Abstract
Functional cardiac tissue was prepared using a modular tissue engineering approach with the goal of creating vascularized tissue. Rat aortic endothelial cells (RAEC) were seeded onto submillimeter-sized modules made of type I bovine collagen supplemented with Matrigel™ (25% v/v) embedded with cardiomyocyte (CM)-enriched neonatal rat heart cells and assembled into a contractile, macroporous, sheet-like construct. Modules (without RAEC) cultured in 10% bovine serum (BS) were more contractile and responsive to external stimulus (lower excitation threshold, higher maximum capture rate, and greater en face fractional area changes) than modules cultured in 10% fetal BS. Incorporating 25% Matrigel in the matrix reduced the excitation threshold and increased the fractional area change relative to collagen only modules (without RAEC). A coculture medium, containing 10% BS, low Mg2+ (0.814 mM), and normal glucose (5.5 mM), was used to maintain RAEC junction morphology (VE-cadherin) and CM contractility, although the responsiveness of CM was attenuated with RAEC on the modules. Macroporous, sheet-like module constructs were assembled by partially immobilizing a layer of modules in alginate gel until day 8, with or without RAEC. RAEC/CM module sheets were electrically responsive; however, like modules with RAEC this responsiveness was attenuated relative to CM-only sheets. Muscle bundles coexpressing cardiac troponin I and connexin-43 were evident near the perimeter of modules and at intermodule junctions. These results suggest the potential of the modular approach as a platform for building vascularized cardiac tissue.
Introduction
Congestive heart failure is a major cause of death in both the developed and developing world. Although the pathophysiologies relating to congestive heart failure are complex, the condition is ultimately caused by the weakening of heart muscle tissue resulting in lowered cardiac output. The decrease in cardiac output causes chronic systemic ischemia that leads to decompensation responses such as ventricular hypertrophy, increased blood volume, and increased blood pressure, which in turn increases cardiac load and leads to further damage to cardiac muscle tissue. As a result, there is considerable interest in fabricating a cardiac tissue substitute using cardiomyocytes (CMs) in vitro. The resulting tissue-engineered cardiac muscle would be expected, at a minimum, to possess similar mechanical and functional properties as native cardiac tissue, but also have the capability to be integrated into native tissue, thus preventing long-term cardiac tissue remodeling.1
Attempts to fabricate functional cardiac tissues in vitro have yielded promising results.2,3 A crucial factor in creating viable three-dimensional tissue in vitro is to achieve adequate perfusion throughout the tissue. This is especially true for cardiac tissue due to its high metabolic rate and oxygen demand. With a view to improving engineered tissue perfusion, Radisic et al.4 incorporated oxygen carriers and flow channels into neonatal rat CM-seeded scaffolds. Aside from nutrients, environmental factors including mechanical5 and electrical6 stimuli are also needed for the proper development of cardiac tissue. Many of these concepts have been incorporated into recent culture systems7 in which CM are incorporated with other materials to form functional tissues.8 For example, a three-dimensional model was used by Zimmermann et al.,5 where they embedded a neonatal rat CM-enriched cell population (also containing fibroblasts and endothelial cells [EC]) into a Matrigel™/collagen gel. In their model, the EC in culture facilitated angiogenesis and host vasculature coupling. Other three-dimensional culture systems rely on cell-secreted extracellular matrix as the major matrix component. Kelm et al.9,10 created microtissues containing CM in a hanging drop culture system. The scale of these microtissues (∼100 μm) has been shown to support core tissue viability. In addition, contractile CM sheets have been cultivated and assembled into multilayer tissues by Shimizu et al.11 These strategies improve tissue perfusion by promoting either angiogenesis of surrounding blood vessels, or neovasculogenesis of embedded EC (part of the CM mixed cell population) via cytokine and chemokine signaling.
The overall goal of this work was to create functional, cardiac tissue using a modular tissue engineering approach with neonatal rat CM embedded in a collagen/Matrigel gel (the module) and seeded with rat aortic EC (RAEC). Modules had a postcontraction length of ∼500 μm and diameter of 300 μm. When assembled into a construct, the resulting interconnected channels are lined with RAEC and perfuseable, mimicking the required vasculature. The small diameter of each module ensures sufficient nutrient delivery to the center by diffusion. Modules may be assembled into larger constructs in vitro followed by implantation, or they may be deployed in vivo and allowed to be remodeled by host tissue. We hypothesized that the modular tissue engineering approach12,13 can be used to produce vascularized cardiac tissue by assembling modules into a macroporous sheet, where EC in the intermodular space can form vessel-like structures. This approach results in uniform, scalable, and vascularized constructs13 and is being explored elsewhere for its utility in pancreatic islet transplantation and fat reconstruction.
In work reported here, we have characterized the in vitro phenotype and functional response of a CM/RAEC coculture system comprised of both individual modules and modules in the form of a contractile sheet. We examined the responses of CM only and RAEC/CM coculture modules to external electrical stimulation, as well as the effect of surface-coated RAEC on selected features of the embedded CM. Key aspects of this study were the definition of a suitable medium for coculture of CM and EC and a means of preparing sheet-like structures from the modules.
Materials and Methods
CM isolation and module fabrication
The protocol for cell isolation was similar to that reported by Radisic et al.6 Hearts from neonatal rat pups (1–2 days old) were harvested and kept in ice-cold Hanks balanced salt solution (HBSS; Gibco). The aortas were trimmed and the remaining heart tissues were quartered followed by serial digestion with bovine pancreas trypsin (0.6 mg/mL in HBSS; Sigma) and collagenase II (1 mg/mL in HBSS; Worthington Biochemical). The extracted cell mixture was preplated onto tissue culture polystyrene for 1 h to enrich its CM content. Harvested cells were maintained in native CM medium consisting of high-glucose (25 mM) Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal bovine serum (FBS; or 10% bovine serum [BS]; Sigma), 1% HEPES buffer (10 mM; Gibco), and 1% penicillin/streptomycin (Gibco). Each CM preparation consisted of cells from an entire litter (10–13 neonatal rats) to enhance consistency. Over the course of the study several litters were used in each set of data. The number of experiments reflects the use of these replicate litters.
The CM-enriched cell mixture was loaded onto a Cytospin® slide and stained for troponin I and prolyl-4-hydroxylase (see below) to identify CM and fibroblasts, respectively: the mixture consisted of 50% CMs and 40% fibroblasts. The remaining 10% was presumed to consist of other cardiac cell types, including EC. The CM-rich cell mixture was embedded in a type I bovine collagen matrix supplemented with 25% Matrigel (v/v; BD Bioscience), and cast into modules using methods as described previously.13 Viability of embedded cells was determined using Live/Dead™ assay kit (Molecular Probes).
EC seeding
RAEC were purchased and cultured in an MCDB-131-based native EC medium (VEC Technologies), supplemented with 10% FBS. In some experiments, RAEC were transduced with a lentiviral-enhanced green fluorescent protein (eGFP) vector14 to enable tracking over time (in collaboration with Dr. J. Medin, Ontario Cancer Institute).
CM-embedded modules were cultured in the native CM medium supplemented with 10% BS for 3 days before EC seeding. To coat the surface of modules, 2–3 million RAEC were suspended in a seeding medium consisting of a 50:50 mixture of the native CM (10% BS) and native EC (10% FBS) medium, and mixed with 1 mL of CM-embedded modules inside a 15 mL centrifuge tube. The tube was agitated for 30 min on a uniaxial rocker at low speed. The mixture was transferred to a nontissue culture-treated six-well plate and kept in th seeding medium overnight to promote RAEC attachment. The seeding medium was used to transit the CM and EC from their separate native media to the coculture medium, described in Table 1. After 1 day, modules were transferred into a new nontissue culture-treated six-well plate to remove any RAEC that did not attach to modules, and the seeding medium was changed to the coculture medium. Modules were maintained in this medium until assayed.
Table 1.
Medium Compositions (Presumed Key Differences) and Serum Types Used in the Study
| Name | Native CM | Coculture | Seeding | Native EC |
|---|---|---|---|---|
| Base medium | DMEM | DMEM | MCDB-131/DMEM (50/50) | MCDB-131 |
| Components (mM) | ||||
| L-Glutamine | 4 | 4 | 2 | 0 |
| Magnesium sulfate | 0.814 | 0.814 | 5.42 | 10.02 |
| D-Glucose | 25 | 5.56 | 15.28 | 5.56 |
| Sodium pyruvate | 0 | 1.28 | 0.5 | 1 |
| HEPES | 10 | 10 | 5 | 0 |
| Serum concentration (%) | ||||
| Bovine serum | 10 | 10 | 5 | 0 |
| Fetal bovine serum | 0 | 0 | 5 | 10 |
CM, cardiomyocyte; DMEM, Dulbecco's modified Eagle's medium; EC, endothelial cells.
Module sheet
Four days after fabrication, CM-embedded modules were assembled into a monolayer sheet. For RAEC/CM modular sheets, CM-embedded modules (3 days after fabrication) were seeded with RAEC and assembled into sheets the next day. Using a 10 mL serological pipette, modules, with or without RAEC, were placed over a sterile nylon mesh (pore size = 100 μm; Millipore) to form a single layer. A dilute alginate solution (1.2% w/w; Sigma) was dripped over the modular sheet followed by a few drops of 50 μM calcium chloride to crosslink the alginate. Once the alginate formed a soft gel (∼5–10 s), the sheet was rinsed in phosphate-buffered saline (1.06 mM KH2PO4, 155.17 mM NaCl, and 2.97 mM Na2HPO4-7H2O) and transferred into the CM or coculture medium. These alginate-embedded sheets were stimulated (see below) for 10 days and then the alginate gel was removed before assay by brief incubation in 100 μM citrate buffer at room temperature.
Electrical response assessment and field stimulation
CM-embedded modules were equilibrated in 1 × Tyrode's salt solution (Sigma) at 37°C for 1 h before measurement. Electrical responses of modules were assessed using custom-made testing chambers. Modules were paced between a pair of graphite electrodes kept 1 cm apart by a polycarbonate bracket. Using a six-well plate made from tissue culture polystyrene, modules and electrodes were placed in an individual well with 1.5 mL of Tyrode's salt solution, which was enough to partially submerge the electrodes. The small chamber volume ensured that most of the modules were located between the electrodes.
Module sheets were assayed using a polydimethylsiloxane (PDMS)/glass testing chamber. A pair of graphite electrodes were kept 1 cm apart by a PDMS bracket and placed over a layer of PDMS poured into a 60 mm glass Petri dish. This allowed the module sheets to be fixed in position relative to the electrode.
For both systems, a signal generator (S88X stimulator; Grass Technologies) connected to the electrodes provided the desired electrical stimulation (biphasic, 2 ms square pulse, up to 12 V at various frequencies). The samples were kept at 37°C during the experiment using a solid-state microscope stage heater. Contractions of modules and module sheets were recorded with a CCD camera (Hitachi Denshi, model KP-M1U) on an inverted phase-contrast microscope (Olympus CX2). The image sequences were analyzed using ImageJ (NIH, version 1.38x).
Excitation threshold (ET) was defined as the minimum voltage required for synchronous contraction at 1 Hz. Maximum capture rate (MCR) was defined as the maximum rate of synchronous contraction under an electric field equivalent to twice the ET. The contractility of modules was defined as the fractional en face area change during one contraction cycle using frame-by-frame video analysis in ImageJ.
Module sheets were electrically conditioned before assay. Briefly, CM-embedded modules were maintained in the native CM medium with 10% BS for 3 days. Then, the modules were fabricated into sheets (see above) and transferred into a well, fitted with a pair of graphite electrodes as above, and stimulated continuously with biphasic pulses at 5 V/cm and 1 Hz for 10 days. The medium was supplemented with 10 μM of ascorbic acid and changed daily to minimize the effect of reactive oxygen species generated at the electrodes. Individual modules were not electrically conditioned because they could not be fixed in position relative to the electrical field over the course of an experiment.
Immunofluorescence staining
Freshly harvested, CM-enriched cell mixtures were analyzed by Cytospin. Slides were stained with rabbit anti-rat troponin I (Santa Cruz Biotechnology) and mouse anti-rat prolyl 4-hydroxylase (Sigma) antibodies to identify CMs and fibroblasts, respectively.
RAEC coverage on modules was determined by whole mount immunofluorescence staining of goat anti-rat VE-cadherin primary antibody (Santa Cruz Biotechnology) followed by rabbit anti-goat immunoglobulin (IgG) AlexaFluor™ 488 secondary antibody (Molecular Probes). Modules were fixed in 4% paraformaldehyde solution and permeabilized using 0.2% Triton-X100 solution. On immunohistochemical sections, CMs were stained using rabbit anti-rat troponin I (Santa Cruz Biotechnology) followed by goat anti-rabbit IgG secondary antibodies conjugated with AlexaFluor 488 (Molecular Probes). Cardiac fibroblasts were stained with mouse anti-rat vimentin antibody (Sigma) followed by goat anti-mouse IgG AlexaFluor 568 (Molecular Probes). In sections that were triple-stained (troponin I, vimentin, and connexin-43), connexin-43 was stained before immunofluorescence staining using rabbit anti-rat Cx43 antibody (Chemicon) coupled by a three-step immunohistochemistry (IHC) chromogenic method. In some experiments, surface-seeded RAEC were tracked by eGFP, where cell nuclei were counterstained with Hoechst nuclear stain (Sigma). Samples were observed using a Zeiss LSM510 confocal microscope.
Statistical analysis
Statistical analyses, including calculations of standard error of means and mean comparisons (t-test or one-way analysis of variance), were performed using Prism (Version 5.0; Graphpad Software).
Results
Contractility of CM-embedded modules
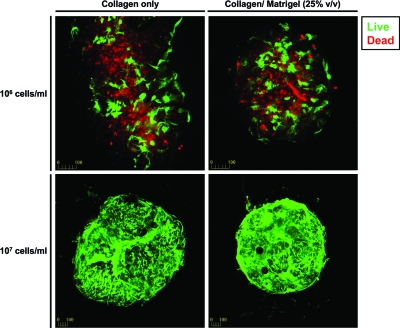
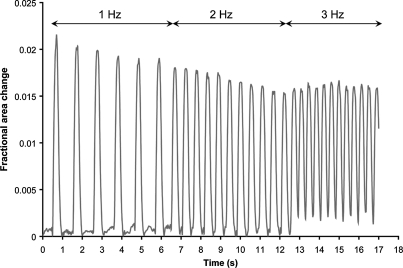
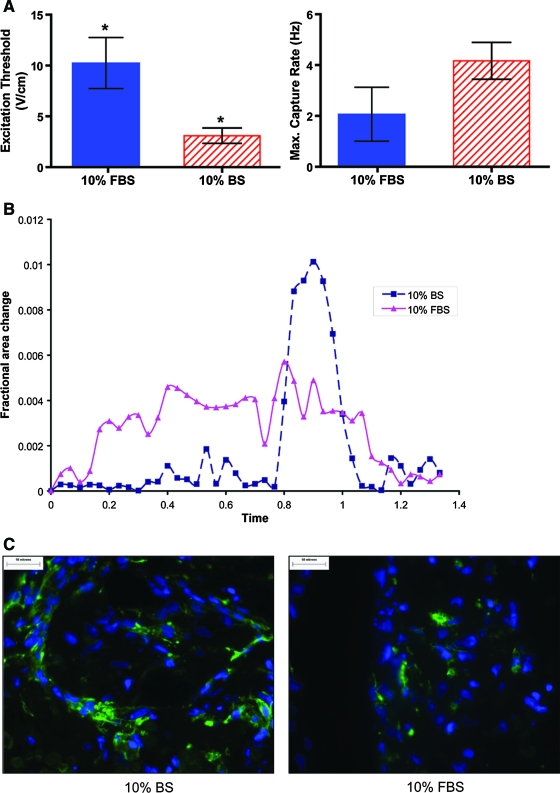
CMs embedded in modules remained viable for at least 14 days in either type I collagen or type I collagen supplemented with 25% Matrigel. However, higher viability was obtained with an initial cell seeding of 107 cells/mL compared to 106 cells/mL (Fig. 1). Modules without RAEC were cultured for 7 days in the CM medium containing either 10% FBS or 10% BS and then assayed for electrical responsiveness. In both cases, modules displayed synchronous contractions corresponding to the external stimulation frequency (Fig. 2). A small proportion of the modules (<10%) contracted spontaneously even in the absence of external electrical stimulation and continued to contract when stimulated. Modules cultured in 10% BS had a lower ET but similar MCR compared to modules cultured in 10% FBS (Fig. 3A). Moreover, modules cultured in 10% BS had better defined peaks with higher maximum contraction amplitudes, compared to modules cultured in 10% FBS; a representative sequence of a single module (from a group of >30) is shown in Figure 3B. Histological sections of modules cultured in 10% BS showed higher troponin I expression and elongated cell morphology compared to modules cultured in 10% FBS (Fig. 3C). These results suggest that BS enhanced the electrical responsiveness and contractile performance of CM-embedded modules, through an effect on the expression or organization of the contractile apparatus within the cells. For this reason, all subsequent experiments were carried out in the 10% BS supplemented medium.
FIG. 1.
Viability (Live/Dead assay) of CMs embedded in modules, cultured in 10% BS, at 14 days postfabrication. Matrix composition did not affect embedded cell viability, whereas increasing seeding density from 106 to 107 cell/mL dramatically improved viability (magnification = 100 × ). BS, bovine serum; CMs, cardiomyocytes. Color images available online at www.liebertonline.com/ten.
FIG. 2.
Fractional area change of a single module when stimulated by external signal. CM-embedded modules (2 × 107 cell/mL collagen supplemented with 25% v/v Matrigel) were cultured in 10% BS medium for 7 days before assay. At a voltage twice the ET, modules displayed synchronous contraction corresponding to the external signal frequency: 1 Hz from 0 to 7 s, 2 Hz from 7 to 13 s, and 3 Hz from 13 to 17 s. ET, excitation threshold.
FIG. 3.
(A) ET and MCR of CM (2 × 107 cell/mL) modules (collagen with 25% Matrigel) cultured in the medium containing 10% FBS (solid) or 10% BS (hatched) for 7 days postfabrication. Maintaining modules in the medium with 10% BS decreased ET (*p < 0.05, n = 3), but did not affect MCR. (B) Fractional area change of single modules (taken from an aliquot of >30) on day 7. Modules cultured in 10% BS or 10% FBS were stimulated at 200% ET voltage (2 ms, 1 Hz) and recorded on video. Fractional area change is normalized against a reference frame in each sample. (C) CM cultured in 10% BS expressed more cardiac troponin I (green) and assumed a more elongated morphology than those cultured in 10% FBS when examined using immunofluorescent microscopy. FBS, fetal bovine serum; MCR, maximum capture rates. Color images available online at www.liebertonline.com/ten.
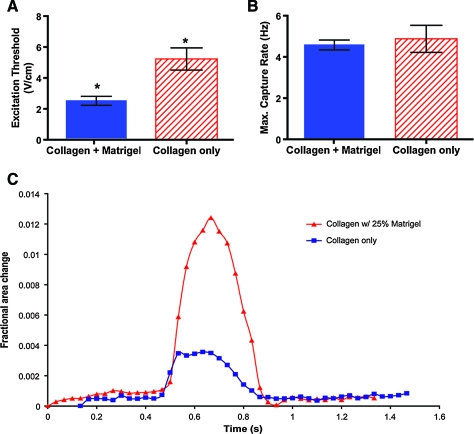
The effect of extracellular matrix on the function of embedded CM (10% BS supplemented medium) was tested by embedding CM in either collagen (type I) or collagen supplemented with Matrigel; the latter choice was based on the work of Zimmerman et al.5 CM embedded in Matrigel-supplemented matrix exhibited lower ET (Fig. 4A, p < 0.05) and higher contractile amplitudes (Fig. 4C) compared to CM embedded in collagen-only modules. However, the MCR were found to be similar for the two groups (Fig. 4B).
FIG. 4.
At day 7 postfabrication, CM (2 × 107 cell/mL) embedded in Matrigel (25% v/v)-supplemented collagen modules, cultured in 10% BS, exhibited a lower ET (A, *p < 0.05, n = 3) and higher contractility (C, single modules taken from an aliquot of >30) compared to collagen only control. However, both collagen only and collagen + Matrigel modules had similar MCR (B).Color images available online at www.liebertonline.com/ten.
Behavior of RAEC-seeded CM-embedded modules
Primary RAEC (2 × 107 cell/mL) were seeded onto modules embedded with CM 3 days after fabrication to form endothelialized modules; ∼25% of the module surface was covered at initial seeding. After 24 h in a seeding medium consisting of a 50:50 mixture of the two native media, the RAEC/CM modules were placed in a coculture medium that had a lower glucose concentration (5.5 mM) than the native CM medium and a much lower Mg2+ concentration than native EC medium (Table 1). These factors, among other perhaps less crucial medium differences, helped maintain the contractility of embedded CM and promoted EC-adherent junction formation. After 14 days in the coculture medium, the surface of the modules was almost completely covered by RAEC with well-developed adherent junctions, confirmed by junction-localized VE-cadherin staining (Fig. 5A), similar to that seen with RAEC seeded on empty collagen modules and cultured in the native EC medium. In contrast, RAEC seeded on modules cultured in the native CM medium showed no morphologically distinguishable junction features (Fig. 5B).
FIG. 5.
(A) Immunofluorescence staining for VE-Cadherin on RAEC/CM modules after 14 days in the coculture medium indicated the presence of a confluent layer of RAEC. (B) In contrast, RAEC seeded on CM modules cultured in the high-glucose native CM medium displayed disorganized junctions. (C) Surface-coated RAEC attenuated the electrical responsiveness of modules, as seen by an increase in ET (***p < 0.0001, n = 3) and decrease in MCR (*p < 0.05, n = 3). (D) With external field stimulation, CM-embedded modules coated with RAEC displayed similar contractility when compared to CM-only modules (single modules taken from an aliquot of >30). Contractility was assessed by comparing the en face fractional area change. RAEC, rat aortic endothelial cells. Color images available online at www.liebertonline.com/ten.
When stimulated by an external electric field, endothelialized modules were able to contract synchronously, but they had a higher ET (p < 0.001) and lower MCR (p < 0.05) compared to CM-only modules (Fig. 5C). In fact their electrical response profile resembled that of CM-only modules cultured in 10% FBS. However, both endothelialized modules and CM-only modules had similar contraction amplitudes (Fig. 5D).
Module sheets
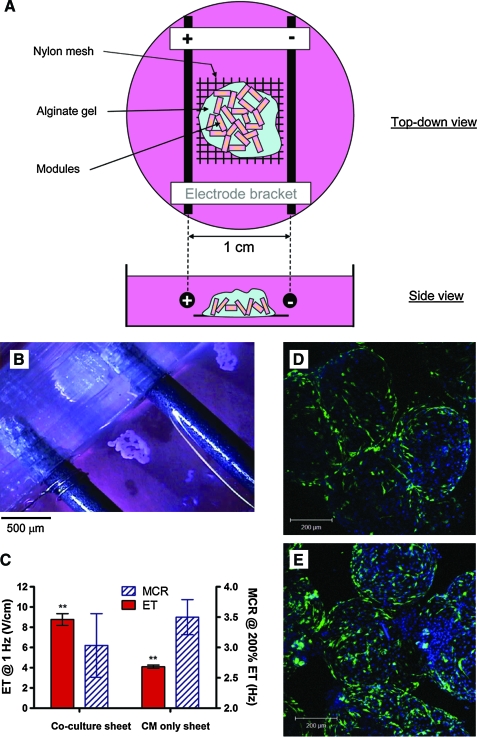
The modular approach mimics the multilayered organization of cardiac muscle by first fusing submillimeter modules into a sheet-like construct with a view to subsequently stacking these sheets together to yield a perfuseable, macroporous, endothelialized three-dimensional tissue. One of the challenges of assembling multiple cell-embedded modules into a sheet is to control the rate and degree of agglomeration caused by remodeling. Modules embedded with contractile cell types, such as smooth muscle cells15 and CM, tend to clump and fuse into a large cell mass spontaneously under static conditions. The size of these nonporous aggregates is expected to impair diffusion and ultimately lead to necrosis of the core. To minimize this, the modules were partially embedded in a thin layer of alginate gel, a noncell adhering matrix, to prevent the spaces between modules from collapsing, while at the same time allowing a limited amount of intermodule fusion to occur (Fig. 6A). In as little as 5 days, a macroporous module sheet was produced, and then the alginate was dissolved, releasing the fragile, yet physically stable sheet from the supporting nylon mesh (Fig. 6B).
FIG. 6.
(A) Schematic diagram of the testing chamber used to condition module sheets. Modules were partially embedded in alginate gel on top of a nylon mesh and conditioned electrically for 10 days. (B) After 10 days of continuous stimulation, macroporous RAEC/CM module sheets were removed from the alginate and nylon mesh and assessed by field stimulation. (C) ET and MCR of RAEC/CM sheets and CM-only sheets; ET was significantly higher in the presence of RAEC (**p < 0.001, n = 3). (D) Enhanced green fluorescent protein-transfected RAEC were uniformly distributed, at a relatively low density, over RAEC/CM modular sheets, 10 days after stimulation. (E) RAEC/CM sheets were reseeded with 3 × 106 RAEC, using the same protocol as in the first seeding, after removing the alginate gel. With this protocol, RAEC surface coverage was increased. Color images available online at www.liebertonline.com/ten.
Both CM only as well as RAEC/CM module sheets were characterized. At 4 days postfabrication, modules were assembled into sheets and stimulated for an additional 10 days. RAEC/CM modules were made by seeding eGFP-transduced RAEC onto modules 1 day before sheet assembly (i.e., on day 3). After 10 days of stimulation, RAEC were tracked and found to be evenly distributed over the surface of module sheets (Fig. 6D). Moreover, the attachment density was enhanced by removing the alginate gel and reseeding the sheet with more RAEC (Fig. 6E), after 10 days of stimulation. When placed in an electrical field (Fig. 6C), both RAEC/CM and CM-only module sheets contracted synchronously. RAEC/CM sheets exhibited a higher ET (p < 0.01), but a roughly similar MCR compared to CM-only sheets (Fig. 6C). When RAEC/CM sheets were not stimulated for the 10 days of culture, they did not contract when placed in an electric field.
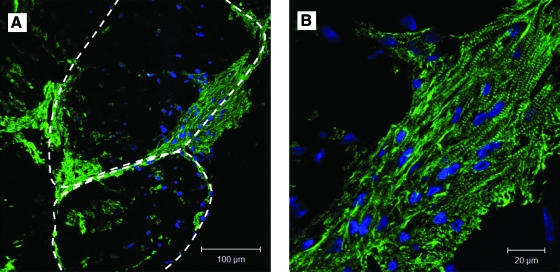
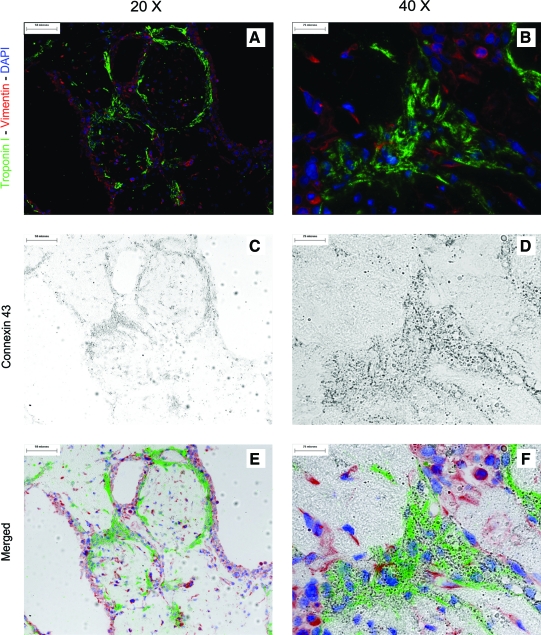
Module sheets with or without RAEC were stained for cardiac troponin I, a protein subunit associated with the troponin–tropomyosin complex in cardiac muscle. Troponin-I-positive cells were found throughout the modules. However, most of the muscle-bundle-like structures were found at the perimeter of the modules or near the intermodule junctions (Fig. 7). Most of these structures were no more than five cell layers thick and covered 12% ± 2% (n = 4) of the cross-sectional area in both RAEC/CM and CM-only sheets (Fig. 7B). When triple-stained with vimentin, troponin I, and connexin-43, we saw a fibroblast-rich layer at the periphery of the modules (Fig. 8A). We also found connexin-43 colocalized with troponin-I-positive structures along the perimeter of modules and intermodular space, just below the fibroblast layer (Fig. 8C, D).
FIG. 7.
(A) Immunofluorescence staining of cardiac troponin I (green) shows a high density of CM near the perimeter of modules and at intermodule junctions (dash lines indicate edges of modules) in a module sheet after 10 days of stimulation. (B) At higher magnification, the CM appeared to form muscle-like bundles, similar to that seen in native cardiac tissue, but such bundles constituted only ∼12% of the field, shown in (A). Color images available online at www.liebertonline.com/ten.
FIG. 8.
(A, B) Triple-stained (vimentin, troponin I, and connexin-43) modular sheets after 10 days of stimulation indicate that surfaces of module sheets were covered by a layer of fibroblasts with an underlying layer of CM. (C, D) Connexin-43-positive structures were also observed. (E, F) Colocalization of connexin-43 and troponin I was confirmed by overlaying high magnification images, suggesting that the CM were electrically coupled. Color images available online at www.liebertonline.com/ten.
Discussion
We have characterized submillimeter-sized modules containing CMs and EC and examined the feasibility of building larger constructs with this modular approach. We focus here on characterizing the individual modules and the assembled sheet-like structures. Future reports will describe the fate of these modules and sheets in cardiac infarct models.
Electrical characterization
CM-embedded modules responded to external field stimulation after 7 days in culture. Addition of Matrigel (25% v/v) to the collagen matrix lowered the ET and improved the contractility of the modules, but did not affect the MCR. This benefit is attributed to the presence of basal lamina proteins that are found in Matrigel, including laminin and collagen type IV, as well as the various growth factors that are found in Matrigel. The presence of Matrigel is presumed to provide an extracellular matrix environment that is more similar to the native myocardium than collagen type I alone, resulting in improved cell organization and tissue remodeling.6 For further development of this approach, an alternative is required since the tumor-derived Matrigel has limited utility in clinical applications.
Culturing modules in 10% BS instead of 10% FBS also resulted in improved electrical performance and contractility, and this was accompanied by an increase in troponin I expression and cell elongation. This observation is consistent with that of Schwarzkopf et al.,16 who showed that the medium supplemented with adult serum (same species) promoted viability and maturation of CMs compared to fetal calf serum. While we did not investigate the effect of adult rat serum on rat CM, we saw a similar improvement in CM maturation with adult BS in comparison to FBS. It was beyond the scope of this study to elucidate the underlying mechanism. Similarly, further optimizations of the matrix or the medium are left for the future although the value of this for rat CM or EC may not be warranted.
We acknowledge that the current method for assessing electrical performance is highly dependent on the materials used and the geometry of the testing chamber, and therefore cannot be viewed as a true analog for representing in vivo electrical performance. Of particular importance is the inability to assess regional differences in contractility and whether action potentials propagated throughout the thickness of the construct or just merely on the surface. Nevertheless, the current method allowed us to compare the overall effects exerted by different environmental signals and enabled us to optimum culture conditions.
Coculture model
One of the main objectives of this study was to develop a means of maintaining a functional RAEC layer on the surface of contractile CM-embedded modules. By adjusting the composition of the medium (Table 1) and particularly lowering the glucose level to that of native EC medium, primary RAEC remained viable and showed typical VE-cadherin expression after 14 days, suggesting that the EC were in a quiescent state17 in the coculture medium. Using the same medium, we were also able to generate viable RAEC/CM coculture modular sheets.
To our knowledge, this is the first study to investigate the effects of surface-coated EC on the electrical performance and responsiveness of underlying CM in a modular construct. A 24 h transition period and seeding medium (a 50:50 mixture of the native media) was used to enable RAEC seeding and coverage. Without this seeding medium, RAEC attachment and coverage was poor. We found that the presence of a quiescent RAEC layer in the coculture medium, on either modules or modular sheets, permitted the contractile response of embedded CM, provided that the Mg2+ concentration was at the low level of the CM medium and not the high value of the native EC medium. However, the RAEC reduced the module and module sheet electrical responsiveness, manifested as an increase in ET and decrease in MCR. These changes in electrical performance were not seen when CM-only modules were cultured in the same coculture medium. We believe that these changes were most likely caused by the presence of the RAEC layer that may have changed the surface conductance of the modules. Such changes would cause the ET to increase compared to CM-only modules. Differences in membrane protein expression (e.g., connexin proteins) may also attenuate the propagation of electrical impulses from the surface to the underlying CM, resulting in a lower MCR.
Judging from VE-cadherin staining, the native cardiac EC embedded in the modules did not form vessel-like structures, nor did they appear to interact with surface seeded RAEC. However, these cardiac EC may still play an important role in CM maturation, as demonstrated in other multiculture cardiac constructs.18–20 For example, Iyer et al. showed the presence of fibroblasts and EC in cardiac tissue constructs enhanced CM elongation and excitability,21 possibly by spatially orienting CM into muscle-like bundles.
Our initial aim was to use the RAEC/CM modules as a vehicle to uniformly deliver viable EC through out an assembled construct containing multiple modules. In a separate study involving RAEC modules (without embedded cells) implanted in a rat omental pouch, we observed that RAEC migrate from the modules and form chimeric (host-donor vessels) functional blood vessels in the intermodular space after 21 days.22 We expect the RAEC on the coculture modules to migrate and form vessels in a similar fashion, when implanted. Plans to evaluate the modular construct in a rat implant model are expected to reveal the fate and remodeling process of RAEC/CM modules in vivo; how this then changes the in vivo electrical properties and morphology of CM remains to be defined.
Sheet formation
A major challenge in creating clinically relevant, thick (1–10 mm) cardiac tissues in vitro is the lack of tissue vasculature. Specifically, the formation of blood vessels, both host and graft derived, can be quite slow and insufficient to meet the high nutrient demands of CMs. There are several approaches designed to circumvent this limitation, including the use of oxygen carriers in the culture medium,23 incorporating vessel-like flow channels in preformed scaffolds,24 and preloading scaffolds with various growth factors and/or functional cell types that promote angiogenesis.25,26
We devised a method to produce a sheet-like structure from an assemblage of modules. A thin layer of modules was laid onto a nylon mesh and partially immobilized in alginate gel. At least some of the CM in these modules matured into muscle-like bundles and formed a physically integrated sheet capable of synchronous contraction when stimulated. Moreover, the CM at intermodular spaces expressed connexin-43, suggesting that the module sheet may be electrically coupled. However, further electrophysiological assays are needed to characterize the electrical coupling between modules.
Despite their morphology and contractile nature, these muscle-like bundles, as observed on histological sections, are relatively thin and only occupy a fraction of the total volume of the module. The lack of troponin-I-positive CM near the core of the module suggests that the CM may have been crowded out by the faster growing fibroblasts. However, this may also be the result of active CM migration and maturation. Further studies at different time points are needed to reveal the mechanism of the remodeling process. Since we assume that these bundles are responsible for generating the contractile forces, it is crucial to increase the proportion of these bundles for the modular construct to produce physiologically relevant force. For instance, in future implementations it may be beneficial to reduce the diameter of modules so that the surface-to-volume ratio is increased, thus increasing the contractile force on a per module basis. It is also conceivable that over a longer incubation period in vitro or when implanted in vivo, more CM may migrate to the surface, thus increasing the thickness of this layer. These issues will be explored in future studies.
In recent years, there has been interest in building cardiac constructs using small functional subunits: for example, microtissues,9 cardiac sheets,11 or other components.27 In the approach most conceptually similar to modules, Kelm et al. demonstrated that human umbilical vein EC-coated microtissue (a “spheroid” without collagen gel) with embedded CM was able to agglomerate to form a patch with functional human umbilical vein EC-lined capillaries.10,28 In all cases, the goal was to create uniform, scalable, vascularized, and functional cardiac tissue, and the merits of one approach relative to the other will be determined by the success of meeting these goals based on in vivo performance. Of particular significance in this work is that coculture conditions that maintained both CM function and RAEC junction morphology were devised. We found, for example, that RAEC/CM sheets that were not stimulated during culture for 10 days did not contract when subject to external field stimulation.
We chose to embed modules in alginate not only because of its ease of application and removal by changing local calcium concentration, but also because it is cell compatible and noncell adherent. A single-module thickness across the sheet ensured that sufficient nutrients could diffuse into the core of each module, at least in vitro. These macroporous module sheets will serve as intermediate components for eventually building thicker tissues in a scalable fashion, where the tissue maturation process can be monitored. When the modules were assembled into sheets, there was a small decrease in the electrical responsiveness relative to modules alone. This is likely the result of differing geometries of the stimulation apparatus resulting in differences in the field perceived by the CM. It is also conceivable that intermodular interactions of surface-seeded RAEC may have lead to a change in tissue conductance.
Fabrication of functional cardiac tissue in vitro depends on many parameters, including medium composition, extracellular matrix geometry, and cell seeding sequence. Native myocardium consists of CMs that form mechanically and electrically coupled bundles. These bundles are encased in extracellular matrix secreted by cardiac fibroblasts, and maintained by capillaries lined with EC. The three cell types exist in their respective microenvironments. The RAEC/CM coculture system described in this study consists of more than two cell types, since the primary CM-enriched cell population contained a significant quantity of fibroblasts and cardiac EC. While in this study, the electrical responsiveness of CM and the adherent junction morphology of RAEC were chosen as the primary endpoints, we must also consider the contribution of cardiac fibroblasts and EC to these measurements. The data presented here suggest that cardiac fibroblasts actively segregate and migrate to the surface of the modular sheet, and potentially play an important role in the formation and remodeling of the modular sheet since the spatio-temporal relationships between CM and the various supporting cell types have been shown to enhance CM maturation and the overall functionality of construct.21,29 Therefore, aside from being a viable platform for in vitro tissue formation, the module system may also serve as a cell delivery vehicle that is capable of providing a supportive environment to multiple cell types in a predetermined seeding ratio and geometry.
Conclusion
We have created RAEC-coated, neonatal rat CM-embedded modules as well as module sheets. In both cases, embedded cells formed troponin-I-positive, native-muscle-like structures (although to a limited extent), and contracted with external field stimulation. In the presence of EC in an appropriate coculture medium, the construct became less responsive, although it continued to be excitable and contractile. While an in vivo study will be needed to elucidate the fate of these modules, this study has demonstrated the feasibility of creating functional cardiac tissue using the modular approach.
Acknowledgments
We acknowledge the financial support from the Canadian Institutes of Health Research through a team grant to R.D. Weisel (The Cardiac Regeneration Project), the National Institutes of Health (EB006903), and through the Training Program in Regenerative Medicine (PI: G.A. Levy). We acknowledge the technical assistance of Chuen Lo in harvesting the necessary tissues. We also acknowledge the many helpful discussions with M. Radisic and her laboratory.
Disclosure Statement
No competing financial interests exist.
References
- 1.Schmidt D. Mol A. Neuenschwander S. Breymann C. Gossi M. Zund G. Turina M. Hoerstrup S.P. Living patches engineered from human umbilical cord derived fibroblasts and endothelial progenitor cells. Eur J Cardiothorac Surg. 2005;27:795. doi: 10.1016/j.ejcts.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 2.Carrier R.L. Papadaki M. Rupnick M. Schoen F.J. Bursac N. Langer R. Freed L.E. Vunjak-Novakovic G. Cardiac tissue engineering: cell seeding, cultivation parameters, and tissue construct characterization. Biotechnol Bioeng. 1999;64:580. doi: 10.1002/(sici)1097-0290(19990905)64:5<580::aid-bit8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Li R.K. Jia Z.Q. Weisel R.D. Mickle D.A. Choi A. Yau T.M. Survival and function of bioengineered cardiac grafts. Circulation. 1999;100:II63. doi: 10.1161/01.cir.100.suppl_2.ii-63. [DOI] [PubMed] [Google Scholar]
- 4.Radisic M. Park H. Chen F. Salazar-Lazzaro J.E. Wang Y. Dennis R. Langer R. Freed L.E. Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann W.H. Fink C. Kralisch D. Remmers U. Weil J. Eschenhagen T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng. 2000;68:106. [PubMed] [Google Scholar]
- 6.Radisic M. Park H. Shing H. Consi T. Schoen F.J. Langer R. Freed L.E. Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grayson W.L. Martens T.P. Eng G.M. Radisic M. Vunjak-Novakovic G. Biomimetic approach to tissue engineering. Semin Cell Dev Biol. 2009;20:665. doi: 10.1016/j.semcdb.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vunjak-Novakovic G. Tandon N. Godier A. Maidhof R. Marsano A. Martens T. Radisic M. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev. 2010;16:169. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelm J.M. Ehler E. Nielsen L.K. Schlatter S. Perriard J.C. Fussenegger M. Design of artificial myocardial microtissues. Tissue Eng. 2004;10:201. doi: 10.1089/107632704322791853. [DOI] [PubMed] [Google Scholar]
- 10.Kelm J.M. Djonov V. Ittner L.M. Fluri D. Born W. Hoerstrup S.P. Fussenegger M. Design of custom-shaped vascularized tissues using microtissue spheroids as minimal building units. Tissue Eng. 2006;12:2151. doi: 10.1089/ten.2006.12.2151. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu T. Yamato M. Isoi Y. Akutsu T. Setomaru T. Abe K. Kikuchi A. Umezu M. Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;90:e40. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 12.McGuigan A.P. Sefton M.V. Design and fabrication of a vascularized tissue engineered construct. Cardiovasc Pathol. 2004;13:S182. [Google Scholar]
- 13.McGuigan A.P. Sefton M.V. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc Natl Acad Sci U S A. 2006;103:11461. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimitsu M. Higuchi K. Ramsubir S. Nonaka T. Rasaiah V.I. Siatskas C. Liang S.B. Murray G.J. Brady R.O. Medin J.A. Efficient correction of Fabry mice and patient cells mediated by lentiviral transduction of hematopoietic stem/progenitor cells. Gene Ther. 2007;14:256. doi: 10.1038/sj.gt.3302839. [DOI] [PubMed] [Google Scholar]
- 15.Leung B.M. Sefton M.V. A modular tissue engineering construct containing smooth muscle cells and endothelial cells. Ann Biomed Eng. 2007;35:2039. doi: 10.1007/s10439-007-9380-0. [DOI] [PubMed] [Google Scholar]
- 16.Schwarzkopf R. Shachar M. Dvir T. Dayan Y. Holbova R. Leor J. Cohen S. Autospecies and post-myocardial infarction sera enhance the viability, proliferation, and maturation of 3D cardiac cell culture. Tissue Eng. 2006;12:3467. doi: 10.1089/ten.2006.12.3467. [DOI] [PubMed] [Google Scholar]
- 17.Scharpfenecker M. Fiedler U. Reiss Y. Augustin H.G. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi H. Shimizu T. Yamato M. Tono K. Masuda H. Asahara T. Kasanuki H. Okano T. Fibroblast sheets co-cultured with endothelial progenitor cells improve cardiac function of infarcted hearts. J Artif Organs. 2008;11:141. doi: 10.1007/s10047-008-0421-8. [DOI] [PubMed] [Google Scholar]
- 19.Iyer R.K. Chui J. Radisic M. Spatiotemporal tracking of cells in tissue-engineered cardiac organoids. J Tissue Eng Regen Med. 2009;3:196. doi: 10.1002/term.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesman A. Habib M. Caspi O. Gepstein A. Arbel G. Levenberg S. Gepstein L. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Part A. 2010;16:115. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- 21.Iyer R.K. Chiu L.L. Radisic M. Microfabricated poly(ethylene glycol) templates enable rapid screening of triculture conditions for cardiac tissue engineering. J Biomed Mater Res A. 2009;89:616. doi: 10.1002/jbm.a.32014. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain M.D. Gupta R. Sefton M.V. Chimeric vessels in allogenic rat tissue engineering driven by endothelialized modules. 2009. (Submitted). [DOI] [PMC free article] [PubMed]
- 23.Iyer R.K. Radisic M. Cannizzaro C. Vunjak-Novakovic G. Synthetic oxygen carriers in cardiac tissue engineering. Artif Cells Blood Substit Immobil Biotechnol. 2007;35:135. doi: 10.1080/10731190600974988. [DOI] [PubMed] [Google Scholar]
- 24.Radisic M. Marsano A. Maidhof R. Wang Y. Vunjak-Novakovic G. Cardiac tissue engineering using perfusion bioreactor systems. Nat Protoc. 2008;3:719. doi: 10.1038/nprot.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaigler D. Wang Z. Horger K. Mooney D.J. Krebsbach P.H. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006;21:735. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 26.Chen L. He Z. Chen B. Yang M. Zhao Y. Sun W. Xiao Z. Zhang J. Dai J. Loading of VEGF to the heparin cross-linked demineralized bone matrix improves vascularization of the scaffold. J Mater Sci Mater Med. 2010;21:309. doi: 10.1007/s10856-009-3827-9. [DOI] [PubMed] [Google Scholar]
- 27.Naito H. Melnychenko I. Didie M. Schneiderbanger K. Schubert P. Rosenkranz S. Eschenhagen T. Zimmermann W.H. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114:I72. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 28.Kelm J.M. Djonov V. Hoerstrup S.P. Guenter C.I. Ittner L.M. Greve F. Hierlemann A. Sanchez-Bustamante C.D. Perriard J.C. Ehler E. Fussenegger M. Tissue-transplant fusion and vascularization of myocardial microtissues and macrotissues implanted into chicken embryos and rats. Tissue Eng. 2006;12:2541. doi: 10.1089/ten.2006.12.2541. [DOI] [PubMed] [Google Scholar]
- 29.van Luyn M.J. Tio R.A. van Seijen X.J. Plantinga J.A. de Leij L.F. DeJongste M.J. van Wachem P.B. Cardiac tissue engineering: characteristics of in unison contracting two- and three-dimensional neonatal rat ventricle cell (co)-cultures. Biomaterials. 2002;23:4793. doi: 10.1016/s0142-9612(02)00230-2. [DOI] [PubMed] [Google Scholar]