Abstract
Completely biological tissue replacements can be fabricated by entrapping cells in a molded fibrin gel. Over time, the fibrin is degraded and replaced with cell-produced extracellular matrix. However, the relationship between fibrin degradation and matrix deposition has not been elucidated. We developed techniques to quantify fibrin degradation products (FDP) and examine plasmin activity in the conditioned medium from fibrin-based constructs. Fibrin-based tissue constructs fabricated with vascular smooth muscle cells (vSMC) were cultured for 5 weeks in the presence of varied concentrations of the fibrinolysis inhibitor ɛ-aminocaproic acid and cellularity, and deposited collagen and elastin were measured weekly. These data revealed that increasing concentrations of ɛ-aminocaproic acid led to delayed and diminished FDP production, lower vSMC proliferation, and decreased collagen and elastin deposition. FDP were shown to have a direct biological effect on vSMC cultures and vSMC within the fibrin-based constructs. Supplementing construct cultures with 250 or 500 μg/mL FDP led to 30% higher collagen deposition than the untreated controls. FDP concentrations as high as 250 μg/mL were estimated to exist within the constructs, indicating that FDP generation during remodeling of the fibrin-based constructs exerted direct biological activity. These results help explain many of the positive outcomes reported with fibrin-based tissue constructs in the literature, as well as demonstrate the importance of regulating plasmin activity during their fabrication.
Introduction
The plasma protein fibrinogen has shown great utility in tissue engineering, being used as the basis for a fibrin gel scaffold for growth of bone marrow stromal cells,1 chondrocytes,2 osteoblasts,3 and nerve axons.4 Fibrin gel has also been used as a scaffold in cardiovascular tissue engineering5,6 and used in conjunction with vascular smooth muscle cells (vSMC) in vascular tissue engineering.7–10 As a biopolymer, fibrin has a number of advantages, including the option for direct cell entrapment during construct fabrication, the availability of sites for cellular adhesion and binding of other matrix molecules and growth factors, and the ability to produce alignment of cells and fibrils in gels that are mechanically constrained during gel compaction by cell traction forces.11,12 Fibrin-based constructs undergo extensive matrix remodeling as the cells degrade fibrin and deposit extracellular matrix (ECM).7,13,14 vSMC secrete more collagen and elastin in fibrin gel than in collagen gel, producing constructs with superior mechanical properties.14,15
Fibrin gel is formed by the thrombin-catalyzed self-assembly of fibrin monomers, derived from fibrinogen, into native protein filaments. This process forms a fibrin gel entrapping the cells suspended in the fibrinogen solution, analogous to the entrapment of platelets in a fibrin clot during wound healing.16,17 The serine protease plasmin acts in vivo to resorb the thrombus, after activation of the zymogen plasminogen by urokinase (uPA) or tissue plasminogen activator (tPA).18 Fibrin degradation can be modulated by the addition of plasmin inhibitors, such as aprotinin or ɛ-aminocaproic acid (ACA). ACA inhibits uPA,19 tPA,20 and plasmin,21,22 and is widely used in tissue engineering to slow fibrin degradation.6,23–26
Controlling the rate of fibrinolysis is of great importance when using fibrin gel as a tissue scaffold. Extensive degradation of the gel before the cells secrete sufficient ECM results in construct failure. However, excessive fibrinolytic inhibition could limit fibrin remodeling into tissue and also result in failure. In fact, fibrin degradation products (FDP) have been shown to have biological activity and may aid in the remodeling of the fibrin constructs. For example, plasmin-generated FDP fragment E and atherosclerotic plaque extracts have shown mitogenic effects on SMC,27 and plasmin-derived FDP stimulated collagen synthesis in the chick chorioallantoic membrane model.28
The goal of this study was to examine how fibrin degradation affects proliferation of and matrix deposition by vSMC in fibrin-based tissue constructs. Enzyme-linked immunosorbent assay (ELISA) and zymography methods were developed to monitor levels of bovine FDP and plasmin activity, respectively, in the medium of fibrin-based tissue constructs. The fibrinolytic inhibitor ACA was utilized to alter fibrin degradation by plasmin and examine the effects on collagen and elastin deposition and cell proliferation in fibrin constructs over long-term culture. FDP concentrations in the interstitial fluid of the constructs were measured to estimate the FDP concentrations in proximity to the vSMC during construct degradation. To determine if these concentrations of FDP were bioactive, cell cultures were supplemented with exogenous FDP over a range of concentrations, and changes in cellularity and collagen content were measured. Finally, to demonstrate that FDP were bioactive in the tissue constructs, exogenous FDP were added under conditions of fibrin degradation inhibition.
Materials and Methods
Cell culture
vSMC were isolated from 1- to 3-day-old Fischer rat aortae, as previously described.13 Cell type was verified by staining with α-SM actin and SM myosin heavy chain antibodies (Abcam Inc., Cambridge, MA). The cells were maintained in Dulbecco's modified Eagle's medium/F12 supplemented with 100 U/mL penicillin, 100 U/mL streptomycin (Invitrogen, Carlsbad, CA), and 15% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA). All culture flasks were seeded with 20,000 cells/cm2, split near confluence, and used at passage 6 for construct fabrication.
Fabrication of fibrin-based tissue constructs
Adherent disc constructs were prepared as previously described.13 For these studies, the initial concentrations were 5 × 105 vSMC/mL and 3.3 mg/mL fibrinogen solid (F4753; Sigma Aldrich, St. Louis, MO); 200 μL of fibrin gel was used for each 1-cm-diameter disc construct. After fibrin gelation, construct medium consisting of Dulbecco's modified Eagle's medium/F12, antibiotic/antimycotic (Invitrogen), 10% FBS, 50 μg/mL ascorbate (Sigma Aldrich), 1 ng/mL transforming growth factor β1 (TGF-β1; R&D Systems, Minneapolis, MN), and 2 μg/mL insulin (Sigma Aldrich), plus 3, 6, or 12 mM ACA (Thermo Fisher Scientific) was added at an initial volume of 2 mL per construct and replaced with 1 mL of this supplemented medium every 2–3 days. A medium sample was collected before each medium change. Constructs were harvested weekly for 5 weeks.
In a separate set of experiments, constructs grown in 3 and 12 mM ACA received 0, 100, 250, or 500 μg/mL bovine plasmin-derived FDP 24 h after casting and again with each medium change. FDP were formed as described in the ELISA for bovine FDP section. Constructs were harvested at 1 week.
ELISA for bovine FDP
FDP present in media were quantified with a competitive ELISA using antibovine fibrinogen (American Diagnostica Inc., Stamford, CT) as the primary antibody and purified bovine fibrinogen (Aniara, Mason, OH) as the coating antigen and concentration standard. This fibrinogen was converted to fibrin using the same conditions as construct preparation, digested overnight at 37°C with human plasmin (Sigma Aldrich) at 0.04 U/mg protein in 50 mM Tris, 100 mM NaCl, and 10 mM CaCl2, pH 7.6. Plasmin was quenched with aprotinin (Sigma Aldrich) at 100 KIU/mL and the digest was adjusted to 1 μg/mL bovine FDP in phosphate-buffered saline.
EIA/RIA plates (Corning Inc., Lowell, MA) were coated overnight at 4°C with bovine FDP. All other incubations were performed for 1 h at room temperature in phosphate-buffered saline with 0.05% Tween-20. The plate was then blocked with 1% bovine serum albumin (immunoglobulin G and protease free; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Solutions of primary antibody (3.33 μg/mL) plus either known amounts of bovine FDP or diluted sample media were incubated, added to the plate, and incubated again to allow free antibody to bind to the FDP coated on the plate. Since the ELISA also detected fibrinogen products in FBS, both media and standards were at kept at the same FBS concentration. Attached primary antibody was bound to an horseradish-peroxidase-conjugated secondary antibody (Jackson ImmunoResearch) used at 1:50,000. The bound horseradish peroxidase was incubated with 3,3′, 5,5′-tetramethylbenzidine substrate solution (TMB, Thermo Fisher Scientific) to yield color inversely proportional to FDP concentration. After quenching with 2.5 M H2SO4, the plates were read at 450 nm with background subtraction (BioTek Instruments, Inc., Winooski, VT). Completely digested vSMC constructs contained 330 μg FDP as measured by ELISA.
In one set of experiments, constructs were harvested daily and pooled to allow for quantification of interstitial FDP. In these studies, 100 KIU/mL aprotinin and 10 μM Galardin (Millipore, Billerica, MA) were added to the medium 1 h before harvest and samples of medium were collected for quantification. Then, all medium was aspirated from the construct surface and the constructs were collected into chilled vials and centrifuged at 10,000 g at 4°C for 15 min. The supernatant was collected and used to quantify FDP within the construct via ELISA.
Fibrinogen zymography
Polyacrylamide gels were prepared with the addition of 375 μg/mL fibrinogen to the resolving phase. Medium, human activated plasmin, or α2-antiplasmin (Abcam Inc.) were loaded as indicated and then separated by electrophoresis. The gel was rinsed, extracted in 2.5% Triton X-100 (Sigma Aldrich), rinsed again, and then incubated in reaction buffer (50 mM Tris pH 7.4, 1 mM CaCl2, and 1 mM MgCl2) at 37°C overnight. ACA was added to the buffer as indicated. Digested gels were stained (Blue BANDit; Amresco Inc., Solon, OH) and then scanned for qualitative analysis.
Biochemical and histochemical analysis
DNA was quantified with a modified Hoechst assay.29 Cellularity was determined assuming 7.6 pg of DNA per cell.30 Collagen content was measured with the hydroxyproline assay of Stegemann and Stadler,31 with collagen per sample calculated using a conversion factor of 7.46 μg of collagen per μg of 4-hydroxyproline.32 Elastin content was measured using the modified ninhydrin assay.14,33
Constructs for histology were fixed in 4% paraformaldehyde for 3 h, followed by infiltration with a solution of 30% sucrose and 5% dimethyl sulfoxide. Samples were frozen in optimal cutting temperature (OCT) compound (Tissue-Tek, Torrance, CA), sectioned into 9-μm-thick cross sections, and then stained with Lillie's trichrome and Picrosirius red.34 Images were taken with an Zeiss Axiovert or Olympus IX70 inverted microscope equipped with a color charge-coupled device (CCD) camera. For Picrosirius red imaging, samples were placed between crossed plane polarizers.
Stimulation of cell monolayers
vSMC were plated in six-well plates at an initial density of 20,000 cells/cm2 and were maintained in the construct medium. At 24 h and again with every feeding, either bovine FDP (0, 100, 250, and 500 μg/mL) or ACA (0, 3, 6, and 12 mM) was added to the medium. The medium was changed on days 3 and 6 and harvested at day 7.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance for cell culture studies conducted with one time point, and two-way analysis of variance for 5-week construct studies and construct studies with combined ACA and FDP treatments in GraphPad Prism software for Windows (GraphPad Software, Inc., San Diego, CA). Tukey post hoc analysis was conducted to evaluate significant differences. A significance level of α = 0.05 was used for all tests. Pearson's correlation coefficient was calculated to examine the correlation between fibrin degradation and ECM deposition or cellularity. Linear regression was performed to examine the relationship between FDP concentration in the culture medium and collagen deposition. The best fit line and 95% confidence intervals are shown in Figure 3D.
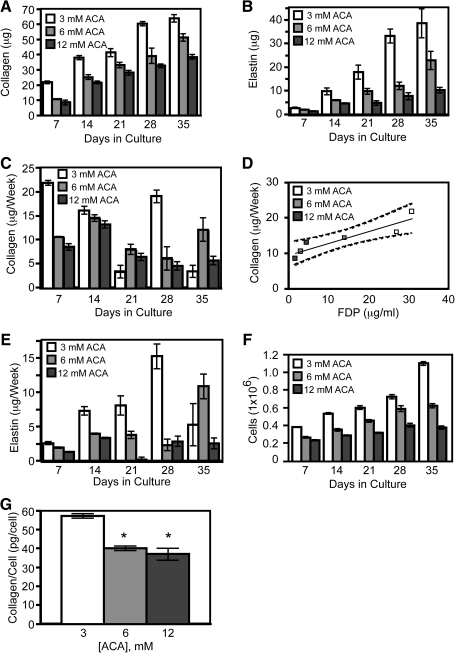
FIG. 3.
Plasmin inhibition decreases collagen and elastin content and cellular proliferation in tissue constructs. (A) Collagen and (B) elastin contents increased with time and were lower with higher ACA treatment. (C) Net collagen deposited per week and (E) net elastin deposited per week are shown to depict times of peak extracellular matrix deposition. (D) A correlation exists between the FDP concentration 24 h before harvest in the medium and the collagen deposited for that week during the first 2 weeks of remodeling (r2 = 0.84, p < 0.01). Dashed lines represent the 95% confidence band for the regression line. (F) Cellularity of constructs decreased with increasing ACA concentrations. (G) Collagen deposited per cell was significantly higher in the low ACA constructs during the first week. *A significant difference (p < 0.05) from low ACA samples. Values are mean ± standard error of the mean (SEM) for five constructs in all panels.
Results
ACA leads to both a delay and decrease in FDP release
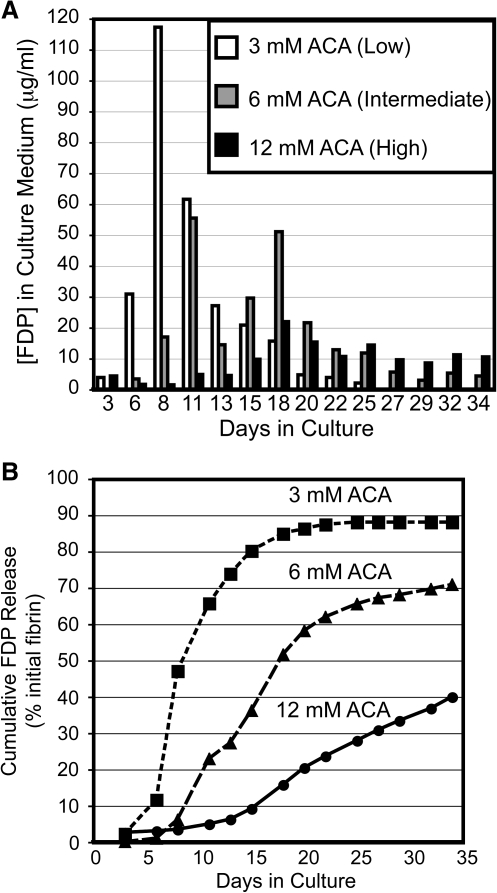
Fibrin-based tissue constructs cultured in the absence of plasmin inhibition are completely degraded within 24 h of casting, which is prevented by supplementation of the medium by ACA, an inhibitor of plasmin activity. To quantify the effect of plasmin inhibition on scaffold digestion, an ELISA sensitive to the presence of bovine FDP in the culture medium of the tissue constructs was developed. Tissue constructs cultured with high ACA released few FDP, never exceeding a medium concentration of ∼20 μg/mL (Fig. 1A). For intermediate and low ACA concentrations, the maximal FDP concentration increased to ∼50 and 120 μg/mL, respectively. Higher ACA concentrations also shifted the time of maximal FDP concentration in the medium. Peaks in FDP generation occurred earliest for the low ACA samples and latest for the high ACA samples, at days 8 and 18, respectively. After 5 weeks in culture, nearly 90% of the initial fibrin had been digested in the low ACA constructs, while in high ACA constructs only 40% of the initial fibrin was digested (Fig. 1B).
FIG. 1.
Plasmin inhibition reduces and delays fibrin degradation product (FDP) release from tissue constructs. (A) FDP concentrations in the culture medium were measured for 5 weeks before each medium change. Each data point represents analysis of pooled medium from five constructs at each ɛ-aminocaproic acid (ACA) concentration (low, intermediate, and high, corresponding to 3, 6, and 12 mM ACA, respectively). (B) Cumulative FDP release over 5 weeks is shown, expressed as a percentage of the initial fibrin that has been degraded.
Fibrinolytic activity in the culture medium is primarily due to plasmin
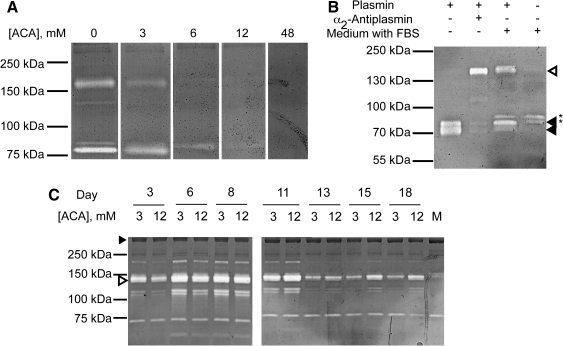
To identify the enzyme(s) relevant to fibrinolysis in the constructs, fibrinogen zymography was used by preparing polyacrylamide gels supplemented with the same fibrinogen used in construct fabrication. Medium from constructs cultured overnight without ACA, representing peak plasmin activity, served as a positive control for this assay. The primary fibrinolytic bands appeared as a doublet near 72/74 kDa and a poorly resolved doublet near 140 kDa (Fig. 2A). Addition of ACA at the levels used in construct culture (Fig. 2A) or the serine protease inhibitor aprotinin (Supplemental Material, Fig. S1, available online at www.liebertonline.com/ten), inhibited digestion by all species, pointing to their identity as complexes of the serine protease plasmin. The broad specificity matrix metalloprotease inhibitor Galardin had no effect on the digest pattern (Supplemental Fig. S1). This study, in agreement with previous work,35 demonstrates that vSMC use endogenous plasminogen activators, such as uPA (Supplemental Fig. S2, available online at www.liebertonline.com/ten), to activate plasminogen present in serum,36 producing plasmin that can be used for fibrinolysis.
FIG. 2.
Plasmin/α2-antiplasmin complexes are detectable in the conditioned medium of tissue constructs. (A) ACA added to the zymogram reaction buffer decreases fibrinolytic activity in a concentration-dependent manner. (B) Plasmin binds to α2-antiplasmin in the fetal bovine serum (FBS)-containing culture medium to form a sodium dodecyl sulfate-resistant 140 kDa complex. Plasmin migrated as a doublet (black arrowheads) that shifted completely or partially to a high molecular weight complex (white arrowhead) in the presence of α2-antiplasmin or serum-containing medium, respectively. *Fibrinolytic activity found in FBS. (C) Plasmin in tissue construct medium decreased over time and in response to plasmin inhibition. “M” denotes fresh construct medium. The white arrowhead indicates the plasmin/α2-antiplasmin complex identified in (B). The black arrowhead indicates a high molecular weight protein found in the culture medium that served as a loading control.
Purified plasmin migrates as a doublet near 72/74 kDa that, in the presence of the serum-containing culture medium, shifts largely to the doublet at 140 kDa, consistent with the migration of plasmin and the plasmin/α2-antiplasmin complex, respectively.37,38 To confirm this, a mixture of plasmin with purified α2-antiplasmin was analyzed by zymography, and this migrated as the same 140 kDa doublet (Fig. 2B). Since plasminogen binds to α2-antiplasmin 1000-fold more weakly than plasmin (KD = 3 nM and 3 μM for plasmin and plasminogen, respectively),39 and assuming a constant α2-antiplasmin serum concentration, this complex should be relatively insensitive to plasminogen and correlate with plasmin activity. In addition, the 72/74 kDa band could easily be confused with a fibrinolytic activity present in serum. Therefore, the 140 kDa species was chosen for following plasmin activity generated by the constructs.
Plasmin activity precedes FDP release into the medium
Medium samples from constructs incubated in varying ACA concentrations were analyzed for plasmin activity. As expected, the 140 kDa bands associated with plasmin complexes were identified in the culture medium, and this plasmin activity appeared as early as day 3, before the peak FDP levels identified by ELISA (Fig. 2C). Constructs grown in low levels of ACA showed the greatest initial fibrinolytic activity in the culture medium, which declined after several medium changes and was at background levels within 2 weeks. In contrast, the culture medium of constructs grown in high levels of ACA showed a more sustained fibrinolytic profile with lower levels of initial activity, consistent with sustained FDP production (Fig. 1A). After 2 weeks, the band intensity in high ACA constructs also decreased, but remained above background levels. Similar patterns of fibrinolytic activity to that seen for the 140 kDa complex were seen with bands at 110/120 and 230 kDa, which are presumably also plasmin complexes.
Reduced ACA leads to higher collagen and elastin deposition and increased cellularity
Constructs were harvested weekly to evaluate composition. Collagen and elastin levels increased over the 5-week culture period (Fig. 3A, B). Collagen content was consistently higher for constructs grown in low ACA, whereas high ACA samples had the lowest collagen content. Elastin levels showed a similar trend. There was a positive correlation between the percentage of fibrin that had been degraded and both the collagen (r2 = 0.81, p < 0.001) and elastin (r2 = 0.69, p < 0.001) contents. The weekly amounts of collagen and elastin deposited were calculated for comparison with the concentration of FDP in the medium. Collagen deposition was at the highest in the low ACA group for the first week and decreased weekly, except for a spike at week 4 (Fig. 3C). Constructs grown in higher concentrations of ACA, which had delayed peaks in fibrin degradation (Fig. 1A), had peak levels of deposited collagen during the second week. A positive correlation was found between the concentration of FDP in the medium and the collagen produced each week during the first 2 weeks of remodeling (r2 = 0.84, p < 0.01) (Fig. 3D). In contrast to the collagen results, weekly elastin deposition in the low ACA group increased over the first 4 weeks of culture, was lower for the higher ACA groups, and did not correlate with FDP concentrations in the medium (Fig. 3E).
Increased collagen and elastin content of the low ACA tissue constructs could be due in part to higher cellularity. By week 1, the vSMC in all constructs had proliferated, with highest proliferation in the constructs grown in the lowest ACA concentration (Fig. 3F). The cellularity increased in all three groups over the course of the 5 weeks, with the majority of the increase occurring in the first week for all ACA concentrations. However, the increased cellularity does not entirely explain the higher collagen in the low ACA group: in the first week, vSMC deposited more collagen per cell in the low ACA constructs than in the intermediate and high ACA constructs (Fig. 3G).
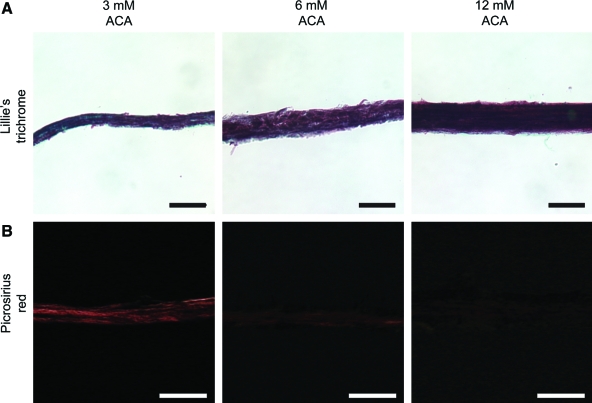
Trichrome and Picrosirius red staining of constructs harvested at 5 weeks revealed the differences in fibrin remodeling that occurred depending on ACA concentration (Fig. 4). The low ACA constructs showed extensive remodeling with visible collagen deposition, whereas the high ACA constructs had more limited remodeling and minimal collagen staining.
FIG. 4.
Histological sections show enhanced remodeling of constructs in low ACA. (A) Lillie's trichrome staining of 5-week constructs shows enhanced fibrin remodeling in the low ACA constructs. Collagen-rich regions stained green, residual fibrin and other noncollagenous protein are red, and nuclei stained black. The 3 mM ACA construct has visible collagen staining and more extensive remodeling than the higher (6 and 12 mM) ACA constructs. (B) Picrosirius red staining indicates increased and/or more mature collagen content in low ACA constructs. Images were exposed identically to observe staining in the 3 mM construct. Scale bar = 50 μm in all images. Color images available online at www.liebertonline.com/ten.
To address the functional consequence of reduced collagen content in fibrin-based constructs, tubular constructs were cultured in varying ACA concentrations and harvested after 5 weeks for mechanical testing. Tubular constructs cultured in 12 mM ACA had lower collagen and cell densities than 6 mM ACA constructs (Supplemental Fig. S3A, B, available online at www.liebertonline.com/ten). In addition, the Young's modulus of high ACA constructs was lower (Supplemental Fig. S3C, available online at www.liebertonline.com/ten).
ACA and FDP affect collagen deposition in vSMC culture
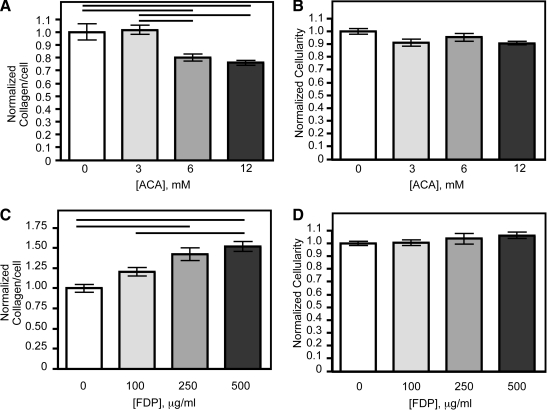
ACA may influence cellularity and collagen deposition in the fibrin-based constructs through a number of mechanisms. To determine if ACA had a direct biological effect on the cells, 0, 3, 6, and 12 mM ACA were used to supplement the medium of vSMC grown on tissue culture plastic, and the cell and collagen contents were quantified after 1 week. As was seen in the constructs (Fig. 3G), significant decreases in collagen deposition per cell occurred at the higher ACA concentrations (Fig. 5A). There was no difference in cellularity between groups (Fig. 5B). To examine potential stimulatory effects of FDP, plasmin-derived FDP were used to supplement the medium of vSMC cultures. There was a 50% increase in collagen deposited per cell when 250 or 500 μg/mL FDP was added (Fig. 5C), but no difference in cellularity (Fig. 5D).
FIG. 5.
Addition of ACA or plasmin-generated FDP to vascular smooth muscle cells on tissue-culture plastic led to altered collagen deposition. (A) Vascular smooth muscle cells deposited less collagen per cell when 6 or 12 mM ACA was added. (C) Addition of 250 or 500 μg/mL FDP led to increased collagen deposition per cell. Cellularity was not altered by addition of (B) ACA or (D) FDP to the medium. Values for cellularity and collagen deposition per cell were normalized to the untreated control. Values are mean ± SEM for six wells for all panels. Solid lines represent a significant difference between groups (p < 0.05).
Higher concentrations of FDP are measured within the construct interstitial fluid than in the overlying medium
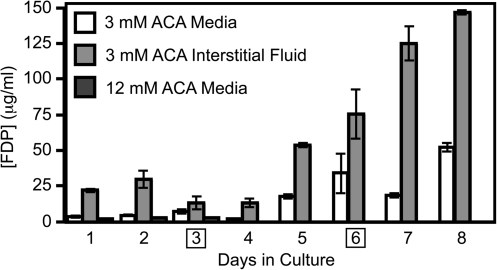
The maximal FDP concentration measured in the construct medium was roughly 120 μg/mL (in the low ACA constructs), which is not in the range shown to provide biological activity in vSMC culture (Fig. 5C). However, the concentration of FDP within the interstitial fluid of the constructs may have been higher due to a high rate of FDP production versus slow diffusion of FDP into the medium and/or binding interactions of FDP with ECM that retarded FDP diffusion into the medium. To examine this possibility, low ACA constructs were harvested for interstitial FDP measurement daily and compared to the medium FDP measurement. There were large differences in these values (Fig. 6). The largest differences occurred on the days after a medium change, when six to ninefold increases in FDP concentration were measured within the interstitial fluid. The average difference over the 8 days was approximately a fivefold increase in FDP measured in the interstitial fluid compared to the medium. After 8 days, sufficient volumes of interstitial fluid were not available for harvest from the constructs. This study was done with a different isolation of vSMC, which most likely accounts for the decreased fibrinolysis in these results as compared to the earlier study (Fig. 1A). However, it proves that substantially higher concentrations of FDP may be present within the construct than are measured within the overlying medium.
FIG. 6.
The FDP concentration measured in the interstitial fluid of the construct is higher than the FDP concentration measured in the overlying medium. FDP were measured in the medium or interstitial fluid daily for 1 week. Higher concentrations of FDP were present in the interstitial fluid than in the medium for constructs in 3 mM ACA based on one-way analysis of variance (p < 0.05). Values are mean ± range/2 for n = 2. Medium changes occurred on days 3 and 6, as indicated by squares in the figure; the FDP sampling was done immediately before the medium change, resulting in medium values that were lower on the following day.
Addition of FDP to construct culture increases collagen deposition
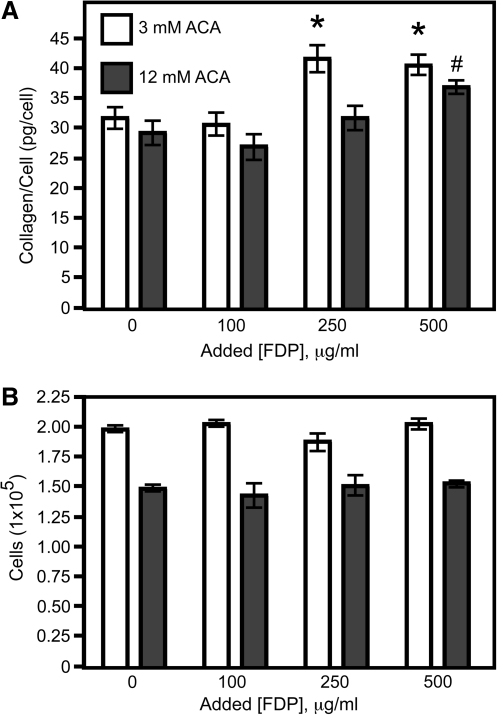
Supplementation of the construct medium with plasmin-derived FDP for 1 week led to increased collagen deposition (Fig. 7A) without affecting cellularity (Fig. 7B). Supplementing with 250 and 500 μg/mL FDP led to increased collagen deposition per cell in low ACA constructs, whereas an increase in collagen deposition in the high ACA constructs only occurred when 500 μg/mL FDP was added. The FDP concentration profiles shown in Figure 6 are from the same second set of experiments. There was no difference in collagen deposited per cell for these controls as there was in previous experiments with a different isolation of vSMC (Fig. 3G). This may be due to the lower fibrinolysis in these constructs during week 1 for both low and high ACA as compared to the earlier experiments (Fig. 1A).
FIG. 7.
Constructs supplemented with high FDP concentrations had increased collagen deposition per cell. (A) Collagen deposited per cell was enhanced when constructs were supplemented with FDP. (B) Cellularity of constructs was not affected by addition of FDP. Values are mean ± SEM for 5 constructs for both panels. *Significant differences (p < 0.05) compared to low ACA control (3 mM ACA). #Significant difference (p < 0.05) compared to high ACA control (12 mM ACA).
Discussion
ACA has documented potency against plasmin, uPA, and tPA over the range of 3–12 mM used in this study.19–21 This potency was confirmed by the effective inhibition of zymography using these concentrations. Plasmin inhibition by ACA, either directly or through plasminogen activators, has broad effects on fibrin remodeling driven by vSMC in fibrin-based tissue constructs. Most directly, inhibition prevents the degradation of the network of fibrin fibrils that entraps the vSMC. Without ACA, activation of plasminogen by uPA expressed by the vSMC led to rapid digestion of the fibrin and resulted in the destruction of the tissue construct because new ECM deposition had not yet occurred. The use of ACA delayed fibrin degradation, with increasing concentrations of ACA leading to delayed and diminished FDP production. While a fibrinolytic inhibitor such as ACA is necessary in the system to control fibrin degradation, this study shows that ACA can also have negative side effects. Increasing the ACA concentration led to lower cellularity and lower collagen and elastin content.
The reasons for these negative outcomes with high ACA usage were explored. During the first 2 weeks of construct incubation, the FDP concentration in the medium was highly correlated with collagen deposition. This indicates two likely possibilities for how ACA affects fibrin remodeling: (1) degradation of fibrin is required for new matrix deposition or cell proliferation and/or (2) FDP have a direct biological effect on vSMC that leads to increased matrix deposition or cell proliferation. In support of the latter, vSMC cultures and fibrin constructs supplemented with 250 or 500 μg/mL FDP deposited more collagen per cell than the untreated controls, indicating that FDP generation during remodeling of fibrin constructs has the potential for direct biological activity. Cellularity in vSMC culture and constructs was not altered by FDP addition. FDP levels within the construct and hence the effective local concentration of FDP seen by the cells were found to be, on average, fivefold higher than the concentration measured in the medium. Constructs cultured in 3 and 6 mM ACA had peak FDP of 120 and 50 μg/mL in the medium and thus could be expected to reach a biologically active FDP concentration within the interstitial fluid of the construct. Since FDP generation is severely reduced when high concentrations of ACA are utilized, FDP-mediated collagen deposition is more likely to occur at lower ACA concentrations.
In addition to inhibition of FDP generation, inhibition of plasminogen activation could have other negative effects on vSMC proliferation and matrix production. In vSMC culture, ACA negatively affected matrix deposition, as treatment with the higher concentrations of ACA (6 and 12 mM) led to decreased collagen deposition per cell. This indicates that ACA has broader effects than just limiting fibrin degradation. Many growth factors are sequestered in the ECM in a latent form; plasmin activity has been shown to release TGF-β, insulin-like growth factor, and fibroblast growth factor-2 from these latent complexes, leading to increased mitogenic activity.40–42 TGF-β in particular has been shown to increase collagen transcription and deposition of collagen and cross-linked elastin by vSMC in fibrin-based tissue constructs.14,43 Thus, the use of ACA may inhibit not only fibrinolysis but also other positive effects of plasmin in our system.
This study demonstrates that one enzyme, plasmin, can have multiple effects with regard to fibrin remodeling by vSMC in fibrin-based tissue constructs. Plasmin acts to degrade the fibrin, which correlates positively with deposition of the ECM proteins collagen and elastin. One key byproduct of plasmin activity is the generation of FDP, which we have shown to increase collagen deposition by vSMC in monolayer culture and in the constructs. Therefore, to ultimately fabricate a fibrin-based tissue construct for implantation, the time course of plasmin activity and the associated rate of FDP generation must be carefully prescribed. This will be of particular importance as new cell types are considered or for the eventual use of autologous cells for implant. Different cell isolations can be expected to have varied fibrinolytic activity; thus, careful monitoring of fibrinolysis will be necessary to allow for appropriate remodeling of fibrin-based tissue constructs.
Supplementary Material
Acknowledgments
We thank Stephen Stephens for performing biochemical assays, Lee Meier for help with sodium dodecyl sulfate–polyacrylamide gel electrophoresis and biochemical assays, and Naomi Ferguson for assistance in maintaining cell cultures. Funding was from NIH R01 HL083880 (to R.T.T.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Itosaka H. Kuroda S. Shichinohe H. Yasuda H. Yano S. Kamei S. Kawamura R. Hida K. Iwasaki Y. Fibrin matrix provides a suitable scaffold for bone marrow stromal cells transplanted into injured spinal cord: a novel material for CNS tissue engineering. Neuropathology. 2009;29:248. doi: 10.1111/j.1440-1789.2008.00971.x. [DOI] [PubMed] [Google Scholar]
- 2.Pelaez D. Huang C.Y. Cheung H.S. Cyclic compression maintains viability and induces chondrogenesis of human mesenchymal stem cells in fibrin gel scaffolds. Stem Cells Dev. 2009;18:93. doi: 10.1089/scd.2008.0030. [DOI] [PubMed] [Google Scholar]
- 3.Osathanon T. Linnes M.L. Rajachar R.M. Ratner B.D. Somerman M.J. Giachelli C.M. Microporous nanofibrous fibrin-based scaffolds for bone tissue engineering. Biomaterials. 2008;29:4091. doi: 10.1016/j.biomaterials.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor S.J. Sakiyama-Elbert S.E. Effect of controlled delivery of neurotrophin-3 from fibrin on spinal cord injury in a long term model. J Control Release. 2006;116:204. doi: 10.1016/j.jconrel.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jockenhoevel S. Zund G. Hoerstrup S.P. Chalabi K. Sachweh J.S. Demircan L. Messmer B.J. Turina M. Fibrin gel—advantages of a new scaffold in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2001;19:424. doi: 10.1016/s1010-7940(01)00624-8. [DOI] [PubMed] [Google Scholar]
- 6.Mol A. van Lieshout M.I. Dam-de Veen C.G. Neuenschwander S. Hoerstrup S.P. Baaijens F.P. Bouten C.V. Fibrin as a cell carrier in cardiovascular tissue engineering applications. Biomaterials. 2005;26:3113. doi: 10.1016/j.biomaterials.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Ross J.J. Tranquillo R.T. ECM gene expression correlates with in vitro tissue growth and development in fibrin gel remodeled by neonatal smooth muscle cells. Matrix Biol. 2003;22:477. doi: 10.1016/s0945-053x(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 8.Cummings C.L. Gawlitta D. Nerem R.M. Stegemann J.P. Properties of engineered vascular constructs made from collagen, fibrin, and collagen-fibrin mixtures. Biomaterials. 2004;25:3699. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 9.Swartz D.D. Russell J.A. Andreadis S.T. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol. 2005;288:H1451. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- 10.Yao L. Swartz D.D. Gugino S.F. Russell J.A. Andreadis S.T. Fibrin-based tissue-engineered blood vessels: differential effects of biomaterial and culture parameters on mechanical strength and vascular reactivity. Tissue Eng. 2005;11:991. doi: 10.1089/ten.2005.11.991. [DOI] [PubMed] [Google Scholar]
- 11.Barocas V.H. Girton T.S. Tranquillo R.T. Engineered alignment in media equivalents: magnetic prealignment and mandrel compaction. J Biomech Eng. 1998;120:660. doi: 10.1115/1.2834759. [DOI] [PubMed] [Google Scholar]
- 12.L'Heureux N. Germain L. Labbe R. Auger F.A. In vitro construction of a human blood vessel from cultured vascular cells: a morphologic study. J Vasc Surg. 1993;17:499. doi: 10.1067/mva.1993.38251. [DOI] [PubMed] [Google Scholar]
- 13.Grassl E.D. Oegema T.R. Tranquillo R.T. A fibrin-based arterial media equivalent. J Biomed Mater Res. 2003;66:550. doi: 10.1002/jbm.a.10589. [DOI] [PubMed] [Google Scholar]
- 14.Long J.L. Tranquillo R.T. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22:339. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 15.Grassl E.D. Oegema T.R. Tranquillo R.T. Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. J Biomed Mater Res. 2002;60:607. doi: 10.1002/jbm.10107. [DOI] [PubMed] [Google Scholar]
- 16.Clark R.A. Fibrin is a many splendored thing. J Invest Dermatol. 2003;121:xxi. doi: 10.1046/j.1523-1747.2003.12575.x. [DOI] [PubMed] [Google Scholar]
- 17.Cox S. Cole M. Tawil B. Behavior of human dermal fibroblasts in three-dimensional fibrin clots: dependence on fibrinogen and thrombin concentration. Tissue Eng. 2004;10:942. doi: 10.1089/1076327041348392. [DOI] [PubMed] [Google Scholar]
- 18.Rijken D.C. Lijnen H.R. New insights into the molecular mechanisms of the fibrinolytic system. J Thromb Haemost. 2009;7:4. doi: 10.1111/j.1538-7836.2008.03220.x. [DOI] [PubMed] [Google Scholar]
- 19.Sun Z. Chen Y.H. Wang P. Zhang J. Gurewich V. Zhang P. Liu J.N. The blockage of the high-affinity lysine binding sites of plasminogen by EACA significantly inhibits prourokinase-induced plasminogen activation. Biochim Biophys Acta. 2002;1596:182. doi: 10.1016/s0167-4838(02)00233-9. [DOI] [PubMed] [Google Scholar]
- 20.Bakker A.H. Weening-Verhoeff E.J. Verheijen J.H. The role of the lysyl binding site of tissue-type plasminogen activator in the interaction with a forming fibrin clot. J Biol Chem. 1995;270:12355. doi: 10.1074/jbc.270.21.12355. [DOI] [PubMed] [Google Scholar]
- 21.Anonick P.K. Vasudevan J. Gonias S.L. Antifibrinolytic activities of alpha-N-acetyl-L-lysine methyl ester, epsilon-aminocaproic acid, and tranexamic acid. Importance of kringle interactions and active site inhibition. Arterioscler Thromb. 1992;12:708. doi: 10.1161/01.atv.12.6.708. [DOI] [PubMed] [Google Scholar]
- 22.Christensen U. Allosteric effects of some antifibrinolytic amino acids on the catalytic activity of human plasmin. Biochim Biophys Acta. 1978;526:194. doi: 10.1016/0005-2744(78)90304-2. [DOI] [PubMed] [Google Scholar]
- 23.Hunter C.J. Levenston M.E. Maturation and integration of tissue-engineered cartilages within an in vitro defect repair model. Tissue Eng. 2004;10:736. doi: 10.1089/1076327041348310. [DOI] [PubMed] [Google Scholar]
- 24.Rowe S.L. Stegemann J.P. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules. 2006;7:2942. doi: 10.1021/bm0602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bian W. Bursac N. Engineered skeletal muscle tissue networks with controllable architecture. Biomaterials. 2009;30:1401. doi: 10.1016/j.biomaterials.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kupcsik L. Alini M. Stoddart M.J. Epsilon-aminocaproic acid is a useful fibrin degradation inhibitor for cartilage tissue engineering. Tissue Eng Part A. 2009;15:2309. doi: 10.1089/ten.tea.2008.0400. [DOI] [PubMed] [Google Scholar]
- 27.Naito M. Stirk C.M. Smith E.B. Thompson W.D. Smooth muscle cell outgrowth stimulated by fibrin degradation products. The potential role of fibrin fragment E in restenosis and atherogenesis. Thromb Res. 2000;98:165. doi: 10.1016/s0049-3848(99)00202-9. [DOI] [PubMed] [Google Scholar]
- 28.Thompson W.D. Evans A.T. Campbell R. The control of fibrogenesis: stimulation and suppression of collagen synthesis in the chick chorioallantoic membrane with fibrin degradation products, wound extracts and proteases. J Pathol. 1986;148:207. doi: 10.1002/path.1711480304. [DOI] [PubMed] [Google Scholar]
- 29.Williams C. Johnson S.L. Robinson P.S. Tranquillo R.T. Cell sourcing and culture conditions for fibrin-based valve constructs. Tissue Eng. 2006;12:1489. doi: 10.1089/ten.2006.12.1489. [DOI] [PubMed] [Google Scholar]
- 30.Kim B.S. Mooney D.J. Engineering smooth muscle tissue with a predefined structure. J Biomed Mater Res. 1998;41:322. doi: 10.1002/(sici)1097-4636(199808)41:2<322::aid-jbm18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Stegemann H. Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 32.Dombi G.W. Haut R.C. Sullivan W.G. Correlation of high-speed tensile strength with collagen content in control and lathyritic rat skin. J Surg Res. 1993;54:21. doi: 10.1006/jsre.1993.1004. [DOI] [PubMed] [Google Scholar]
- 33.Starcher B. A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal Biochem. 2001;292:125. doi: 10.1006/abio.2001.5050. [DOI] [PubMed] [Google Scholar]
- 34.Kiernan J.A. Histological and Histochemical Methods: Theory and Practice. Oxford, UK: Butterworth-Heinemann; 1999. [Google Scholar]
- 35.Ugwu F. Lemmens G. Collen D. Lijnen H.R. Matrix metalloproteinase deficiencies do not impair cell-associated fibrinolytic activity. Thromb Res. 2001;102:61. doi: 10.1016/s0049-3848(01)00218-3. [DOI] [PubMed] [Google Scholar]
- 36.Cederholm-Williams S.A. Concentration of plasminogen and antiplasmin in plasma and serum. J Clin Pathol. 1981;34:979. doi: 10.1136/jcp.34.9.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green K.A. Almholt K. Ploug M. Rono B. Castellino F.J. Johnsen M. Bugge T.H. Romer J. Lund L.R. Profibrinolytic effects of metalloproteinases during skin wound healing in the absence of plasminogen. J Invest Dermatol. 2008;128:2092. doi: 10.1038/jid.2008.54. [DOI] [PubMed] [Google Scholar]
- 38.Yan D. Urano T. Takada Y. Takada A. Dissociation of alpha 2-plasmin-inhibitor-plasmin complex and regeneration of plasmin activity by SDS treatment. Thromb Res. 1993;69:491. doi: 10.1016/0049-3848(93)90053-q. [DOI] [PubMed] [Google Scholar]
- 39.Christensen U. Clemmensen I. Purification and reaction mechanisms of the primary inhibitor of plasmin from human plasma. Biochem J. 1978;175:635. doi: 10.1042/bj1750635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell P.G. Novak J.F. Yanosick T.B. McMaster J.H. Involvement of the plasmin system in dissociation of the insulin-like growth factor-binding protein complex. Endocrinology. 1992;130:1401. doi: 10.1210/endo.130.3.1371448. [DOI] [PubMed] [Google Scholar]
- 41.George S.J. Johnson J.L. Smith M.A. Angelini G.D. Jackson C.L. Transforming growth factor-beta is activated by plasmin and inhibits smooth muscle cell death in human saphenous vein. J Vasc Res. 2005;42:247. doi: 10.1159/000085657. [DOI] [PubMed] [Google Scholar]
- 42.George S.J. Johnson J.L. Smith M.A. Jackson C.L. Plasmin-mediated fibroblast growth factor-2 mobilisation supports smooth muscle cell proliferation in human saphenous vein. J Vasc Res. 2001;38:492. doi: 10.1159/000051082. [DOI] [PubMed] [Google Scholar]
- 43.Weinbaum J.S. Qi J. Tranquillo R.T. Monitoring collagen transcription by vascular smooth muscle cells in fibrin-based tissue constructs. Tissue Eng Part C Methods. 2010;16:459. doi: 10.1089/ten.tec.2009.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.