Abstract
The aim of this study was to understand the skin irritation effects of saturated aliphatic hydrocarbons (HCs), C9–C16, found jet fuels using in vitro 3-dimensional EpiDerm full thickness-300 (EFT-300) skin cultures. The EFT-300 cultures were treated with 2.5 µl of HCs and the culture medium and skin samples were collected at 24 and 48 h to measure the release of various inflammatory biomarkers (IL-1α, IL-6 and IL-8). To validate the in vitro results, in vivo skin irritation studies were carried out in hairless rats by measuring trans epidermal water loss (TEWL) and erythema following un-occlusive dermal exposure of HCs for 72 h. The MTT tissue viability assay results with the EFT-300 tissue show that 2.5 µl/tissue (≈4.1 µl/cm2) of the HCs did not induce any significant changes in the tissue viability for exposure times up to 48 h of exposure. Microscopic observation of the EFT-300 cross-sections indicated that there were no obvious changes in the tissue morphology of the samples at 24 h, but after 48 h of exposure, tridecane, tetradecane and hexadecane produced a slight thickening and disruption of stratum corneum. Dermal exposures of C12–C16 HCs for 24 h significantly increased the expression of IL-1α in the skin as well as in the culture medium. Similarly, dermal exposure of all HCs for 24 h significantly increased the expression of interleukin-6 (IL-6) and IL-8 in the skin as well as in the culture medium in proportion to the HC chain length. As the exposure time increased to 48 h, IL-6 concentrations increased 2-fold compared to the IL-6 values at 24 h. The in vivo skin irritation data also showed that both TEWL and erythema scores increased with increased HCs chain length (C9–C16). In conclusion, the EFT-300 showed that the skin irritation profile of HCs was in the order of C9 ≤ C10 ≤ C11 ≤ C12 < C13 ≈ C14 ≈ C16 and that the tissue was an excellent in vitro model to predict in vivo irritation and to understand the structural activity relationship of HCs.
Keywords: EFT-300, Skin irritation, In vitro, Aliphatic hydrocarbons, Jet fuels
1. Introduction
Skin irritation is a reversible inflammatory response observed following exposure to noxious stimuli, which causes the release of various inflammatory cytokines and chemokines by skin keratinocytes and fibroblast cells (Kinkead et al., 1992; Kanikkannan et al., 2000). Kerosene based fuels are widely used as jet propellants (JPs) and several studies have shown that dermal exposure of these fuels can cause skin irritation and/or sensitization. Occupational exposure to jet fuels may occur during fuel transport, aircraft fueling and de-fueling, and maintenance of equipment (Subcommittee on Jet-Propulsion 8 Fuel of Committee on Toxicology, 2003). Jet fuels used in commercial and military aviation are complex multi-component mixtures consisting of hundreds of aromatic and aliphatic hydrocarbons. The skin absorption and permeation of aromatics and aliphatic compounds depend on the chemical nature of the individual component. However, aliphatic hydrocarbons (HCs) have very high skin partitioning and retention compared to aromatics mostly due to their longer residence time in skin (McDougal et al., 2000). Our earlier in vivo studies with nonane, dodecane and tetradecane showed that these chemicals can cause skin irritation following a single occlusive dose and that nonane was the most irritating of these three chemicals (Babu et al., 2004a). Very few studies have been carried out to evaluate the structural activity relationship and skin irritation of HCs and it is very important to understand the skin toxicological behavior of individual aliphatic components of jet fuels.
Most of the reported jet fuel skin irritation studies have been carried out using laboratory animals such as rabbits, rats and mice. The main problems with in vivo animal experiments are that: (a) these animal models produce higher skin permeation and irritation than human skin, (b) there are differences between human and animal skin, (c) a large number of animals are required to produce unbiased results, and (d) in vivo protocols are cumbersome to carry out in industrial setting due to restrictions placed on the use of animals, especially in European countries. Hence, there is a need to look for alternate in vitro toxicity screening models to minimize the animal use in the skin toxicological studies. We already have demonstrated that three dimensional skin models (EpiDerm EPI-200, MatTek Corporation, Ashland, MA) can be used to study the skin irritation of jet fuels (Chatterjee et al., 2006); however, because of absence of dermis in the EPI-200 skin cultures, we were unable to measure changes in cytokine and chemokine levels following exposure to jet fuels that completely reflect the in vivo situation. This study utilizes the EFT-300 tissue which is a full thickness skin model and contains well-differentiated stratum corneum, epidermis and dermis. There are many other skin cultures such as Alloderm®, Apligraft®, Biobrane®, Celaderm™, Dermagraft®, Epicel®, EZ Derm™, Laserskin®, OrCel®, TransCyte®, etc. are available from various commercial sources. These skin substitutes are widely used for skin burns, wound healing and diabetic ulcer applications, whereas EFT-300 model can be used for studying the skin permeation and irritation applications of drug products and chemicals.
The EFT-300 cultures are derived from human neonatal foreskin tissue and consist of normal human-derived epidermal keratinocytes and fibroblasts, which are cultured to form a multilayered, highly differentiated model of human dermis. The dermal compartment is composed of a collagen matrix containing viable normal human dermal fibroblasts and keratinocytes are cultured atop the dermal component to form the epidermis. Ultra structurally, the EFT-300 skin model closely resembles human skin, thus providing a useful in vitro means to assess dermal irritation and skin toxicity (Asbill et al., 2000; Hayden et al., 2009). EFT-300 model consists of organized basal, spinous, granular, and cornified epidermal layers analogous to those of human skin. Epidermal and dermal interactions will have significant effect on cytokine and chemokine secretion following exposure to an irritant signal and these interactions are important in understanding the skin irritation processes. Significant differences in pro-inflammatory mediator secretion exist, depending on the presence or absence of dermal fibroblasts. According to Welss et al. (2004) the epidermal skin equivalents such as EPI-200 skin are unable to release the secondary cytokines (such as IL-6), whereas due to presence of fibroblasts the EFT-300 full thickness skin model is known to release range of pro-inflammatory markers including IL-1α, IL-6, IL-8, IL-10 and GM-CSF (Bernhofer et al., 1999).
In vitro skin irritation testing methods could be useful in preclinical safety screening as well in ranking chemicals for their irritation potential even at the low end of irritation spectrum (Hayden et al., 2003). Human epidermal keratinocytes (NHEK) exposed to three jet fuels, jet A, JP-8 and JP-8+100 in a culture medium demonstrated that these chemicals induce the release of pro-inflammatory cytokines as TNF-α and IL-8 (Allen et al., 2000). Similar results were obtained with porcine keratinocytes (PKC) exposed to jet fuels; both TNF-α and IL-8 were up-regulated (Allen et al., 2001). Jet fuel aliphatic hydrocarbons (C6–C16) were dosed on NHEK to evaluate their effect on cytotoxicity and IL-8 expression in the tissue. Short chain hydrocarbons (C6–C11) were more cytotoxic, while C9–C13 hydrocarbons were more effective in inducing proinflammatory cytokine IL-8 in the cultures (Chou et al., 2002). The exposure of the aromatic hydrocarbon components of jet fuels (e.g. cyclohexylbenzene, trimethylbenzene, xylene, dimethylnaphthalene, ethylbenzene, toluene and benzene) to NHEK resulted in a dose-related differential response in IL-8 release (Chou et al., 2003). All the above studies utilized monolayer keratinocytes growing submerged in a culture medium. In contrast to cells in monolayer culture, engineered skin equivalents mimic human epidermis in terms of tissue architecture and barrier function (Andreadis et al., 2001).
In this study, we have used 3-dimensional EFT-300 skin culture to systematically study the structural activity relationship (SAR) of saturated HCs (from nonane (C9) to hexadecane (C16)) and their effect on tissue viability, skin morphology and cytokine release. In addition, this study assessed if the aliphatic HC structure–dermal irritancy relationship obtained by EFT-300 is comparable to that produced in a rodent (rat) model. Therefore, we have conducted in vivo skin irritation (TEWL and erythema) studies of these chemicals in hairless rats and compared the in vivo and in vitro results.
2. Materials and methods
2.1. Materials
The EpiDerm full thickness-300 (EFT-300) was obtained from MatTek Corporation (Ashland, MA). A Dulbecco’s Modified Eagle (DME) based medium for maintaining cultures was supplied by manufacturer (EFT-300-MM). Aliphatic hydrocarbons (nonane, decane, dodecane, tridecane, tetradecane and hexadecane) were obtained from Wright Patterson AFB, OH. Human IL-1α, IL-6 and IL-8 enzyme immunoassay (EIA) kits were procured from Pierce Biotechnology Inc., Rockford, IL. All chemicals used in these studies were analytical grade.
2.2. Animals
CD® (SD) hrBi hairless rats (250–300 g; Charles River Laboratories) were utilized for the animal studies. The protocol for in vivo experiments was approved by the Animal Care and Use Committee, Florida A&M University. The animals were given standard animal chow and water ad libitum and were acclimated to laboratory conditions for one week prior to experiments. The temperature of the room was maintained at 22 ± 1 °C and the relative humidity varied between 35% and 50%. After completion of the study animals were sacrificed with an overdose of halothane anesthesia.
2.3. Chemicals exposure
EFT-300 culture inserts were placed in 6-well plates and equilibrated with 1 ml of EFT-300-MM medium at 37 °C. Following overnight pre-incubation, the culture medium was replaced with fresh 5 ml of medium and skin cultures were placed on top of two stainless steel washers in 6-well plates. Tissues were treated by topically applying 2.5 µl of HCs (C9–C16) for 24 and 48 h and at each time interval culture medium and tissues were collected for analysis. To spread the chemical evenly on the surface of the tissue, the chemical was mixed with equal amount of Johnsons® Baby Oil (Johnson and Johnson Co., Langhorne, PA). This mixture equal to 2.5 µl of the HC chemical was applied on the tissue. The control samples were treated with Baby Oil alone. Tissue samples were either used for the MTT tissue viability assay or harvested and stored in buffered formalin for histological and biomarkers analyses.
2.4. MTT tissue viability assay
The MTT assay (MTT-100, MatTek Corporation) was carried out as per manufacturer’s instructions. In brief, at the end of 24 and 48 h of treatment, EFT-300 tissue samples were washed twice with PBS and placed in a fresh 24-well plate containing 300 µl/well of MTT solution. After 3 h of incubation at 37 °C, each insert was removed carefully, the bottom was blotted with Kimwipes™ and the insert was transferred into a fresh 24-well plate. The culture inserts were then immersed in 2 ml/well of extraction solution. The plates covered to reduce evaporation and incubated overnight at room temperature in the dark. After overnight extraction, inserts were discarded and the contents of each well were mixed thoroughly before transferring 200 µl of the sample into 96-well plates. The optical density of the samples was read at 570 nm. Background readings for all the samples were determined at 650 nm and were subtracted to obtain the correct O.D. The % viability was determined for each tissue using the equation
2.5. Histological studies
The EFT-300 cultures were collected at the end of the study and fixed in 10% neutral phosphate buffered formalin for at least 24 h at room temperature. Following fixation, samples were dehydrated, and embedded in paraffin. Five micrometer microtomed sections of the skin tissue samples were stained with hematoxylin and eosin according to the common histological procedures (Matsui et al., 1996a,b). The stained slides were examined under an Olympus BX40 microscope and assessed for histo-pathological changes associated with chemical exposure.
2.6. Enzyme immunoassay of inflammatory biomarkers
The culture media was quantitatively analyzed using EIA kits for IL-1α, IL-6 and IL-8 according to manufacturer’s protocol. The tissue homogenates were prepared by homogenizing the skin tissue samples at 4 °C with a tissue homogenizer (Fisher Scientific, Suwannee, GA) in 200 µl of lysis buffer [15 mmol/l MgCl2, 50 mmol/l HEPES (pH 7.4), 150 mmol/l NaCl, 8 mol/l urea, 0.1% Triton X-100] and a cocktail of protease inhibitors [10 µl/g tissue, leupeptin, pepstatin A, aprotinin, bestatin hydrochloride, N-(trans-epoxysuccinyl)-l-leucine 4-guanidinbutylamide, 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride], and centrifuged at 13,200 rpm for 20 min. Supernatants were frozen at −80 °C. The protein concentration of the samples was determined according to the Bradford method.
Levels of cytokines and chemokine in the skin lysates were determined using the bead-based suspension microarray technology (AssayGate, Ijamsville, MD) (Opalka et al., 2003).
2.7. In vivo skin irritation studies in hairless rats
CD hairless rats obtained from Charles River Laboratories (Wilmington, MA) were utilized for the studies. The control and treatment areas were marked as a circular area (~3 cm2) on dorsal surface of the animal. The chemicals were applied un-occlusively using a micropipette at a dose of 15 µl every 2 h for 8 h a day over a 3 day period on the marked skin surface. Measurements of trans epidermal water loss (TEWL) were taken for all treatments and control sites for up to 72 h using Tewameter TM 210 (Courage + Khazaka, Koln, Germany). The erythema was measured by visual scoring by a modified method of Draize et al. (1944). The scores ranging from 0 to 4 were assigned depending on degree of erythema.
2.8. Statistical analysis
The amount of cytokines (IL-1α and IL-6) and chemokine (IL-8) released into the culture medium is presented as pg/ml; cytokines in the skin are presented as pg/mg of protein of the tissue. The differences between multiple groups were examined using analysis of variance (ANOVA) and Tukey multiple comparison test. Mean differences with P < 0.05 were considered to be significant.
3. Results
3.1. Cell viability and histological changes
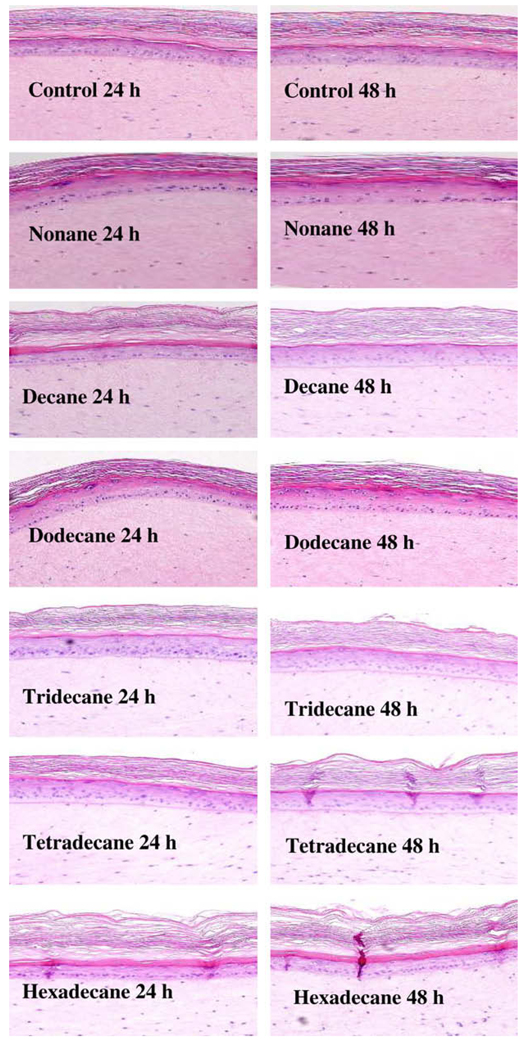
Initial experiments with the HCs indicated that a dose volume of 2.5 µl per EFT-300 tissue did not result in any loss of tissue viability (Table 1). Fig. 1 presents the morphological changes in EFT- 300 skin following exposure to HC for 24 and 48 h. After 24 h of exposure HCs did not induce any significant structural change to the tissue. After 48 h of chemicals exposure, C13–C16 treated tissues induced no significant changes in the skin structure except slight thickening and disruption of the stratum corneum (Fig. 1).
Table 1.
Physico-chemical properties of various aliphatic hydrocarbons and the tissue viability of EFT-300 after dermal exposures with various aliphatic hydrocarbons as measured by MTT assay.
| Hydrocarbon chemical | Molecular weight | Flash point (°C c.c) | Partition coefficient* (log P o/w) | % Cell viability | |
|---|---|---|---|---|---|
| 24 h | 48 h | ||||
| Control | 100 ± 0.0 | 101 ± 0.0 | |||
| Nonane (C9) [CH3(CH2)7 CH3] | 128.2 | 31 | 5.65 | 110 ± 0.01 | 103 ± 2.03 |
| Decane (C10) [CH3(CH2)8 CH3] | 142.3 | 46 | 6.25 | 98.2 ± 1.69 | 102 ± 1.01 |
| Dodecane (C12) [CH3(CH2)10 CH3] | 168.3 | 77 | 7.24 | 99.8 ± 1.61 | 101 ± 1.20 |
| Tridecane (C13) [CH3(CH2)11 CH3] | 184.4 | 102 | 7.57 | 108 ± 0.12 | 98.5 ± 0.91 |
| Tetradecane (C14) [CH3(CH2)12 CH3] | 198.4 | 99 | 7.6 | 103 ± 0.23 | 97.8 ± 0.43 |
| Hexadecane (C16) [CH3(CH2)14 CH3] | 226.4 | 135 | 8.63 | 101 ± 0.18 | 98.5 ± 0.24 |
Fig. 1.
Effect of aliphatic hydrocarbon (C9–C16) treatments on the EFT-300 tissue morphology. Images were obtained at 10x magnification.
3.2. Enzyme immunoassay of biomarkers
3.2.1. In the EFT-300 skin tissues
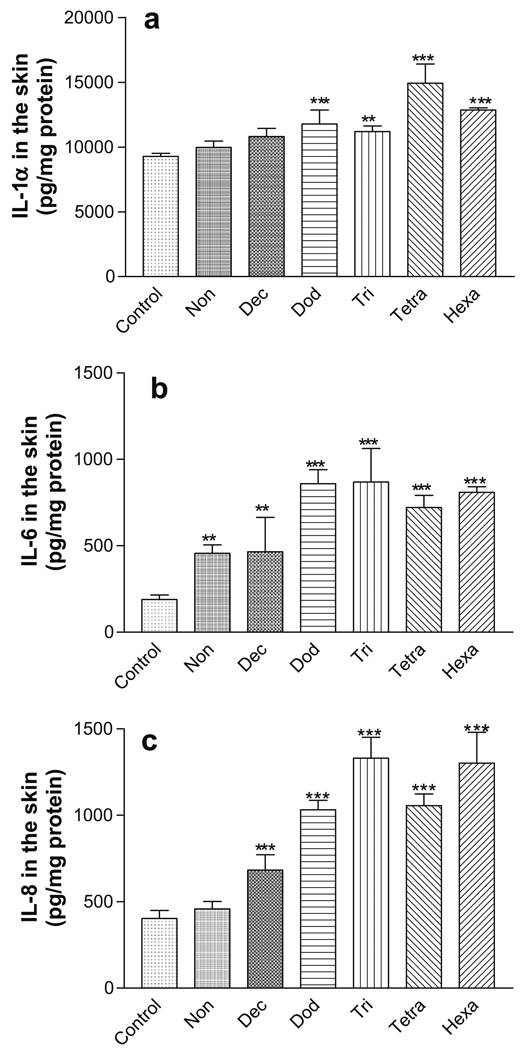
Although there was no tissue damage, the C12–C16 HCs induced statistically significant increases in the IL-1α levels (P < 0.01) in the EFT-300 skin tissues following 24 h chemical treatments compared with the negative control tissues (Fig. 2a). All HCs (C9–C16) induced statistically significant increases in IL-6 levels compared to the negative control (P < 0.01). IL-6 levels increased with increasing hydrocarbon chain length, C9–C16 (Fig. 2b). Similarly, the statistically significant increases in IL-8 compared to the negative control tissues (P < 0.001) were observed and IL-8 levels increased with increasing hydrocarbon chain length from C9 to C16 (Fig. 2c).
Fig. 2.
Inflammatory biomarkers in the EFT-300 skin cultures following 24 h of exposure to aliphatic hydrocarbons. (a) IL-1α; (b) IL-6; (c) IL-8. **P < 0.01; ***P < 0.001.
3.2.2. In culture medium
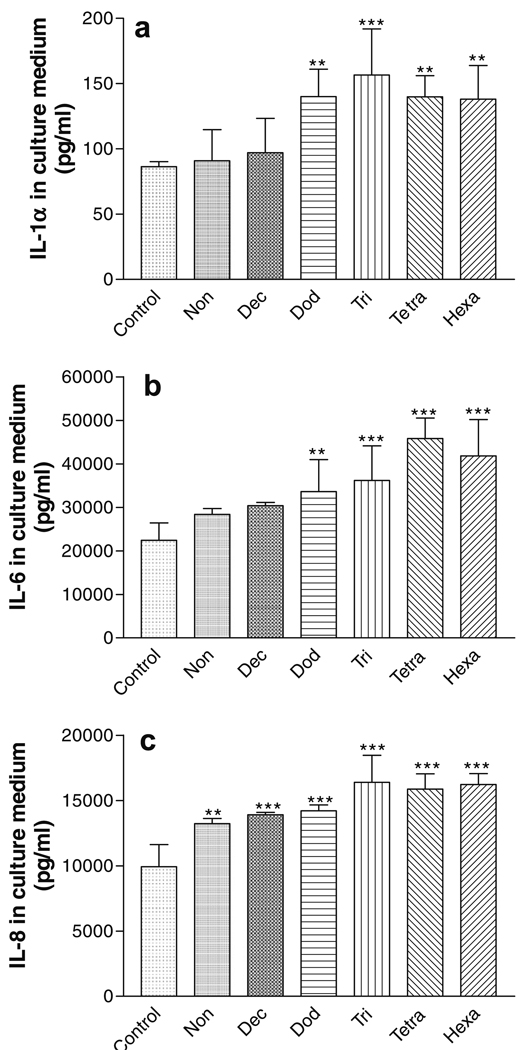
The effect of chemical exposure (24 h) on the release of inflammatory markers into the culture medium is shown in Fig. 3. The C12–C16 HCs induced statistically significant increases in the IL-1α release compared to the negative control (P < 0.001; Fig. 3a). Likewise, statistically significant increases of IL-6 and IL-8 were observed following 24 h exposure to the C9–C16 HCs. In general, the biomarker release increased as the HC chain length increased.
Fig. 3.
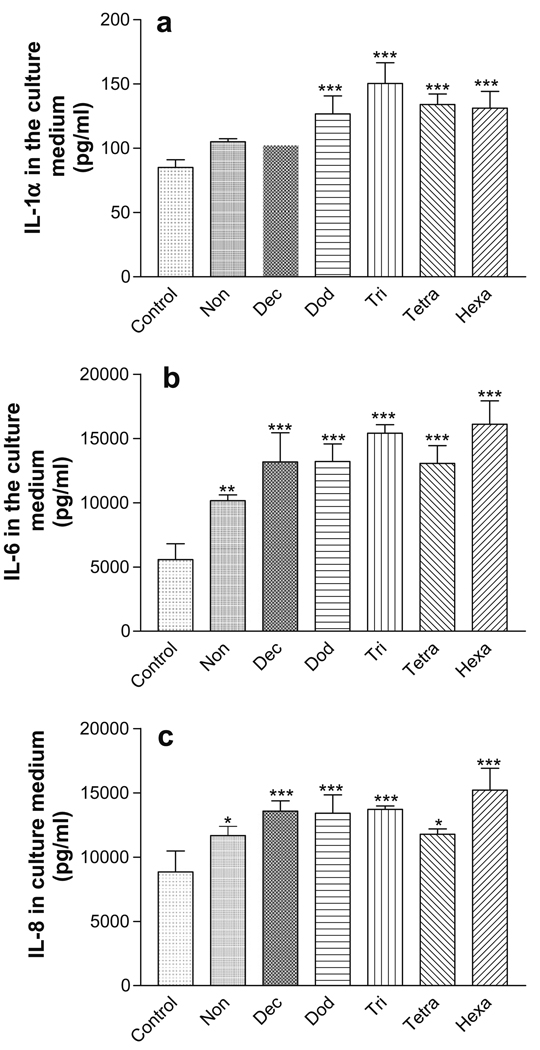
Release of inflammatory biomarkers from EFT-300 skin cultures into the culture medium following 24 h exposure to aliphatic hydrocarbons. (a) IL-1α; (b) IL-6; (c) IL-8. *P < 0.05; **P < 0.01; ***P < 0.001.
The effect of chemical exposure (48 h) on the release of inflammatory markers from the EFT-300 tissue into the culture medium is shown in Fig. 4. The C12–C16 HCs induced statistically significant increases in the IL-1α and IL-6 release compared to the negative control (P < 0.01; Fig. 4a and b). Similarly, the C9–C16 HCs induced statistically significant increases in IL-8 release significantly compared to the negative control (P < 0.001; Fig. 4c). In all cases, cytokine release increased with increasing HC chain length. In addition, the exposure time influenced the release of IL-6 into the medium; after 48 h of HCs exposure, the IL-6 levels in the culture medium were approximately twice as high as the levels after 24 h of exposure (Figs. 3b and 4b).
Fig. 4.
Release of inflammatory biomarkers from EFT-300 skin cultures into the culture medium following 48 h exposure to aliphatic hydrocarbons. (a) IL-1α; (b) IL-6; (c) IL-8. **P < 0.01; ***P < 0.001.
3.3. Skin irritation in hairless rats
The effect of prolonged dermal exposure of HCs on the TEWL and erythema in hairless rats is shown in Table 2. Un-occlusive dermal exposure of HCs produced elevated levels of TEWL and increased erythema at all time points measured. The TEWL and erythema values increased with increasing HC chain length. At the end of 72 h the TEWL values of tridecane, tetradecane and hexadecane were found to be 7.2, 6.8, and 10.4 fold higher, respectively, than the negative control (no treatment). Erythema scores also significantly increased due to the un-occlusive dermal exposure of these hydrocarbons (Table 2).
Table 2.
Skin irritation effects of un-occlusive dermal exposures of aliphatic hydrocarbons in hairless rats.
| Aliphatic hydrocarbon (HC) | TEWL (g/m2/h) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| TEWL (n = 5–6) | |||
| Control | 5.6 ± 1.16 | 4.78 ± 0.66 | 5.04 ± 0.58 |
| Nonane | 8.37 ± 1.20 | 10.33 ± 3.84 | 11.04 ± 3.58 |
| Decane | 7.20 ± 2.98 | 7.75 ± 2.04 | 10.99 ± 2.76 |
| Dodecane | 9.08 ± 2.25 | 10.60 ± 2.05 | 11.14 ± 1.11 |
| Tridecane | 17.47 ± 4.88 | 23.54 ± 4.62 | 36.20 ± 9.54 |
| Tetradecane | 11.86 ± 1.72 | 22.15 ± 6.13 | 34.04 ± 8.19 |
| Hexadecane | 19.63 ± 3.06 | 47.30 ± 7.96 | 52.61 ± 8.45 |
| Aliphatic hydrocarbon (HC) | Erythema | ||
| 24 h | 48 h | 72 h | |
| Erythema (n = 6) | |||
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Nonane | 0.50 ± 0.55 | 0.50 ± 0.53 | 0.88 ± 0.64 |
| Decane | 0.00 ± 0.00 | 0.63 ± 0.52 | 0.50 ± 0.53 |
| Dodecane | 0.83 ± 0.75 | 1.13 ± 0.35 | 1.25 ± 0.46 |
| Tridecane | 1.00 ± 0.00 | 2.00 ± 0.00 | 2.83 ± 0.41 |
| Tetradecane | 1.33 ± 1.03 | 2.50 ± 0.53 | 2.88 ± 0.64 |
| Hexadecane | 3.00 ± 0.00 | 3.00 ± 0.00 | 4.00 ± 0.00 |
3.4. Correlation of in vitro and in vivo results
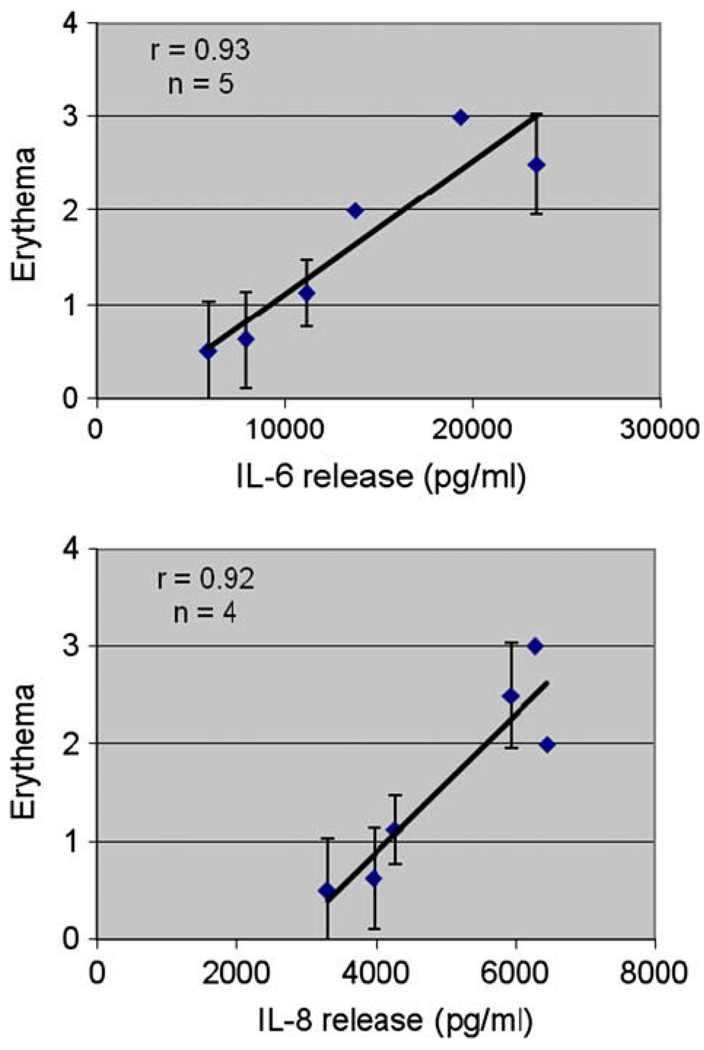
Fig. 5 shows the plots of erythema versus IL-6 and IL-8 release by the EFT-300 tissues after 48 h of un-occlusive dermal exposure of the HCs. A least-squares, linear fit of the in vivo erythema and IL-6 and IL-8 release data gave correlation coefficients, r, of 0.93 and 0.92, respectively. A similar plot for IL-1α gave an r value of 0.74 (data not shown).
Fig. 5.
Correlation of in vitro cytokine release from the EFT-300 skin cultures and in vivo erythema after 48 hours of un-occlusive exposure to aliphatic hydrocarbons: (a) IL-6; (b) IL-8. A least-squares, linear fit of the in vitro and in vivo data were used to determine the correlation coefficient, r.
4. Discussion
Development of new in vitro skin models to evaluate the skin irritation of hazardous chemicals as an alternative to the use of animals is highly desirable in toxicological research. The in vitro skin models such as EPI-200 have been evaluated with many cosmetic products and our earlier studies with jet fuels demonstrated that these skin cultures is an excellent model to evaluate skin irritation (Chatterjee et al., 2006). EFT-300 skin cultures may offer advantages over the EPI-200 epidermal model for skin toxicological studies of irritant chemicals because of: (a) the presence of dermis (b) its ability to express secondary inflammatory biomarkers such as cytokines and chemokines. Most skin irritation studies with jet fuels and HCs have focused on the evaluation of pathological changes in the skin following repeat dermal exposure to chemicals in animal models (Monteiro-Riviere et al., 2004; Kanikkannan et al., 2002). In this study, we utilized EFT-300 as a cultured skin model, to evaluate the HC structure and skin irritation relationship of HCs at cellular level by measuring various cytokines and chemokines. Chou et al. (2003) evaluated the LD(50), the highest non-cytotoxic dose, and interleukin-8 (IL-8) release activity of nine major jet fuel aromatic hydrocarbons in human epidermal keratinocyte (HEK) cell cultures in flasks. Similarly, Chou et al. (2002) assessed acute cytotoxicity and IL-8 release induced by individual aliphatic hydrocarbons using HEK cell cultures in 96-well plates. These two studies are different that they are cell culture suspensions, whereas our current study utilizes fully differentiated full thickness 3D skin grown on the air–liquid interface on the culture medium. The advantage of EFT-300 is that this is a fully differentiated full thickness skin and the chemical can be directly applied on the surface of the cultured skin to study the structural changes in the skin and the expression of various biological markers.
In this study, we have evaluated the effect of HC chain length (C9–C16) on the tissue viability, histological appearance and cytokine (IL-1α and IL-6) and chemokine (IL-8) release in the EFT-300 cultures. The treatment dose and exposure time were selected based on a previously study utilizing the partial thickness (epidermis only) EPI-200 skin cultures where the tissues were exposed to varying amounts of jet fuels (1.25–50 µl/tissue or 2.0–80 µl/cm2). In that study, dose levels 2–4 µl/cm2 did not result in significant loss of tissue viability after 24 and 48 h of treatment (MTT assay), whereas 8 µl/cm2 and higher doses showed considerable cytotoxicity and tissue damage (Chatterjee et al., 2006). Results from preliminary experiments in this study (data not shown) showed that similar doses (2.5 µl = 4.0 µl/cm2) were necessary to avoid cytotoxicity in the full thickness EFT-300 tissue.
Microscopic observation of hematoxylin and eosin (H&E) stained EFT-300 skin sections indicate that HCs induced no significant changes in the skin structure except slight thickening and disruption of the stratum corneum with C13–C16 HC treated tissue samples after 48 h exposures (Fig. 1). Similar observations were made by (Muhammad et al., 2005) with HCs, where sub corneal micro abscesses and macroscopic lesions were observed in pig skin following the dermal exposures of tridecane and tetradecane.
Observation of morphological changes and tissue viability assays alone are not sufficient to determine the skin irritation potential of noxious chemicals. During skin irritation process the normal skin functions are interrupted and there is an imbalance in the production of various inflammatory biomarkers. Determination of these biomarkers will give a complete understanding of skin irritation process. In the present study we measured the release of cytokines (IL-1α and IL-6) and a chemokine (IL-8) in both the skin and the culture medium following 24 and 48 h of chemicals exposures.
Epidermal keratinocytes produce keratins and lipids, which contributed to the structural integrity and barrier formation of skin (Nickoloff, 1992). They produce cytokines which are key mediators of inflammatory and immunologic reactions throughout the body and play an important role in initiating and amplifying the cutaneous inflammatory response during allergic and irritant contact dermatitis (Nickoloff, 1998; Barker et al., 1991). IL-1α is an important inflammatory mediator in the skin and is believed to initiate inflammatory responses (Coquette et al., 2000; Welss et al., 2004). Following topical application of irritant chemicals, the chemicals breach the stratum corneum and enter the epidermis where they can stimulate the release of cytokines and chemokines. Within the epidermis, keratinocytes are the major source of cytokines, along with Langerhans cells and melanocytes (Williams and Kupper, 1996; Mizutani et al., 1991; Kupper and Groves, 1995). In this study, when EFT-300 cultures were treated with HCs for 24 h, C12–C16 HCs enhanced the IL-1α levels in the skin and increased release into the culture medium (P < 0.01); further, IL-1α levels increased with increasing HC chain length (Figs. 2a and 3a).
The reasons for differences in irritation the various HCs tested relates to differences in skin permeation and retention of HC in the skin. Permeation of HC into the skin is inversely proportional to the HC chain length, while the skin retention is directly proportional to the HC chain length (Babu et al., 2004b; Baxter and Miller, 1987). Further, the skin permeation and retention properties depend on the lipid solubility and octanol–water partition coefficient and these properties are proportional to the carbon chain length (Table 1). Brown and Box (1970) observations also strongly suggest that increasing HC chain length increases the skin irritation.
Modulation and regulation of an inflammatory response depends on the communication between keratinocytes and fibroblasts (Boxman et al., 1996). In this study, we observed that after 24 h of HC exposure, all HCs significantly increased IL-6 release compared to the negative control (P < 0.01) (Figs. 2b and 3b). IL-6 is produced by fibroblasts in the dermis and is released after stimulation of IL-1α and TNF-α (Welss et al., 2004). Several studies using in vitro skin cultures demonstrated that the secondary cytokines (IL-6 and GM-CSF) were released only in presence of fibroblast cells in the full thickness skin models only (Bernhofer et al., 1999; Ponec and Kempenaar, 1995; Boxman et al., 1996). In addition to cytokines, there is an evidence that chemokines are also involved in the skin inflammation process. In vitro skin irritation assessment of various facial creams using epidermal cultures and full thickness models indicated that full thickness skin models release IL-8 at 100–150 times higher than the epidermal equivalent skin models (Bernhofer et al., 1999). Enhanced IL-8 expression, a non-specific response, indicates skin damage (Coquette et al., 2003). In this study, we observed increased IL-8 release with increased carbon chain length (Figs. 2c and 3c).
We measured the IL-1α, IL-6 and IL-8 concentrations in the culture medium after HC exposure for 24 and 48 h. The result shows that as the time of exposure increased from 24 to 48 h, all three inflammatory markers were released into the medium (Fig. 4) and in the skin the levels fell to base line values (data not shown). However, after 48 h of chemicals exposure the biomarker expression pattern was similar to that of 24 h exposures. The IL-6 was an exception, the levels increased by about 4-fold at 48 h versus 24 h. The possible explanation for decline in release of skin inflammatory markers in the skin tissue after 48 h of exposure is that the in vitro skin cultures may not retain the biomarkers for longer periods. Further, not only IL-1α stimulates the IL-6 release but other cytokines such as TNF-α and MCP-1 are also involved in the release of IL-6. This possibly led to increased IL-6 levels in receptor fluid at 48 h post exposure. Another possible reason for enhanced IL-6 release over the time is active involvement of IL-6 in the wound healing process (Gallucci et al., 2004).
In this study, EFT-300 cultures were exposed to a non-cytotoxic dose of HCs for 24 and 48 h which induced skin irritation in the order of C9 ≤ C10 ≤ C11 ≤ C12 < C13 ≈ C14 ≈ C16. The observed variation in skin irritation profile of individual HC is mainly due to differences in the skin permeation and retention of HCs due to varied physicochemical properties among HCs. Under equal thermodynamic activities, liquid fluxes are often several folds higher than vapor fluxes across human skin for various penetrants (Barry et al., 1985), and these differences were reflected by the partition coefficients and the amount of penetrant entering the stratum corneum. For instance nonane has flash point of 31 °C and upon un-occlusive exposure, the chemical evaporates very rapidly from the skin and probably causing less skin irritation, whereas dodecane has flash point of 77 °C with higher tissue partition coefficient and evaporates very slowly and associates with the skin tissues for longer period and possibly leads to higher skin irritation. Baker et al. (1999) studied the skin irritation of JP-8 and JP-4 by applying chemicals on the dorsal surface of the rats once daily for 7–28 days, and observed that the skin irritation effect of JP-8 was more than that of JP-4. JP-4 contains more of the volatile lower molecular weight HCs and many of these are potentially irritating chemicals and probably evaporate quickly after the dermal exposures. In this study, the higher molecular weight, longer carbon chain length HC caused higher levels of cytokine release. However, there are some differences exist in the release of cytokines from the skin into the culture medium. Among IL-1α, IL-6 and IL-8 inflammatory markers, the IL-1α showed minimal release into the medium. The reason might be IL-1α is released by the keratinocytes and resides in the upper epidermal layers as a membrane-bond form (Dinarello, 1998). In an intact epidermis the IL-1α reservoir is naturally eliminated by desquamation, due to the fact that IL-1α has no hydrophobic leader sequence for transmembrane secretion. Therefore, IL-1α is only released from leaky cells following cell injury or membrane perturbation.
To further investigate our in vitro results, we evaluated the skin irritation of HC by measuring the TEWL and erythema following low level prolonged exposure of hairless rats to the HCs. Rat skin though has anatomical differences compared to human skin, due to availability and feasibility, this model is still being used to assess and rank the irritation potential of compounds of a chemical series (Kanikkannan et al., 2002; Muhammad et al., 2005). In the present study TEWL and erythema values of aliphatic chemicals were in the order of C9 ≤ C10 ≤ C11 ≤ C12 < C13 ≈ C14 ≈ C16. Because of very high partition coefficient (Table 1) hexadecane will have more affinity towards stratum corneum and this might be the one of the reasons for observed increased skin irritation of hexadecane. The TEWL and erythema values also increased with increased exposure time from 24 to 72 h, especially with C13–C16 HCs (Table 2). Chou et al. (2002) studied acute cytotoxicity and IL-8 release induced by individual aliphatic hydrocarbons (C6–C16) on human epidermal keratinocyte suspensions in a culture medium. IL-8 concentration was increased by 3- to 10-fold, with the highest increase found after exposure to hydrocarbons in the C9–C13 HCs. Allen et al. (2001) examined the effects of individual hydrocarbon components (C11–C16) on the expression if IL-8 by human epidermal keratinocyte suspensions in a culture medium and found that C13 and C16 HCs produced highest level of IL-8 expression at their subtoxic concentrations. These results are in agreement with the finding of this study using EFT-300. The IL-6 and IL-8 cytokine release data showed a high level of correspondence with the in vivo erythema measurements (Fig. 5). Therefore, it seems likely that such in vitro results could be used to predict in-vivo un-occlusive prolonged HC exposure results.
In conclusion, our results indicate that EFT-300 skin cultures are an excellent model to evaluate the skin irritation of chemicals. Further, these models are sensitive to damage produced by the chemicals and can be used to understand the structure activity relationship of various irritant chemicals. The results obtained from in vitro cultures are in agreement with the earlier in vivo studies conducted in our laboratory. We are planning to conduct gene microarray studies and perform RT-PCR to further utilize these cultures for skin irritation evaluation.
Acknowledgements
This work was supported by the International Foundation of Ethical Research (IFER), Department of Defense (DOD, W911NF-04-1-0368). The research assistance of Department of Pharmacal Sciences, Auburn University, AL is appreciated.
References
- Allen DG, Riviere JE, Monteiro-Riviere NA. Identification of early biomarkers of inflammation produced by keratinocytes exposed to jet fuels jet A, JP-8, and JP-8(100) J. Biochem. Mol. Toxicol. 2000;14:231–237. doi: 10.1002/1099-0461(2000)14:5<231::AID-JBT1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Allen DG, Riviere JE, Monteiro-Riviere NA. Cytokine induction as a measure of cutaneous toxicity in primary and immortalized porcine keratinocytes exposed to jet fuels, and their relationship to normal human epidermal keratinocytes. Toxicol. Lett. 2001;119:209–217. doi: 10.1016/s0378-4274(00)00316-7. [DOI] [PubMed] [Google Scholar]
- Andreadis ST, Hamoen KE, Yarmush ML, Morgan JR. Keratinocyte growth factor induces hyperproliferation and delays differentiation in a skin equivalent model system. FASEB J. 2001;15:898–906. doi: 10.1096/fj.00-0324com. [DOI] [PubMed] [Google Scholar]
- Asbill CS, El-Kattan AF, Michniak B. Enhancement of transdermal drug delivery: chemical and physical approaches. Crit. Rev. Ther. Drug Carrier Syst. 2000;17:621–658. [PubMed] [Google Scholar]
- Babu RJ, Chatterjee A, Singh M. Assessment of skin irritation and molecular responses in rat skin exposed to nonane, dodecane and tetradecane. Toxicol. Lett. 2004a;153:255–266. doi: 10.1016/j.toxlet.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Babu RJ, Chatterjee A, Ahaghotu E, Singh M. Percutaneous absorption and skin irritation upon low-level prolonged dermal exposure to nonane, dodecane and tetradecane in hairless rats. Toxicol. Ind. Health. 2004b;20:109–118. doi: 10.1191/0748233704th197oa. [DOI] [PubMed] [Google Scholar]
- Baker W, Dodd D, McDougal JN, Miller TE. Repeated dose skin irritation study on jet fuels – definitive study. Wright Patterson AFB, Air Force Research Laboratory Technical Report, AFRL-HE-WPTR-1999–0022. 1999 [Google Scholar]
- Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ. Keratinocytes as initiators of inflammation. Lancet. 1991;337:211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- Barry BW, Harrison SM, Dugard PH. Vapour and liquid diffusion of model penetrants through human skin; correlation with thermodynamic activity. J. Pharm. Pharmacol. 1985;37:226–236. doi: 10.1111/j.2042-7158.1985.tb05050.x. [DOI] [PubMed] [Google Scholar]
- Baxter CS, Miller ML. Mechanism of mouse skin tumor promotion by n-dodecane. Carcinogenesis. 1987;8:1787–1790. doi: 10.1093/carcin/8.12.1787. [DOI] [PubMed] [Google Scholar]
- Bernhofer LP, Seiberg M, Martin KM. The influence of the response of skin equivalent systems to topically applied consumer products by epithelial mesenchymal interactions. Toxicol. In Vitro. 1999;13:219–229. doi: 10.1016/s0887-2333(98)00087-3. [DOI] [PubMed] [Google Scholar]
- Boxman IL, Ruwhof C, Boerman OC, Lowik CW, Ponec M. Role of fibroblasts in the regulation of proinflammatory interleukin IL-1, IL-6 and IL-8 levels induced by keratinocyte derived IL-1. Arch. Dermatol. Res. 1996;288:391–398. doi: 10.1007/BF02507108. [DOI] [PubMed] [Google Scholar]
- Brown VKH, Box VL. Skin arginase activity as a measure of skin change under the influence of some alkanes and alkenes. Br. J. Dermatol. 1970;82:606–612. doi: 10.1111/j.1365-2133.1970.tb06102.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Babu RJ, Klausner M, Singh M. In vitro and in vivo comparison of dermal irritancy of jet fuel exposure using EpiDerm™ (EPI-200) cultured human skin and hairless rats. Toxicol. Lett. 2006;167:85–94. doi: 10.1016/j.toxlet.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Chou CC, Riviere JE, Monteiro-Riviere NA. Differential relationship between the carbon chain length of jet fuel aliphatic hydrocarbons and their ability to induce cytotoxicity vs. interleukin-8 release in human epidermal keratinocytes. Toxicol. Sci. 2002;69:226–233. doi: 10.1093/toxsci/69.1.226. [DOI] [PubMed] [Google Scholar]
- Chou CC, Riviere JE, Monteiro-Riviere NA. The cytotoxicity of jet fuel aromatic hydrocarbons and dose-related interleukin-8 release from human epidermal keratinocytes. Arch. Toxicol. 2003;77:384–391. doi: 10.1007/s00204-003-0461-z. [DOI] [PubMed] [Google Scholar]
- Coquette A, Berna S, Poumay Y, Pittelkow MR. The keratinocyte in cutaneous irritation and sensitization. In: Kydonieus AF, Wille JJ, editors. Biochemical Modulation of Skin Reactions. Boca Raton, FL: CRC Press; 2000. pp. 125–143. [Google Scholar]
- Coquette A, Berna N, Vandenbosch A, Rosdy M, De Wever B, Poumay Y. Analysis of interleukin-1alpha (IL-1alpha) and interleukin-8 (IL-8) expression and release in in-vitro reconstructed human epidermis for the prediction of in vivo skin irritation and/or sensitization. Toxicol. In Vitro. 2003;17:311–321. doi: 10.1016/s0887-2333(03)00019-5. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1, interleukin-1 receptor and interleukin-1 receptor antagonist. Intern. Rev. Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- Draize J, Woodard G, Calvery H. Methods for the study of irritation and toxicity of substances topically applied to skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944;82:377–390. [Google Scholar]
- Gallucci RM, Sloan DK, Heck JM, Murray AR, O’Dell SJ. Interleukin 6 indirectly induces keratinocyte migration. J. Invest. Dermatol. 2004;122:764–772. doi: 10.1111/j.0022-202X.2004.22323.x. [DOI] [PubMed] [Google Scholar]
- Hayden PJ, Ayehunie S, Jackson GR, Kupfer-Lamore S, Last TJ, Klausner M, Kubilus J. In vitro skin equivalent models for toxicity testing. In: Salem H, Katz SA, editors. Alternative Toxicological Methods. Boca Raton, FL: CRC Press LLC; 2003. pp. 229–247. [Google Scholar]
- Hayden PJ, Petrali JP, Stolper G, Hamilton TA, Jackson GR, Jr, Wertz PW, Ito S, Smith WJ, Klausner M. Vesicating effects of sulfur mustard on an in vitro human skin model. Toxicol. In Vitro. 2009;23:1396–1405. doi: 10.1016/j.tiv.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Kanikkannan N, Jackson T, Shaik MS, Singh M. Evaluation of skin sensitization potential of jet fuels by murine local lymph node assay. Toxicol. Lett. 2000;116:165–170. doi: 10.1016/s0378-4274(00)00212-5. [DOI] [PubMed] [Google Scholar]
- Kanikkannan N, Locke BR, Singh M. Effect of jet fuels on the skin morphology and irritation in hairless rats. Toxicology. 2002;14:35–47. doi: 10.1016/s0300-483x(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Kinkead ER, Salins SA, Wolfe RE. Acute irritation and sensitization potential of JP-8 jet fuel. Acute Tox. Data. 1992;11:700. [Google Scholar]
- Kupper TS, Groves RW. The interleukin-1 axis and cutaneous inflammation. J. Invest. Dermatol. 1995;105 Suppl.:62S–66S. doi: 10.1111/1523-1747.ep12316087. [DOI] [PubMed] [Google Scholar]
- Matsui R, Okura N, Osaki K, Konishi J, Ikegami K, Koide M. Histological evaluation of skin reconstruction using artificial dermis. Biomaterials. 1996a;17:995–1000. doi: 10.1016/0142-9612(96)84674-6. [DOI] [PubMed] [Google Scholar]
- Matsui R, Osaki K, Konishi J, Ikegami K, Koide M. Evaluation of an artificial dermis full-thickness skin defect model in the rat. Biomaterials. 1996b;17:989–994. doi: 10.1016/0142-9612(96)84673-4. [DOI] [PubMed] [Google Scholar]
- McDougal JN, Pollard DL, Wiesman W, Garrett CM, Miller TE. Assessment of skin absorption and penetration of JP-8 jet fuel and its components. Toxicol. Sci. 2000;55:247–255. doi: 10.1093/toxsci/55.2.247. [DOI] [PubMed] [Google Scholar]
- Mizutani H, Black R, Kupper TS. Human keratinocytes produce but do not process pro-interleukin-1 (IL-1) beta. Different strategies of IL-1 production and processing in monocytes and keratinocytes. J. Clin. Invest. 1991;82:1066–1071. doi: 10.1172/JCI115067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro-Riviere NA, Inman AO, Riviere JE. Skin toxicity of jet fuels: ultrastructural studies and the effects of substance P. Toxicol. Appl. Pharmacol. 2004;195:339–347. doi: 10.1016/j.taap.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Muhammad F, Monteiro-Riviere A, Riviere JE. Comparative in vivo toxicity of topical JP-8 jet fuel and its individual hydrocarbon components: identification of tridecane and tetradecane as key constituents responsible for dermal irritation. Toxicol. Pathol. 2005;33:258–266. doi: 10.1080/01926230590908222. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ. Pathophysiology of cutaneous inflammation. Arch. Dermatol. Res. 1992;284:1S10–1S11. doi: 10.1007/BF00638233. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ. Immunologic reactions triggered during irritant contact dermatitis. Am. J. Contact Dermat. 1998;9:107–110. [PubMed] [Google Scholar]
- Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, Esser MT. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin. Diagn. Lab. Immunol. 2003;10:108–115. doi: 10.1128/CDLI.10.1.108-115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponec M, Kempenaar J. Use of human skin recombinants as an in vitro model for testing the irritation potential of cutaneous irritants. Skin Pharmacol. 1995;8:49–59. doi: 10.1159/000211330. [DOI] [PubMed] [Google Scholar]
- Subcommittee on Jet-Propulsion 8 Fuel of Committee on Toxicology. NRC Toxicologic Assessment of Jet-Propulsion Fuel. vol. 8. Washington, DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- Welss T, Basketter DA, Schroder KR. In vitro skin irritation: facts and future. State of the art review of mechanisms and models. Toxicol. In Vitro. 2004;18:231–243. doi: 10.1016/j.tiv.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Williams IR, Kupper TS. Immunity at the surface: homeostatic mechanisms of the skin immune system. Life Sci. 1996;58:1485–1507. doi: 10.1016/0024-3205(96)00042-2. [DOI] [PubMed] [Google Scholar]