Abstract
The recruitment of myeloid cells has been consistently associated with the formation of new blood vessels during pathological angiogenesis. However, the participation of myeloid cells in bioengineered vascular networks remains unclear. Therefore, we tested whether host myeloid cells play a role in the formation of bioengineered vascular networks that occurs in vivo upon coimplantation of blood-derived endothelial progenitor cells and bone-marrow-derived mesenchymal progenitor cells, suspended as single cells in Matrigel, into immune-deficient mice. We observed an influx of spatially organized host CD11b+ myeloid cells into the Matrigel implant 1 to 3 days after implantation, which was shown to be cell mediated rather than a nonspecific response. Myeloid cells were significantly reduced once the implants were fully vascularized at days 6 and 7, suggesting an active role during steps that precede formation of functional anastomoses and perfused vessels. Importantly, depletion of circulating myeloid cells resulted in a significant reduction in microvessel density in the implants. In summary, the recruitment of myeloid cells occurs rapidly after coimplantation of endothelial and mesenchymal progenitor cells and is necessary for full vascularization in this model. This is the first demonstration of a role for recruited myeloid cells in the formation of bioengineered vascular networks.
Introduction
The participation of bone-marrow-derived mononuclear cells (MNCs) in pathological neovascularization has been well studied. For example, numerous clinical and experimental reports have shown that infiltrated accessory myeloid cells, including monocyte/macrophages, neutrophils, eosinophils, mast cells and dendritic cells, actively contribute to tumor progression by modulating angiogenesis.1–6 Less well-studied is the role of myeloid cells in nonneoplastic neovascularization; however, experimental hind limb ischemia models suggest that the initiation of angiogenesis is related to a neutrophil-mediated increase in matrix metalloproteinases (MMP)-2 and -9 activity.7 In other studies, subpopulations of myeloid cells were observed at the tips of nascent capillaries in neonatal murine retina8 and in experimental models of growth factor-induced angiogenesis and tissue regeneration.9–12 Taken together, these studies suggest that myeloid cells facilitate the comigration and the spatial arrangement of multiple cell types and assist progenitor cells during neovascularization in health and disease.
The pro-angiogenic features of subpopulations of peripheral blood MNCs have been recognized,13,14 and even though their participation during neovascularization has led to some confusion over the definition of endothelial progenitor cells (EPCs), there is now a better consensus on the distinction between the accessory role of myeloid cells and the lumen forming, structural role of true EPCs.15 Another example of the pro-angiogenic role is the apparent beneficial effect of autologous bone-marrow-derived MNCs administered to ischemic tissues,16,17 findings that have prompted clinical trials.18 Finally, myeloid cells have also been shown to influence neo-vessel formation by paracrine mechanisms when recruited to perivascular sites of neovascularization.19
We and others have proposed the combined use of EPCs and mesenchymal progenitor cells (MPCs) to engineer vascular networks in vivo.20–22 Here we show for the first time that a population of recruited CD11b+ myeloid cells constitutes an important cellular component of this vasculogenic process and that their recruitment should be considered a necessary step during the early events that take place after implantation of endothelial and mesenchymal progenitors.
Materials and Methods
In vivo vasculogenesis assay
EPC/MPC-driven vasculogenesis in vivo was evaluated using a previously described xenograft model.21,23 Briefly, EPCs and MPCs (40:60 ratio; 1.9 × 106 cells total) were resuspended in 200 μL of Matrigel and injected subcutaneously into 6-week-old male athymic nu/nu mice (n = 4 or n = 5, as indicated, for each experimental condition). (The immune-deficient nu/nu mice are lacking in B-cells.) The following implants served as controls: (a) Matrigel without cells, (b) Matrigel with EPCs alone, (c) Matrigel with MPCs alone, and (d) Matrigel with human MNCs (hMNCs; 3 × 106 in 200 μL of Matrigel). Additional controls were performed using mouse dermal endothelial cells and mouse bone-marrow-derived MPCs isolated from C57BL/6 mice24 (40:60 ratio; 1.9 × 106 cells total) injected into either (a) 6-week-old male athymic nu/nu mouse or (b) 6-week-old male immune-competent C57BL/6 mice.
Myeloid depletion experiment
Rat Ly-6G (Gr-1) (herein referred to as Gr-1) or control (rat immunoglobulin G [IgG]2b) antibodies at 200 mg/mouse were administered intraperitoneally as follows: 2 days before Matrigel-EPC/MPC implantation, the same day of Matrigel implantation, and 3 days postimplantation. Each group was performed with five mice. Myeloid cell depletion was confirmed on blood samples by flow cytometry (FC) using Gr-1 antibody. Implants from each group were harvested at days 2 and 7 and analyzed by histology. Blood was withdrawn from the retro orbital sinus for complete blood count (CBC) with differential analyses (Department of Laboratory Medicine at Children's Hospital Boston).
Analysis
The presence of myeloid cells was evaluated by both FC and immunohistochemistry using antibodies against CD11b and Gr-1. EPCs were observed using a human-specific antibody against CD31 (hCD31). Evaluation of explant microvessel density (MVD) was carried out as described.23,25
Statistical analysis
The data were expressed as mean ± standard error of mean. Unless otherwise stated, all p-values reported were generated by two-tailed Student's unpaired t-tests. Additionally, multiple comparisons were performed where appropriate by one-way analysis of variance followed by Tukey's multiple comparison tests. p-Values <0.05 were considered statistically significant.
An Expanded Methods section (Supplemental Material, available online at www.liebertonline.com/ten) describes cell isolation and expansion, cell retrieval from Matrigel explants, FC, histology and immunohistochemistry, retroviral transduction, and MVD.
Results
Progressive vascularization of implants
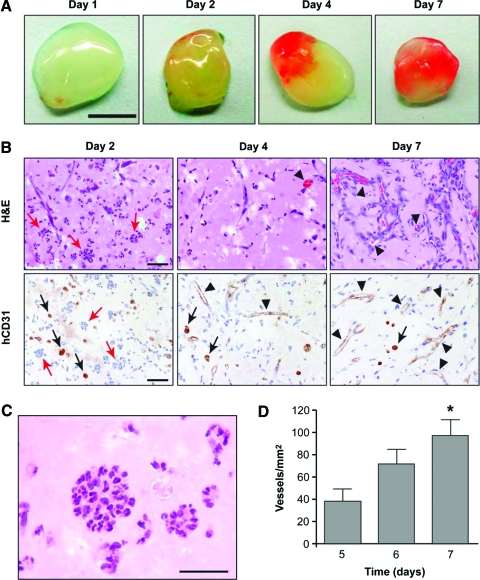
We previously showed that coimplantation of EPCs and MPCs in Matrigel into immunodeficient mice leads to extensive networks of human blood vessels with functional anastomoses to the host circulatory system.21 In particular, vascular networks contained erythrocytes by day 7 and implants remained vascularized for up to 4 weeks. We have now studied time points <7 days to analyze cellular events before the onset of connections between human and murine vessels. Human EPCs and MPCs were implanted, and explants examined at daily intervals (n = 4, each day) (Fig. 1). Gross examination at the time of harvest revealed partial vascularization of some implants (judged by the red color) at time points earlier than day 7 (Fig. 1A), suggesting that the formation of functional anastomoses occurs progressively during the first week. Histological analyses supported progressive appearance of functional blood vessels as shown by the presence of erythrocyte-filled vessels in hematoxylin and eosin (H&E)–stained sections and immunohistochemical staining of the lumenal structures with hCD31 antibody (Fig. 1B). Some implants showed partial vascularization at day 3 to 5. Specifically, one of four implants at day 3, two of four at day 4, and three of four at day 5 were vascularized. Finally, all implants were perfused at days 6 and 7. Quantification of the average MVD in implants that were vascularized (Fig. 1D) revealed a significant increase between day 5 (37 ± 11 vessels/mm2, average ± standard deviation) and day 7 (97 ± 14 vessels/mm2). These results suggested that from day 5 to 7, vascularization was still in progress.
FIG. 1.
Time course of bioengineered vascularization. EPCs/MPCs were implanted in nu/nu mice by subcutaneous injection and harvested at daily intervals (n = 4). (A) Macroscopic view of explanted Matrigel (scale bar, 500 μm). (B) H&E and hCD31 immunohistochemical staining of explants harvested at days 2, 4, and 7. The presence of blood vessels containing erythrocytes was evident from day 4 (black arrowheads). Both microvessels and individual EPCs (black arrows) stained positive for hCD31. In addition, day 2 explants contained circular clusters of polymorphonuclear cells (red arrows). Scale bar, 50 μm. (C) High-power image of implant removed at day 2 to show morphology of circular clusters (scale bar, 30 μm). (D) Microvessel density quantification was performed by counting erythrocyte-filled vessels in all implants. Each bar represents the mean ± standard deviation (vessels/mm2) obtained from only vascularized implants. *p < 0.05 compared to implants harvested at day 5. EPCs, endothelial progenitor cells; MPCs, mesenchymal progenitor cells; H&E, hematoxylin and eosin; hCD31, human-specific antibody against CD31. Color images available online at www.liebertonline.com/ten.
Early presence of infiltrated host myeloid cells
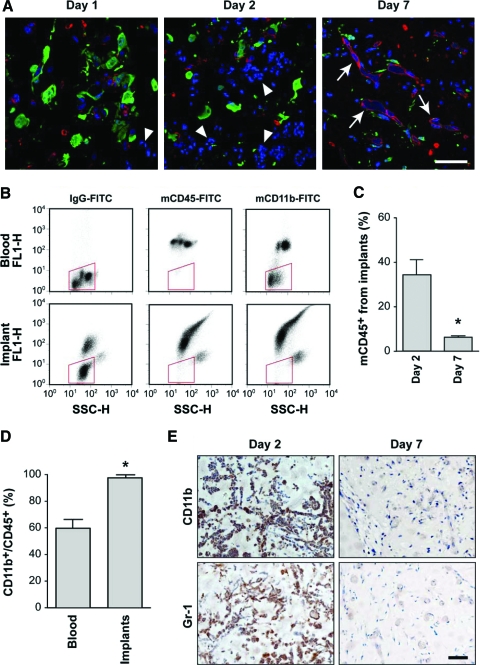
Histological examination of the implants at daily intervals also revealed what appeared to be polymorphonuclear leukocytes at day 2 (red arrows in Fig. 1B). These cells were spatially organized into bundles of cells with some arranged into cellular cords and/or circular clusters. This observation suggested an involvement of host-derived leukocytes early in the vasculogenic process. To confirm the contribution of murine cells at different stages of vascularization, we implanted green fluorescent protein (GFP)–transfected MPCs together with unlabeled EPCs and examined the explants at daily intervals (n = 4, each day) (Fig. 2A). This combination of GFP-MPCs and EPCs allowed unequivocal tracking of both cell types by confocal microscopy. MPCs were identified as GFP positive (green staining in Fig. 2A), whereas EPCs were observed using a hCD31 followed by a Texas Red–conjugated secondary antibody (red staining in Fig. 2A). This analysis allowed for the identification of unstained nucleated murine cells that had infiltrated the implants (i.e., 4′,6-diamidino-2-phenylindole (DAPI+), GFP−, and hCD31− cells in Fig. 2A). Murine cells infiltrated as early as day 1 (Fig. 2A, left panel), with increased abundance at day 2 (middle panel), and a progressive decrease thereafter. Of note, at day 7, few murine cells remained, and as expected based on our previous work, EPCs and MPCs were found at lumenal and perivascular locations, respectively (Fig. 2A, right panel).
FIG. 2.
Early infiltration of host myeloid cells. Matrigel implants containing EPCs and either GFP-MPCs (A) or unlabeled MPCs (B–E). (A) Confocal microscopy of immunostained sections from implants harvested at different time points (green, red, and blue staining correspond to GFP, hCD31, and DAPI, respectively). Host cells (DAPI+, GFP−, hCD31−; white arrowheads) were first seen at day 1 (left panel), were abundant at day 2 (middle panel), and declined thereafter. Perfused human vessels (white arrows) were observed at day 7 with EPCs (red) and MPCs (green) found at lumenal and perivascular locations, respectively (right panel). Scale bar, 50 μm. (B, C) Flow cytometry analysis of cells obtained from both implants and peripheral blood were carried out at days 2 and 7 (n = 4 at each time point) with antibodies against mCD45 and mCD11b. Representative dot-plot diagrams from day 2 are depicted. Red box shows region of nonstained hematopoietic cells. (C) Quantitative analyses showed that the number of infiltrated mCD45+ cells at day 2 was significantly higher than at day 7 (*p < 0.05, n = 4). (D) Quantitative analyses at day 2 revealed that the ratio CD11b+/CD45+ cells in the implants was significantly higher than in peripheral blood (*p < 0.05, n = 4). (E) The presence and absence of myeloid cells at days 2 and 7, respectively, were confirmed using antibodies against CD11b and Gr-1. Images at each time point are representative of implants harvested from four different mice (scale bars, 50 μm). GFP, green fluorescent protein; mCD45, murine CD45; IgG, immunoglobulin G; FITC, fluorescein isothiocyanate. DAPI, 4′,6-diamidino-2-phenylindole; FL1-H, fluorescence channel-1 for FITC; SSC-H, side light scatter. Color images available online at www.liebertonline.com/ten.
To confirm the presence of infiltrated murine leukocytes, we carried out flow cytometric analysis of the cells retrieved from the implants by enzymatic digestion at days 2 and 7. Using a murine-specific antibody against the pan-hematopoietic marker CD45 (see Supplemental Fig. S1, available online at www.liebertonline.com/ten for antibody specificity), we found that implants contained 34.2% murine CD45+ (mCD45+) cells at day 2. This number dropped to 6.1% by day 7 (Fig. 2B, C), confirming the histological observation of more murine cells present at the early time points. At the same time, the percentage of hCD31+ cells (EPCs) increased from 44.7% (day 2) to 54.2% (day 7), whereas the percentage of hCD90+ cells (MPCs) increased from 18.1% at day 2 to 36.7% at day 7 (Supplemental Fig. S2, available online at www.liebertonline.com/ten). These increases in the percentages of EPCs and MPCs at day 7 are in part explained by the less prominent presence of murine hematopoietic cells.
To specifically examine the presence of myeloid cells we used an antibody against mCD11b. Myeloid lineage cells, including monocytes, polymorphonucleated granulocytes (i.e., neutrophils, eosinophils, and basophils), and mast cells,1 express CD11b in addition to CD45. In contrast, lymphocytes (the other major nucleated cell population) are negative for CD11b, with the exception of natural killer cells. Since all CD11b+ myeloid cells are also CD45+, we quantified the CD11b+/CD45+ cell ratio in the implants and compared it to the ratio found in the peripheral blood of the implant-bearing mice (Fig. 2B, D) at day 2. The peripheral blood ratio was 58.2%. In contrast, the implant ratio was 98.1%, indicating that myeloid cells preferentially migrated into the implants during the early days of the vasculogenic process. Immunostaining with an antibody against CD11b (see Supplemental Fig. S3, available online at www.liebertonline.com/ten for controls) supported the observation that infiltrated CD11b+ cells were abundant at day 2 but barely detectable at day 7 (Fig. 2E). In fact, the low detection of CD11b+ cells throughout the tissue at day 7 suggested that the number of CD11b+ found by FC (Fig. 2C) likely corresponded to myeloid cells present in the peripheral blood in the implant vessels. Similar results were found using an antibody against Gr-1, a marker shared by granulocytes and some monocytes.1 Infiltrated Gr-1+ cells were abundant at day 2 but less so at day 7 once the implants became fully vascularized (Fig. 2E).
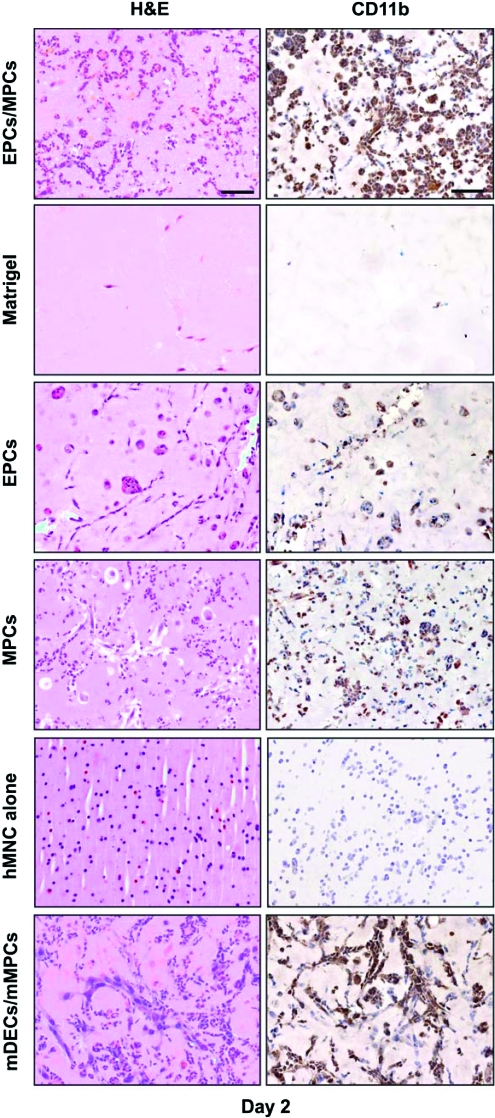
To elucidate the cellular components of the implant responsible for the early infiltration of murine myeloid cells, we compared the following (n = 4 for each group): (a) our standard EPCs/MPCs in Matrigel, (b) Matrigel without cells, (c) EPCs alone in Matrigel, (d) MPCs alone in Matrigel, and (e) hMNC alone in Matrigel. Histological and anti-CD11b staining of the explants at day 2 revealed that both EPCs and MPCs alone were able to instigate the recruitment of myeloid cells (Fig. 3). In contrast, implants with Matrigel alone showed minimal cell infiltration, suggesting that Matrigel itself was inert and the presence of infiltrated myeloid cells was cell mediated. Medium components such as fetal bovine serum in the implant did not cause myeloid cell recruitment, since Matrigel implants spiked with culture medium also lacked murine cell infiltration (data not shown). To elucidate whether the recruitment of myeloid cells was due to the fact that our model uses human cells, hMNCs in Matrigel were tested, but showed no signs of CD11b+ murine cell infiltration by immunostaining (Fig. 3) or by FC (not shown). Thus, the lack of mCD11b+ cells indicated specificity; human cells per se were not sufficient to instigate the recruitment of host myeloid cells into Matrigel. In another test, we substituted human EPCs and MPCs with murine dermal endothelial cells and murine MPCs, both cell types isolated from C57BL/6 mice,24 and implanted into either nu/nu mice (Fig. 3 bottom panels) or into immune-competent C57BL/6 mice (n = 4 each group; see Supplemental Fig. S4, available online at www.liebertonline.com/ten). At day 2, implants from both groups of mice presented a large number of infiltrated myeloid cells as observed by H&E and CD11b staining, again indicating that recruitment was not due to a reaction against human cells, or a consequence of using immune-deficient mice as an animal model.
FIG. 3.
Cell-mediated infiltration of CD11b+ cells. Matrigel implants containing either EPCs and MPCs, no cells, EPCs alone, MPCs alone, human MNCs alone, or mDECs and mMPCs. Implants were harvested at day 2 (n = 4) and stained by H&E (left column) and CD11b immunohistochemistry (right column). Images are representative of explants harvested from four different mice (scale bars, 50 μm). MNCs, mononuclear cells; mDECs, mouse dermal endothelial cells; mMPCs, mouse bone-marrow-derived mesenchymal progenitor cells. Color images available online at www.liebertonline.com/ten.
MMP-9 and -2 expression by infiltrated murine myeloid cells
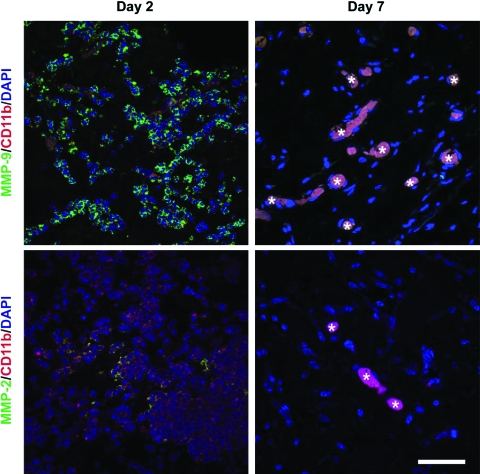
The infiltration of myeloid cells at sites of neovascularization has been associated with expression of MMPs. To investigate whether the infiltrated myeloid cells expressed MMP-9 and -2, we carried out double-label immunofluorescence (CD11b/MMP-9 and CD11b/MMP-2) on sections taken from explants at days 2 and 7. As depicted in Figure 4, CD11b+ cells expressing MMP-9 were very abundant at day 2, but negligible at day 7 (the fluorescent signal detected within the lumen of multiple blood vessels at day 7 was an artifact caused by erythrocyte autofluorescence). Expression of MMP-2 by some infiltrated CD11b+ cells was also evident at day 2, although MMP-9-expressing cells appeared to predominate. Expression of both CD11b and MMP-2 at day 7 was absent, suggesting the absence of infiltrated myeloid cells at later stages of the vasculogenic process. Control staining for MMP-9 and -2 expression was carried out with sections from mouse liver and spleen (Supplemental Fig. S5, available online at www.liebertonline.com/ten).
FIG. 4.
MMP-9 and -2 expression by infiltrated myeloid cells. Matrigel implants containing EPCs and MPCs were harvested at day 2 (left column) and day 7 (right column). Double immunofluorescent staining was carried out using antibodies against MMP-9 (green) and CD11b (red) or MMP-2 (green) and CD11b (red) (top and bottom panels, respectively). Confocal microscopy revealed abundant CD11b+ cells expressing MMP-9 at day 2, and negligible expression at day 7. Expression of MMP-2 by some infiltrated CD11b+ cells was also evident at day 2, but absent at day 7. The fluorescent signal detected within lumens at day 7 was due to erythrocyte autofluorescence (asterisks). Images at each time point are representative of implants harvested from four different mice (scale bars, 50 μm). MMP, matrix metalloproteinase. Color images available online at www.liebertonline.com/ten.
Depletion of circulating myeloid cells impairs vasculogenesis
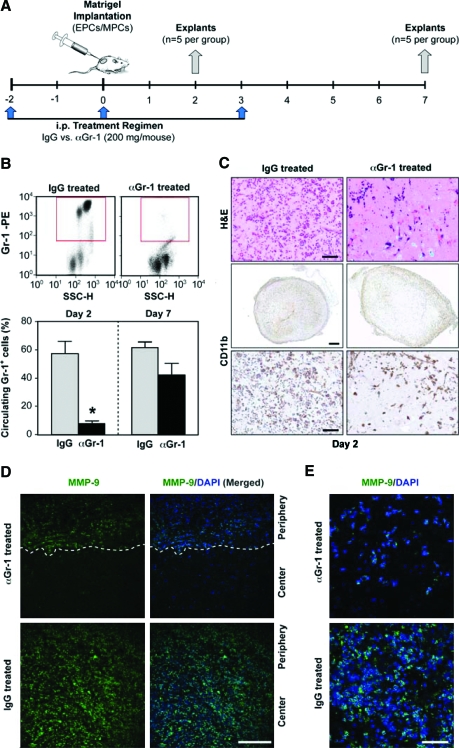
To elucidate whether the infiltrated myeloid cells are necessary during the vascularization process, we implemented a strategy that has proved effective in the depletion of circulating Gr-1+ cells from the peripheral blood of mice.26 Two groups of mice (n = 5 each) were given, by intraperitoneal injection, 200 mg/mouse of either anti-Gr-1 or IgG control antibodies 2 days before, the day of, and 3 days after EPCs/MPCs implantation (Fig. 5A). The successful depletion of Gr-1+ cells from the mouse peripheral blood is shown in Figure 5B by FC analysis: IgG-treated mice presented 57.3% Gr-1+ cells at day 2, whereas the αGr-1-treated group had only 7.9%. Myeloid depletion was also evident from the CBC with differential analyses of the blood from each group of mice. The CBC showed a significantly reduced level of both neutrophils and monocytes in the αGr-1-treated group (see full analysis in Supplemental Fig. S6, available online at www.liebertonline.com/ten). Depletion of circulating myeloid cells affected the number of infiltrated CD11b+ cells seen in the implants. As expected, both H&E- and CD11b-stained sections of explants harvested at day 2 from the IgG-treated mice showed abundant and uniform presence of infiltrated myeloid cells (Fig. 5C). In contrast, the number of infiltrated CD11b cells in the αGr-1-treated group was significantly reduced and their presence was limited to the periphery of the implants, suggesting that remaining circulating myeloid cells were insufficient to infiltrate the implant. This pattern of impaired myeloid cell recruitment correlated with expression of MMP-9 as observed by immunofluorescent staining: IgG-treated mice showed uniform expression of MMP-9, whereas implants from αGr-1-treated mice showed MMP-9 limited to the periphery (Fig. 5D, E).
FIG. 5.
Systemic depletion of circulating myeloid cells. (A) Two groups of mice received 200 mg/mouse of either anti Gr-1 (αGr-1 treated) or control IgG (IgG treated) antibodies by intraperitoneal injection at three time points as shown. Matrigel implants containing EPCs and MPCs were injected subcutaneously and harvested at days 2 and 7 (n = 5). (B) Flow cytometry analyses of peripheral blood MNCs from implant-bearing mice were carried out with anti-Gr-1 to confirm myeloid depletion. Representative dot-plot diagrams from day 2 analyses are depicted. Red boxes indicate region of Gr-1-positive cells. Quantitative cytometric analyses from both groups of mice (n = 5) were compared at days 2 and 7 (*p < 0.05; n = 5). (C) H&E and CD11b immunostaining carried out at day 2 showed a reduced number of infiltrated CD11b+ cells in the αGr-1-treated group compared to IgG-treated mice (scale bars, 50 μm). (D, E) Immunostaining carried out at day 2 showed that infiltrated MMP-9+ cells were reduced and preferentially located in the periphery of the implants in the αGr-1-treated group; in contrast, MMP-9+ cells were abundant and uniformly distributed in implants from IgG-treated mice (E: scale bars, 200 μm; D: scale bar, 50 μm). D: White dashed line indicates demarcation between the center and periphery of the Matrigel implant. All images are representative of implants harvested from five different mice. Color images available online at www.liebertonline.com/ten.
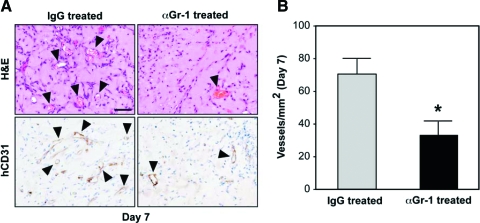
We then evaluated the effect of myeloid cell depletion on the formation of blood vessels at day 7. As expected, systemic treatment with IgG had no detrimental effect on the formation of vascular networks. A large number of human-specific lumenal structures containing erythrocytes were observed in H&E- and hCD31-stained sections; however, explants taken from the αGr-1-treated mice showed a reduction in the number of blood vessels (Fig. 6). MVD quantification revealed a statistically significant (p = 0.01) reduction of 52.8% in the number of blood vessels found in the αGr-1-treated mice (33 + 8 vessels/mm2) as compared to the IgG-treated mice (71 + 9 vessels/mm2), indicating that depletion of circulating myeloid cells impaired the process of vasculogenesis.
FIG. 6.
Effect of myeloid cell depletion on posttransplantation vascularization. (A) Implants harvested at day 7 from both αGr-1- and IgG-treated mice. H&E and hCD31 immunohistochemistry revealed a reduced number of blood vessels (arrowheads) in the αGr-1-treated group. Images are representative of implants harvested from five different mice (scale bar, 50 μm). (B) Microvessel density quantification of αGr-1-treated mice as compared to the IgG-treated animals. Each bar represents the mean ± standard deviation (vessels/mm2) (*p < 0.05; n = 5). Color images available online at www.liebertonline.com/ten.
Discussion
Since EPCs were first identified in peripheral blood,27 there has been great motivation to apply these cells to new therapies for vascularization. Recently, we and other authors have proposed the combined use of EPCs and perivascular cells to engineer vascular networks in vivo,20–22 but none of these studies investigated the participation of host cells as the nascent vessels form.
Using our vasculogenic model of subcutaneous coimplantation of human EPCs and MPCs into immune-deficient mice, we examined the contribution of host cells from the time of subcutaneous injection to the time at which blood vessels were fully formed. As expected, the formation of functional anastomoses was found to be progressive: perfused blood vessels were seen in the implants as early as day 3 (partial) with full vascularization achieved by day 7.21 These results were similar to those reported by other authors using different perivascular cells20,22; however, it was the examination of early time points, before the appearance of perfused vessels, that revealed an abundant and unexpected presence of infiltrated murine myeloid (CD45+, CD11b+, Gr-1+) cells. The myeloid cells were spatially organized in bundles and arranged into cellular cords and/or circular clusters. Two important aspects of our initial observations are the following: (1) the proportion of CD11b+/CD45+ was significantly higher in the implants (98%) than in peripheral blood (57%), suggesting recruitment of myeloid cells rather than a nonspecific diffusion of total white blood cells from potentially leaky vasculature, and (2) the presence of infiltrated myeloid cells was temporary; their disappearance coincided with the onset of anastomoses formation.
Even though myeloid cells have frequently been observed at sites of neovascularization, their presence often provokes debate about possible nonspecific inflammatory reactions. In this regard, we have demonstrated that the recruitment of host hematopoietic cells was specific and cell mediated based on the following observations: (1) both EPCs and MPCs, independently and in combination, were able to recruit mCD11b+ cells to the Matrigel implant; (2) there was no such recruitment when Matrigel alone (with and without culture medium) was injected or when hMNCs were implanted; (3) murine endothelial and mesenchymal cells produced recruitment of myeloid cells similar to the human counterparts, ruling out the possibility of an inflammatory reaction driven by species differences. Past studies have shown that the role of recruited myeloid cells at sites of neovascularization is often multifaceted, and their presence has been associated with the production of vast array of pro-angiogenic cytokines and vascular-modulating enzymes, including MMPs.1,3,28,29 In this context, we found that the large majority of infiltrated CD11b+ cells uniformly expressed MMP-9, and to a lesser extent MMP-2. The potential for MMP-mediated matrix remodeling may in part explain the necessity of early host cellular support. Collectively, these observations suggest a purposeful recruitment of host circulating CD11b+ cells by the implanted vasculogenic cells. Further work is needed to elucidate the molecular mechanism by which myeloid cells are recruited during the early steps of bioengineered vasculogenesis.
The definitive confirmation of active myeloid cell participation was found by carrying out depletion experiments. By significantly reducing the number of circulating myeloid cells available in the peripheral blood, implants recruited fewer CD11b+ cells. Importantly, this reduction in myeloid cells correlated with a significant decrease in the number of perfused blood vessels found at day 7 (53% reduction in implants from myeloid-depleted mice). This finding clearly indicated an active and necessary involvement of host myeloid cells since their absence led to impaired (or at least delayed) vasculogenesis. In previous work, we showed that optimal vasculogenesis occurs when EPCs are combined with an appropriate source and proportion of perivascular cells (e.g., MPCs), and that neither cell type alone can produce high MVDs in Matrigel.21 We have now found that both cell types are capable of host myeloid cell recruitment and this recruitment is necessary, but not sufficient to achieve rapid neovascularization at high density.
In summary, we demonstrate for the first time that a population of recruited CD11b+ myeloid cells plays an active role in the formation of bioengineered vascular networks using EPC and MPC. Myeloid cells were found to be increased at early time points, but their number was reduced when functional anastomoses between the newly formed lumens and the host circulation formed. Depletion of myeloid cells from peripheral blood before implantation resulted in a reduced number of blood vessels, indicating that the presence of myeloid cells is a necessary step during the early stages of vasculogenesis.
Supplementary Material
Acknowledgments
We would like to thank Dr. Dipak Panigrahy for advice with myeloid cell depletion experiments, Dr. Soo-Young Kang for assistance with retroviral transfections, Dr. Harry Kozakewich, Department of Pathology, Children's Hospital Boston, for initially helping us to identify the infiltrating cells, Jill Wylie-Sears for technical assistance, and Kristin Johnson for figure preparation. This research was supported by the Specialized Histopathology Services, Longwood Facility of the Dana-Farber/Harvard Cancer Center (P30 CA06516). This work was supported by the U.S. Army Medical Research and Materiel Command (W81XWH-05-1-0115).
Disclosure Statement
No competing financial interests exist.
References
- 1.Murdoch C. Muthana M. Coffelt S.B. Lewis C.E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill F. Charles K.A. Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Coussens L.M. Werb Z. Inflammation and cancer. Nature. 2002;420:860. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollard J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F. Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 6.De Palma M. Venneri M.A. Galli R. Sergi Sergi L. Politi L.S. Sampaolesi M. Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Muhs B. Gagne P. Plitas G. Shaw J. Shamamian P. Experimental hindlimb ischemia leads to neutrophil-mediated increases in gastrocnemius MMP-2 and-9 …. J Surg Res. 2004;117:249. doi: 10.1016/j.jss.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Naug H.L. Browning J. Gole G.A. Gobe G. Vitreal macrophages express vascular endothelial growth factor in oxygen-induced retinopathy. Clin Exp Ophthalmol. 2000;28:48. doi: 10.1046/j.1442-9071.2000.00226.x. [DOI] [PubMed] [Google Scholar]
- 9.Anghelina M. Krishnan P. Moldovan L. Moldovan N.I. Monocytes and macrophages form branched cell columns in Matrigel: implications for a role in neovascularization. Stem Cells Dev. 2004;13:665. doi: 10.1089/scd.2004.13.665. [DOI] [PubMed] [Google Scholar]
- 10.Tigges U. Hyer E.G. Scharf J. Stallcup W.B. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development. 2008;135:523. doi: 10.1242/dev.002071. [DOI] [PubMed] [Google Scholar]
- 11.Anghelina M. Krishnan P. Moldovan L. Moldovan N.I. Monocytes/macrophages cooperate with progenitor cells during neovascularization and tissue repair: conversion of cell columns into fibrovascular bundles. Am J Pathol. 2006;168:529. doi: 10.2353/ajpath.2006.050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmeisser A. Garlichs C.D. Zhang H. Eskafi S. Graffy C. Ludwig J. Strasser R.H. Daniel W.G. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;49:671. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 13.Gulati R. Jevremovic D. Peterson T.E. Chatterjee S. Shah V. Vile R.G. Simari R.D. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 14.Rehman J. Li J. Orschell C.M. March K.L. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 15.Yoder M.C. Mead L.E. Prater D. Krier T.R. Mroueh K.N. Li F. Krasich R. Temm C.J. Prchal J.T. Ingram D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalka C. Masuda H. Takahashi T. Kalka-Moll W.M. Silver M. Kearney M. Li T. Isner J.M. Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kocher A.A. Schuster M.D. Szabolcs M.J. Takuma S. Burkhoff D. Wang J. Homma S. Edwards N.M. Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 18.Schachinger V. Assmus B. Britten M.B. Honold J. Lehmann R. Teupe C. Abolmaali N.D. Vogl T.J. Hofmann W.K. Martin H. Dimmeler S. Zeiher A.M. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Grunewald M. Avraham I. Dor Y. Bachar-Lustig E. Itin A. Jung S. Chimenti S. Landsman L. Abramovitch R. Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 20.Traktuev D.O. Prater D.N. Merfeld-Clauss S. Sanjeevaiah A.R. Saadatzadeh M.R. Murphy M. Johnstone B.H. Ingram D.A. March K.L. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res. 2009;104:1410. doi: 10.1161/CIRCRESAHA.108.190926. [DOI] [PubMed] [Google Scholar]
- 21.Melero-Martin J. De Obaldia M.E. Kang S.Y. Khan Z.A. Yuan L. Oettgen P. Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Au P. Daheron L. Duda D.G. Cohen K.S. Tyrrell J.A. Lanning R.M. Fukumura D. Scadden D.T. Jain R.K. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111:1302. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melero-Martin J. Bischoff J. Chapter 13. An in vivo experimental model for postnatal vasculogenesis. Methods Enzymol. 2008;445:303. doi: 10.1016/S0076-6879(08)03013-9. [DOI] [PubMed] [Google Scholar]
- 24.Dudley A.C. Khan Z.A. Shih S.C. Kang S.Y. Zwaans B.M. Bischoff J. Klagsbrun M. Calcification of multipotent prostate tumor endothelium. Cancer Cell. 2008;14:201. doi: 10.1016/j.ccr.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melero-Martin J. Khan Z.A. Picard A. Wu X. Paruchuri S. Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 26.Kaipainen A. Kieran M. Huang S. Butterfield C. Bielenberg D. Mostoslavsky G. Mulligan R. Folkman J. Panigrahy D. PPARalpha deficiency in inflammatory cells suppresses tumor growth. PLoS ONE. 2007;2:e260. doi: 10.1371/journal.pone.0000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 28.Coussens L.M. Tinkle C.L. Hanahan D. Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coussens L.M. Raymond W.W. Bergers G. Laig-Webster M. Behrendtsen O. Werb Z. Caughey G.H. Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.