Abstract
Tooth infections or injuries involving dental pulp are treated routinely by root canal therapy. Endodontically treated teeth are devitalized, susceptible to re-infections, fractures, and subsequent tooth loss. Here, we report regeneration of dental-pulp-like tissue by cell homing and without cell transplantation. Upon in vivo implantation of endodontically treated real-size, native human teeth in mouse dorsum for the tested 3 weeks, delivery of basic fibroblast growth factor and/or vascular endothelial growth factor (bFGF and/or VEGF) yielded re-cellularized and revascularized connective tissue that integrated to native dentinal wall in root canals. Further, combined delivery of bFGF, VEGF, or platelet-derived growth factor (PDGF) with a basal set of nerve growth factor (NGF) and bone morphogenetic protein-7 (BMP7) generated cellularized and vascularized tissues positive of VEGF antibody staining and apparent neo-dentin formation over the surface of native dentinal wall in some, but not all, endodontically treated teeth. Newly formed dental pulp tissue appeared dense with disconnected cells surrounded by extracellular matrix. Erythrocyte-filled blood vessels were present with endothelial-like cell lining. Reconstructed, multiple microscopic images showed complete fill of dental-pulp-like tissue in the entire root canal from root apex to pulp chamber with tissue integration to dentinal wall upon delivery of bFGF, VEGF, or PDGF with a basal set of NGF and BMP7. Quantitative ELISA showed that combinatory delivery of bFGF, VEGF, or PDGF with basal NGF and BMP7 elaborated von Willerbrand factor, dentin sialoprotein, and NGF. These findings represent the first demonstration of regenerated dental-pulp-like tissue in endodontically treated root canals of real-size, native human teeth. The present chemotaxis-based approach has potent cell homing effects for re-cellularization and revascularization in endodontically treated root canals in vivo, although in an ectopic model. Regeneration of dental pulp by cell homing, rather than cell delivery, may accelerate clinical translation.
Introduction
Dental pulp is the only vascularized dental tissue encapsulated in highly mineralized structures, including dentin, enamel, and cementum, and maintains homeostasis of the tooth as a viable organ.1 The overall health of the tooth is compromised upon dental pulp trauma or infections, frequently manifested as pulpitis.2 A typical endodontic treatment or root canal therapy for irreversible pulpitis is pulpectomy, involving pulp extirpation followed by root canal enlargement and obturation with gutta percha, a bioinert thermoplastic material.2 Despite reported clinical success, endodontically treated teeth become de-vitalized and brittle, susceptible to postoperative fracture and other complications, including re-infections due to coronal leakage or microleakage.2,3 A substantial amount of tooth structures, including enamel and dentin, is removed during endodontic treatment, potentially leading to posttreatment tooth fracture and trauma.2–4 Endodontically treated teeth have lost pulpal sensation, and are deprived of the ability to detect secondary infections.2,3,5 The complications of current endodontic treatment are inevitable because of pulp devitalization or the loss of the tooth's innate homeostasis and defense mechanisms.
Existing effort in dental pulp regeneration has focused on cell transplantation (reviews6–8). Several reports have documented regeneration of dental-pulp-like tissue in vitro or ectopically by transplantation of dental pulp stem cells.9–11 Deciduous and adult dental pulp stem cells seeded in a self-assembling peptide-amphiphile hydrogel showed distinctive behavior: greater proliferative rate for deciduous cells but greater osteogenic differentiation potential for adult cells.7,8 Delivery of collagen scaffolds with dental pulp stem cells and dentin matrix protein-1 in dentin slices in mice led to ectopic formation of pulp-like tissue.12 Deciduous dental pulp stem/progenitor cells seeded in Matrigel in 1.5-mm cross-sectional tooth slices regenerated vascular pulp-like tissue after ectopic implantation in SCID mice.13 Similarly, stem/progenitor cells from apical papilla and dental pulp in root fragments yielded vascularized pulp-like tissue after ectopic implantation also in SCID mice.12 Despite its scientific validity, dental pulp regeneration by dental pulp stem cells encounters clinical and commercialization hurdles. Pulpectomy, the most common endodontic treatment, involves extirpation of dental pulp, and therefore leaves no dental pulp stem cells in the same tooth for pulp regeneration. For a patient who requires endodontic treatment in a given tooth but has intact dentition otherwise, no healthy tooth is to be sacrificed for isolation of dental pulp stem cells. Even in patients whose autologous dental pulp stem cells can be harvested, for example, from extracted wisdom teeth, clinical therapy of dental pulp regeneration is difficult to develop due to excessive costs, including cell isolation, handling, storage, and shipping, ex vivo manipulation, immune rejection (for allogeneic cells), not to mention liabilities of potential contamination, pathogen transmission, and tumorigenesis that may be associated with cell transplantation.14,15
Cell homing has been regarded as a process of exit of hematopoietic stem cells from blood vessels by transendothelialization and subsequent migration.16 In tissue regeneration, cell homing is dubbed as active recruitment of endogenous cells, including stem/progenitor cells, into an anatomic compartment.17,18 The hypothesis of this study is whether dental-pulp-like tissue can be regenerated in endodontically treated root canals of real-size, native human teeth by chemotaxis-induced cell homing, rather than cell transplantation. Our motivation for the study is to explore whether chemotaxis-induced cell homing is sufficient for the regeneration of dental-pulp-like tissue in endodontically treated root canals of real-size, native human teeth.
Materials and Methods
Endodontic treatment in extracted human teeth
After IRB approval, intact permanent maxillary and mandibular incisors and cuspids that have been freshly extracted during dental treatment were collected. Residual periodontal and peri-apical soft tissues were removed with a scalpel and cleaned with 70% ethanol. The teeth were disinfected further in 10% NaOCl for at least 1 week. Endodontic treatment was performed as in patients.2 An access opening was made through the lingual crown into pulp chamber. Pulpal tissue was extirpated, followed by cleaning and shaping the root canal with hand files and rotary instruments, but without obturation of root canal with gutta percha. All teeth were then autoclaved to eliminate any biological tissue.
Cytokine delivery
All cytokines in this study are recombinant human growth factors. Vascular endothelial growth factor (VEGF-2) at a dose of 10 ng/mL (R&D, Minneapolis, MN) was adopted for its chemotactic, mitogenic, and angiogenic roles in dental pulp cells.19,20 Basic fibroblast growth factor (bFGF) at a dose of 100 ng/mL (R&D) was adopted for its chemotactic and angiogenic roles.21,22 Platelet-derived growth factor (PDGF) at a dose of 10 ng/mL (R&D) was adopted for its roles in promoting angiogenesis, especially sprouting of existing blood vessels.23,24 Nerve growth factor (NGF) at a dose of 50 ng/mL (R&D) was adopted for its roles in promoting the survival and growth of nerve fibers.25 Bone morphogenetic protein-7 (BMP7) at a dose of 100 ng/mL was adopted for its roles in promoting mineralized tissue formation.26,27 Cytokines were adsorbed in 2 mg/mL neutralized collagen gel solution (BD Biosciences, Bedford, MA) that was injected into pulp chambers and root canals of endodontically treated human teeth. A total of 100–150 μL collagen gel solution was needed for each pulp chamber and root canal. Cytokine-free collagen gels served as controls. Endodontically treated human teeth treated with cytokine-loaded or cytokine-free collagen gel were incubated at 37°C for 1 h to induce gelation.
In vivo implantation
After IACUC approval, 5–7-week-old male mice (Harlan Laboratories, Boston, MA) were anesthetized with 1%–5% isoflurane. A linear incision was made in the dorsum to create a subcutaneous pocket. Endodontically treated human teeth with cytokine-adsorbed or cytokine-free collagen gel were implanted subcutaneously for 3 weeks.
Hisotology, immunohistochemistry, and ELISA
Retrieved implants were fixed in 10% buffered formalin, demineralized with 10% formic acid, and embedded in paraffin. Histological sections (5 μm thickness) were stained with hematoxylin-eosin or unstained for immunohistochemistry. In separate implants, soft tissue in pulp chamber was isolated for ELISA with antibodies conjugated using Lightning-Link HRP Conjugation Kit (Innova Biosciences, Cambridge, United Kingdom) and following manufacture's protocol (Santa Cruz Biotechnology, Santa Cruz, CA) to detect von Willerbrand factor (vWF), dentin sialoprotein (DSP), and NGF. Immunolocalization of anti-VEGF (Abcam, Cambridge, MA) was used.22,28 After overnight incubation with primary antibodies in a humidity chamber, sections were rinsed with phosphate-buffered saline and incubated with a secondary antibody. Sections were then incubated with streptavidin–horseradish peroxidase conjugate for 30 min in humidity chamber. After washing in phosphate-buffered saline, the double linking procedure with the secondary antibody was repeated. Slides were developed with diaminobenzadine solution and counterstained with Mayer's hematoxylin for 3–5 min. Counterstained slides were dehydrated in graded ethanol and cleared in xylene. The same procedures were performed for negative controls except for the omission of the primary antibodies. All immunohistochemistry procedures followed our previous methods.22
Statistical analysis
One-way analysis of variance and post hoc LSD tests were used with α level at 0.05 upon confirmation of normal data distribution.
Results
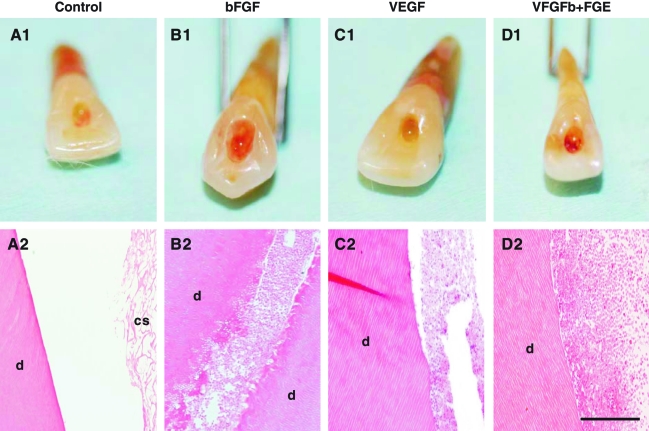
Upon retrieval of in-vivo-implanted human teeth, we observed typical pale access opening in teeth implanted with collagen scaffold alone (Fig. 1A1), as well as pale root apex opening (but impossible to image due to its pointed morphology). Microscopically, root canals filled with collagen scaffold alone showed residual amount of collagen scaffold (cs) that is adjacent to dentin (d), but little sign of cell ingrowth (Fig. 1A2). Contrastingly, bFGF delivery yielded red pigmentation in access opening upon in vivo harvest (Fig. 1B1), suggesting re-vascularization of endodontically treated human teeth, with a representative microscopic section showing abundant cells and some extracellular matrix that integrated with dentinal wall of root canal (Fig. 1B2). Similarly, VEGF delivery yielded vascularization in the representative gross specimen (Fig. 1C1) and connective tissue with abundant cells that integrated with dentinal wall (Fig. 1C2). Combined bFGF and VEGF delivery also generated red pigmentation in access opening (Fig. 1D1) and abundant cells that integrated with dentinal wall (Fig. 1D2). In absence of cell delivery, all the cells present in endodontically treated root canals after in vivo implantation are host derived.
FIG. 1.
Re-cellularization and revascularization of endodontically treated root canals in real-size human teeth. Clinically extracted human teeth were treated endodontically as in patients, with the exception of filling with gutta percha, a bioinert thermoplastic material. Instead, collage scaffolds were implanted in endodontically treated root canals with or without delivery of basic fibroblast growth factor (bFGF) and/or vascular endothelial growth factor (VEGF) followed by 3-week in vivo implantation. (A1) Endodontically treated root canals with collagen scaffold alone showed pale access opening. (A2) Microscopically, root canals with collagen scaffold alone showed residual collagen scaffold (cs) that is adjacent to native dentin (d), but little cell ingrowth. (B1) bFGF delivery in collagen scaffold yielded red pigmentation. (B2) Corresponding micrograph showing re-cellularization of endodontically treated root canal with abundant cells and some extracellular matrix that integrated with the wall of native dentin (d). (C1) VEGF delivery yielded re-cellularization with connective tissue within root canal (C2). Separation of soft tissue with dentinal wall is likely due to artifacts in histology processing, in consideration of tissue integration with dentin in other groups and also in Figure 2 below. Combined bFGF and VEGF delivery also generated red pigmentation (D1) and abundant cells within root canal (D2). Scale bars (A2, B2, C2, D2): 500 μm. Color images available online at www.liebertonline.com/ten.
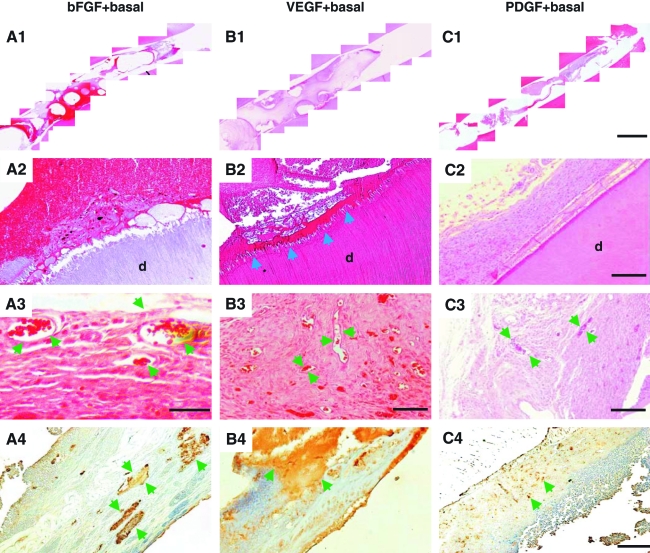
Given the initial positive data with delivery of bFGF and/or VEGF, two potent angiogenic cytokines, we followed up with an exploration of the efficacy of additional cytokines, with or without bFGF or VEGF, in pulp regeneration. A basal combination consisted of NGF and BMP7 due to its potency on induction of odontoblast/osteoblast differentiation. Combined delivery of bFGF with basal cytokines (NGF and BMP7) also yielded re-cellularization in the entire endodontically treated root canal of real-size human tooth as shown by reconstructed, multiple microscopic images (Fig. 2A1). A representative close-up image shows abundant cells in the endodontically treated root canal and apparent integration of regenerated tissues with dentinal wall in root canal (Fig. 2A2). A representative higher magnification image further shows the presence of erythrocyte-filled, blood-vessel-like structures (arrows in Fig. 2A3). Scattered islands of positive VEGF antibody staining were found in association with combined bFGF + basal cytokine delivery (Fig. 2A4). Further, combined delivery of VEGF with basal cytokines (NGF and BMP7) induced re-cellularization in endodontically treated root canal from root apex to pulp chamber as shown by reconstructed, multiple microscopic images (Fig. 2B1). Abundant cells were present in the endodontically treated root canal and showed apparent integration with dentinal wall (Fig. 2B2). Remarkably, a layer of amorphous dentin-like tissue was present on the surface of native dentinal wall (arrows in Fig. 2B2), resembling newly formed secondary dentin. Multiple blood vessel-like structures were present in regenerated soft tissue in root canal (arrows in Fig. 2B3). Areas of positive VEGF antibody staining were readily identified in association with VEGF + basal cytokine delivery (Fig. 2B4). Combined delivery of PDGF with basal cytokines (NGF and BMP7) in an endodontically treated root canal resulted in re-cellularization along the entire root canal as shown by reconstructed, multiple microscopic images (Fig. 2C1). A representative close-up image shows abundant cells and integration of regenerated tissue with dentinal wall in root canal (Fig. 2C2). A representative higher magnification image further shows the presence of blood vessel-like structures (arrows in Fig. 2C3) with abundant cells and some extracellular matrix. VEGF antibody staining revealed scattered positive locations in association with PDGF + basal delivery (Fig. 2C4). Given all human teeth utilized in the present work were endodontically treated and autoclaved to remove any biological tissue, all regenerated tissues, including vasculature, in the root canal are derived from host endogenous cells.
FIG. 2.
Combinatory cytokine delivery and chemotactic effects on pulp regeneration from host endogenous cells. (A1) Combined delivery of bFGF with basal cytokines of nerve growth factor (NGF) and bone morphogenetic protein-7 (BMP7) yielded re-cellularization in the entire endodontically treated root canal as shown by reconstructed, multiple microscopic images from root apex to pulp chamber. (A2) Close-up image showing abundant cells and integration with native dentin (d) in endodontically treated root canal. (A3) Higher magnification showing erythrocyte-filled blood vessels (arrows) in connective tissue. (A4) Scattered islands of positive VEGF antibody staining (arrows). (B1) Combined delivery of VEGF with basal cytokines of NGF and BMP7 induced re-cellularization in the entire endodontically treated root canal. (B2) Connective tissue with abundant cells integrated with native dentin (d), including a layer of dentin-like tissue between abundant cells and dentinal wall (arrows). (B3) Multiple blood-vessel-like structures in regenerated connective tissue (arrows). (B4) Positive VEGF antibody staining. Arrows indicate positive VEGF staining. (C1) Combined delivery of platelet-derived growth factor (PDGF) with basal cytokines of NGF and BMP7 yielded fully regenerated tissues in the entire endodontically treated root canal. (C2) Abundant cells in connective tissue that integrated to native dentin (d). (C3) Blood-vessel-like structures (arrows) in connective tissue. (C4) VEGF antibody staining. Arrows indicate positive VEGF staining. Scale bars (A1, B1, C1): 1 mm; (A2, B2, C2): 100 μm; (A3): 100 μm; (B3, C3): 300 μm; (A4, B4, C4): 300 μm. Color images available online at www.liebertonline.com/ten.
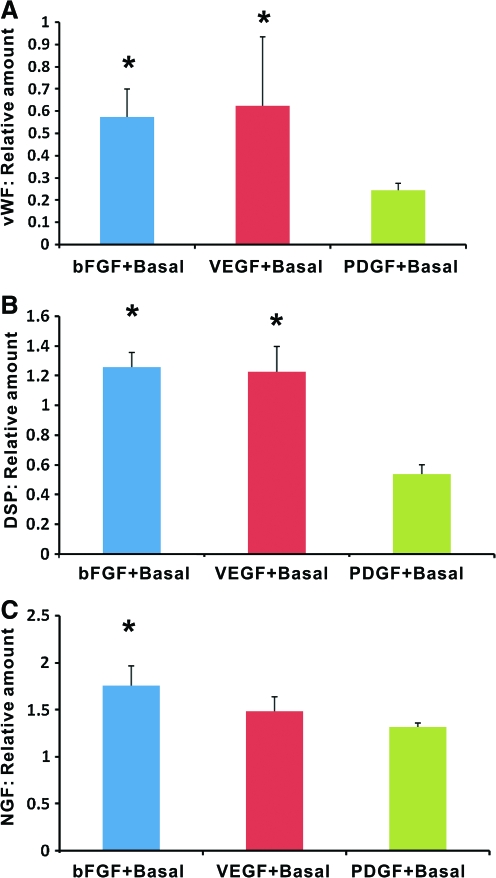
The regenerated tissue in endodontically treated root canal of human teeth after in vivo implantation in mice was retrieved for ELISA relative to collagen scaffold only group. Quantitatively, vWF, an endothelium-elaborated glycoprotein in blood plasma, was present in bFGF, VEGF, or PDGF in conjunction with basal cytokines of NGF and BMP7, with significantly higher content in association with VEGF + basal cytokines and bFGF + basal cytokines than PDGF + basal cytokines (Fig. 3A). Further, delivery of VEGF + basal cytokines or bFGF + basal cytokines induced significantly more DSP, an odontoblastic marker,29 than PDGF + basal cytokines (Fig. 3B). Also, combined delivery of bFGF + basal cytokines yielded significantly more NGF than VEGF + basal cytokines or PDGF + basal cytokines (Fig. 3C). These ELISA data suggest that ectopically homed cells, in mouse dorsum, elaborate angiogenesis, mineralization, and neurogenesis in regenerated dental pulp-like tissue in endodontically treated, real-size human teeth. Given delivery of exogenous NGF, ELISA-quantified total NGF includes both exogenous and endogenous NGF.
FIG. 3.
ELISA of von Willerbrand factor (vWF), dentin sialoprotein (DSP), and NGF in regenerated dental-pulp-like tissue relative to collagen scaffold only group. (A) Codelivery of bFGF or VEGF with basal cytokines of NGF and BMP7 yielded significantly more vWF than PDGF with NGF and BMP7. (B) Codelivery of bFGF or VEGF with basal cytokines of NGF and BMP7 yielded significantly more DSP than PDGF with NGF and BMP7. (C) Codelivery of bFGF or VEGF with basal cytokines of NGF and BMP7 yielded significantly more NGF than PDGF with NGF and BMP7. n = 6; *p < 0.05. Color images available online at www.liebertonline.com/ten.
Discussion
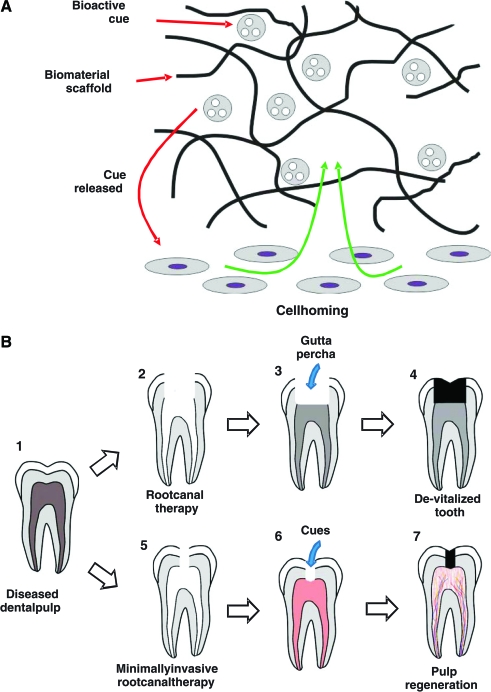
The present findings demonstrate regeneration of dental-pulp-like tissue in endodontically treated root canals of real-size human teeth after in vivo ectopic implantation. This is another step forward and scale up from previous approaches of regeneration of ectopic-pulp-like tissue in 1.5-mm cross-sectional tooth slices or root fragments.12,13 As a departure from previous reports of dental pulp regeneration by cell transplantation (e.g., Refs.7–13), we have taken a cell homing approach by chemotaxis of host's endogenous cells. As compared to incurable and life-threatening diseases such as diabetes, Parkinson's, muscular dystrophy, and Alzheimer's, for which cell transplantation is warranted,14,30 dental pulp regeneration by cell transplantation likely will not be economically viable or competitive with current root canal therapy or dental implants. Cell transplantation and cell homing are both scientifically meritorious approaches for dental pulp regeneration, and should both continue. However, cell homing represents a novel concept for dental pulp regeneration and may offer a clinically translatable approach. Bioactive cues can be adsorbed, tethered, or encapsulated in biomaterials (Fig. 4A).31 Upon release of bioactive cues in vivo, adjacent and/or systemic cells adjacent to root apices of endodontically treated root canals, including stem/progenitor cells, migrate into an anatomic compartment (Fig. 4A), which in this case, is root canal that serves as a native scaffold.
FIG. 4.
Schemes of cell homing for dental pulp regeneration and clinical translation. (A) Bioactive cues can be adsorbed, tethered, or encapsulated in biomaterial scaffolds. Upon release of bioactive cues, such as from endodontically treated root canals in this work, local and/or systemic cells, including stem/progenitor cells, can be homed in vivo into an anatomic compartment, which in this case, is root canal that serves as a native scaffold. Current root canal treatment of diseased dental pulp (B1) necessitates removal of substantial enamel and dentin structures because obturation of gutta percha requires unobstructed access (B2, B3), yet leading to a de-vitalized tooth (B4). We propose that a diseased dental pulp (B1) can be treated with a revised, minimally invasive root canal therapy (B5) on the basis that delivery of injectable bioactive cues does not require unobstructed access to pulp chamber and root canal (B6). Although residual inflammation in endodontically treated root canal and peri-apical region are anticipated to present challenges for pulp regeneration, chemotaxis-induced angiogenesis as shown in the present may provide the potential for native defense mechanisms that may counteract residue infection in the root canal (B6), leading to a vital tooth with regenerated dental pulp (B7). Color images available online at www.liebertonline.com/ten.
The rationale for delivery of multiple cytokines, either singularly or in combination, is to broadly survey their individual and combined effects on dental pulp regeneration. Our present data show that multiple cytokines, bFGF, VEGF, or PDGF, alone or in combination with NGF and BMP7, induce cell homing, angiogenesis, and mineralized tissue formation. It appears that not all presently tested cytokines are needed in a single formulation for dental pulp regeneration. Our ongoing work takes a reduction approach to determine whether a minimum subset of cytokines, or even a single cytokine, can regenerate dental pulp. This reduction approach is necessary to minimize the cost of dental pulp regeneration in translational approaches. Our data showing bFGF's potency in pulp-like tissue formation in endodontically treated root canals are consistent with dentinal bridge formation by bFGF in a pulp capping model.21 Our data showing VEGF-mediated vascularization and pulp-like tissue genesis are supported by its potent chemotactic effects on dental pulp cells and expression in primary and permanent tooth pulps.19,20 Host endogenous cells, some of which are likely vasculature-elaborating cells, are capable of homing into and populate root canals of endodontically treated human teeth. Although bFGF and VEGF in conjunction with a basal set of NGF and BMP7 induced significantly more vWF, DSP, and NGF than PDGF plus the same basal set of NGF and BMP7, it remains possible that the amount of PDGF-elaborated vWF, DSP, and NGF is sufficient for dental pulp regeneration, which can only be ascertained in an orthotopic model. Angiogenesis is indicated by the presence of vWF in endodontically treated root canals upon delivery of bFGF, VEGF, or PDGF. The present two-step experiments, first by delivery of angiogenic and chemotactic cytokines, bFGF and VEGF, and then combined delivery of bFGF, VEGF, or PDGF with a basal set of NGF and BMP7, are based on our working hypothesis that angiogenesis is a priority in dental pulp regeneration, followed by regeneration of odontoblastic and neural tissue.
Dental pulp regeneration by chemotactic cell homing represents a variation from the mainstream approach of pulp regeneration by cell transplantation. During the course of our experiments, an interesting model was reported to seed dental pulp stem cells and/or endothelial progenitor cells in 1.5-mm cross-sectional human tooth slices, followed by in vivo ectopic implantation.13,32 Tissue regeneration and vascularization are apparent in harvested tooth slices. However, the acellular control group also reveals tissue regeneration and vascularization in tooth slices,13,32 suggesting the potential of re-cellularization and revascularization without cell delivery. The pulp regeneration model created in this work, that is, endodontically treated root canals of real-size human teeth, represents a scale up from previous tooth slice or root fragment models.12,32 Substantial tissue formation in endodontically treated root canals in this study suggests the potency of delivered cytokines to induce cell homing and regeneration in a presumably anaerobic environment in root canals (up to 8–10 mm in length) of in vivo implanted, endodontically treated human teeth, consistent with the observed proliferation of human dental pulp cells in hypoxia.33 Although angiogenesis is primary, dental pulp regeneration likely is incomplete without regeneration of odontoblasts and nerve fibers. BMP7, when delivered to the site of pulp exposure, elaborates dentinogenesis and regeneration of dentin-like tissue.27,34,35 Our data showing putative dentin formation by combinatory cytokines including BMP7 is consistent with its illustrated roles in pulp capping.8,26,27,36–39 The presence of DSP in this work serves as another indication of mineralization in endodontically root canals, in addition to apparent mineralized neo-dentin formation on native dental wall (Fig. 2B2). However, other members of the transforming growth factor-beta/BMP superfamily such as BMP2 or transforming growth factor-beta isoforms may also induce dentin regeneration, which warrants additional studies. Most of the nerve fibers in dental pulp are nociceptive and vasoregulatory, signifying the importance of pain as a defense mechanism.40 Several neurotrophic factors, especially NGF, promote the outgrowth and sprouting of sensory nerve endings.25 Therefore, NGF and other neurotrophic factors are viable candidates for nerve regeneration in dental pulp, as shown by NGF assay in the present work. Given that NGF was delivered in equal amounts in bFGF, VEGF, and PDGF groups, significantly different NGF content in regenerated pulp-like tissue among bFGF, VEGF, and PDGF groups indicates that endogenously produced NGF differs among these groups.
We propose that endogenous cells of peri-apical regions in the jaw bone in an orthotopic model such as endodontic patients can be recruited by chemotactic effects of released bioactive cues and differentiate along odontoblastic, angiogenic, and neurogenic directions that initiate dental pulp regeneration (Fig. 4A). This hypothesis obviously needs to be tested in orthotopic dental pulp regeneration models. One of the potential beneficial factors for orthotopic dental pulp regeneration includes available stem/progenitor cells and blood vessels in the peri-apical region in the marrow of the trabecular bone of the maxilla or mandible, in comparison with the ectopic environment of the dorsum. These data showing cell homing into endodontically treated root canals of real-size, native human teeth suggest that chemotaxis has induced cell recruitment, survival, and retention for no <3–4 mm (½ of human incisor/cuspid root length due to bidirectional opening in crown and apex) into a presumably anaerobic root canal environment over the tested 3 weeks. This is remarkable and provides the rationale to test dental pulp regeneration orthotopically in vivo in our ongoing work. The present translational approach is perhaps counterintuitive to mechanistic investigations to study one biomolecule at a time, but paves foundation for our ongoing reduction approach to regenerate dental pulp in a large animal model and subsequently in endodontic patients, utilizing a translatable approach that may serve as an alternative to gutta percha for endodontic treatment (Fig. 4B). Current root canal treatment of a diseased dental pulp (Fig. 4B1) necessitates removal of substantial enamel and dentin structures because filling of gutta percha requires unobstructed access (Fig. 4B2, 4B3), and yet leads to a de-vitalized tooth (Fig. 4B4). We propose that a diseased dental pulp can be treated with a revised, minimally invasive root canal therapy (Fig. 4B5) on the basis that delivery of injectable bioactive cues does not require unobstructed access to pulp chamber and root canal (Fig. 4B6). Although residual inflammation in endodontically treated root canal and peri-apical region may present additional challenges for pulp regeneration, chemotaxis-induced angiogenesis in this study provides the potential for native defense mechanisms that may counteract any residue infection in the root canal (Fig. 4B6, 4B7). Further, some of the cytokines that are endogenously elaborated during inflammation are also those capable of chemotaxis in tissue regeneration.41,42 Taken together, this study represents the first step toward perhaps a clinically translatable approach of dental pulp regeneration by cell homing.
Acknowledgments
The authors thank F. Guo and K. Hua for technical and administrative assistance. This research was supported by USPHS Research Grant RC2DE020767 from the National Institute of Dental and Craniofacial Research (NIDCR), National Institutes of Health Bethesda, MD 20892.
Disclosure Statement
No competing financial interests exist.
References
- 1.Ten Cate A.R. Dentin and pulp. In: Ten Cate A.R., editor. Oral Histology. London: Mosby; 1998. pp. 246–278. [Google Scholar]
- 2.Ingle J.I. Bakland L.K. Structure and function of the dentin-pulp complex. In: Ingle J.I., editor; Bakland L.K., editor. Endodontics. Hamilton, Ontario, London: BC Decker, Inc.; 2002. pp. 121–143. [Google Scholar]
- 3.Dammaschke T. Steven D. Kaup M. Ott K.H. Long-term survival of root-canal-treated teeth: a retrospective study over 10 years. J Endod. 2003;29:638. doi: 10.1097/00004770-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Andreasen J.O. Farik B. Munksgaard E.C. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 5.Caplan D.J. Cai J. Yin G. White B.A. Root canal filled versus non-root canal filled teeth: a retrospective comparison of survival times. J Public Health Dent. 2005;65:90. doi: 10.1111/j.1752-7325.2005.tb02792.x. [DOI] [PubMed] [Google Scholar]
- 6.Hargreaves K.M. Giesler T. Henry M. Wang Y. Regeneration potential of the young permanent tooth: what does the future hold? J Endod. 2008;34:S51. doi: 10.1016/j.joen.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Murray P.E. Garcia-Godoy F. Hargreaves K.M. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Sloan A.J. Smith A.J. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis. 2007;13:151. doi: 10.1111/j.1601-0825.2006.01346.x. [DOI] [PubMed] [Google Scholar]
- 9.Galler K.M. Cavender A. Yuwono V. Dong H. Shi S. Schmalz G. Hartgerink J.D. D'Souza R.N. Self-assembling peptide amphiphile nanofibers as a scaffold for dental stem cells. Tissue Eng Part A. 2008;14:2051. doi: 10.1089/ten.tea.2007.0413. [DOI] [PubMed] [Google Scholar]
- 10.Gotlieb E.L. Murray P.E. Namerow K.N. Kuttler S. Garcia-Godoy F. An ultrastructural investigation of tissue-engineered pulp constructs implanted within endodontically treated teeth. J Am Dent Assoc. 2008;139:457. doi: 10.14219/jada.archive.2008.0189. [DOI] [PubMed] [Google Scholar]
- 11.Prescott R.S. Alsanea R. Fayad M.I. Johnson B.R. Wenckus C.S. Hao J. John A.S. George A. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 2008;34:421. doi: 10.1016/j.joen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang G.T. Yamaza T. Shea L.D. Djouad F. Kuhn N.Z. Tuan R.S. Shi S. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010;16:605. doi: 10.1089/ten.tea.2009.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordeiro M.M. Dong Z. Kaneko T. Zhang Z. Miyazawa M. Shi S. Smith A.J. Nor J.E. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Ahsan T. Bellamkonda R. Nerem R.M. Tissue engineering and regenerative medicine: advancing towards clinical therapies. In: Mao J.J., editor; Vunjak-Novakovic G., editor; Mikos A.M., editor; Atala A., editor. Translational Approaches in Tissue Engineering and Regenerative Medicine. Boston, MA: Artech House; 2007. pp. 3–16. [Google Scholar]
- 15.Mao J.J. Stem cells and the future of dental care. N Y State Dent J. 2008;74:20. [PubMed] [Google Scholar]
- 16.Quesenberry P.J. Becker P.S. Stem cell homing: rolling, crawling, and nesting. Proc Natl Acad Sci U S A. 1998;95:15155. doi: 10.1073/pnas.95.26.15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao J.J. Stosich M.S. Moioli E.K. Lee C.H. Fu S.Y. Bastian B. Eisig S.B. Zemnick C. Ascherman J. Wu J. Rohde C. Ahn J. Facial reconstruction by biosurgery: cell transplantation versus cell homing. Tissue Eng Part B Rev. 2010;16:257. doi: 10.1089/ten.teb.2009.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laird D.J. von Andrian U.H. Wagers A.J. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 19.Grando Mattuella L. Poli de Figueiredo J.A. Nor J.E. de Araujo F.B. Medeiros Fossati A.C. Vascular endothelial growth factor receptor-2 expression in the pulp of human primary and young permanent teeth. J Endod. 2007;33:1408. doi: 10.1016/j.joen.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Matsushita K. Motani R. Sakuta T. Yamaguchi N. Koga T. Matsuo K. Nagaoka S. Abeyama K. Maruyama I. Torii M. The role of vascular endothelial growth factor in human dental pulp cells: induction of chemotaxis, proliferation, and differentiation and activation of the AP-1-dependent signaling pathway. J Dent Res. 2000;79:1596. doi: 10.1177/00220345000790081201. [DOI] [PubMed] [Google Scholar]
- 21.Ishimatsu H. Kitamura C. Morotomi T. Tabata Y. Nishihara T. Chen K.K. Terashita M. Formation of dentinal bridge on surface of regenerated dental pulp in dentin defects by controlled release of fibroblast growth factor-2 from gelatin hydrogels. J Endod. 2009;35:858. doi: 10.1016/j.joen.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 22.Stosich M.S. Bastian B. Marion N.W. Clark P.A. Reilly G. Mao J.J. Vascularized adipose tissue grafts from human mesenchymal stem cells with bioactive cues and microchannel conduits. Tissue Eng. 2007;13:2881. doi: 10.1089/ten.2007.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooke J.W. Sarment D.P. Whitesman L.A. Miller S.E. Jin Q. Lynch S.E. Giannobile W.V. Effect of rhPDGF-BB delivery on mediators of periodontal wound repair. Tissue Eng. 2006;12:1441. doi: 10.1089/ten.2006.12.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimokado K. Higaki M. Signal transduction for PDGF-induced chemotaxis of vascular smooth muscle cells. Ann N Y Acad Sci. 1997;811:130. doi: 10.1111/j.1749-6632.1997.tb51995.x. [DOI] [PubMed] [Google Scholar]
- 25.Micera A. Lambiase A. Stampachiacchiere B. Bonini S. Levi-Schaffer F. Nerve growth factor and tissue repair remodeling: trkA(NGFR) and p75(NTR), two receptors one fate. Cytokine Growth Factor Rev. 2007;18:245. doi: 10.1016/j.cytogfr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Nakashima M. Nagasawa H. Yamada Y. Reddi A.H. Regulatory role of transforming growth factor-beta, bone morphogenetic protein-2, and protein-4 on gene expression of extracellular matrix proteins and differentiation of dental pulp cells. Dev Biol. 1994;162:18. doi: 10.1006/dbio.1994.1063. [DOI] [PubMed] [Google Scholar]
- 27.Rutherford B. Fitzgerald M. A new biological approach to vital pulp therapy. Crit Rev Oral Biol Med. 1995;6:218. doi: 10.1177/10454411950060030401. [DOI] [PubMed] [Google Scholar]
- 28.Jinga V.V. Gafencu A. Antohe F. Constantinescu E. Heltianu C. Raicu M. Manolescu I. Hunziker W. Simionescu M. Establishment of a pure vascular endothelial cell line from human placenta. Placenta. 2000;21:325. doi: 10.1053/plac.1999.0492. [DOI] [PubMed] [Google Scholar]
- 29.Paine M.L. Luo W. Wang H.J. Bringas P., Jr. Ngan A.Y. Miklus V.G. Zhu D.H. MacDougall M. White S.N. Snead M.L. Dentin sialoprotein and dentin phosphoprotein overexpression during amelogenesis. J Biol Chem. 2005;280:31991. doi: 10.1074/jbc.M502991200. [DOI] [PubMed] [Google Scholar]
- 30.Yang R. Chen M. Lee C.H. Yoon R. Lal S. Mao J.J. Clones of ectopic stem cells in the regeneration of muscle defects in vivo. Plos One. 2010 doi: 10.1371/journal.pone.0013547. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moioli E.K. Clark P.A. Xin X. Lal S. Mao J.J. Matrices and scaffolds for drug delivery in dental, oral and craniofacial tissue engineering. Adv Drug Deliv Rev. 2007;59:308. doi: 10.1016/j.addr.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goncalves S.B. Dong Z. Bramante C.M. Holland G.R. Smith A.J. Nor J.E. Tooth slice-based models for the study of human dental pulp angiogenesis. J Endod. 2007;33:811. doi: 10.1016/j.joen.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Sakdee J.B. White R.R. Pagonis T.C. Hauschka P.V. Hypoxia-amplified proliferation of human dental pulp cells. J Endod. 2009;35:818. doi: 10.1016/j.joen.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Jernvall J. Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92:19. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- 35.Nakashima M. Reddi A.H. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21:1025. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 36.Goldberg M. Lacerda-Pinheiro S. Jegat N. Six N. Septier D. Priam F. Bonnefoix M. Tompkins K. Chardin H. Denbesten P. Veis A. Poliard A. The impact of bioactive molecules to stimulate tooth repair and regeneration as part of restorative dentistry. Dent Clin North Am. 2006;50:277. doi: 10.1016/j.cden.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg M. Six N. Decup F. Buch D. Soheili Majd E. Lasfargues J.J. Salih E. Stanislawski L. Application of bioactive molecules in pulp-capping situations. Adv Dent Res. 2001;15:91. doi: 10.1177/08959374010150012401. [DOI] [PubMed] [Google Scholar]
- 38.Rutherford R.B. Wahle J. Tucker M. Rueger D. Charette M. Induction of reparative dentine formation in monkeys by recombinant human osteogenic protein-1. Arch Oral Biol. 1993;38:571. doi: 10.1016/0003-9969(93)90121-2. [DOI] [PubMed] [Google Scholar]
- 39.Six N. Decup F. Lasfargues J.J. Salih E. Goldberg M. Osteogenic proteins (bone sialoprotein and bone morphogenetic protein-7) and dental pulp mineralization. J Mater Sci Mater Med. 2002;13:225. doi: 10.1023/a:1013846516693. [DOI] [PubMed] [Google Scholar]
- 40.Diogenes A. Akopian A.N. Hargreaves K.M. NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res. 2007;86:550. doi: 10.1177/154405910708600612. [DOI] [PubMed] [Google Scholar]
- 41.Guo S. Dipietro L.A. Factors affecting wound healing. J Dent Res. 2010;89:219. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mountziaris P.M. Mikos A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14:179. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]