (VSL#3 acts here); 4) Translocation of NF-κB to the nucleus, now in active form; 5) Binding of NF-κB to pro-inflammatory gene targets in the nucleus; 6) Production of the gene products of inflammatory gene targets (e.g., MCP-1, IL-8) (E. coli Nissle, L. reuteri act here)
Acknowledgments
I would like to acknowledge Dr. J. Sun for her suggestions and help with the manuscript, Dr. E. B. Chang and NIH grant DK42086 for support with the S. boulardii experiments and Dr. C. Pothoulakis for providing the toxin A used in Fig. (2). Probiotic research in our laboratory is supported by the National Institutes of Health (AT004044-01A2) and by the Crohn's and Colitis Foundation of Canada.
Abbreviations
- AAD
Antibiotic associated diarrhea
- CDAD
Clostridium difficile associated disease
- CM
Conditioned media
- DAI
Disease activity index
- DSS
Dextran sodium sulfate
- Hsp
Heat shock protein
- HSF-1
Heat shock factor - 1
- IBD
Inflammatory bowel disease
- IkappaB
Inhibitor of NF-kappa B
- LGG
Lactobacillus rhamnosus GG
- MCP-1
Monocyte chemoattractant protein -1
- MPO
Myeloperoxidase activity
- NF-kappaB
Nuclear factor kappa B
- NNT
Number needed to treat
- TER
Transepithelial resistance
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
- UC
Ulcerative colitis
References
- 1.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 3.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 4.Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168(1):171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 5.Stecher B, Hardt WD. The role of microbiota in infectious disease. Trends Microbiol. 2008;16(3):107–114. doi: 10.1016/j.tim.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Riley MA, Wertz JE. Bacteriocins: evolution, ecology, and application. Annu Rev Microbiol. 2002;56:117–137. doi: 10.1146/annurev.micro.56.012302.161024. [DOI] [PubMed] [Google Scholar]
- 7.Corr SC, Li Y, Riedel CU, O'Toole PW, Hill C, Gahan CG. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci USA. 2007;104(18):7617–7621. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton-Miller JM, Shah S. Deficiencies in microbiological quality and labelling of probiotic supplements. Int J Food Microbiol. 2002;72(1-2):175–176. doi: 10.1016/s0168-1605(01)00703-6. [DOI] [PubMed] [Google Scholar]
- 9.Hibberd PL, Davidson L. Probiotic foods and drugs: impact of US regulatory status on design of clinical trials. Clin Infect Dis. 2008;46(Suppl 2):S137–140. doi: 10.1086/523321. discussion S144-51. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman FA, Heimbach JT, Sanders ME, Hibberd PL. Executive summary: scientific and regulatory challenges of development of probiotics as foods and drugs. Clin Infect Dis. 2008;46(Suppl 2):S53–57. doi: 10.1086/523342. [DOI] [PubMed] [Google Scholar]
- 11.Reid G. The importance of guidelines in the development and application of probiotics. Curr Pharm Des. 2005;11(1):11–16. doi: 10.2174/1381612053382395. [DOI] [PubMed] [Google Scholar]
- 12.Rioux KP, Madsen KL, Fedorak RN. The role of enteric microflora in inflammatory bowel disease: human and animal studies with probiotics and prebiotics. Gastroenterol Clin North Am. 2005;34(3):465–482. ix. doi: 10.1016/j.gtc.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16(4):658–672. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126(6):1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, Matteuzzi D, Campieri M. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther. 1999;13(8):1103–1108. doi: 10.1046/j.1365-2036.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 16.Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind placebo-controlled trial. Gastroenterology. 2000;119(2):305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 17.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Gut. 1. Vol. 53. 2004. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis; pp. 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124(5):1202–1209. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 19.Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100(7):1539–1546. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 20.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121(3):580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 21.Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol. 2004;286(4):G613–626. doi: 10.1152/ajpgi.00341.2003. [DOI] [PubMed] [Google Scholar]
- 22.Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127(5):1474–1487. doi: 10.1053/j.gastro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Jijon H, Backer J, Diaz H, Yeung H, Thiel D, McKaigney C, De Simone C, Madsen K. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. 2004;126(5):1358–1373. doi: 10.1053/j.gastro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126(2):520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Nissle A. Mutaflor and its medical significance. Z Klin Med. 1951;2(3-4):68. [PubMed] [Google Scholar]
- 26.Schultz M. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(7):1012–1018. doi: 10.1002/ibd.20377. [DOI] [PubMed] [Google Scholar]
- 27.Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M, Kamm MA, Weismueller J, Beglinger C, Stolte M, Wolff C, Schulze J. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53(11):1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kruis W, Schutz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 1997;11(5):853–858. doi: 10.1046/j.1365-2036.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- 29.Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354(9179):635–639. doi: 10.1016/s0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- 30.Kamada N, Inoue N, Hisamatsu T, Okamoto S, Matsuoka K, Sato T, Chinen H, Hong KS, Yamada T, Suzuki Y, Suzuki T, Watanabe N, Tsuchimoto K, Hibi T. Nonpathogenic Escherichia coli strain Nissle1917 prevents murine acute and chronic colitis. Inflamm Bowel Dis. 2005;11(5):455–463. doi: 10.1097/01.mib.0000158158.55955.de. [DOI] [PubMed] [Google Scholar]
- 31.Grabig A, Paclik D, Guzy C, Dankof A, Baumgart DC, Erckenbrecht J, Raupach B, Sonnenborn U, Eckert J, Schumann RR, Wiedenmann B, Dignass AU, Sturm A. Escherichia coli strain Nissle 1917 ameliorates experimental colitis via toll-like receptor 2- and toll-like receptor 4-dependent pathways. Infect Immun. 2006;74(7):4075–4082. doi: 10.1128/IAI.01449-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz M, Strauch UG, Linde HJ, Watzl S, Obermeier F, Gottl C, Dunger N, Grunwald N, Scholmerich J, Rath HC. Preventive effects of Escherichia coli strain Nissle 1917 on acute and chronic intestinal inflammation in two different murine models of colitis. Clin Diagn Lab Immunol. 2004;11(2):372–378. doi: 10.1128/CDLI.11.2.372-378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamada N, Maeda K, Inoue N, Hisamatsu T, Okamoto S, Hong KS, Yamada T, Watanabe N, Tsuchimoto K, Ogata H, Hibi T. Nonpathogenic Escherichia coli strain Nissle 1917 inhibits signal transduction in intestinal epithelial cells. Infect Immun. 2008;76(1):214–220. doi: 10.1128/IAI.01193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9(3):804–816. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 35.Wehkamp J, Harder J, Wehkamp K, Wehkamp-von Meissner B, Schlee M, Enders C, Sonnenborn U, Nuding S, Bengmark S, Fellermann K, Schroder JM, Stange EF. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun. 2004;72(10):5750–5758. doi: 10.1128/IAI.72.10.5750-5758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlee M, Wehkamp J, Altenhoefer A, Oelschlaeger TA, Stange EF, Fellermann K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect Immun. 2007;75(5):2399–2407. doi: 10.1128/IAI.01563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramasundara M, Leach ST, Lemberg DA, Day AS. Defensins and inflammation: the role of defensins in inflammatory bowel disease. J Gastroenterol Hepatol. 2009;24(2):202–208. doi: 10.1111/j.1440-1746.2008.05772.x. [DOI] [PubMed] [Google Scholar]
- 38.Walter J. Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl Environ Microbiol. 2008;74(16):4985–4996. doi: 10.1128/AEM.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conway PL, Gorbach SL, Goldin BR. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J Dairy Sci. 1987;70(1):1–12. doi: 10.3168/jds.S0022-0302(87)79974-3. [DOI] [PubMed] [Google Scholar]
- 40.Goldin BR, Gorbach SL, Saxelin M, Barakat S, Gualtieri L, Salminen S. Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig Dis Sci. 1992;37(1):121–128. doi: 10.1007/BF01308354. [DOI] [PubMed] [Google Scholar]
- 41.Saxelin M, Pessi T, Salminen S. Fecal recovery following oral administration of Lactobacillus strain GG (ATCC 53103) in gelatine capsules to healthy volunteers. Int J Food Microbiol. 1995;25(2):199–203. doi: 10.1016/0168-1605(94)00091-j. [DOI] [PubMed] [Google Scholar]
- 42.Vanderhoof JA, Whitney DB, Antonson DL, Hanner TL, Lupo JV, Young RJ. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135(5):564–568. doi: 10.1016/s0022-3476(99)70053-3. [DOI] [PubMed] [Google Scholar]
- 43.Szajewska H, Kotowska M, Mrukowicz JZ, Armanska M, Mikolajczyk W. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. J Pediatr. 2001;138(3):361–365. doi: 10.1067/mpd.2001.111321. [DOI] [PubMed] [Google Scholar]
- 44.Majamaa H, Isolauri E, Saxelin M, Vesikari T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1995;20(3):333–338. doi: 10.1097/00005176-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, Kolacek S, Massar K, Micetic-Turk D, Papadopoulou A, de Sousa JS, Sandhu B, Szajewska H, Weizman Z. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30(1):54–60. doi: 10.1097/00005176-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 46.Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics. 1991;88(1):90–97. [PubMed] [Google Scholar]
- 47.Arvola T, Laiho K, Torkkeli S, Mykkanen H, Salminen S, Maunula L, Isolauri E. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104(5):e64. doi: 10.1542/peds.104.5.e64. [DOI] [PubMed] [Google Scholar]
- 48.Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361(9372):1869–1871. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 49.Ellison RT., 3rd Review: probiotics are effective for prevention of antibiotic-associated diarrhea and treatment of Clostridium difficile disease. ACP J Club. 2006;145(2):46. [PubMed] [Google Scholar]
- 50.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101(4):812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 51.D'Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. BMJ. 2002;324(7350):1361. doi: 10.1136/bmj.324.7350.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szajewska H, Setty M, Mrukowicz J, Guandalini S. Probiotics in gastrointestinal diseases in children: hard and not-so-hard evidence of efficacy. J Pediatr Gastroenterol Nutr. 2006;42(5):454–475. doi: 10.1097/01.mpg.0000221913.88511.72. [DOI] [PubMed] [Google Scholar]
- 53.Doron SI, Hibberd PL, Gorbach SL. Probiotics for prevention of antibiotic-associated diarrhea. J Clin Gastroenterol. 2008;42(Suppl 2):S58–63. doi: 10.1097/MCG.0b013e3181618ab7. [DOI] [PubMed] [Google Scholar]
- 54.Floch MH, Walker WA, Guandalini S, Hibberd P, Gorbach S, Surawicz C, Sanders ME, Garcia-Tsao G, Quigley EM, Isolauri E, Fedorak RN, Dieleman LA. Recommendations for probiotic use--2008. J Clin Gastroenterol. 2008;42(Suppl 2):S104–108. doi: 10.1097/MCG.0b013e31816b903f. [DOI] [PubMed] [Google Scholar]
- 55.Silva M, Jacobus NV, Deneke C, Gorbach SL. Antimicrobial substance from a human Lactobacillus strain. Antimicrob Agents Chemother. 1987;31(8):1231–1233. doi: 10.1128/aac.31.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta P, Andrew H, Kirschner BS, Guandalini S. Is lactobacillus GG helpful in children with Crohn's disease? Results of a preliminary, open-label study. J Pediatr Gastroenterol Nutr. 2000;31(4):453–457. doi: 10.1097/00005176-200010000-00024. [DOI] [PubMed] [Google Scholar]
- 57.Isolauri E, Majamaa H, Arvola T, Rantala I, Virtanen E, Arvilommi H. Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterology. 1993;105(6):1643–1650. doi: 10.1016/0016-5085(93)91059-q. [DOI] [PubMed] [Google Scholar]
- 58.Johnson-Henry KC, Donato KA, Shen-Tu G, Gordanpour M, Sherman PM. Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157:H7-induced changes in epithelial barrier function. Infect Immun. 2008;76(4):1340–1348. doi: 10.1128/IAI.00778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from the probiotic Lactobacillus GG activate MAP kinases and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2005;290(4):C1018–1030. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 60.Musch MW, Sugi K, Straus D, Chang EB. Heat-shock protein 72 protects against oxidant-induced injury of barrier function of human colonic epithelial Caco2/bbe cells. Gastroenterology. 1999;117(1):115–122. doi: 10.1016/s0016-5085(99)70557-3. [DOI] [PubMed] [Google Scholar]
- 61.Seth A, Yan F, Polk DB, Rao RK. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2008;294(4):G1060–1069. doi: 10.1152/ajpgi.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277(52):50959–50965. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lan PT, Hayashi H, Sakamoto M, Benno Y. Phylogenetic analysis of cecal microbiota in chicken by the use of 16S rDNA clone libraries. Microbiol Immunol. 2002;46(6):371–382. doi: 10.1111/j.1348-0421.2002.tb02709.x. [DOI] [PubMed] [Google Scholar]
- 64.Naito S, Hayashidani H, Kaneko K, Ogawa M, Benno Y. Development of intestinal lactobacilli in normal piglets. J Appl Bacteriol. 1995;79(2):230–236. doi: 10.1111/j.1365-2672.1995.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 65.Molin G, Johansson ML, Stahl M, Ahrne S, Andersson R, Jeppsson B, Bengmark S. Systematics of the Lactobacillus population on rat intestinal mucosa with special reference to Lactobacillus reuteri. Antonie Van Leeuwenhoek. 1992;61(3):175–183. doi: 10.1007/BF00584224. [DOI] [PubMed] [Google Scholar]
- 66.Shornikova AV, Casas IA, Isolauri E, Mykkanen H, Vesikari T. Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. J Pediatr Gastroenterol Nutr. 1997;24(4):399–404. doi: 10.1097/00005176-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Shornikova AV, Casas IA, Mykkanen H, Salo E, Vesikari T. Bacteriotherapy with Lactobacillus reuteri in rotavirus gastroenteritis. Pediatr Infect Dis J. 1997;16(12):1103–1107. doi: 10.1097/00006454-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116(5):1107–1114. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 69.Petrof EO, Claud EC, Sun J, Abramova T, Guo Y, Waypa TS, He SM, Nakagawa Y, Chang EB. Bacteria-free solution derived from lactobacillus plantarum inhibits multiple NF-kappaB pathways and inhibits proteasome function. Inflamm Bowel Dis. 2009 doi: 10.1002/ibd.20930. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin YP, Thibodeaux CH, Pena JA, Ferry GD, Versalovic J. Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm Bowel Dis. 2008;14(8):1068–1083. doi: 10.1002/ibd.20448. [DOI] [PubMed] [Google Scholar]
- 71.Iyer C, Kosters A, Sethi G, Kunnumakkara AB, Aggarwal BB, Versalovic J. Probiotic Lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NF-kappaB and MAPK signalling. Cell Microbiol. 2008;10(7):1442–1452. doi: 10.1111/j.1462-5822.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- 72.Spinler JK, Taweechotipatr M, Rognerud CL, Ou CN, Tumwasorn S, Versalovic J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe. 2008;14(3):166–171. doi: 10.1016/j.anaerobe.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Surawicz CM. Probiotics, antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in humans. Best Pract Res Clin Gastroenterol. 2003;17(5):775–783. doi: 10.1016/s1521-6918(03)00054-4. [DOI] [PubMed] [Google Scholar]
- 74.Blehaut H, Massot J, Elmer GW, Levy RH. Disposition kinetics of Saccharomyces boulardii in man and rat. Biopharm Drug Dispos. 1989;10(4):353–364. doi: 10.1002/bdd.2510100403. [DOI] [PubMed] [Google Scholar]
- 75.Bleichner G, Blehaut H, Mentec H, Moyse D. Saccharomyces boulardii prevents diarrhea in critically ill tube-fed patients: a multicenter, randomized, double-blind placebo-controlled trial. Intensive Care Med. 1997;23(5):517–523. doi: 10.1007/s001340050367. [DOI] [PubMed] [Google Scholar]
- 76.Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P. Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli-infected T84 cells. Infect Immun. 2000;68(10):5998–6004. doi: 10.1128/iai.68.10.5998-6004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dahan S, Dalmasso G, Imbert V, Peyron JF, Rampal P, Czerucka D. Saccharomyces boulardii interferes with enterohemorrhagic Escherichia coli-induced signaling pathways in T84 cells. Infect Immun. 2003;71(2):766–773. doi: 10.1128/IAI.71.2.766-773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kimmey MB, Elmer GW, Surawicz CM, McFarland LV. Prevention of further recurrences of Clostridium difficile colitis with Saccharomyces boulardii. Dig Dis Sci. 1990;35(7):897–901. doi: 10.1007/BF01536805. [DOI] [PubMed] [Google Scholar]
- 79.McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol. 1995;90(3):439–448. [PubMed] [Google Scholar]
- 80.McFarland LV, Surawicz CM, Greenberg RN, Fekety R, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL, Noorani Z. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271(24):1913–1918. [PubMed] [Google Scholar]
- 81.Surawicz CM, Elmer GW, Speelman P, McFarland LV, Chinn J, van Belle G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology. 1989;96(4):981–988. doi: 10.1016/0016-5085(89)91613-2. [DOI] [PubMed] [Google Scholar]
- 82.Surawicz CM, McFarland LV, Greenberg RN, Rubin M, Fekety R, Mulligan ME, Garcia RJ, Brandmarker S, Bowen K, Borjal D, Elmer GW. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis. 2000;31(4):1012–1017. doi: 10.1086/318130. [DOI] [PubMed] [Google Scholar]
- 83.Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S12–18. doi: 10.1086/521863. [DOI] [PubMed] [Google Scholar]
- 84.Surawicz CM. Treatment of recurrent Clostridium difficile-associated disease. Nat Clin Pract Gastroenterol Hepatol. 2004;1(1):32–38. doi: 10.1038/ncpgasthep0018. [DOI] [PubMed] [Google Scholar]
- 85.Cassone M, Serra P, Mondello F, Girolamo A, Scafetti S, Pistella E, Venditti M. Outbreak of Saccharomyces cerevisiae subtype boulardii fungemia in patients neighboring those treated with a probiotic preparation of the organism. J Clin Microbiol. 2003;41(11):5340–5343. doi: 10.1128/JCM.41.11.5340-5343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Munoz P, Bouza E, Cuenca-Estrella M, Eiros JM, Perez MJ, Sanchez-Somolinos M, Rincon C, Hortal J, Pelaez T. Saccharomyces cerevisiae fungemia: an emerging infectious disease. Clin Infect Dis. 2005;40(11):1625–1634. doi: 10.1086/429916. [DOI] [PubMed] [Google Scholar]
- 87.Herbrecht R, Nivoix Y. Saccharomyces cerevisiae fungemia: an adverse effect of Saccharomyces boulardii probiotic administration. Clin Infect Dis. 2005;40(11):1635–1637. doi: 10.1086/429926. [DOI] [PubMed] [Google Scholar]
- 88.Castagliuolo I, LaMont JT, Nikulasson ST, Pothoulakis C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect Immun. 1996;64(12):5225–5232. doi: 10.1128/iai.64.12.5225-5232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Castagliuolo I, Riegler MF, Valenick L, LaMont JT, Pothoulakis C. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect Immun. 1999;67(1):302–307. doi: 10.1128/iai.67.1.302-307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Buts JP, Bernasconi P, Vaerman JP, Dive C. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci. 1990;35(2):251–256. doi: 10.1007/BF01536771. [DOI] [PubMed] [Google Scholar]
- 91.Qamar A, Aboudola S, Warny M, Michetti P, Pothoulakis C, LaMont JT, Kelly CP. Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to Clostridium difficile toxin A in mice. Infect Immun. 2001;69(4):2762–2765. doi: 10.1128/IAI.69.4.2762-2765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson S, Sypura WD, Gerding DN, Ewing SL, Janoff EN. Selective neutralization of a bacterial enterotoxin by serum immunoglobulin A in response to mucosal disease. Infect Immun. 1995;63(8):3166–3173. doi: 10.1128/iai.63.8.3166-3173.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rautio M, Jousimies-Somer H, Kauma H, Pietarinen I, Saxelin M, Tynkkynen S, Koskela M. Liver abscess due to a Lactobacillus rhamnosus strain indistinguishable from L. rhamnosus strain GG. Clin Infect Dis. 1999;28(5):1159–1160. doi: 10.1086/514766. [DOI] [PubMed] [Google Scholar]
- 94.Borriello SP, Hammes WP, Holzapfel W, Marteau P, Schrezenmeir J, Vaara M, Valtonen V. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis. 2003;36(6):775–780. doi: 10.1086/368080. [DOI] [PubMed] [Google Scholar]
- 95.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, Rosman C, Ploeg RJ, Brink MA, Schaapherder AF, Dejong CH, Wahab PJ, van Laarhoven CJ, van der Harst E, van Eijck CH, Cuesta MA, Akkermans LM, Gooszen HG. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9613):651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 96.Temmerman R, Pot B, Huys G, Swings J. Identification and antibiotic susceptibility of bacterial isolates from probiotic products. Int J Food Microbiol. 2003;81(1):1–10. doi: 10.1016/s0168-1605(02)00162-9. [DOI] [PubMed] [Google Scholar]
- 97.Tannock GW. Exploring the relationships between intestinal microflora and inflammatory conditions of the human bowel and spine. Antonie Van Leeuwenhoek. 2002;81(1-4):529–535. doi: 10.1023/a:1020517603993. [DOI] [PubMed] [Google Scholar]
- 98.Tannock GW. The search for disease-associated compositional shifts in bowel bacterial communities of humans. Trends Microbiol. 2008;16(10):488–495. doi: 10.1016/j.tim.2008.07.005. [DOI] [PubMed] [Google Scholar]


