Abstract
Concern over the adequacy of biosafety training and incident-reporting practices within biological laboratories in the United States has risen in recent years due to the increase in research on infectious diseases and the concomitant rise in the number of biocontainment laboratories. Reports of laboratory-acquired infections and delays in reporting such incidents have also contributed to the concern. Consequently, biosafety training and incident-reporting practices are being given considerable attention by both the executive branch and Congress. We conducted a 51-question survey of biosafety professionals in June 2008 to capture information on methods used to train new laboratory workers within biosafety level 2 (BSL-2) laboratories, animal biosafety level 2 (ABSL-2) laboratories, biosafety level 3 (BSL-3) laboratories, and animal biosafety level 3 (ABSL-3) laboratories. The survey results suggest nearly all senior scientists, faculty, staff, and students working in these biocontainment laboratories are required to have biosafety training, and three-quarters of respondents indicated a biosafety or environmental health and safety professional provides explicit instructions on reporting incidents to each new lab worker. Only half of the respondents with BSL-2/ABSL-2 laboratories at their institution and 59% of respondents from institutions with BSL-3/ABSL-3 laboratories indicated custodial or maintenance workers are required to receive biosafety training at the BSL-2/ABSL-2 and BSL-3/ABSL-3 levels, respectively. Opportunities for targeted improvement such as providing training to non-traditional laboratory workers (e.g., custodians, maintenance workers) and posting laboratory incident-reporting protocols on institutional environmental health and safety websites may exist. Variations in biosafety training requirements, incident-reporting practices, and attitudes towards laboratory safety revealed through this survey of biosafety professionals also support the development of core competencies in biosafety practice that could lead to more uniform practices and robust safety cultures.
Background
In recent years, an increase in biodefense research, including research on emerging infectious diseases, has led to concomitant increases in the number of biocontainment laboratories in the United States and persons needed to staff them. In addition, the select agent legislation subsequent to the anthrax attacks provided more rigorous regulation of biosafety and biosecurity; the select agent regulations prior to this time had primarily regulated shipment and transfer of select agents (Dembek, 2007). Biosafety has become a highly visible issue nationally, especially for laboratories working on pathogens designated as select agents. Universities have received adverse publicity following reports of occupationally-acquired illnesses among laboratory workers and a significant delay in reporting those illnesses to public health authorities (Enserink, 2007; Kaiser, 2007; Lawler, 2005). Concerns that biosafety training is inadequate and reporting systems for laboratory incidents are insufficient have also increased. Both the executive and legislative branches of government have been considering actions to ensure biosafety and biosecurity in U.S. laboratories and have been considering how best to implement a reporting system for laboratory incidents (Executive Order, 2009; Graham et al., 2008; Rhodes, 2007; S.3127, 2008).
In an effort to assess laboratory incident-reporting practices, the Policy, Ethics and Law (PEL) Core of the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense (SERCEB) convened a workshop on January 30, 2008. As one of 11 regional centers of excellence devoted to emerging infectious disease and biodefense research, the SERCEB consortium conducts research with infectious agents in laboratories meeting BSL-2 or BSL-3 criteria (National Institutes of Health, 2009). Biosafety professionals and researchers from seven SERCEB institutions, including University of North Carolina at Chapel Hill, Duke University, Vanderbilt University, Emory University, University of Florida, University of Alabama at Birmingham, and Wake Forest University, came together to review their laboratory incident-reporting structures and to collectively assess ways to improve reporting—not only to improve operations but also to enhance the support and management of biosafety in their institutions and the communities in which they operate.
To facilitate discussion at the workshop, a working definition of “incident” as it relates to laboratories working with infectious agents was provided to participants. This definition was “any occurrence that has the potential to lead to unintended exposure of the agent to humans, animals or the environment.” Workshop participants noted that this broad definition encompasses a range of events which often vary among institutions. Each institution can also have different reporting channels depending on the type of incident. Workshop participants did not reach consensus on what incident-reporting practices are considered standard or sufficient. Each representative described different methods used for encouraging laboratory workers to report incidents. Upon examination of the institutions’ incident-reporting protocols, the majority of participants agreed that a strong link exists between incident-reporting and biosafety training. If incidents can be utilized as lessons learned, they can serve to strengthen biosafety training and overall laboratory safety. Participants at the workshop believed the need to improve biosafety cultures across BSL-2 and BSL-3 laboratories regardless of the nature of the research is paramount. Participants agreed more data needed to be collected from biosafety professionals and laboratory workers in order to identify mechanisms that might improve biosafety training and incident-reporting practices, both within life science laboratories and across institutions.
This 51-question anonymous survey of biosafety professionals in the United States was developed to obtain information about biosafety training and incident-reporting practices within BSL-2 and BSL-3 laboratories. The goal was to identify aspects of the biosafety training and incident-reporting feedback loop that could inform industry-wide best practices to strengthen overall biosafety practices and reporting procedures.
Methods
This survey addressed biosafety training practices, biosafety compliance and oversight practices, incident-reporting and incident-reporting protocols, information sharing, and attitudes towards biosafety at respondents’ institutions. The survey allowed respondents to use their own institutions’ criteria for what constitutes an incident. Data were collected on BSL-2/ABSL-2 and BSL-3/ABSL-3 laboratories. No data were collected on BSL-4/ABSL-4 level laboratories; biosafety officials convened separately to address the biosafety training issues for these maximum containment facilities (Le Duc et al., 2008).
The survey was approved by the Emory University Institutional Review Board and piloted in April 2008 at the Emory Biosafety Leadership Institute in Atlanta, Georgia. The national survey was administered through the web-based survey tool SurveyMonkey.com (Finley, 2009) and was distributed by the American Biological Safety Association (ABSA) to its membership of approximately 1,700 individuals on June 16, 2008. The survey remained open for one month, until July 16, 2008. Eligible respondents were individuals at least 18 years of age and practicing biosafety professionals employed within the United States. The total number of ABSA members who meet these criteria is unknown.
Data were analyzed using SAS version 9.2 statistical software (SAS Institute, Cary, NC). Statistical analyses included χ2 and Fisher’s Exact for cell values <5. A P-value < 0.05 was considered significant.
Results
Demographics
Approximately 19% (318/1700) of the total ABSA membership entered the online survey, and of these 318 individuals, 81% (258) met the survey’s eligibility requirements. Of these 258, 93% (240) provided responses to survey questions.
Fifty-two percent of the 240 respondents were from academia, 23% were employed by private companies or industry, 16% worked for U.S. Government agencies, and 10% worked for non-profit organizations. The majority of respondents (over 70%) categorized themselves as biosafety officers, environmental health and safety officers, or directors of environmental health and safety divisions. Approximately one-fourth provided other job titles, including occupational health and safety specialists, laboratory managers, and scientists.
Approximately 30% of respondents indicated they have at least one professional biosafety certification. Twelve percent of respondents indicated they are a Registered Biosafety Professional (RBP), and 11% indicated they are a Certified Biological Safety Professional (CBSP). Another 8% indicated they are both RBP- and CBSP-certified.
Regarding full-time equivalent (FTE) employees devoted to biosafety, most respondents indicated their institution has less than three FTEs devoted to biosafety (Table 1). Nearly all respondents (94%) from institutions with BSL-2/ABSL-2 or lower biocontainment laboratories reported having less than three FTEs devoted to biosafety. Of respondents from institutions with BSL-3/ABSL-3 laboratories, 64% indicated operating with less than three FTEs devoted to biosafety. For de-identification purposes, data were not collected on institution size or number of biocontainment laboratories; no correlations between number of FTEs devoted to biosafety and size of facilities were drawn.
Table 1.
Number of full-time equivalent (FTE) employees devoted to biosafety at institutions with and without BSL-3/ABSL-3 laboratories.
| Number of FTEs devoted to biosafety | Institutions with BSL-2/ABSL-2 laboratories; no BSL-3/ABSL-3 laboratories (n=69) |
Institutions with both BSL-2/ABSL-2 and BSL-3/ABSL-3 laboratories (n=151) |
|---|---|---|
| < 1 | 36 (52%) | 21 (14%) |
| 1 - <3 | 29 (42%) | 75 (50%) |
| ≥ 3 | 4 (6%) | 55 (36%) |
Biosafety Training Practices
Training Requirements by Biosafety Level
Of 136 respondents with BSL-3/ABSL-3 laboratories at their institutions, nearly all indicated that senior scientists, faculty, laboratory staff, and students working in BSL-3/ABSL-3 laboratories are required to take biosafety training (Table 2). Among 202 respondents with BSL-2/ABSL-2 laboratories at their institutions, 85% and 91% indicated biosafety training at the BSL-2/ABSL-2 level is required of senior laboratory scientists/faculty and laboratory staff/students, respectively. At both levels, a lower percentage of respondents indicated biosafety training requirements for other types of laboratory workers such as visiting scientists and custodial or maintenance workers.
Table 2.
Types of laboratory workers required to take biosafety training by biosafety level of laboratory.
| BSL-2/ABSL-2 laboratories (n=202) | BSL-3/ABSL-3 laboratories (n=136) | |
|---|---|---|
| Senior laboratory scientists and/or faculty | 85% | 99% |
| Laboratory staff and/or students | 91% | 99% |
| Visiting: scientists, students, and/or faculty | 72% | 86% |
| Custodial or maintenance workers | 50% | 59% |
| Biosafety training is not required at this level | 8% | 0% |
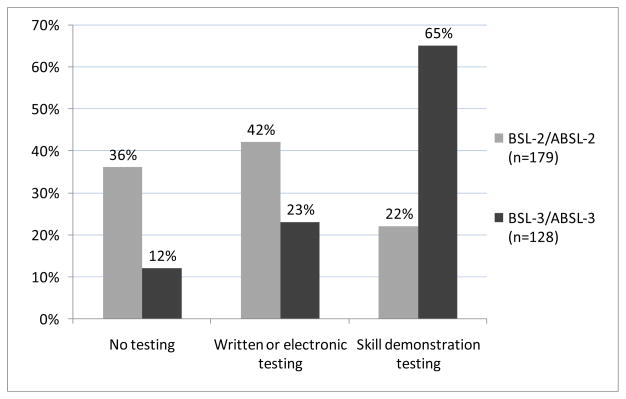
Among 179 respondents who indicated their institutions require biosafety training at the BSL-2/ABSL-2 level, 64% (115/179) indicated new BSL-2/ABSL-2 laboratory workers are tested on their knowledge of biosafety techniques after having received training (Figure 1). At the BSL-3/ABSL-3 level, 88% (112/128) of respondents indicated new workers at the BSL-3/ABSL-3 level are tested on knowledge of biosafety techniques.
Figure 1.
Methods used to test new laboratory workers’ knowledge of biosafety techniques after having received training.
Didactic vs. Hands-on Training: Biohazardous Spills and Needlesticks
Of 188 respondents with BSL-2 laboratories at their institutions, 23% (44/188) indicated drills or activities simulating biohazardous spills are included in the required biosafety training of new laboratory workers entering BSL-2/ABSL-2 laboratories (Table 3). Spill training involving drills and simulations for BSL-2 laboratory workers was used more frequently if the institution also had a BSL-3/ABSL-3 laboratory.
Table 3.
Hands-on training for biohazardous spills and needlestick incidents for new laboratory workers at the BSL-2/ABSL-2 according to the presence of a BSL-3/ABSL-3 laboratory.
| Institutions with BSL-2/ABSL-2 laboratories; no BSL-3/ABSL-3 laboratories | Institutions with both BSL-2/ABSL-2 and BSL-3/ABSL-3 laboratories | |
|---|---|---|
| Requires hands-on training for biohazardous spills for new workers at the BSL-2/ABSL-2 level | 18% (12/66) | 26% (32/122) |
| Provides training on responses to needlesticks via simulations or drills to new workers at BSL-2/ABSL-2 level | 1% (1/65) | 9% (12/119) |
Regarding hands-on training for response to needle-stick incidents, 7% (13/184) of respondents from institutions with BSL-2/ABSL-2 laboratories indicated hands-on training is used to teach new laboratory workers how to respond to needlestick incidents at the BSL-2/ABSL-2 level. The percentage utilizing hands-on training for needlesticks was higher among those respondents whose institutions also have BSL-3/ABSL-3 laboratories. For training new laboratory workers entering BSL-3/ABSL-3 laboratories, 64% (82/128) and 25% (30/121) of respondents indicated drills or simulations are included in the required biosafety training for biohazardous spills and needlestick incidents, respectively.
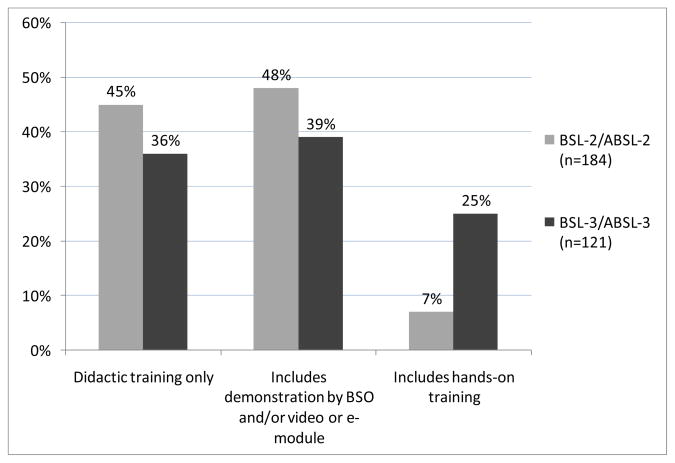
For workers in both BSL-2/ABSL-2 and BSL-3/ABSL-3 laboratories, didactic training is the most common method for needlestick training, with 45% (83/184) and 36% (44/121) of respondents indicating didactic training is the only method used to train new laboratory workers about needlestick incidents at the BSL-2/ABSL-2 and BSL-3/ABSL-3 levels, respectively (Figure 2). Respondents indicated that demonstrations by video or e-module are used more frequently at the BSL-2/ABSL-2 level, whereas demonstrations by the biosafety officer are used more often at the BSL-3/ABSL-3 level.
Figure 2.
Methods used to train new laboratory workers on needlestick incidents.
Annual Laboratory Inspections and Renewal of Biosafety Training Requirements
Ninety-three percent (119/128) of respondents with BSL-3/ABSL-3 laboratories at their institutions indicated that these laboratories receive an annual inspection by a biosafety officer. Of the 119 respondents, 87% (104/119) indicated their institutions have one or more FTE employees dedicated to biosafety, whereas 44% (4/9) of the respondents who indicated the level-3 laboratories at their institutions do not receive an annual inspection by a biosafety officer have one or more FTE employees dedicated to biosafety. This correlation between number of FTEs devoted to biosafety and a yearly BSL-3/ABSL-3 laboratory inspection is significant (P=0.0048).
At the BSL-2/ABSL-2 level, no correlation between number of FTEs devoted to biosafety and yearly laboratory inspection was found. Seventy-three percent (141/193) of respondents with BSL-2/ABSL-2 laboratories at their institutions indicated each BSL-2/ABSL-2 laboratory undergoes an annual inspection by a biosafety officer. Of these 141 respondents, 73% (103/141) have one or more FTE employees devoted to biosafety. Sixty-nine percent (36/52) of respondents who indicated their level-2 laboratories do not get inspected annually by a biosafety officer indicated their institutions also have one or more FTE employees devoted to biosafety.
Forty-seven percent of respondents (93/197) indicated their institutions require all laboratory workers in the life sciences to renew their biosafety training requirements annually. Thirty-one percent of respondents (62/197) indicated selected laboratory workers are required to renew their biosafety training annually, with the majority of these workers identified as those involved in blood-borne pathogen research, select agent research, or working within BSL-3/ABSL-3 laboratories. Twenty-one percent of respondents (42/197) indicated annual renewal of biosafety training is not a requirement at their institutions.
Of the 93 respondents who indicated that their institutions require all laboratory workers to renew biosafety training annually, 87% (81) indicated having a positive perception of overall safety within the laboratories they work with or oversee. Perception of safety was determined by degree of agreement or disagreement with the statement, “I feel like the laboratories I oversee or work with are safe places to work.” Of the 62 respondents who indicated that selected laboratory workers are required to renew their biosafety training annually, 69% (43) indicated having a positive perception of overall safety; of the 42 respondents who indicated that annual biosafety training renewal is not required of any laboratory workers, 62% (26) indicated having a positive perception of overall laboratory safety in the laboratories they work with or oversee.
Biosafety Compliance and Annual Individual Performance Evaluation
Of 174 individuals who indicated they oversee a BSL-2 laboratory or higher:
35% (61) indicated that some or all faculty or senior researchers are evaluated annually on their compliance with safety protocols
50% (87) indicated that at some or all of the staff within the laboratories that they work with or oversee are evaluated annually on their compliance with safety protocols
21% (36) indicated biosafety compliance is part of the annual performance evaluation for all students working in laboratories which they work with or oversee
34% (60) indicated that compliance with biosafety measures is not part of any laboratory workers’ annual individual performance evaluations
13% (23) were not sure whether or not compliance with safety measures is part of the annual individual performance evaluation
Incident-reporting Protocols
Developing Incident-reporting Protocols
Approximately 69% of respondents (132/190) indicated their biosafety office and/or environmental health and safety office creates and maintains their institutions’ formal written protocols for how to report incidents across all laboratories. One-quarter of respondents (47/190) indicated creating and maintaining incident-reporting protocols was the responsibility of another administrative division (e.g., occupational health and safety), and about 6% (11/190) revealed each research division or individual laboratory is responsible for creating and maintaining its own incident-reporting protocols.
Seventy-seven percent of respondents (156/201) indicated that a biosafety/environmental health and safety professional at their institutions provides explicit instructions on reporting incidents to each new laboratory worker.
Communicating Incident-reporting Protocols and Lessons Learned
Approximately three-quarters of respondents indicated protocols for reporting biohazardous spills and needlestick incidents are posted directly on their biosafety/environmental health and safety websites or accessible via a clear link that is provided on the safety site (Table 4). Approximately 44% of respondents indicated they post or provide links for reporting protocols on theft or vandalism to a life science laboratory.
Table 4.
Posting formal incident reporting protocols on biosafety/environmental health and safety websites.
| Does your institution’s biosafety/environmental health & safety website provide formal reporting protocols for the following incidents? (n = 196) | ||||
|---|---|---|---|---|
| Yes | No, but a clear link to the guidance is provided | No | Not sure/ No response |
|
| Biohazardous spills | 70% (138) | 5% (9) | 19% (38) | 6% (11) |
| Any needlesticks | 70% (137) | 8% (15) | 18% (35) | 5% (9) |
| Contaminated needlesticks | 66% (129) | 7% (14) | 18% (36) | 9% (17) |
| Laboratory-acquired infections | 59% (115) | 8% (15) | 24% (48) | 9% (18) |
| Animal bites or scratches | 54% (106) | 8% (16) | 27% (53) | 11% (21) |
| Equipment failure that results in potential loss of containment | 49% (96) | 6% (12) | 35% (68) | 10% (20) |
| Break or tear in Personal Protective Equipment (PPE) | 42% (83) | 5% (10) | 39% (76) | 14% (27) |
| Unauthorized entry to a life sciences laboratory | 38% (75) | 9% (18) | 38% (75) | 14% (28) |
| Theft or vandalism within a life sciences laboratory | 35% (68) | 9% (18) | 39% (77) | 17% (33) |
Sharing Lessons Learned
Forty-four percent of respondents (90/207) indicated that within the last 12 months, their institutions have created and disseminated at least one newsletter, e-newsletter, or flyer that addressed biosafety issues. Seventy percent (63/90) of these respondents indicated their newsletter is used to share information about laboratory incidents or lessons-learned from incidents. Sixty of these 63 respondents also responded to the question on perception of safety. Ninety percent (54/60) of these individuals indicated a positive perception of safety within their laboratories versus 69% (11/16) of those whose institutions do not share lessons learned in their newsletter (P = 0.047).
Relationship Between Life Science Laboratories and Medical Support Staff
Nearly half of survey respondents (93/196) characterize the relationship between the life science laboratories and medical support staff at their institutions to be “close” or “very close.” The remaining 53% (103/196) of respondents gave ratings of “somewhat close,” “not close,” or “distant.”
Of the 93 respondents who indicated a “close” or “very close” relationship between the life science laboratories and the medical support staff, 92% (86) gave favorable ratings regarding their perception of overall safety within the laboratories they work with or oversee. In contrast, 64% (66/103) of the respondents who rated their relationship with medical support staff as “somewhat close,” “not close,” or “distant” gave favorable ratings on their perception of overall safety within the laboratories they work with or oversee (P < 0.0001).
Discussion
Because this survey was distributed only to members of the American Biological Safety Association and some respondents’ surveys were not entirely complete, the results may not be representative of the larger community of biosafety professionals or biocontainment laboratories. This survey also does not present perspectives from other cohorts of individuals involved in the oversight of laboratory research, such as laboratory workers.
Additionally, the survey was limited to biosafety professionals working within the United States, so no international perspectives are represented. Nevertheless, survey findings are sufficiently robust to demonstrate both substantial attention to biosafety and opportunities to strengthen specific aspects of biosafety training and incident-reporting practices.
The results from this survey suggest that nearly all senior scientists, faculty, staff, and students working in BSL-2/ABSL-2 and BSL-3/ABSL-3 laboratories are required to have biosafety training. The vast majority of BSL-3/ABSL-3 laboratories get inspected at least once per year by a biosafety officer, and nearly three-quarters of respondents indicated their biosafety or environmental health and safety website posts incident-reporting protocols on biohazardous spills and needle-sticks. Over three-quarters of respondents also indicated a biosafety or environmental health and safety professional provides explicit instructions on reporting incidents to each new laboratory worker. At the same time, the results identified biosafety training and incident-reporting practices could be improved in many laboratories through targeted measures at the institutional level.
Considering Workers’ Roles, Training Priorities, and Core Biosafety Competencies
Determining which individuals within a research institution should receive biosafety training and instruction on incident-reporting is a necessary component of any biosafety program. As biological research programs increase in number and size, new and larger cohorts of people, including maintenance workers, custodians, and visiting scientists, require access to laboratories. However, due to their varied roles within a laboratory, each of these groups is likely to require tailored biosafety training. Determining adequate training for these individuals is a challenge for biosafety professionals. Results from this survey indicate many institutions do not require custodial or maintenance workers to have biosafety training. Over one-quarter of institutions with BSL-2/ABSL-2 laboratories also do not require visiting scientists to have biosafety training. While this survey did not inquire about biosafety training for local police, fire, or EMS responders, increasing numbers of laboratories are beginning to provide training for these individuals as well (Kaufman et al., 2009).
Adequate training on biosafety concepts also involves determining which training methodologies result in the greatest absorption and retention of concepts taught. While certain activities such as biohazardous spill training may benefit the worker most when taught via hands-on simulations, other training components may be readily retained when described verbally by a biosafety professional or other appropriate instructor. While the 5th edition of Biosafety in Microbiological and Biomedical Laboratories (BMBL) guidance encourages evaluation of staff proficiency regarding safe practices in BSL-2 through BSL-4 laboratories, it neither describes effective ways to train on specific concepts, nor suggests ways to assess those competencies (U.S. Department of Health and Human Services, CDC & NIH, 2007). To date, these decisions have been left up to individual laboratories. Such autonomy may have been adequate in past decades, but with growth in the number of laboratories and laboratory personnel, more standardization around core competencies at various biosafety levels would be useful.
The data from this survey also suggest that biosafety professionals believe the laboratories they work with or oversee are safer places to work when laboratory workers are required to renew their biosafety training requirements annually. Periodic renewal of biosafety training may be important in reinforcing institutional safety expectations and providing an opportunity to review new safety measures (Isouard, 1988). While proactive outreach by laboratory workers to biosafety professionals is ideal, many laboratory workers may not seek clarification on safety-related concerns from institutional biosafety or environmental health and safety offices. Establishing periodic renewal periods can enable biosafety professionals to reserve time with busy laboratory workers to clarify concepts and answer safety-related questions.
Encouraging Incident-reporting at the Institutional Level Through Translation into Lessons Learned
Studies conducted throughout the 20th century indicated that surveillance systems for laboratory-acquired infections and incidents leading to possible exposures are not robust (Pike, 1976; Pike, 1979; Sejvar et al., 2005; Sewell, 1995). However, incident reports begin at the institutional level, and the lack of reporting at various levels both within the institution and external to the institution can be due to a variety of reasons separate from the existence and availability of standardized reporting protocols. Laboratory workers may be reluctant to report due to embarrassment, fear of retribution, or the belief that an incident was not worthy of reporting. Other studies have also cited inadequate feedback to the persons reporting incidents as a major deterrent to reporting incidents in the workplace (Evans et al., 2006; Handler et al., 2007). While there are many ways to provide feedback, turning incidents into lessons learned is one method. This survey inquired about whether or not lessons learned from incident reports are ever shared in biosafety newsletters or flyers. A higher percentage of survey respondents indicated a positive perception of laboratory safety if they used their newsletters to share lessons learned from incidents versus those who indicated that their newsletters were not used to share lessons learned. Reporting incidents is a behavior, and determining ways to turn reported incidents into lessons learned may be one way to decrease fear or negative stigma associated with reporting.
Promoting Buy-in and Support of Biosafety Practices from Laboratory Directors and Principal Investigators
The commitment of senior leadership to workplace safety practices is well regarded as an important factor in cultivating and promoting workplace safety (Andriessen, 1978; DeJoy, 2005; Simard & Marchard, 1995; Thompson et al., 1998). If senior leadership is not committed to safety or if senior leadership is not held to the same safety standards as its staff, workers may have less motivation to adhere to more stringent safety requirements. While 50% of survey respondents who oversee a BSL-2/ABSL-2 or higher laboratory indicated that some or all staff within the laboratories they work with or oversee are evaluated annually on their compliance with safety protocols, only 35% (61/174) indicated that some or all faculty or senior researchers are evaluated annually on their compliance with safety measures. Moreover, survey data suggest that slightly more laboratory staff and students are required to have biosafety training at the BSL-2/ABSL-2 level than senior laboratory scientists or faculty at the same level.
Keeping job requirements in mind is important when considering who should be required to take biosafety training, when they should renew, and whether or not it should be an element built into their performance evaluations. With some rarely engaging in laboratory activities, many senior scientists and principal investigators (PIs) commonly delegate research duties to their laboratory staff. Moreover, evaluations of research PIs may be done by department chairpersons who may or may not have the ability to evaluate adherence to biosafety practices. Regardless, it remains important to acknowledge senior scientists’ roles both within their laboratories and within their institutions broadly. Within their laboratories, despite their presence or absence from the bench, many senior scientists are considered the responsible representative for their laboratories. They are held accountable for ensuring that their staff is properly trained, and they are responsible for preventing and responding to incidents within their laboratories. Within their institutions, they are the key link between the laboratory and institutional leadership and play a key role in conveying the importance of a well-supported laboratory safety program.
Examining and Improving Laboratory Relationships with Medical Support Staff
The BMBL puts a significant emphasis on the role of medical support services in the promotion of a safe working environment within laboratories. Section VII of the BMBL focuses on occupational health, citing that optimal worker protection depends on the effective, ongoing collaboration among principal investigators, laboratory directors, safety specialists, and healthcare providers (U.S. Department of Health and Human Services, CDC & NIH, 2007). Approximately half of the biosafety professionals who responded to this survey described the working relationship between life science laboratories and health care providers (i.e., medical support service) at their institution as “somewhat close,” “not close,” or “distant.” Considering that this survey asked only one question about this relationship, this would be an interesting topic that would benefit from further inquiry and to determine measures that could strengthen this critical relationship.
Although this survey did not address it, a related laboratory issue of increasing concern is the interpersonal or emotional well-being of laboratory workers. Guidance on how to report and manage mental, emotional, or behavioral concerns in laboratory colleagues is becoming an important topic for biosafety officers, especially at institutions with biocontainment facilities. Further research and surveys are needed to explore the methods different institutions use to enhance personnel reliability.
Conclusion
This survey represents a cursory exploration into the biosafety training and incident-reporting practices within biological laboratories in the United States. Every institution that conducts biological research has different needs for biosafety, but certain practices such as posting incident-reporting protocols in accessible places such as biosafety websites and turning laboratory incidents into shared lessons learned are likely to improve biosafety regardless of the biosafety level of the laboratory. Engaging laboratory leadership in biosafety training activities and providing job-specific training to all persons entering biocontainment laboratories are also important to promoting a collective responsibility towards safety. Given the variety of individuals working in biological laboratories now, perhaps the time is right to develop industry-wide biosafety competencies that offer more detailed and uniform guidance about what each laboratory worker should be expected to know and demonstrate. As government and institutional leaders look for ways to make biological laboratories safer, these concepts are ones that deserve more attention, promotion, and research.
Acknowledgments
Funding for this paper was supported by the National Institutes of Health (Grant Number 5 U54 AI057 157-02) and the O. Wayne Rollins Foundation (grant to the Center for Public Health Preparedness and Research, Rollins School of Public Health, Emory University). The authors would like to thank the National Institutes of Health for its support through the Southeast Regional Center of Excellence for Emerging Infections and Biodefense (SERCEB) and the SERCEB Policy, Ethics, and Law Core. We recognize and thank the American Biological Safety Association (ABSA) for permitting us to survey its membership and Maureen Thompson, RN, from Emory University for her input during survey development and previous drafts of this paper.
References
- Andriessen JHTH. Safe behaviour and safety motivation. Journal of Occupational Accidents. 1978;1(4):363–376. [Google Scholar]
- DeJoy DM. Behavior change versus culture change: Divergent approaches to managing workplace safety. Safety Science. 2005;43(2):105–129. [Google Scholar]
- Dembek ZF. Medical aspects of biological warfare. Textbooks of military medicine. Washington, DC: Office of the Surgeon General; 2007. [Google Scholar]
- Enserink M. BIOSECURITY: Reports blame animal health lab in foot-and-mouth whodunit. Science. 2007;317(5844):1486. doi: 10.1126/science.317.5844.1486. [DOI] [PubMed] [Google Scholar]
- Evans SM, Berry JG, Smith BJ, Esterman A, Selim P, O’Shaughnessy J, et al. Attitudes and barriers to incident-reporting: A collaborative hospital study. Quality and Safety in Health Care. 2006;15(1):39–43. doi: 10.1136/qshc.2004.012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Executive Order from The White House. Strengthening laboratory biosecurity in the United States. Washington, DC: Federal Register; 2009. Available at: http://edocket.access.gpo.gov/2009/pdf/E9-818.pdf. [Google Scholar]
- Finley R. Portland, Oregon, USA: 2009. Owner. www.SurveyMonkey.com. [Google Scholar]
- Graham B, Talent J. The world at risk: The report of the Commission on the Prevention of WMD Proliferation and Terrorism. New York: Vintage Books; 2008. [Google Scholar]
- Handler SM, Perera S, Olshansky EF, Studenski SA, Nace DA, Fridsma DB, et al. Identifying modifiable barriers to medication error reporting in the nursing home setting. Journal of the American Medical Directors Association. 2007;8(9):568–574. doi: 10.1016/j.jamda.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isouard G. Biosafety practices in pathology laboratories. Australian Health Review. 1988;11(2):122–129. [PubMed] [Google Scholar]
- Kaiser J. BIODEFENSE: Lawmakers worry that lab expansion poses risks. Science. 2007;318(5848):182. doi: 10.1126/science.318.5848.182. [DOI] [PubMed] [Google Scholar]
- Kaufman SG, Alderman LM, Mathews HM, Augustine JJ, Berkelman RL. Review of the Emory University Applied Laboratory Emergency Response Training (ALERT) program. Applied Biosafety: Journal of the American Biological Safety Association. 2009;14(1):22–32. [Google Scholar]
- Lawler A. BIODEFENSE LABS: Boston University under fire for pathogen mishap. Science. 2005;307(5709):501. doi: 10.1126/science.307.5709.501a. [DOI] [PubMed] [Google Scholar]
- Le Duc JW, Anderson K, Bloom M, Estep JE, Feldmann H, Geisbert JB, et al. Framework for leadership and training of Biosafety Level 4 laboratory workers. Emerging Infectious Diseases. 2008;14(11) doi: 10.3201/eid1411.080741. [serial on the Internet]. Available at: www.cdc.gov/EID/content/14/11/1685.htm. [DOI] [PMC free article] [PubMed]
- National Insitutues of Health, National Institute for Allergy and Infectious Diseases. Regional centers of excellence for biodefense and emerging infectious diseases (RCEs) 2009 Available at www3.niaid.nih.gov/LabsAndResources/resources/rce/
- Pike RM. Laboratory-associated infections: Summary and analysis of 3921 cases. Health Laboratory Sciences. 1976;13(2):105–114. [PubMed] [Google Scholar]
- Pike RM. Laboratory-associated infections: Incidence, fatalities, causes, and prevention. Annual Review of Microbiology. 1979;33:41–66. doi: 10.1146/annurev.mi.33.100179.000353. [DOI] [PubMed] [Google Scholar]
- Rhodes K. U.S. House of Representatives’ Subcommittee on Oversight and Investigations, Committee on Energy and Commerce. Washington, DC: Government Accountability Office; 2007. High-containment biosafety laboratories: Preliminary observations on the oversight of the proliferation of BSL-3 and BSL-4 laboratories in the United States. [Google Scholar]
- Burr R, Kennedy E. Select Agent Program and Biosafety Improvement Act of 2008 (S.3127). U.S. Senate. 110th Congress; 2008. Available at: http://frwebgate.access.gpo.gov/cgi-bin/getpage.cgi?dbname=2008_record&page=S5686&position=all. [Google Scholar]
- Sejvar JJ, Johnson D, Popovic T, Miller JM, Downes F, Somsel P, et al. Assessing the risk of laboratory-acquired meningococcal disease. Journal of Clinical Microbiology. 2005;43(9):4811–4814. doi: 10.1128/JCM.43.9.4811-4814.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell DL. Laboratory-associated infections and biosafety. Clinical Microbiology Review. 1995;8(3):389–405. doi: 10.1128/cmr.8.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, Marchand A. A multilevel analysis of organisational factors related to the taking of safety initiatives by work groups. Safety Science. 1995;21(2):113–129. [Google Scholar]
- Thompson RC, Hilton TF, Witt LA. Where the safety rubber meets the shop floor: A confirmatory model of management influence on workplace safety. Journal of Safety Research. 1998;29(1):15–24. [Google Scholar]
- U.S. Department of Health and Human Services, Centers for Disease Control and Prevention & National Institutes of Health. Biosafety in microbiological and biomedical laboratories. 5. Washington, DC: U.S. Government Printing Office; 2007. Available at: www.cdc.gov/OD/ohs/biosfty/bmbl5/BMBL_5th_Edition.pdf. [Google Scholar]