Abstract
Neuroimaging studies have indicated abnormalities in cortico-striatal-thalamo-cortical circuits in patients with obsessive–compulsive disorder compared with controls. However, there are inconsistencies between studies regarding the exact set of brain structures involved and the direction of anatomical and functional changes. These inconsistencies may reflect the differential impact of environmental and genetic risk factors for obsessive–compulsive disorder on different parts of the brain. To distinguish between functional brain changes underlying environmentally and genetically mediated obsessive–compulsive disorder, we compared task performance and brain activation during a Tower of London planning paradigm in monozygotic twins discordant (n = 38) or concordant (n = 100) for obsessive–compulsive symptoms. Twins who score high on obsessive–compulsive symptoms can be considered at high risk for obsessive–compulsive disorder. We found that subjects at high risk for obsessive–compulsive disorder did not differ from the low-risk subjects behaviourally, but we obtained evidence that the high-risk subjects differed from the low-risk subjects in the patterns of brain activation accompanying task execution. These regions can be separated into those that were affected by mainly environmental risk (dorsolateral prefrontal cortex and lingual cortex), genetic risk (frontopolar cortex, inferior frontal cortex, globus pallidus and caudate nucleus) and regions affected by both environmental and genetic risk factors (cingulate cortex, premotor cortex and parts of the parietal cortex). Our results suggest that neurobiological changes related to obsessive–compulsive symptoms induced by environmental factors involve primarily the dorsolateral prefrontal cortex, whereas neurobiological changes induced by genetic factors involve orbitofrontal–basal ganglia structures. Regions showing similar changes in high-risk twins from discordant and concordant pairs may be part of compensatory networks that keep planning performance intact, in spite of cortico-striatal-thalamo-cortical deficits.
Keywords: discordant/concordant monozygotic twin design, obsessive–compulsive symptoms, fMRI, planning
Introduction
Obsessive–compulsive symptoms are characterized by recurrent, persistent and intrusive anxiety-provoking thoughts or images (obsessions) and subsequent repetitive behaviours (compulsions) performed to reduce anxiety and/or distress caused by the obsessions (American Psychiatric Association, 1994). Common obsessions include fear of contamination, fixation on symmetry and orderliness and somatic and aggressive obsessions. Well-known compulsions are excessive hand washing, counting and detailed and rigid rituals or habits, such as excessive checking or specific morning or eating routines. When a person performs these obsessions and/or compulsions for >1 h a day and these thoughts and rituals significantly interfere with routines of daily life, the person fulfils the criteria for obsessive–compulsive disorder. Obsessive–compulsive disorder is generally assessed by clinical interviews, e.g. Diagnostic and Statistical Manual of Mental Disorders [DSM-IV, 4th edn. (American Psychiatric Association, 1994)]. Questionnaires, such as the Padua Inventory (Sanavio, 1988) and quantitative versions of the Yale–Brown Obsessive–Compulsive Scale (Goodman et al., 1989a, b) can be utilized to explore obsessive–compulsive symptomatology on a more quantitative scale. While the estimates of the prevalence of lifetime obsessive–compulsive disorder are found to be as high as 0.5–2% (American Psychiatric Association, 1994; Grabe et al., 2000), the prevalence of obsessive–compulsive symptoms in the general population is much higher, with estimates up to 72% as reported by Rachman and de Silva (1978).
Neuropsychological studies have shown that patients with obsessive–compulsive disorder suffer from deficits in executive function, including cognitive planning, response inhibition, set-switching, working memory and sustained attention (for review see Schultz et al., 1999; Chamberlain et al., 2005; Menzies et al., 2008). Recent neuroimaging studies have indicated several neurobiological changes associated with obsessive–compulsive disorder. Structural MRI has revealed brain volume changes in the orbitofrontal cortex, dorsolateral prefrontal cortex, basal ganglia, anterior cingulate cortex, parietal cortex and thalamus (Pujol et al., 2004; Valente Jr et al., 2005; Menzies et al., 2007; Radua and Mataix-Cols, 2009; Rotge et al., 2009; van den Heuvel et al., 2009), in line with the hypothesis of a disturbed cortico-striato-thalamo-cortical (CSTC) network. Functional neuroimaging studies have also shown altered activation in the abovementioned brain structures during performance of cognitive tasks and after symptoms provocation (Breiter et al., 1996; Ursu et al., 2003; Maltby et al., 2005; Rauch et al., 2007; Menzies et al., 2008; Chamberlain and Menzies, 2009). Although the overall picture points to a deficit in CSTC processing, there are considerable inconsistencies across studies regarding the brain areas involved and the direction of anatomical and functional changes. A possible explanation for this relates to the presence of methodological differences between studies, such as heterogeneity of patient groups and differences in sample size, scanning modalities/parameters and analysis methods. However, there may also be ‘true’ variability in the underlying neurobiology of obsessive–compulsive disorder, that is, it may be that dysfunction of different brain regions leads to highly comparable changes at the behavioural level, because these regions are part of the same brain network involved in the regulation of anxiety and safety behaviours. Such heterogeneity in affected brain regions may, for instance, reflect the differential influence of environmental and genetic risk factors for obsessive–compulsive disorder that may impact on different parts of the brain.
Family studies (Nestadt et al., 2000; Hettema et al., 2001) and twin studies (Jonnal et al., 2000; van Grootheest et al., 2005) have indicated the importance of genetic as well as environmental risk factors with regard to the aetiology of obsessive–compulsive disorder. Heritability for obsessive–compulsive disorder has been estimated to be between 27% and 47% in adults and between 45% and 65% in children (Jonnal et al., 2000; van Grootheest et al., 2005), and linkage and association studies have pointed towards mainly functional deficits of genes involved in serotonergic, glutamatergic and dopaminergic neural signalling (Billett et al., 1998; Bengel et al., 1999; Enoch et al., 2001; Nicolini et al., 2009). Given this moderate heritability, as much as 35–73% of the risk for obsessive–compulsive disorder should be accounted for by environmental stressors and/or adverse gene–environment interactions. Potential environmental risk factors for obsessive–compulsive disorder include traumatic life experiences, perinatal problems, streptococcal infection, psychosocial stress, aspects of parenting (e.g. parental overprotection), pregnancy, divorce and emotional neglect (Albert et al., 2000; Alonso et al., 2004; Lin et al., 2007; Cath et al., 2008; Geller et al., 2008; Wilcox et al., 2008).
Most brain imaging studies apply a group comparison of affected individuals with healthy controls. These standard case–control designs cannot disentangle differences in brain function that are due to environmental risk factors from those that are due to genetic risk factors. A design that makes a distinction between genetically and environmentally mediated neurobiological changes underlying the development of behavioural traits such as obsessive–compulsive disorder is the so-called discordant/concordant monozygotic twin design (de Geus et al., 2007; Wolfensberger et al., 2008; van’t Ent et al., 2009). As nearly all monozygotic twins begin life with identical genomes, discordance at the behavioural level is likely to arise from differential exposure to environmental influences. Consequently, differences in brain function between the high-risk twin and the low-risk co-twin from discordant pairs reflect environmental effects on the brain, rather than effects of genetic variation, although these environmental stressors may ultimately act through modification of gene expression (Heijmans et al., 2009).
In contrast, to maximize detection of the effects of genetic risk factors, neuroimaging results can be compared between monozygotic twins who both score high on obsessive–compulsive symptoms and monozygotic twins who both score very low on obsessive–compulsive symptoms. These monozygotic concordant high- and low-scoring twins are likely to come from families with either high or low vulnerability for obsessive–compulsive disorder. This familial vulnerability may consist of shared environmental or genetic vulnerability. However, since no influence of shared family environment on obsessive–compulsive behaviour was found in any of the studies in adult twins (Clifford et al., 1984; Jonnal et al., 2000; van Grootheest et al., 2007), familial vulnerability for this trait translates entirely to genetic vulnerability. Therefore, a comparison between monozygotic twins scoring both high (concordant-high) on obsessive–compulsive symptoms and monozygotic twins scoring both low (concordant-low) on obsessive–compulsive symptoms will reveal functional activation differences due to influences of genetic risk factors. Furthermore, comparing the regions affected in the high-risk discordant twins with those in high-risk concordant twins allows for the identification of regions commonly affected in all high-risk subjects. These regions may be most closely correlated with the observed behavioural deficits of the disorder.
In the present study, the discordant/concordant monozygotic twin design was used to assess differences in functional brain activation during cognitive planning with the Tower of London paradigm (Shallice, 1982). The Tower of London paradigm has been found to activate the dorsolateral prefrontal cortex, anterior cingulate cortex, caudate nucleus, (pre)cuneus, supramarginal and angular gyrus of the parietal lobe, and frontal opercular areas of the insula (Dagher et al., 1999; Lazeron et al., 2000; Newman et al., 2003; van den Heuvel et al., 2003). Several neuropsychological studies have used a computerized version of the Tower of London to assess problem solving and planning ability in patients with obsessive–compulsive disorder (Kuelz et al., 2004; Menzies et al., 2008). Some studies revealed that deviant performance on the Tower of London was evident not so much as a deficit in planning accuracy, but rather that patients were slower to recover from an incorrect move (Veale et al., 1996) or had longer movement times (Purcell et al., 1998a,b) compared with healthy controls. Chamberlain and colleagues (2007) further revealed that patients with obsessive–compulsive disorder required more attempts to obtain a correct response on the Tower of London, but only for the highest difficulty levels (4–6 moves).
Importantly, Delorme and colleagues (2007) found that unaffected relatives of patients with obsessive–compulsive disorder had significantly lower scores and increased response times on the Tower of London task compared with controls, which suggests genetic contribution to the behavioural planning deficits. A neuroimaging study further demonstrated that behavioural impairment on the Tower of London task in patients with obsessive–compulsive disorder was associated with decreased functional MRI activation in the dorsolateral prefrontal cortex and caudate nucleus as well as increased activation in the anterior cingulate cortex (van den Heuvel et al., 2005). This differential brain activation does not only reflect a genetic aetiology, since we replicated the reduced dorsolateral prefrontal cortex activation in 12 monozygotic twin pairs discordant for obsessive–compulsive symptoms (den Braber et al., 2008). No obsessive–compulsive symptom-related changes were found for the caudate nucleus or the anterior cingulate cortex, which may be more specific to obsessive–compulsive symptoms caused by genetic factors.
Here, we aimed to extend our previous findings and to specifically examine whether different brain regions are affected in subjects at high risk for obsessive–compulsive disorder due to adverse environmental influences or to genetic influences. For this, we compared performance and functional MRI data during the Tower of London task between twins scoring low and high on obsessive–compulsive symptoms from discordant monozygotic pairs, and between concordant pairs where both twins scored low or both scored high on obsessive–compulsive symptoms. Furthermore, we explicitly tested for the presence of overlap in the regions that were affected by both environmental and genetic risk for obsessive–compulsive disorder.
Materials and methods
Subjects
The twin pairs in this study were recruited from the Netherlands Twin Register (Boomsma et al., 2006). In 2002, surveys were sent to twin families that included the Padua Inventory Abbreviated. The Padua Inventory Abbreviated is derived from the Padua Inventory–Revised version, a widely used self-report inventory on obsessive–compulsive symptoms (Sanavio, 1988; van Oppen, 1992). The Padua Inventory–Revised measures obsessive–compulsive symptoms on a scale from 0 to 4, and contains five subcategories: washing, checking, rumination, precision and impulses (van Oppen et al., 1995). The Padua Inventory–Revised correlates moderately with the Yale–Brown Obsessive–Compulsive Scale symptom checklist, a clinician-derived inventory on obsessive–compulsive symptoms (Denys et al., 2004). Reduction of the Padua Inventory–Revised to 12 items was implemented by selecting two items from each of the five Padua Inventory–Revised subscales with highest factor loadings in a previous validation study (van Oppen et al., 1995) and adding another two items for each of the more equivocal obsession subscales: rumination and impulses.
Completed Padua Inventory Abbreviated questionnaires were returned by 815 monozygotic twin pairs (222 male, 593 female). From this sample, we selected twin pairs with an age range between 18 and 60 years who scored discordant, concordant-high or concordant-low for obsessive–compulsive symptoms. A twin pair was classified as discordant for obsessive–compulsive symptoms if one twin scored high (>16) and the co-twin scored low (≤7). A twin pair was classified as concordant-high for obsessive–compulsive symptoms if both twins scored ≥15, with at least one twin scoring ≥16. A twin pair was classified as concordant-low for obsessive–compulsive symptoms if both twins scored ≤7. These Padua Inventory Abbreviated cut-off scores were derived from sensitivity and specificity measurements in a sample of patients with obsessive–compulsive disorder compared with clinical controls [n = 120; mean scores 20.7, standard deviation (SD) 8.1; sensitivity 0.74 and specificity 0.72 at the best cut-off point of 16 (Cath et al., 2008)]. This initial selection yielded 32 discordant monozygotic twin pairs, 40 concordant-high monozygotic twin pairs and 269 concordant-low monozygotic twin pairs for obsessive–compulsive symptoms. From the large sample of concordant-low twin pairs, a selection was made to optimally match the concordant-high twin pairs by sex and age that resulted in a final concordant-low sample of 41 twin pairs. Two concordant-high twin pairs were omitted from the selection; in one pair, both twins were treated for severe anorexia and had indicated that they were not willing to participate in research projects; in the other pair, the twins indicated that they were not willing to participate in research projects other than the filling out of questionnaires. The remaining 111 twin pairs were invited by letter. Exclusion criteria were neurological damage, colour blindness and contraindications for MRI (e.g. pregnancy, metal artefacts in the body and claustrophobia). From this group, 69 monozygotic twin pairs finally participated in our MRI study, including 19 discordant (seven pairs newly enrolled), 22 concordant-high and 28 concordant-low twin pairs (Table 1). Of this final population, two twins with high obsessive–compulsive symptoms scores from the discordant group and five twins with high obsessive–compulsive symptoms scores from the concordant-high group met clinical diagnosis for obsessive–compulsive disorder. Furthermore, three twins with high obsessive–compulsive symptoms scores and one twin with a low obsessive–compulsive symptoms score from the discordant group, and six twins from the concordant-high group used selective serotonin reuptake inhibitors.
Table 1.
Twin sample demographics
|
Twin pairs |
||||
|---|---|---|---|---|
|
Discordant |
Concordant |
|||
| High (13 female, 6 male) | Low (13 female, 6 male) | High (17 female, 5 male) | Low (20 female, 8 male) | |
| Age at MRI scan (SD) | 35.58 (8.92) | 36.23 (10.87) | 37.50 (8.87) | |
| Obsessive–compulsive symptoms | ||||
| PI-R-ABBR 2002 | 19.55 (3.99) | 4.53 (2.17)a | 20.62 (4.56) | 4.18 (2.19)b |
| PI-R-ABBR current | 12.63 (7.34) | 6.84 (4.15)a | 15.27 (5.58) | 4.43 (3.00)b |
| Y-BOCS severity lifetime | 7.74 (5.85) | 7.00 (8.29) | 10.66 (7.21) | 3.18 (4.54)b |
| Y-BOCS severity current | 5.42 (5.78) | 1.47 (2.25)a | 7.64 (5.95) | 0.95 (2.13)b |
| Y-BOCS symptom lifetime | 22.11 (25.32) | 7.11 (7.17)a | 30.09 (27.34) | 4.82 (6.15)b |
| Y-BOCS symptom current | 24.32 (30.37) | 7.26 (9.61)a | 22.82 (20.64) | 3.25 (5.11)b |
| Agressive/checking lifetime | 5.84 (7.34) | 2.11 (2.73)a | 9.43 (9.36) | 1.79 (2.16)b |
| Agressive/checking current | 5.74 (7.82) | 2.00 (3.59)a | 6.89 (7.61) | 1.05 (1.54)b |
| Hoarding/saving lifetime | 1.16 (1.38) | 0.26 (0.56)a | 1.48 (1.84) | 0.36 (0.70)b |
| Hoarding/saving current | 1.21 (1.44) | 0.37 (0.68)a | 1.23 (1.64) | 0.39 (0.78)b |
| Symmetry/ordering lifetime | 1.68 (3.48) | 0.84 (1.64) | 2.64 (3.44) | 0.43 (1.29)b |
| Symmetry/ordering current | 1.58 (3.63) | 0.68 (1.49) | 2.02 (3.14) | 0.23 (0.63)b |
| Washing/cleaning lifetime | 5.11 (8.09) | 0.95 (2.39)a | 4.84 (6.39) | 0.77 (1.90)b |
| Washing/cleaning current | 5.21 (6.70) | 1.32 (2.83)a | 3.43 (4.74) | 0.63 (1.74)b |
| Comorbidity | ||||
| Comorbidity lifetime (MINI) | 1.58 (1.39) | 0.74 (1.10)a | 1.45 (1.42) | 0.41 (0.78)b |
| Comorbidity current (MINI) | 0.63 (1.71) | 0.00 (0.00)a | 0.27 (0.50) | 0.02 (0.13) |
| Tic | 0.37 (0.76) | 0.16 (0.37) | 0.27 (0.66) | 0.05 (0.23)b |
| BDI | 4.58 (6.61) | 2.95 (2.84) | 3.57 (3.22) | 1.38 (2.18)b |
| STAI | 13.95 (8.77) | 12.53 (6.17) | 13.64 (7.36) | 8.55 (7.36)b |
| STAS | 0.21 (0.71) | 0.00 (0.00) | 0.48 (2.14) | 0.11 (0.49) |
a Significant difference between discordant-high and discordant-low-scoring twins.
b Significant difference between concordant-high and concordant-low-scoring twins.
PI-R-ABBR 2002 = mean Padua Inventory Abbreviated scores (SD) assessed in 2002; PI-R-ABBR = mean Padua Inventory abbreviated scores (SD) at time of MRI examination; Y-BOCS severity lifetime/current = mean Yale–Brown Obsessive–Compulsive Scale severity scores (SD) across whole life span and at the time of MRI; Y-BOCS symptom lifetime/current: mean compound Yale–Brown Obsessive–Compulsive Scale symptom scores (SD) across whole life span and at the time of MRI; aggressive/checking, hoarding/saving, symmetry/ordering and washing/cleaning lifetime/current = mean Yale–Brown Obsessive–Compulsive Scale subcategory scores (SD) across the life span or at time of MRI (assessed using the Yale–Brown Obsessive–Compulsive Scale symptoms list).
Comorbidity lifetime/current (MINI) = mean comorbidity scores (SD) across whole life span or at the time of MRI (measured using the Mini International Neuropsychiatric Interview); Tic = mean tic scores (SD) at time of MRI; BDI = mean Beck Depression Inventory scores (SD) at time of MRI; STAI = mean State Trait Anxiety inventory scores (SD) at time of MRI; STAS = mean State Trait Anxiety Inventory scores (SD) at time of MRI.
The MRI protocol could not be completed by one of the twins from a concordant-low pair due to a metal artefact at the eyebrow level and by one of the twins from a concordant-high pair due to a panic attack.
Protocol
A self-report questionnaire, consisting of demographic questions, life events, comparative twin rating (Reynolds et al., 2005), the 13-item Beck Depression Inventory Short Form (Beck et al., 1961, 1974) and the 12-item Padua Inventory Abbreviated, was sent to the subjects at home to be filled in before the day of MRI scanning. On the day of scanning, the following diagnostic interviews and questionnaires were administered: (i) an adapted form of the Yale–Brown Obsessive–Compulsive Scale, to measure both lifetime and current obsessive–compulsive symptoms and severity; (ii) the State Trait Anxiety Inventory; (iii) the State Trait Anger Scale (Spielberger et al., 1970, 1983); and (iv) the Mini International Neuropsychiatric Interview (Sheehan et al., 1998) to test for possible comorbidities. Comorbidities tested by the Mini International Neuropsychiatric Interview include depression, panic disorder, agoraphobia, social phobia, post traumatic stress disorder and generalized anxiety disorder. In addition, subjects were screened for the eight most common tics (head shaking, eye blinking, other facial tics, shoulder raising, expressing swear words/foul language/dirty words, sound making, growling and throat clearing/coughing/sniffing), since high comorbidity rates have been found between obsessive–compulsive disorder and chronic tic disorders (Cath et al., 2001). The subjects were asked to indicate whether they were familiar with one of these tics by answering ‘yes’ or ‘no’.
All subjects were asked to collect mucosal cell samples for DNA extraction to test zygosity. The ethical review board of the VU University medical centre approved the study and all subjects provided written informed consent.
Tower of London
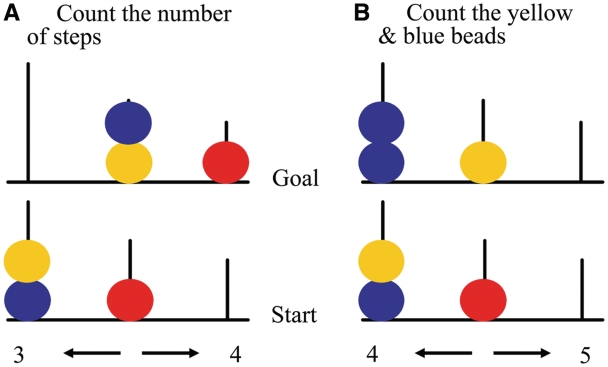
Stimuli for the Tower of London task consisted of images of three coloured beads (red, blue and yellow) placed on three vertical rods of decreasing height (Fig. 1). In each trial, a start configuration (Fig. 1, bottom) and final target configuration (Fig. 1, top) were simultaneously displayed. During planning trials (Fig. 1A), subjects were requested to count the number of steps to get from the start to final target configuration, with the restrictions that only one bead could be moved at a time and that a bead could be moved only if there was no other bead on top. Five planning difficulty levels were included corresponding to the minimum number of moves (1–5) needed to achieve the target configuration. In addition, baseline stimuli were included (Fig. 1B) during which subjects only had to count the total number of yellow and blue beads. With each stimulus presentation, two possible answers (one correct and one incorrect) were presented at the bottom left and right of the screen. The correct answer had to be indicated by pressing the corresponding left or right hand button. No feedback regarding the correct answer was provided.
Figure 1.
Examples of Tower of London stimuli used in the present study. (A) Planning condition; (B) baseline condition [adapted from van den Heuvel et al. (2005)].
The stimuli were presented in an event-related design of 17 min with self-paced stimulus timing, i.e. a subsequent trial was presented on the screen immediately after the response on a previous trial, or directly after the maximum reaction time limit of 60 s. Presentation order of the stimuli was pseudo-random with distribution frequency of the six stimulus types similar to van den Heuvel et al. (2005). The stimulus presentation order was the same for all subjects; however, the total number of trials completed by each subject depended on the subject’s reaction times.
Stimuli were projected on a screen at the end of the MRI scanner table and viewed by the subject through a mirror. Two MRI compatible response boxes were used to record the subject’s performance. Prior to performance of the Tower of London task within the scanner, subjects practiced the task on a personal computer outside the scanner. Furthermore, subjects performed a number of practice trials while in the scanner, immediately before the actual task.
Image acquisition
The MRI session consisted of a structural part of ∼6 min and a functional part of ∼17 min. Subjects remained inside the scanner and were asked to minimize head movements during and between consecutive runs. To reduce motion artefacts, subjects’ heads were immobilized using foam pads.
MRI was performed on a 3.0 T Intera MR system (Philips, Medical Systems, Best) with a standard SENSE receiver head coil. The anatomical scan consisted of 182 coronal slices with a 3D gradient-echo T1-weighted sequence (flip angle 8°; repetition time = 9.69 ms; echo time = 4.60 ms, matrix, 256 × 256 pixels; voxel size, 1.00 mm × 1.00 mm × 1.20 mm). For functional MRI, an echo planar imaging sequence (flip angle 80°; repetition time = 2300 ms; echo time = 30 ms, matrix, 96 × 96 pixels; field of view 220 × 220 mm) was used, covering the whole brain (40 axial slices; 2.29 mm × 2.29 mm in-plane resolution; 3.0 mm slice thickness). A total of 440 echo planar imaging volumes were collected per subject.
Data analysis
MRI data were analysed using Statistical Parametric Mapping version 5 (SPM5) (Wellcome Department of Imaging Neuroscience, London, UK). Echo planar imaging scans were slice time corrected, realigned and normalized to the standard Montreal Neurological Institute (MNI) brain of SPM. Subsequently, data were re-sliced to 3 mm × 3 mm × 3 mm voxels and spatially smoothed using an 8 mm isotropic Gaussian kernel. After high-pass filtering (cut-off 128 s), functional scans were analysed in the context of the general linear model using delta functions convolved with a canonical haemodynamic response function. Event duration, computed as the time between stimulus and response onset, was included in the model to account for haemodynamic responses of varying lengths to each type of stimulus. Error trials and head-movement parameters were modelled as regressors of no interest. Post hoc analysis of subject motion during the scans, based on the functional scan realignment parameters, indicated that subjects with high obsessive–compulsive symptoms scores did not exhibit significantly larger head movements compared with those with low obsessive–compulsive symptoms scores. For each subject, a ‘planning versus baseline’ main effect was computed in which brain activation during all planning trials was compared with brain activation during baseline trials. In addition, a main effect of ‘task load’ was computed using a linear contrast to identify brain regions that showed magnetic resonance signal intensity variation correlated with task difficulty (van den Heuvel et al., 2005).
Statistical tests
Differences in survey- and interview-based variables were tested using a mixed-model ANOVA [mixed models linear menu item in statistical package for the social sciences (SPSS; Chicago, IL, USA)] with twin pair type (discordant versus concordant) and obsessive–compulsive symptoms score (high versus low) as two fixed factors and family as a random factor to account for within-twin pair dependence. For the analysis of task performance data, a similar mixed-model ANOVA was used, with task load (planning difficulty levels 1–5) as an additional repeated measures factor. Preplanned contrasts of significant ‘task load’ × ‘obsessive–compulsive symptoms score’ × ‘twin pair type’ interactions compared the discordant and concordant-high and low groups for each of the task load levels. Statistical results with regard to questionnaire and task performance data were considered significant at P < 0.05, Bonferroni corrected.
First-level functional MRI contrast estimates for ‘planning versus baseline’ and ‘task load’ were entered into second-level analyses available in SPM5. Differences in contrast estimates between twins scoring high or low on obsessive–compulsive symptoms from discordant pairs were investigated by paired sample t-test. Differences in contrast estimates between concordant twin pairs scoring high or low on obsessive–compulsive symptoms were assessed using an ANOVA group comparison. To account for within-twin pair correlations of functional MRI signals, first-level results of the twin and co-twin of each concordant pair were entered as repeated measures. For main task effects of selected contrasts, we set an individual voxel threshold of P < 0.05, corrected for multiple comparisons (false discovery rate), with a minimal cluster extent of 10 voxels. Group differences, masked with the appropriate main task effect (mask thresholded at P < 0.05, uncorrected), are reported at an uncorrected individual voxel threshold of P < 0.005 with a minimal cluster extent of five voxels.
Post hoc region of interest based comparison
After an independent assessment of obsessive–compulsive symptom-related differences across the whole brain in discordant high–low and concordant-high versus concordant-low twins, we performed an additional region of interest analysis to directly compare functional brain activation differences observed in both types of twin contrasts. That is, we tested for increased (or decreased) functional brain activation in concordant-high versus concordant-low twin pairs specifically in spherical regions of interest (radius 10 mm) centred on the coordinates where discordant-high twins showed maximally increased (or decreased) functional activation relative to discordant-low twins. Conversely, we tested for increased (or decreased) functional brain activation in discordant-high versus discordant-low twins in spherical regions of interest centred on the coordinates where concordant-high twins showed maximally increased (or decreased) functional activation relative to concordant-low twins. For these post hoc regions of interest analyses, we applied an individual voxel P-value threshold of P < 0.05, corrected for multiple comparisons (false discovery rate).
Results
Questionnaire and interview data
Demographics and data on obsessive–compulsive symptoms of the subjects are summarized in Table 1. Significant main effects of ‘obsessive–compulsive symptoms score’ were found for the Padua Inventory Abbreviated obtained in 2002 [F(1,120.66) = 579.32, P < 0.001], Padua Inventory Abbreviated current scores [F(1,122.19) = 87.91, P < 0.001], lifetime and current Yale–Brown Obsessive–Compulsive Scale symptoms scores [F(1,124.23) = 34.26, P < 0.001; F(1,122.31) = 34.95, P < 0.001] as well as lifetime and current Yale–Brown Obsessive–Compulsive Scale severity scores [F(1,135.67) = 14.34, P < 0.001; F(1,134.54) = 50.27, P < 0.001]. Furthermore, an interaction between ‘obsessive–compulsive symptoms score’ and ‘twin pair type’ (discordant/concordant) was found for Padua Inventory Abbreviated current scores [F(1,122.19) = 8.12, P = 0.005] and lifetime Yale–Brown Obsessive–Compulsive Scale severity scores [F(1,135.67) = 9.66, P = 0.002]. In both cases, this was due to larger differences between high- and low-scoring twins in concordant compared with discordant groups. There was no significant ‘obsessive–compulsive symptoms score’ by ‘twin pair type’ interaction for the Yale–Brown Obsessive–Compulsive Scale subcategories aggressive/checking, hoarding/saving, symmetry/ordering and washing/cleaning, either across the whole life span [aggressive/checking: F(1,126.32) = 3.04, P = 0.084; hoarding/saving: F(1,128.86) = 0.01, P = 0.929; symmetry/ordering: F(1,126.35) = 2.19, P = 0.141; washing/cleaning: F(1,130.15) = 0.00, P = 0.962] or at the time of MRI scanning [aggressive/checking: F(1,126.49) = 1.13, P = 0.289; hoarding/saving: F(1,115.37) = 0.00, P = 0.987; symmetry/ordering: F(1,120.28) = 1.09, P = 0.299; washing/cleaning: F(1,131.56) = 0.60, P = 0.439].
Table 1 also shows scores on questionnaires measuring comorbidities in the discordant and concordant twin pairs. Significant main effects of ‘obsessive–compulsive symptoms score’, were found for lifetime and current comorbidity scores measured with the Mini International Neuropsychiatric Interview [F(1,132.70) = 21.60, P < 0.001; F(1,116.75) = 11.48, P < 0.001], tic scores [F(1,118.47) = 4.92, P = 0.028], Beck Depression Inventory scores [F(1,136.69) = 8.67, P = 0.004] and State Trait Anxiety Inventory scores [F(1,134.43) = 6.27, P = 0.013]. There was no significant main effect of ‘obsessive–compulsive symptoms score’ with regard to State Trait Anxiety Inventory scores [F(1,122.61) = 2.09, P = 0.150]. Significant ‘obsessive–compulsive symptoms score’ by ‘twin pair type’ interactions were absent for all comorbidity measures.
Task performance
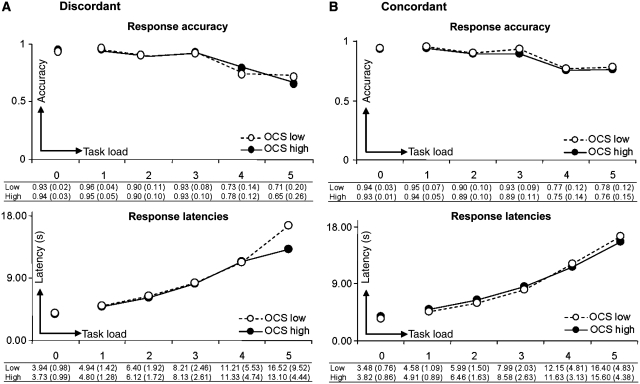
Figure 2 indicates Tower of London task response accuracy (top) and response latency (bottom) as a function of task load for twins scoring high and low on obsessive–compulsive symptoms in both the discordant (Fig. 2A) and concordant groups (Fig. 2B). Significant main effects of variable ‘task load’ across groups indicated that reaction accuracy decreased and reaction times increased with increasing task difficulty [response accuracy: F(1,221.14) = 89.37, P < 0.001; response latency: F(1,168) = 263.70, P < 0.001]. There was no significant main effect of ‘obsessive–compulsive symptoms score’ for either the baseline condition [accuracy: F(1,126.80) = 0.23, P = 0.632; latency: F(1,134.85) = 0.23, P = 0.629] or during planning [accuracy: F(1,181.76) = 0.51, P = 0.477; latency: F(1,285.81) = 0.94, P = 0.332]. In addition, there was no significant interaction between ‘task load’ and ‘obsessive–compulsive symptoms score’ [accuracy: F(1,221.14) = 0.94, P = 0.440; latency: F(1,168) = 1.09, P = 0.365], or a significant ‘task load’ by ‘obsessive–compulsive symptoms score’ by ‘twin pair type’ interaction [accuracy: F(1,221.14) = 0.69, P = 0.600; latency: F(1,168) = 0.51, P = 0.728]. In short, high-scoring twins of either discordant or concordant pairs did not perform differently to the low-scoring twins.
Figure 2.
Tower of London task performance. (Top): Response accuracy (between 0 and 1) as a function of task load levels 1, 2, 3, 4 and 5 (task load 0 = baseline condition) in the (A) discordant group and (B) concordant group. (Bottom): Mean latencies (s) of correct responses as a function of task load. Data for twins scoring high and low on obsessive–compulsive symptoms (OCS) are indicated by filled and open circles, respectively.
Functional imaging
Main task effect
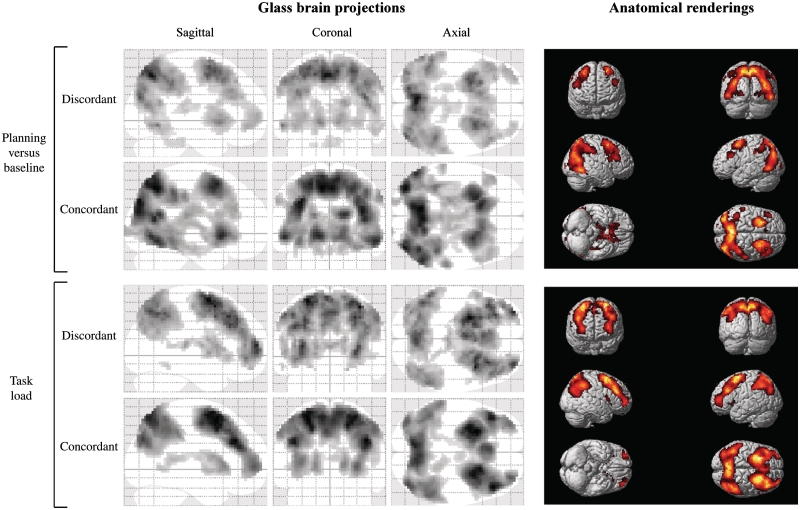
Activated brain regions for the ‘planning versus baseline’ and ‘task load’ contrasts are summarized in Fig. 3 and Table 2. In both the discordant and concordant groups, clusters of increased activation associated with Tower of London planning were noted in the parietal cortex [Brodmann areas (BA) 7 and 40], (pre)frontal cortex (BA 6, 8, 9, 10 and 46), anterior cingulate cortex (BA 32), caudate nucleus and thalamus pulvinar. For the ‘task load’ contrast, relative to ‘planning versus baseline’, there was a tendency for more robust task-related activation in regions of the inferior frontal lobes (BA 44 and 47) as well as left and right frontopolar areas (compare the anatomical renderings in the top and bottom panels of Fig. 3).
Figure 3.
Brain regions showing increased functional MRI signal during Tower of London cognitive planning. Glass brain overviews depict brain activity patterns for ‘planning versus baseline’ (top) and ‘task load’ (bottom) contrasts in discordant and concordant twins. Anatomical renderings on the right illustrate locations of functional brain activation for the ‘planning versus baseline’ (top) and ‘task load’ (bottom) contrasts, across all concordant twins.
Table 2.
Brain activity for ‘planning versus baseline’ and ‘task load’ contrasts
| Contrast | Anatomical location | Side | BA |
Discordant (n = 38) |
Concordant (n = 98) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
MNI coordinates |
Z-score |
MNI coordinates |
Z-score | ||||||||
| x | y | z | x | y | z | ||||||
| ‘Planning versus baseline’ | Parietal cortex | L | 7 | −6 | −66 | 51 | Inf | −9 | −60 | 51 | Inf |
| R | 7 | 9 | −69 | 57 | 7.30 | 3 | −60 | 51 | Inf | ||
| L | 40 | −60 | −36 | 36 | 5.36 | −63 | −33 | 36 | 4.72 | ||
| R | 40 | 42 | −42 | 42 | 6.54 | 45 | −42 | 48 | 6.97 | ||
| Frontal cortex | L | 6 | −30 | 0 | 51 | 7.10 | −21 | 9 | 57 | Inf | |
| R | 6 | 27 | 9 | 57 | 7.11 | 21 | 12 | 54 | 7.34 | ||
| L | 8 | −30 | 15 | 48 | 5.40 | −30 | 15 | 48 | 6.26 | ||
| R | 8 | 33 | 12 | 51 | 5.80 | 21 | 12 | 54 | 7.34 | ||
| L | 10 | −42 | 48 | −6 | 5.29 | ||||||
| R | 10 | 30 | 60 | −3 | 4.60 | ||||||
| L | 9/46 | −48 | 24 | 36 | 5.55 | −48 | 33 | 27 | 5.00 | ||
| R | 9/46 | 45 | 30 | 36 | 5.97 | 45 | 27 | 24 | 4.38 | ||
| Occipital cortex | L | 18 | −33 | −69 | 0 | 5.14 | |||||
| R | 18 | 21 | −99 | 3 | 4.45 | ||||||
| Anterior cingulate | L | 32 | −6 | 21 | 48 | 5.41 | −9 | 21 | 45 | 3.95 | |
| R | 32 | 9 | 21 | 48 | 4.46 | ||||||
| Caudate nucleus | L | – | −12 | 15 | −3 | 6.25 | −12 | 15 | −3 | Inf | |
| R | – | 12 | 9 | 0 | 5.81 | 15 | 18 | −3 | 7.02 | ||
| Thalamus pulvinar | L | – | −15 | −30 | 12 | 2.72 | −9 | −30 | 6 | 3.03 | |
| R | – | 9 | −27 | 12 | 4.07 | 3 | −21 | 12 | 4.27 | ||
| ‘Task load’ | Parietal cortex | L | 7 | −3 | −69 | 51 | 6.04 | −9 | −72 | 60 | Inf |
| R | 7 | 6 | −66 | 63 | 5.35 | 12 | −66 | 66 | Inf | ||
| L | 40 | −45 | −60 | 48 | 6.05 | −42 | −57 | 48 | 7.24 | ||
| R | 40 | 57 | −54 | 42 | 5.52 | 54 | −54 | 45 | 7.60 | ||
| Frontal cortex | L | 6 | −27 | 3 | 63 | 6.95 | −27 | 12 | 60 | Inf | |
| R | 6 | 36 | 9 | 57 | 6.81 | 30 | 6 | 60 | Inf | ||
| L | 8 | −30 | 15 | 48 | 5.52 | −3 | 27 | 45 | Inf | ||
| R | 8 | 33 | 14 | 51 | 5.62 | 21 | 15 | 51 | Inf | ||
| L | 9 | −42 | 27 | 33 | 6.24 | −42 | 30 | 33 | Inf | ||
| R | 9 | 45 | 30 | 33 | 5.61 | 45 | 33 | 33 | Inf | ||
| L | 10 | −33 | 60 | 12 | 6.51 | −36 | 51 | 9 | 7.08 | ||
| R | 10 | 33 | 60 | 6 | 6.21 | 33 | 54 | 3 | Inf | ||
| L | 44 | −51 | 9 | 12 | 3.53 | ||||||
| R | 44 | 54 | 9 | 12 | 3.85 | ||||||
| L | 47 | −51 | 18 | 0 | 2.95 | −48 | 15 | 0 | 3.94 | ||
| R | 47 | 51 | 18 | 0 | 3.14 | 33 | 24 | −6 | 3.70 | ||
| Temporal cortex | L | 37 | −57 | −48 | −12 | 3.37 | |||||
| Anterior cingulate | L | 32 | −6 | 24 | 36 | 5.90 | −6 | 24 | 39 | 6.52 | |
| R | 32 | 9 | 33 | 30 | 5.30 | 9 | 24 | 36 | 4.32 | ||
| Caudate nucleus | L | – | −15 | 12 | 12 | 5.65 | −18 | 18 | 6 | 6.57 | |
| R | – | 18 | 21 | 6 | 4.87 | 18 | 18 | 6 | 6.71 | ||
| Globus pallidus | L | – | −12 | 3 | 0 | 3.41 | −15 | 0 | −3 | 5.03 | |
| R | – | 12 | 3 | −3 | 2.31 | ||||||
| Thalamus pulvinar | L | – | −9 | −24 | 12 | 2.62 | −12 | −27 | 15 | 2.66 | |
| R | – | 9 | −27 | 12 | 4.14 | 9 | −27 | 12 | 3.08 | ||
Brain regions showing significant functional MRI signal increase for the ‘planning versus baseline’ and ‘task load’ contrasts in the discordant and concordant twin groups. Anatomical location = activated brain region; L = left hemisphere; R = right hemisphere; BA = Brodmann area; MNI coordinates (mm) = location of voxel with largest effect size; Z-score: z-value of voxel with largest effect size; Inf = infinite.
Environmental risk: high- versus low-scoring twins from discordant pairs
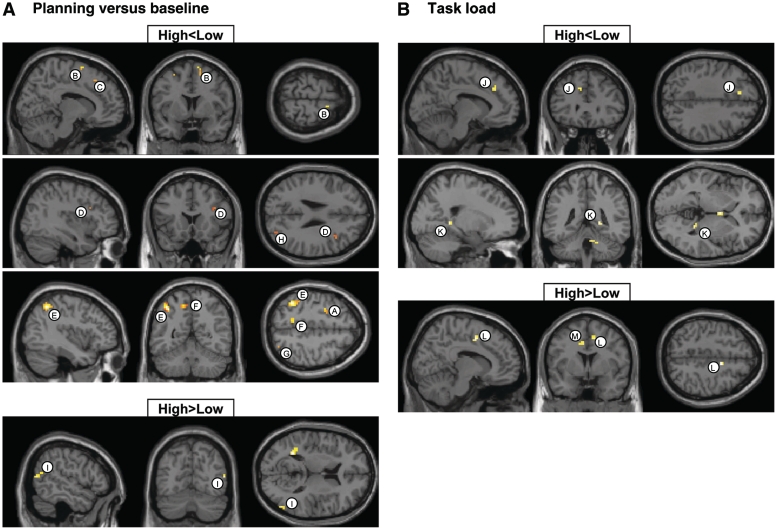
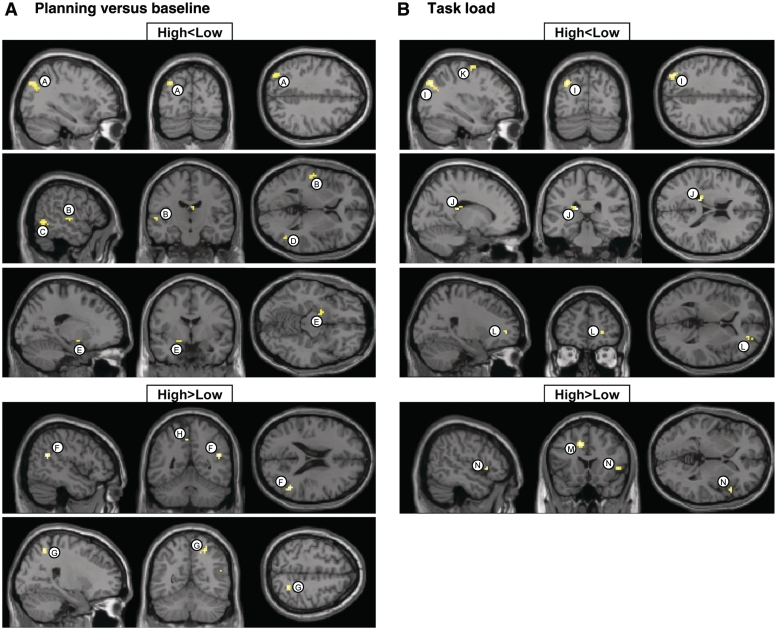
Table 3, left, and Fig. 4 show clusters of obsessive–compulsive symptoms-related differences in brain activation between the discordant-high and -low twins. For the ‘planning versus baseline’ contrast (Fig. 4A), twins scoring high on obsessive–compulsive symptoms compared with their low-scoring co-twins exhibited clusters of decreased brain activation in the premotor cortex (clusters labelled A and B in Table 3, left and Fig. 4A) and superior parietal cortex (Clusters F–H), both bilaterally, and right medial frontal cortex (Cluster C), right dorsolateral prefrontal cortex (Cluster D) and left inferior parietal cortex (Cluster E). Increased brain activation for twins scoring high on obsessive–compulsive symptoms was observed in the right middle temporal cortex (Cluster I). For the ‘task load’ contrast (Fig. 4B), clusters of decreased brain activation in twins scoring high on obsessive–compulsive symptoms relative to twins scoring low were noted in the left dorsolateral prefrontal cortex (cluster labelled J in Table 3, left and Fig. 4B) and right lingual cortex (Cluster K). Increased brain activation for the twins scoring high on obsessive–compulsive symptoms was observed bilaterally in the cingulate cortex (Clusters L and M).
Table 3.
Brain activation differences between twins scoring high and low on obsessive–compulsive symptoms from the discordant group
| Test | Cluster labela | Anatomical location | BA |
MNI coordinates (19 pairs) |
Z-score | P-value | No. of voxels |
MNI coordinates (8 pairs)b |
Z-score | No. of voxels | Euclidean distance (mm) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||||||||||
| ‘Planning versus baseline’ | |||||||||||||||
| High < low | A | Left premotor cortex | 6 | −27 | 3 | 54 | 3.41 | <0.001 | 15 | −30 | 0 | 48 | 3.84 | 9 | 7.3 |
| B | Right premotor cortex | 6 | 12 | 6 | 69 | 3.33 | <0.001 | 9 | 12 | 9 | 60 | 2.98 | 1 | 9.5 | |
| C | Right medial frontal cortex | 8 | 15 | 27 | 48 | 3.20 | 0.001 | 8 | – | – | – | – | – | – | |
| D | Right dorsolateral prefrontal cortex | 9 | 39 | 18 | 27 | 2.85 | 0.002 | 5 | 33 | 21 | 21 | 2.82 | 1 | 9 | |
| E | Left inferior parietal cortex | 40 | −39 | −54 | 51 | 4.33 | <0.001 | 46 | −39 | −54 | 51 | 2.70 | 4 | 0 | |
| 40 | −57 | −36 | 45 | 3.18 | 0.001 | 9 | −57 | −42 | 45 | 2.66 | 1 | 6 | |||
| F | Left superior parietal cortex | 7 | −12 | −54 | 54 | 3.40 | <0.001 | 14 | – | – | – | – | – | – | |
| 7 | −18 | −69 | 42 | 2.87 | 0.002 | 6 | 0 | −69 | 51 | 2.86 | 5 | 20.1 | |||
| G | Right superior parietal cortex | 7 | 30 | −72 | 48 | 2.93 | 0.002 | 5 | – | – | – | – | – | – | |
| H | Right superior parietal cortex | 19 | 30 | −78 | 30 | 3.36 | <0.001 | 9 | 39 | −84 | 33 | 3.40 | 1 | 11.2 | |
| High > low | I | Right middle temporal cortex | 39 | 54 | −69 | 12 | 3.44 | <0.001 | 12 | 57 | −60 | 21 | 3.02 | 4 | 13.1 |
| ‘Task load’ | |||||||||||||||
| High < low | J | Left dorsolateral prefrontal cortex | 9 | −9 | 30 | 36 | 3.09 | 0.001 | 9 | −21 | 15 | 48 | 2.61 | 1 | 22.6 |
| K | Right lingual cortex | 30 | 21 | −42 | 0 | 3.21 | 0.001 | 8 | – | – | – | – | – | – | |
| High > low | L | Right cingulate cortex | 24 | 12 | 0 | 48 | 3.46 | <0.001 | 10 | 12 | 0 | 48 | 2.76 | 1 | 0 |
| M | Left cingulate cortex | 24 | −9 | 0 | 42 | 3.01 | 0.001 | 9 | −3 | 3 | 42 | 2.99 | 3 | 6.7 | |
a Clusters with regional brain activation differences between discordant-high and discordant-low twins for the ‘planning versus baseline’ and ‘task load’ contrasts.
Test = test for significant decreases (high < low) or increases (high > low) in twins with high relative to low obsessive–compulsive symptoms scores; cluster label = alphabetical cluster label as displayed in anatomical overlays of Fig. 4A (‘planning versus baseline’ contrast) and B (‘task load’ contrast); BA = Brodmann area; MNI coordinates (19 pairs) (mm) = location of voxel with largest effect size for the 19-pair comparison; Z-score = z-value of voxel with largest effect size; P-value = cluster P-value; no. of voxels = number of voxels in cluster.
b Montreal Neurological Institute coordinates (mm) of voxel with largest effect size for the post hoc within-pair comparison in the 8 pairs still extremely discordant at the time of scanning; Z-score = z-value of voxel with largest effect size for the post hoc 8 pair comparison; no. of voxels = number of voxels in cluster for the post hoc 8 pair comparison; Euclidean distance (mm) = distance between the coordinates derived from the original 19 pair comparison and the coordinates derived from the additional 8 pair comparison.
Figure 4.
Brain regions showing reduced (top: high < low) and increased (bottom: high > low) functional MRI signal in twins with high obsessive–compulsive symptoms scores versus twins with low scores from the discordant group. (A) ‘Planning versus baseline’ contrast; (B) ‘task load’ contrast.
Genetic risk: concordant-high- versus concordant-low-scoring twins
Table 4, left, and Fig. 5 show clusters of obsessive–compulsive symptoms-related differences in brain activation between the concordant-high and -low twin pairs. For the ‘planning versus baseline’ contrast (Fig. 5A), concordant-high-scoring twins compared with concordant-low twins exhibited clusters of decreased brain activation, bilaterally, in the temporal cortex (clusters labelled B, C and D in Table 4, left and Fig. 5A), left globus pallidus (cluster E) and left superior parietal cortex (Cluster A). Clusters of increased brain activation for twins scoring high on obsessive–compulsive symptoms were noted in the right parietal cortex (Clusters F and G) and left cingulate cortex (Cluster H). For the ‘task load’ contrast (Fig. 5B), clusters of decreased brain activation in concordant-high twins were found in the left premotor cortex (cluster labelled K in Table 4, left and Fig. 5B), right frontopolar cortex (Cluster L), left superior parietal cortex (Cluster I) and left caudate tail (Cluster J). Increased brain activation for the concordant-high twins was observed in the left cingulate cortex (Cluster M) and right inferior frontal cortex (Cluster N).
Table 4.
Brain activation differences between twins scoring high and low on obsessive–compulsive symptoms from the concordant group
| Test | Cluster labela | Anatomical location | BA |
MNI coordinates (22/28pairs) |
Z-score | P-value | No. of voxels |
MNI coordinates (10/23 pairs)b |
Z-score | No. of voxels | Euclidean distance (mm) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||||||||||
| ‘Planning versus baseline’ | |||||||||||||||
| High < low | A | Left superior parietal cortex | 19 | −33 | −78 | 39 | 3.34 | <0.001 | 35 | −36 | −72 | 39 | 2.80 | 4 | 6.7 |
| B | Left superior temporal cortex | 22 | −54 | −15 | 3 | 3.10 | 0.001 | 9 | −57 | −12 | 6 | 3.24 | 13 | 5.2 | |
| C | Left inferior temporal cortex | 37 | −48 | −51 | −3 | 3.78 | <0.001 | 29 | −48 | −51 | −3 | 4.22 | 84 | 0 | |
| D | Right middle temporal cortex | 37 | 48 | −63 | 0 | 3.27 | 0.001 | 23 | – | – | – | – | – | – | |
| E | Left globus pallidus | −18 | −3 | −12 | 3.00 | 0.001 | 5 | – | – | – | – | – | – | ||
| High > low | F | Right inferior parietal cortex | 40 | 48 | −51 | 21 | 3.28 | 0.001 | 15 | 63 | −54 | 21 | 2.66 | 1 | 15.3 |
| G | Right superior parietal cortex | 7 | 24 | −57 | 54 | 3.14 | 0.001 | 15 | 27 | −54 | 57 | 3.75 | 47 | 5.2 | |
| H | Left cingulate cortex | 31 | −6 | −45 | 48 | 3.08 | 0.001 | 12 | – | – | – | – | – | – | |
| ‘Task load’ | |||||||||||||||
| High < low | I | Left superior parietal cortex | 19 | −33 | −75 | 39 | 3.54 | <0.001 | 39 | −33 | −72 | 39 | 3.15 | 10 | 3 |
| J | Left caudate tail | −15 | −30 | 18 | 3.65 | <0.001 | 14 | −24 | −27 | 18 | 3.05 | 20 | 9.5 | ||
| K | Left premotor cortex | 6 | −33 | −6 | 66 | 3.19 | 0.001 | 11 | – | – | – | – | – | – | |
| L | Right frontopolar cortex | 10 | 24 | 48 | 3 | 3.11 | 0.001 | 7 | 24 | 48 | 0 | 3.00 | 15 | 3 | |
| High > low | M | Left cingulate cortex | 32 | −9 | 18 | 39 | 3.81 | <0.001 | 26 | −12 | 18 | 39 | 3.29 | 10 | 3 |
| N | Right inferior frontal cortex | 47 | 51 | 18 | 0 | 3.50 | <0.001 | 7 | 51 | 18 | 0 | 2.65 | 5 | 0 | |
a Clusters with regional brain activation differences between concordant-high and concordant-low twins for the ‘planning versus baseline’ and ‘task load’ contrasts.
Test = test for significant decreases (high < low) or increases (high > low) in twins with high relative to low obsessive–compulsive symptoms scores; Cluster label = alphabetical cluster label as displayed in anatomical overlays of Fig. 5A (‘planning versus baseline’ contrast) and B (‘task load’ contrast); BA = Brodmann area; MNI coordinates (22/28 pairs) (mm) = location of voxel with largest effect size for the 22-high to 28-low twin pair comparison; Z-score = z-value of voxel with largest effect size; P-value = cluster P-value; no. of voxels = number of voxels in cluster.
b Montreal Neurological Institute coordinates (mm) of voxel with largest effect size for the post hoc comparison between the 10 twin pairs that scored high at the time of scanning and the 23 twin pairs that scored low at the time of scanning; Z-score = z-value of voxel with largest effect size for the post hoc 10-high to 23-low-scoring twin pair comparison; no. of voxels = number of voxels in cluster for the post hoc 10-high to 23-low scoring twin pair comparison; Euclidean distance (mm) = distance between the coordinates derived from the original 22-high to 28-low-twin pair comparison and the coordinates derived from our additional 10-high to 23-low-scoring twin pair comparison.
Figure 5.
Brain regions showing reduced (top: high < low) and increased (bottom: high > low) functional MRI signal in concordant-high versus concordant-low twins. (A) ‘Planning versus baseline’ contrast; (B) ‘task load’ contrast.
Post hoc region of interest comparisons
Post hoc tests revealed no significant differences in brain activation for concordant-high versus concordant-low twin pairs in regions of interest centred around the clusters with functional activation differences in the whole-brain discordant twin comparison (i.e. spherical regions of interest placed on each of the cluster peak coordinates from the discordant comparison listed in Table 3, left). There were also no differences in brain activation in discordant-high versus discordant-low twin pairs in regions of interest centred around the clusters with functional activation differences in the whole brain concordant twin comparison (i.e. spherical regions of interest placed on each of the cluster peak coordinates from the concordant comparison listed in Table 4, left).
Post hoc analyses using obsessive–compulsive symptoms scores at the time of scanning
This study had a prospective design in that selection of the twins preceded the actual MRI scans by 4–7 years. As a consequence, many of the discordant pairs and some of the concordant pairs no longer met the criteria at the time of scanning. We therefore conducted new analyses on our data to test if a focus on the obsessive–compulsive symptoms scores at the time of scanning would affect our results significantly. We re-run the analysis on a group of eight discordant pairs who still met the criteria at the time of MRI scanning, [high obsessive–compulsive symptom score: mean (SD) = 17.75 (7.6); low obsessive–compulsive symptom score: mean (SD) = 4.75 (3.1)] and on those concordant pairs with a mean obsessive–compulsive symptoms score meeting the cut-off criteria at the time of scanning [10 concordant-high twin pairs with mean (SD) = 19.30 (5.1) and 23 concordant-low twin pairs with mean (SD) = 3.76 (2.2)]. To directly compare functional brain activation differences observed from the original analysis in 19 discordant pairs with those obtained from the analysis in the selected eight pairs, we tested for increased (or decreased) functional brain activation (P < 0.005, uncorrected) in our 8 pair comparison specifically at the coordinates where the analysis on 19 pairs showed maximally increased (or decreased) functional activation. If no significant cluster was found at the exact coordinate derived from our 19 pair comparison, we searched for the nearest local maxima within that anatomical location. Results are reported in Table 3, right. The same analysis was performed for the concordant group, in which we tested for increased (or decreased) functional brain activation (P < 0.005, uncorrected) in our 10 concordant-high to 23 concordant-low pair comparison specifically at the coordinates, where the analysis on the original 22 concordant-high to 28 concordant-low pair comparison showed maximally increased (or decreased) functional activation. Results are reported in Table 4, right. Post hoc analyses in both the discordant and concordant groups revealed highly similar results compared with those obtained from the original analyses, although a few areas were lost due to reduced statistical power.
Discussion
In the present study, task performance and brain activation during a Tower of London cognitive planning paradigm were compared within monozygotic twin pairs discordant for obsessive–compulsive symptoms and between monozygotic twin pairs who scored concordant-low or concordant-high for obsessive–compulsive symptoms. No differences were found in response accuracy or latency measures between discordant twins, which implies that the environmentally mediated risk for obsessive–compulsive disorder did not influence behavioural task performance. Likewise, concordant-high twins did not perform worse than concordant-low twins, suggesting that the genetically mediated risk for obsessive–compulsive disorder did not interfere with actual task performance. These results partly disagree with studies comparing Tower of London performance in patients with obsessive–compulsive disorder versus controls. Purcell and colleagues (1998a) found no significant differences in response accuracy in Tower of London task performance between patients with obsessive–compulsive disorder and controls, but the patients with obsessive–compulsive disorder reacted significantly slower. In addition, van den Heuvel and colleagues (2005) found patients with obsessive–compulsive disorder to be significantly less accurate and slower. It is unclear whether the absence of performance deficits in our study reflects the lower severity of obsessive–compulsive symptoms in this largely non-clinical sample, the fact that only few of our subjects had a history of anti-depressant medication (in contrast to the studies with patient groups), or a combination.
Although their performance remained intact, there was evidence that the high-risk subjects in our study deviated from the low-risk subjects in the patterns of brain activation accompanying execution of the Tower of London task. The brain regions in which subjects with high obsessive–compulsive symptoms scores differed from subjects with low obsessive–compulsive symptoms scores can be separated into regions that were mainly affected by environmental risk [dorsolateral prefrontal cortex (BA 9) and lingual cortex (BA 30)], genetic risk [frontopolar cortex (BA 10), inferior frontal cortex (BA 47), globus pallidus and caudate nucleus] and both environmental and genetic risk factors [cingulate cortex (BA 24, 31 and 32), premotor cortex (BA 6) and parts of the parietal cortex (BA 7, 19 and 40)]. We discuss these findings in more detail below.
Regions affected by environmental risk
Brain regions showing different activation patterns in twins with high obsessive–compulsive symptoms scores compared with those with low obsessive–compulsive symptoms scores that were present in only the discordant group and, therefore, are probably related to environmental risk factors for obsessive–compulsive disorder include the dorsolateral prefrontal cortex (BA 9) (‘planning versus baseline’ and ‘task load’) and right lingual cortex (BA 30) (‘task load’). Our findings of decreased ‘planning versus baseline’ and ‘task load’ associated dorsolateral prefrontal cortex activity in the twins with high obsessive–compulsive symptoms scores compared with those with low obsessive–compulsive symptoms scores replicates our previous findings in a subsample of the present discordant twin population (den Braber et al., 2008). In addition, these results are in line with the findings of a study in patients with obsessive–compulsive disorder (van den Heuvel et al., 2005). The dorsolateral prefrontal cortex has been related to executive processing, including attention, response inhibition, cognitive planning and decision making (Faw, 2003; Newman et al., 2003; Ridderinkhof et al., 2004). In addition, neuropsychological studies have typically associated dysfunction of this brain structure with perseverative, disinhibited behaviours, which patients with obsessive–compulsive disorder particularly show during the completion of their compulsions (Friedlander and Desrocher, 2006). Reduced activity in the dorsolateral prefrontal cortex also agrees with the commonly accepted neurobiological model of CSTC abnormalities in obsessive–compulsive disorder (Insel and Winslow, 1992; Graybiel and Rauch, 2000; Menzies et al., 2008).
In line with our results, a decrease in lingual cortex activity (‘task load’) in patients with obsessive–compulsive disorder compared with unaffected controls has been found in a symptom provocation study by Mataix-Cols and colleagues (2004). In their study, patients with obsessive–compulsive disorder and controls were presented with emotional (e.g. washing-related, checking-related) pictures during functional MRI scanning. The observed decrease in lingual activity was specifically associated with the checking symptom dimension. The lingual cortex is part of the occipital cortex, which is involved in visual processing. The authors suggested that the patients with obsessive–compulsive disorder directed their attention more to the emotional salience of the pictures rather than focusing on the visual details, which would explain the decrease in activation of the occipital cortex.
Regions affected by genetic risk
Brain regions showing different activation patterns in twins with high obsessive–compulsive symptoms scores compared with those with low obsessive–compulsive symptoms scores that were present in only the concordant group and therefore are suggested to be related to genetic risk factors for obsessive–compulsive disorder include the right frontopolar cortex (BA 10) (‘task load’), the right inferior frontal cortex (BA 47) (‘task load’), the left caudate nucleus (‘task load’) and the left globus pallidus (‘planning versus baseline’). The ‘task load’-related decrease in frontopolar activity (BA 10) in twins with high obsessive–compulsive symptoms scores is in agreement with lower activity in this area in patients with obsessive–compulsive disorder after performing a set switching paradigm (Gu et al., 2008). Although its specific role in cognitive functioning is not yet clearly understood, the frontopolar region appears to be engaged in a wide variety of higher order cognitive functions, such as learning and exploration, memory retrieval, relational reasoning, multitasking behaviour and ‘the human ability to hold in mind goals while exploring and processing secondary goals’ (Ramnani and Owen, 2004; Burgess et al., 2007; Koechlin and Hyafil, 2007). This region is connected to areas in the CSTC network, including the prefrontal cortex and cingulate cortex (Ramnani and Owen, 2004; Koechlin and Hyafil, 2007) and may influence obsessive–compulsive disorder through these connections.
Our finding of increased ‘task load’-related activity in the inferior frontal cortex is in line with findings in patients with obsessive–compulsive disorder (van den Heuvel et al., 2005). The inferior frontal cortex has been implicated in a wide range of cognitive processes, including task switching, reversal learning and cognitive and emotional inhibition (Ramnani and Owen, 2004; Dillon and Pizzagalli, 2007). Furthermore, this region is involved in regulating socially appropriate behaviours and, when impaired, a patient may show tactless, impulsive and disinhibited behaviour (Friedlander and Desrocher, 2006).
Our findings of decreased caudate nucleus (‘task load’) and globus pallidus (‘planning versus baseline’) activity are consistent with several neuroimaging studies (Giedd et al., 2000; Szeszko et al., 2004; van den Heuvel et al., 2005; Mataix-Cols and van den Heuvel, 2006). Reduced activity patterns in these basal ganglia structures agree with the general theory of a dysfunction in the CSTC circuitry in obsessive–compulsive disorder (Graybiel and Rauch, 2000; Menzies et al., 2008). The basal ganglia have strong connections with associative, orbitofrontal and sensorimotor cortices and participate in many neuronal pathways implicated in motor, emotional, motivational, associative and cognitive functions (Herrero et al., 2002). In addition, the basal ganglia play a role in reinforcing wanted behaviours and suppressing unwanted behaviours (Schultz et al., 1997). A dysfunction in the globus pallidus and/or caudate nucleus might therefore result in the behavioural deficits seen in obsessive–compulsive disorder, which is supported by the fact that focal lesions in the caudate nucleus or globus pallidus produce striking obsessive–compulsive disorder-like behaviour (Laplane et al., 1989).
Taken together, our findings of altered prefrontal and striatal activity in twins with high obsessive–compulsive symptoms scores compared with those with low scores fit very well with a model of neurobiological changes due to the genetic risk for obsessive–compulsive disorder. Since family and twin studies have shown that obsessive–compulsive disorder is heritable (van Grootheest et al., 2005), several studies have tried to identify genetic variants involved in obsessive–compulsive disorder aetiology (Nicolini et al., 2009). Glutamine and serotonin system genes are among the candidate genes for which replication has most often been reported (Nicolini et al., 2009). In prefrontal regions and their projection areas in the striatum, both glutamatergic and serotonergic neurotransmission is highly abundant (Carlsson, 2001; Fineberg et al., 2010). Interestingly, pharmacological studies have indicated glutamate/serotonin interactions in these particular regions, which are further supported by PET and magnetic resonance spectroscopy studies (Carlsson, 2001).
Regions affected by environmental and genetic risk
The additional regions of interest analysis employed in this study, testing the presence of overlap in brain activation changes observed in our discordant and concordant twins, did not reveal any significant results after correction for multiple testing. Nonetheless, there was an implication that some areas in the uncorrected whole-brain analyses were affected by both environmental and genetic risk factors for obsessive–compulsive disorder. These regions included the cingulate, premotor and parietal cortices.
In agreement with our findings, increased activity in the cingulate cortex (‘task load’) was also found in patients with obsessive–compulsive disorder (van den Heuvel et al., 2005). A priori, we hypothesized that regions affected by both environmental and genetic risk factors for obsessive–compulsive disorder should be most closely related to the behavioural abnormalities characteristic of the disorder. At first sight, this appears to make sense for the cingulate cortex, as this brain region, through its connections with other regions of the limbic system, is implicated in the assessment of emotional information and the regulation of emotional responses, and thereby might mediate the anxiety-provoking thoughts and subsequent repetitive behaviours seen in obsessive–compulsive disorder (Aouizerate et al., 2004).
However, in view of the full pattern of our results, we a posteriori favour the alternative explanation that the regions found to be affected by both environmental and genetic risk factors for obsessive–compulsive disorder, including the cingulate cortex, act to compensate for the disturbances in CSTC circuits rather than playing a central role in obsessive–compulsive symptomatology. The cingulate cortex is related to performance monitoring (MacDonald, III et al., 2000) and error signalling (Magno et al., 2006), and the high obsessive–compulsive symptoms group may feel a strong need to perform well and avoid errors, as perfectionism is highly associated with obsessive–compulsive disorder (Frost and Steketee, 1997). This is in line with our finding that subjects with high obsessive–compulsive symptoms scores in both discordant and concordant groups kept their performance intact.
Decreases in brain activity in the high-scoring compared with low-scoring twins from both groups were found in the premotor cortex (BA 6) and regions of the parietal cortex (BA 7, 19 and 40). Activation decreases in these regions are almost exclusively in the ‘planning versus baseline’ contrast, are in line with our previous findings (den Braber et al., 2008) and those from van den Heuvel and colleagues (2005). Since these areas are involved in basic functions of motion processing (Rowe et al., 2001), motor preparation (Hoshi and Tanji, 2000; Mars et al., 2007) and visuospatial processing (Cabeza and Nyberg, 2000), they may support mainly proper task execution (e.g. analysis of planning stimulus, imaginary movement of the beads, executing a response) rather than higher order planning.
Obsessive–compulsive disorder-related abnormalities in superior and inferior parietal regions have also been found (Lucey et al., 1995; Kwon et al., 2003; Ciesielski et al., 2005; Szeszko et al., 2005; Valente Jr et al., 2005; Kitamura et al., 2006; Menzies et al., 2007, 2008). While the decrease in brain activation in the parietal cortex in the high obsessive–compulsive symptoms group might indicate a deficit in visual processing, there could also be another explanation. The superior and inferior parietal cortices are connected to each other, and results from animal studies have shown that these structures are strongly interconnected with the prefrontal cortex, dorsal premotor area, supplementary motor area and anterior cingulate cortex (Petrides and Pandya, 1984; Goldman-Rakic, 1988; Diwadkar et al., 2000; Faw, 2003). The superior parietal cortex also has major subcortical connections with the claustrum, caudate nucleus and putamen (Yeterian and Pandya, 1993; Leichnetz, 2001). These considerations indicate that the parietal cortex and dorsolateral prefrontal cortex (or caudate nucleus) do not act independently but influence each other. Therefore, the decrease in parietal activity found in our study might be directly related to the decreased activity observed in the dorsolateral prefrontal cortex and caudate nucleus. This is in line with recent evidence that the underlying pathology of obsessive–compulsive disorder is not limited to orbitofrontal–striatal regions and associated limbic structures, but also involves parietal lobe abnormalities (Menzies et al., 2008).
This study had a prospective design in that selection of the twins preceded the actual scans by 4–7 years. As a consequence, some of the discordant and concordant pairs no longer matched the stringent selection criteria at the time of MRI scanning, which could have influenced our results adversely. Nevertheless, the within-pair difference in the discordant group and the between-pair difference in the concordant high–low group were still significant at the time of scanning and the post hoc analysis; comparing only those twins that matched selection criteria at the time of scanning revealed highly comparable results. These results indicate that environmentally or genetically mediated functional brain alterations in obsessive–compulsive symptoms remain unchanged regardless of having present obsessive–compulsive symptoms, suggesting that these brain alterations are trait-like in nature. This is consistent with conclusions drawn by others (Bannon et al., 2006; Rao et al., 2008) that used neuropsychological tests rather than functional MRI.
To summarize, the present results suggest that brain regions affected by the environmental risk for obsessive–compulsive disorder are partly distinct from brain regions affected by the genetic risk for obsessive–compulsive disorder. Regions with neurobiological changes induced by environmental risk factors include the dorsolateral prefrontal cortex and lingual cortex, which are part of the dorsolateral prefrontal–subcortical loop (Cummings, 1995) of the CSTC network in which several imaging studies have reported abnormalities (Menzies et al., 2008). Disturbances in the dorsolateral prefrontal–subcortical loop may result in perseveration, reduced mental control and impaired response inhibition, as seen in obsessive–compulsive disorder. Regions with neurobiological changes induced by genetic factors include orbitofrontal–basal ganglia structures that are part of the orbitofrontal–basal ganglia loop of the CSTC network (Menzies et al., 2008). Disturbances in the orbitofrontal–basal ganglia loop may result in the tactless, impulsive and disinhibited behaviour seen in obsessive–compulsive disorder (Graybiel and Rauch, 2000). Regions that show similar decreases in activity in discordant and concordant groups, such as superior and inferior parietal regions, may indirectly reflect the deficits in dorsolateral prefrontal and orbitofrontal–striatal networks to which they are highly connected. Regions that show similar increases in activity in discordant and concordant groups, such as the cingulate cortex, may be part of compensatory networks that keep planning performance intact, at least during a relatively unchallenging task like the Tower of London.
Funding
This work was supported by the The Netherlands Organisation for Scientific Research (NWO) [Medical Sciences (MW): grant no. 904-61-193; Social Sciences (MaGW): grant no. 400-07-080; Social Sciences (MaGW): grant no. 480-04-004]. Funding to pay the Open Access publication charges for this article was provided by The Netherlands Organisation for Scientific Research (NWO).
Acknowledgements
We thank Gabriëlla Blokland, Myrle Kemperman and Mira Geirnaert for help with MRI data collection.
Glossary
Abbreviation
- CSTC
cortico-striato-thalamo-cortical
References
- Albert U, Maina G, Bogetto F, Ravizza L. The role of recent life events in the onset of obsessive-compulsive disorder. CNS Spectr. 2000;5:44–50. doi: 10.1017/s109285290000780x. [DOI] [PubMed] [Google Scholar]
- Alonso P, Menchon JM, Mataix-Cols D, Pifarre J, Urretavizcaya M, Crespo JM, et al. Perceived parental rearing style in obsessive-compulsive disorder: relation to symptom dimensions. Psychiatry Res. 2004;127:267–78. doi: 10.1016/j.psychres.2001.12.002. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorder: DSM-IV. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Aouizerate B, Guehl D, Cuny E, Rougier A, Bioulac B, Tignol J, et al. Pathophysiology of obsessive-compulsive disorder: a necessary link between phenomenology, neuropsychology, imagery and physiology. Prog Neurobiol. 2004;72:195–221. doi: 10.1016/j.pneurobio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bannon S, Gonsalvez CJ, Croft RJ, Boyce PM. Executive functions in obsessive-compulsive disorder: state or trait deficits? Aust N Z J Psychiatry. 2006;40:1031–8. doi: 10.1080/j.1440-1614.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rial WY, Rickels K. Short form of depression inventory: cross-validation. Psychol Rep. 1974;34:1184–6. [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bengel D, Greenberg BD, Cora-Locatelli G, Altemus M, Heils A, Li Q, et al. Association of the serotonin transporter promoter regulatory region polymorphism and obsessive-compulsive disorder. Mol Psychiatry. 1999;4:463–6. doi: 10.1038/sj.mp.4000550. [DOI] [PubMed] [Google Scholar]
- Billett EA, Richter MA, Sam F, Swinson RP, Dai XY, King N, et al. Investigation of dopamine system genes in obsessive-compulsive disorder. Psychiatr Genet. 1998;8:163–9. doi: 10.1097/00041444-199800830-00005. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, de Geus EJ, Vink JM, Stubbe JH, Distel MA, Hottenga JJ, et al. Netherlands Twin Register: from twins to twin families. Twin Res Hum Genet. 2006;9:849–57. doi: 10.1375/183242706779462426. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Gilbert SJ, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Philos Trans R Soc Lond B Biol Sci. 2007;362:887–99. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Carlsson ML. On the role of prefrontal cortex glutamate for the antithetical phenomenology of obsessive-compulsive disorder and attention deficit hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:5–26. doi: 10.1016/s0278-5846(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Cath DC, Spinhoven P, Hoogduin CA, Landman AD, van Woerkom TC, van de Wetering BJ, et al. Repetitive behaviors in Tourette's syndrome and OCD with and without tics: what are the differences? Psychiatry res. 2001;101:171–85. doi: 10.1016/s0165-1781(01)00219-0. [DOI] [PubMed] [Google Scholar]
- Cath DC, van Grootheest DS, Willemsen G, van Oppen P, Boomsma DI. Environmental factors in obsessive-compulsive behavior: evidence from discordant and concordant monozygotic twins. Behav Genet. 2008;38:108–20. doi: 10.1007/s10519-007-9185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive-compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. A neuropsychological comparison of obsessive-compulsive disorder and trichotillomania. Neuropsychologia. 2007;45:654–62. doi: 10.1016/j.neuropsychologia.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Menzies L. Endophenotypes of obsessive-compulsive disorder: rationale, evidence and future potential. Expert Rev Neurother. 2009;9:1133–46. doi: 10.1586/ern.09.36. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Hamalainen MS, Lesnik PG, Geller DA, Ahlfors SP. Increased MEG activation in OCD reflects a compensatory mechanism specific to the phase of a visual working memory task. Neuroimage. 2005;24:1180–91. doi: 10.1016/j.neuroimage.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Clifford CA, Murray RM, Fulker DW. Genetic and environmental influences on obsessional traits and symptoms. Psychol Med. 1984;14:791–800. doi: 10.1017/s0033291700019760. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Anatomic and behavioral aspects of frontal-subcortical circuits. Ann N Y Acad Sci USA. 1995;769:1–13. doi: 10.1111/j.1749-6632.1995.tb38127.x. [DOI] [PubMed] [Google Scholar]
- Dagher A, Owen AM, Boecker H, Brooks DJ. Mapping the network for planning: a correlational PET activation study with the Tower of London task. Brain. 1999;122 (Pt 10):1973–87. doi: 10.1093/brain/122.10.1973. [DOI] [PubMed] [Google Scholar]
- de Geus EJ, van’t Ent D, Wolfensberger SP, Heutink P, Hoogendijk WJ, Boomsma DI, et al. Intrapair differences in hippocampal volume in monozygotic twins discordant for the risk for anxiety and depression. Biol Psychiatry. 2007;61:1062–71. doi: 10.1016/j.biopsych.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Delorme R, Gousse V, Roy I, Trandafir A, Mathieu F, Mouren-Simeoni MC, et al. Shared executive dysfunctions in unaffected relatives of patients with autism and obsessive-compulsive disorder. Eur Psychiatry. 2007;22:32–8. doi: 10.1016/j.eurpsy.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Braber A, van’t Ent D, Blokland GA, van Grootheest DS, Cath DC, Veltman DJ, et al. An fMRI study in monozygotic twins discordant for obsessive-compulsive symptoms. Biol Psychol. 2008;79:91–102. doi: 10.1016/j.biopsycho.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Denys D, De Geus F, van Megen HJ, Westenberg HG. Symptom dimensions in obsessive-compulsive disorder: factor analysis on a clinician-rated scale and a self-report measure. Psychopathology. 2004;37:181–9. doi: 10.1159/000079509. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Pizzagalli DA. Inhibition of action, thought, and emotion: a selective neurobiological review. Appl Prev Psychol. 2007;12:99–114. doi: 10.1016/j.appsy.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Carpenter PA, Just MA. Collaborative activity between parietal and dorso-lateral prefrontal cortex in dynamic spatial working memory revealed by fMRI. Neuroimage. 2000;12:85–99. doi: 10.1006/nimg.2000.0586. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Greenberg BD, Murphy DL, Goldman D. Sexually dimorphic relationship of a 5-HT2A promoter polymorphism with obsessive-compulsive disorder. Biol Psychiatry. 2001;49:385–8. doi: 10.1016/s0006-3223(00)01040-4. [DOI] [PubMed] [Google Scholar]
- Faw B. Pre-frontal executive committee for perception, working memory, attention, long-term memory, motor control, and thinking: a tutorial review. Conscious Cogn. 2003;12:83–139. doi: 10.1016/s1053-8100(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, et al. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander L, Desrocher M. Neuroimaging studies of obsessive-compulsive disorder in adults and children. Clin Psych Rev. 2006;26:32–49. doi: 10.1016/j.cpr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Frost RO, Steketee G. Perfectionism in obsessive-compulsive disorder patients. Behav Res Ther. 1997;35:291–6. doi: 10.1016/s0005-7967(96)00108-8. [DOI] [PubMed] [Google Scholar]
- Geller DA, Wieland N, Carey K, Vivas F, Petty CR, Johnson J, et al. Perinatal factors affecting expression of obsessive-compulsive disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2008;18:373–9. doi: 10.1089/cap.2007.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry. 2000;157:281–3. doi: 10.1176/appi.ajp.157.2.281. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–56. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The Yale-Brown Obsessive-compulsive Scale. II. Validity. Arch Gen Psychiatry. 1989a;46:1012–6. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive-compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989b;46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Meyer C, Hapke U, Rumpf HJ, Freyberger HJ, Dilling H, et al. Prevalence, quality of life and psychosocial function in obsessive-compulsive disorder and subclinical obsessive-compulsive disorder in northern Germany. Eur Arch Psychiatry Clin Neurosci. 2000;250:262–8. doi: 10.1007/s004060070017. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–7. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Gu BM, Park JY, Kang DH, Lee SJ, Yoo SY, Jo HJ, et al. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 2008;131:155–64. doi: 10.1093/brain/awm277. [DOI] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Lumey LH, Slagboom PE. The epigenome: archive of the prenatal environment. Epigenetics. 2009;4:526–31. doi: 10.4161/epi.4.8.10265. [DOI] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158:1568–78. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Integration of target and body-part information in the premotor cortex when planning action. Nature. 2000;408:466–70. doi: 10.1038/35044075. [DOI] [PubMed] [Google Scholar]
- Insel TR, Winslow JT. Neurobiology of obsessive-compulsive disorder. Psychiatr Clin North Am. 1992;15:813–24. [PubMed] [Google Scholar]
- Jonnal AH, Gardner CO, Prescott CA, Kendler KS. Obsessive and compulsive symptoms in a general population sample of female twins. Am J Med Genet. 2000;96:791–6. doi: 10.1002/1096-8628(20001204)96:6<791::aid-ajmg19>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Shioiri T, Kimura T, Ohkubo M, Nakada T, Someya T. Parietal white matter abnormalities in obsessive-compulsive disorder: a magnetic resonance spectroscopy study at 3-Tesla. Acta Psychiatr Scand. 2006;114:101–8. doi: 10.1111/j.1600-0447.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–8. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol. 2004;65:185–236. doi: 10.1016/j.biopsycho.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Kim JJ, Lee DW, Lee JS, Lee DS, Kim MS, et al. Neural correlates of clinical symptoms and cognitive dysfunctions in obsessive-compulsive disorder. Psychiatry Res. 2003;122:37–47. doi: 10.1016/s0925-4927(02)00104-x. [DOI] [PubMed] [Google Scholar]
- Laplane D, Levasseur M, Pillon B, Dubois B, Baulac M, Mazoyer B, et al. Obsessive-compulsive and other behavioural changes with bilateral basal ganglia lesions. A neuropsychological, magnetic resonance imaging and positron tomography study. Brain. 1989;112 (Pt 3):699–725. doi: 10.1093/brain/112.3.699. [DOI] [PubMed] [Google Scholar]
- Lazeron RH, Rombouts SA, Machielsen WC, Scheltens P, Witter MP, Uylings HB, et al. Visualizing brain activation during planning: the tower of London test adapted for functional MR imaging. AJNR Am J Neuroradiol. 2000;21:1407–14. [PMC free article] [PubMed] [Google Scholar]
- Leichnetz GR. Connections of the medial posterior parietal cortex (area 7 m) in the monkey. Anat Rec. 2001;263:215–36. doi: 10.1002/ar.1082. [DOI] [PubMed] [Google Scholar]
- Lin H, Katsovich L, Ghebremichael M, Findley DB, Grantz H, Lombroso PJ, et al. Psychosocial stress predicts future symptom severities in children and adolescents with Tourette syndrome and/or obsessive-compulsive disorder. J Child Psychol Psychiatry. 2007;48:157–66. doi: 10.1111/j.1469-7610.2006.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey JV, Costa DC, Blanes T, Busatto GF, Pilowsky LS, Takei N, et al. Regional cerebral blood flow in obsessive-compulsive disordered patients at rest. Differential correlates with obsessive-compulsive and anxious-avoidant dimensions. Br J Psychiatry. 1995;167:629–34. doi: 10.1192/bjp.167.5.629. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H. The anterior cingulate and error avoidance. J Neurosci. 2006;26:4769–73. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Mars RB, Bestmann S, Rothwell JC, Haggard P. Effects of motor preparation and spatial attention on corticospinal excitability in a delayed-response paradigm. Exp Brain Res. 2007;182:125–9. doi: 10.1007/s00221-007-1055-4. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, van den Heuvel OA. Common and distinct neural correlates of obsessive-compulsive and related disorders. Psychiatr Clin North Am. 2006;29:391–410. doi: 10.1016/j.psc.2006.02.006. viii. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–76. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen CH, Del Campo N, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130(Pt12):3223–36. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–49. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestadt G, Samuels J, Riddle M, Bienvenu OJ, III, Liang KY, LaBuda M, et al. A family study of obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57:358–63. doi: 10.1001/archpsyc.57.4.358. [DOI] [PubMed] [Google Scholar]
- Newman SD, Carpenter PA, Varma S, Just MA. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia. 2003;41:1668–82. doi: 10.1016/s0028-3932(03)00091-5. [DOI] [PubMed] [Google Scholar]
- Nicolini H, Arnold P, Nestadt G, Lanzagorta N, Kennedy JL. Overview of genetics and obsessive-compulsive disorder. Psychiatry Res. 2009;170:7–14. doi: 10.1016/j.psychres.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–16. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchon JM, Deus J, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:720–30. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- Purcell R, Maruff P, Kyrios M, Pantelis C. Cognitive deficits in obsessive-compulsive disorder on tests of frontal-striatal function. Biol Psychiatry. 1998a;43:348–57. doi: 10.1016/s0006-3223(97)00201-1. [DOI] [PubMed] [Google Scholar]
- Purcell R, Maruff P, Kyrios M, Pantelis C. Neuropsychological deficits in obsessive-compulsive disorder: a comparison with unipolar depression, panic disorder, and normal controls. Arch Gen Psychiatry. 1998b;55:415–23. doi: 10.1001/archpsyc.55.5.415. [DOI] [PubMed] [Google Scholar]
- Rachman S, de Silva P. Abnormal and normal obsessions. Behav Res Ther. 1978;16:233–48. doi: 10.1016/0005-7967(78)90022-0. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–94. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Rao NP, Reddy YC, Kumar KJ, Kandavel T, Chandrashekar CR. Are neuropsychological deficits trait markers in OCD? Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1574–9. doi: 10.1016/j.pnpbp.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Wedig MM, Wright CI, Martis B, McMullin KG, Shin LM, et al. Functional magnetic resonance imaging study of regional brain activation during implicit sequence learning in obsessive-compulsive disorder. Biol Psychiatry. 2007;61:330–6. doi: 10.1016/j.biopsych.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Reynolds CA, Turner A, Gatz M, Pedersen NL. Comparative rating measures of health and environmental exposures: how well do twins agree? Twin Res Hum Genet. 2005;8:113–9. doi: 10.1375/1832427053738791. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–40. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rotge JY, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M, et al. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol Psychiatry. 2009;65:75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Owen AM, Johnsrude IS, Passingham RE. Imaging the mental components of a planning task. Neuropsychologia. 2001;39:315–27. doi: 10.1016/s0028-3932(00)00109-3. [DOI] [PubMed] [Google Scholar]
- Sanavio E. Obsessions and compulsions: the Padua Inventory. Behav Res Ther. 1988;26:169–77. doi: 10.1016/0005-7967(88)90116-7. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Evans DW, Wolff M. Neuropsychological models of childhood obsessive-compulsive disorder. Child Adolesc Psychiatr Clin N Am. 1999;8:513–31. viii. [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 (Suppl 20):22–33. [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lusehe RE. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Spielberger CD, Jacobs GA, Russell S, Crane RS, Butcher J, Spielberger CD, editors. Assessment of anger: The State Trait Anger Scale. Hillsdale NJ: Lawrence Erlbaum Associates; 1983. [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Malhotra AK, Robinson DG, Bilder RM, et al. White matter abnormalities in obsessive-compulsive disorder: a diffusion tensor imaging study. Arch Gen Psychiatry. 2005;62:782–90. doi: 10.1001/archpsyc.62.7.782. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, MacMillan S, McMeniman M, Chen S, Baribault K, Lim KO, et al. Brain structural abnormalities in psychotropic drug-naive pediatric patients with obsessive-compulsive disorder. Am J Psychiatry. 2004;161:1049–56. doi: 10.1176/appi.ajp.161.6.1049. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci. 2003;14:347–53. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- Valente AA, Jr, Miguel EC, Castro CC, Amaro E, Jr, Duran FL, Buchpiguel CA, et al. Regional gray matter abnormalities in obsessive-compulsive disorder: a voxel-based morphometry study. Biol Psychiatry. 2005;58:479–87. doi: 10.1016/j.biopsych.2005.04.021. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Groenewegen HJ, Barkhof F, Lazeron RH, van Dyck R, Veltman DJ. Frontostriatal system in planning complexity: a parametric functional magnetic resonance version of Tower of London task. Neuroimage. 2003;18:367–74. doi: 10.1016/s1053-8119(02)00010-1. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Remijnse PL, Mataix-Cols D, Vrenken H, Groenewegen HJ, Uylings HB, et al. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132:853–68. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJ, van Hartskamp J, et al. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry. 2005;62:301–9. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- van Grootheest DS, Cath DC, Beekman AT, Boomsma DI. Twin studies on obsessive-compulsive disorder: a review. Twin Res Hum Genet. 2005;8:450–8. doi: 10.1375/183242705774310060. [DOI] [PubMed] [Google Scholar]
- van Grootheest DS, Cath DC, Beekman AT, Boomsma DI. Genetic and environmental influences on obsessive-compulsive symptoms in adults: a population-based twin-family study. Psychol Med. 2007;37:1635–44. doi: 10.1017/S0033291707000980. [DOI] [PubMed] [Google Scholar]
- van Oppen P. Obsessions and compulsions: dimensional structure, reliability, convergent and divergent validity of the Padua Inventory. Behav Res Ther. 1992;30:631–7. doi: 10.1016/0005-7967(92)90008-5. [DOI] [PubMed] [Google Scholar]
- van Oppen P, Hoekstra RJ, Emmelkamp PM. The structure of obsessive-compulsive symptoms. Behav Res Ther. 1995;33:15–23. doi: 10.1016/0005-7967(94)e0010-g. [DOI] [PubMed] [Google Scholar]
- van‘t Ent D, van Beijsterveldt TC, Derks EM, Hudziak JJ, Veltman DJ, Todd RD, et al. Neuroimaging of response interference in twins concordant or discordant for inattention and hyperactivity symptoms. Neuroscience. 2009;164:16–29. doi: 10.1016/j.neuroscience.2009.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veale DM, Sahakian BJ, Owen AM, Marks IM. Specific cognitive deficits in tests sensitive to frontal lobe dysfunction in obsessive-compulsive disorder. Psychol Med. 1996;26:1261–9. doi: 10.1017/s0033291700035984. [DOI] [PubMed] [Google Scholar]
- Wilcox HC, Grados M, Samuels J, Riddle MA, Bienvenu OJ, III, Pinto A, et al. The association between parental bonding and obsessive-compulsive disorder in offspring at high familial risk. J Affect Disord. 2008;111:31–9. doi: 10.1016/j.jad.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Wolfensberger SP, Veltman DJ, Hoogendijk WJ, Boomsma DI, de Geus EJ. Amygdala responses to emotional faces in twins discordant or concordant for the risk for anxiety and depression. Neuroimage. 2008;41:544–52. doi: 10.1016/j.neuroimage.2008.01.053. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Striatal connections of the parietal association cortices in rhesus monkeys. J Comp Neurol. 1993;332:175–97. doi: 10.1002/cne.903320204. [DOI] [PubMed] [Google Scholar]