Abstract
The preBötzinger complex (preBötC) is essential for normal respiratory rhythm generation in rodents, for which the underlying mechanisms remain unknown. Excitatory preBötC pacemaker neurons are proposed to be necessary for rhythm generation. Here we report the presence of a population of preBötC glycinergic pacemaker neurons. We used rhythmic in vitro transverse slice preparations from transgenic mice where neurons expressing the glycine transporter 2 (GlyT2) gene coexpress enhanced green fluorescent protein (EGFP). We combined epifluorescence and whole-cell patch-clamp recording to study preBötC EGFP-labeled, i.e., glycinergic, inspiratory-modulated neurons with pacemaker properties. We defined glycinergic pacemaker neurons as those preBötC EGFP neurons that exhibited the following: (1) ectopic bursting in rhythmic slices when depolarized during their normally silent period and (2) bursting when depolarized in nonrhythmic slices (following AMPA receptor blockade). Forty-two percent of EGFP-labeled neurons were inspiratory (n = 48 of 115), of which 23% (n = 11 of 48 inspiratory; 10% of the total recorded) were pacemakers. We conclude that there is a population of preBötC inspiratory-modulated glycinergic, presumably inhibitory, pacemaker neurons that constitute a substantial fraction of all preBötC pacemaker neurons. These findings challenge contemporary models for respiratory rhythmogenesis that assume the excitatory nature of preBötC pacemaker neurons. Testable and nontrivial predictions of the functional role of excitatory and inhibitory pacemaker neurons need to be proposed and the necessary experiments performed.
Introduction
The preBötzinger complex (preBötC) is essential for normal breathing in rodents (Tan et al., 2008) and is postulated to be an essential site for respiratory rhythm generation (Smith et al., 1991). In brainstem–spinal cord (en bloc) and transverse slice in vitro preparations, respiratory rhythm persists in the absence of postsynaptic inhibition (Feldman and Smith, 1989; Onimaru et al., 1990; Shao and Feldman, 1997; Brockhaus and Ballanyi, 1998). This observation led to the hypothesis that intrinsically rhythmic excitatory pacemaker neurons drive the respiratory rhythm (Smith et al., 1991); preBötC neurons with pacemaker properties dependent on persistent sodium current (INaP) or Ca2+-activated nonspecific cationic current (ICAN) of undetermined neurotransmitter phenotype were subsequently identified (Johnson et al., 1994; Thoby-Brisson and Ramirez, 2001; Del Negro et al., 2002; Peña et al., 2004). Many models for rhythmogenesis predicate a significant role, often obligatory, for excitatory, presumably glutamatergic, pacemaker neurons (Butera et al., 1999a,b; Smith et al., 2000; Del Negro et al., 2001; Rybak et al., 2003, 2008). Pharmacological studies suggest that pacemaker neurons are not obligatory for rhythmogenesis (Del Negro et al., 2002, 2005; Feldman and Del Negro, 2006). Regardless, they could play a role in modulating/stabilizing the rhythm (Purvis et al., 2007). Recently, preBötC pacemakers expressing the vesicular glutamate transporter 2 (VGluT2) and presumably glutamatergic were identified, but they were few in number and may not possess pacemaking properties under normal conditions (St-John et al., 2009).
The preBötC contains excitatory and inhibitory neurons (Stornetta et al., 2003; Winter et al., 2009). How pacemakers are distributed between these two populations is unknown. PreBötC rhythm is abolished in vitro (Greer et al., 1991) and in vivo (Chitravanshi and Sapru, 1996) after blockade of glutamatergic transmission. Although inhibition is not necessary for rhythmogenesis in the preBötC in in vitro slices (Feldman and Smith, 1989; Del Negro et al., 2009), inhibition is important in respiratory patterning (Feldman and Smith, 1989; Bianchi et al., 1995; Shao and Feldman, 1997; Brockhaus and Ballanyi, 1998; Büsselberg et al., 2001; Richter and Spyer, 2001; Ren and Greer, 2006). In some invertebrates, inhibitory pacemakers play a fundamental role in rhythmic behavior (Cardi and Nagy, 1994; Mamiya and Nadim, 2004). Here, we sought to determine whether there are inhibitory neurons with pacemaker properties within the preBötC. We recorded from inspiratory-modulated glycinergic neurons in an in vitro transverse slice preparation from mice with EGFP expressed in GlyT2-containing neurons (Zeilhofer et al., 2005). We used whole-cell recording and epifluorescence to test for two distinct pacemaker properties: (1) ectopic bursting induced by depolarization during their silent period in rhythmic slices and (2) bursting induced by depolarization in nonrhythmic slices (following AMPA receptor blockade). Approximately 23% of these inspiratory-modulated glycinergic neurons had both pacemaker properties. We conclude that the presumption that all preBötC pacemaker neurons are excitatory is incorrect. Establishing the neurotransmitter(s) used by preBötC pacemaker neurons is essential for understanding their functional role, if any, in generating or modulating respiratory pattern, and a prerequisite for validating models that stipulate pacemakers as an essential element of the rhythm generating mechanism. The inspiratory glycinergic, presumably inhibitory, pacemaker neurons in preBötC are a novel class of neurons that may modulate the respiratory network.
Materials and Methods
Medullary slice preparation.
Experiments were performed on transverse brainstem slices generating respiratory-related motor output (Smith et al., 1991) from GlyT2-EGFP mice (Zeilhofer et al., 2005). The Office for the Protection of Research Subjects, University of California Research Committee approved all protocols. Mice (n = 26, P0–P7 from 10 litters) were anesthetized with isoflurane and decerebrated, and the neuraxis was isolated. The brainstem was serially sectioned (Vibratome) in the transverse plane until the nucleus ambiguus and inferior olive were visible. A slice (450–500 μm) containing the preBötC was cut (Del Negro et al., 2002; Ruangkittisakul et al., 2006). The dissection was performed in artificial CSF (ACSF) containing (in mm) 128 NaCl, 3 KCl, 1.5 CaCl2, 1 MgSO4, 23.5 NaHCO3, 0.5 NaH2PO4, and 30 glucose, bubbled with 95% O2/5% CO2 at 27°C. The slice was perfused with ACSF (6 ml/min) in a 1 ml recording chamber.
Electrophysiological recording.
Respiratory-related motor output was recorded from hypoglossal nerves (XIIn) using suction electrodes. To obtain a robust, stable rhythm, ACSF K+ concentration was elevated to 9 mm. Slices were perfused for 30 min before experimental manipulations. XIIn activity was amplified, bandpass filtered (0.3–1 kHz), rectified, and integrated (τ = 20 ms; ∫XIIn). Whole-cell patch-clamp recordings were performed using an Axopatch 200A amplifier (Molecular Devices) in current-clamp mode. preBötC inspiratory neurons were visualized using infrared-enhanced differential interference contrast (IR-DIC) video microscopy. Electrodes were pulled from borosilicate glass (outer diameter, 1.5 mm; inner diameter, 0.86 mm) and filled with solution containing the following (in mm): 130 K-gluconate, 10 NaCl, 10 HEPES, 0.1 CaCl2, 1.1 EGTA, 2 Mg-ATP, and 0.3 GTP-Na, pH 7.3; in some experiments, 0.01% rhodamine was added. Electrophysiological signals were low-pass filtered and digitized at 4 kHz using pCLAMP software and a Digidata 1320 AD/DA board (Molecular Devices).
Neuron visualization.
We detected EGFP-labeled neurons using an upright microscope (DMLFS, Leica) equipped for epifluorescence, a 63× objective (HCX/APO 0.90 numerical aperture, Leica), and dichroics (I3 and N2.1, Leica). In some experiments, 15–30 images were acquired with a CCD camera (Watec), digitized (Scion LG-3), and averaged (Scion Image). Image processing was performed in ImageJ (National Institutes of Health).
Drugs.
Drugs were bath applied. 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μm) and flufenamic acid (FFA, 10–500 μm) were obtained from Sigma Chemical. Riluzole (10–20 μm) was obtained from Tocris Bioscience.
Immunohistochemistry.
Mice (n = 6, P4–P21 from 3 litters) were anesthetized and transcardially perfused with 4% paraformaldehyde in PBS (PFA/PBS). The brainstems were dissected and placed in 4% PFA/PBS overnight, cryoprotected in 30% sucrose/PBS, embedded in OCT medium, and cut in 40 μm transverse sections with a freezing microtome. Freely floating sections were processed for neurokinin-1 receptor (NK1R) immunolabeling (Gray et al., 1999). The preBötC was identified in transverse sections as the NK1R-immunoreactive (ir) zone ventral to the subcompact region of the nucleus ambiguus (Gray et al., 2001),
Imaging.
Confocal image stacks were acquired with a Zeiss LSM 510 microscope and software. Lasers (488 and 543 nm) and appropriate filters were used to visualize EGFP and NK1R-ir. To avoid cross talk between channels, and therefore false colocalization of EGFP and NK1R-ir signals, we acquired images in multitrack mode or ensured that the NK1R-ir signal was unaltered after modifications of the power of the 488 nm laser. We used 40× and 63× objectives to estimate soma size and determine colocalization of the EGFP and NK1R-ir signals.
No striking qualitative differences between the shapes of EGFP-labeled and NK1R-ir somas were found. Somas were approximately spherical. Therefore we measured the diameter at the largest cross-sectional region of the neuron using confocal stacks. We did not explore differences in their neuropil nor attempt other measures. Data are expressed as the mean ± SEM. A t test was used to determine statistical differences between mean values.
Results
Glycinergic inspiratory neurons
Under epifluorescence microscopy and regardless of size or shape, EGFP-labeled neurons (n = 115) from P0–P7 GlyT2-EGFP neonatal mice were whole-cell patch clamped at their somas. Using current-clamp mode, we maintained neuronal Vm at approximately −60 mV during the period between XIIn bursts. Fifty-eight percent (n = 67/115) of these neurons were either silent or had an irregular firing pattern, i.e., were non-respiratory-modulated, and 42% (n = 48/115) had inspiratory-modulated membrane depolarization and spiking. Of these inspiratory-modulated neurons, 27% (13/48) showed delayed excitation when depolarized by a square pulse from a hyperpolarized (−70 mV) membrane potential, a signature of type 1 neurons (Rekling et al., 1996; Gray et al., 1999), and the remaining 73% (35/48) showed a sag during a hyperpolarizing square pulse and postinhibitory rebound, a signature of type 2 neurons.
Pacemaker properties in EGFP-labeled neurons
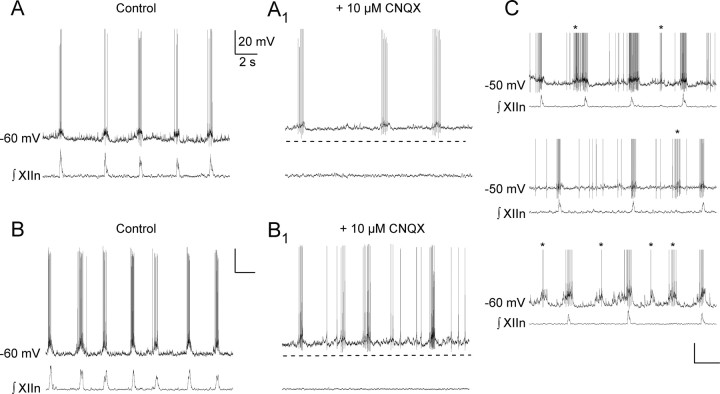
In rhythmic slices, when EGFP-labeled inspiratory neurons (n = 48) were depolarized by current injection, 37% (n = 18/48) produced ectopic bursts of action potentials, i.e., burst out of phase with XIIn inspiratory bursts (Fig. 1C). For these latter neurons, rhythm in the slice was abolished by bath application of CNQX (10 μm), blocking fast glutamatergic transmission; upon depolarization, 61% (n = 11/18) showed voltage-dependent intrinsic bursting (Fig. 1A,B).
Figure 1.
Examples of preBötC GlyT2-EGFP pacemaker neurons. A, Respiratory-modulated discharge in a GlyT2-EGFP pacemaker neuron current clamped at interburst Vm ≈ −60 mV. A1, Bursting activity in 10 μm CNQX bath applied. Network activity is blocked. B, B1, Another example of a respiratory-modulated discharge and bursting in a GlyT2-EGFP neuron. C, Examples of respiratory-modulated discharge and “ectopic bursts” (asterisks) after depolarizing Vm. Action potentials have been truncated.
To determine the presence of INaP or ICAN, we bath applied the ICAN blocker FFA or the INaP blocker riluzole. EGFP-labeled preBötC pacemaker neurons were sensitive to 10 μm riluzole, which abolished bursting within 3 min (n = 7/7; included in this group are two neurons that were insensitive to 10 μm FFA, but further application of 10 μm riluzole abolished intrinsic bursting). In a small sample, EGFP-labeled inspiratory preBötC pacemaker neurons were also extremely sensitive to FFA, which rapidly abolished bursting at 500 μm (n = 2/2 tested) or 100 μm (n = 2/2 tested).
There is an early postnatal age dependence of ICAN-dependent pacemaker activity (Peña et al., 2004; Del Negro et al., 2005). Our small dataset suggests a developmental dependence of glycinergic pacemakers. We infrequently found EGFP-labeled inspiratory preBötC pacemaker neurons in transverse slices from P0–P3 mice (n = 3 pacemaker neurons from 11 slices). The likelihood of finding EGFP-labeled pacemaker neurons tripled in P4–P7 mice (n = 8 neurons from 10 slices).
NK1R expression in EGFP-labeled inspiratory neurons
The preBötC contains a high density of NK1R-ir neurons (Gray et al., 1999; Wang et al., 2001; Pagliardini et al., 2005). Less than 1% (1/140) of preBötC EGFP-labeled neurons were NK1R-ir (n = 140) (Fig. 2B).
Figure 2.
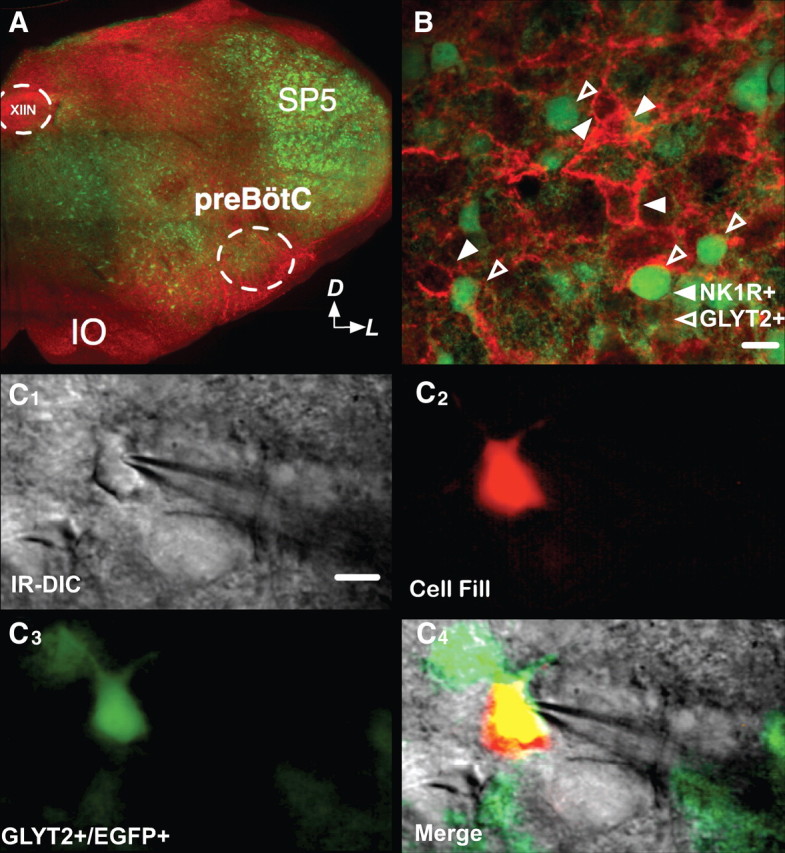
NK1R and GlyT2-EGFP do not colocalize in the preBötC. A, EGFP-labeled neurons in transverse medullary slices of neonatal GlyT2-EGFP mice. NK1R-ir (red) was used to identify the preBötC. Arrows indicate dorsal (D) and lateral (L) orientation of slice. B, We observed little to no colocalization between NK1R-ir and EGFP signal. Note the absence of yellow in the merged channels. C1–C4, Whole-cell patch recordings were made under IR-DIC (C1). Patched cells were tested electrophysiologically for pacemaker properties and filled with rhodamine via the patch electrode (C2). We confirmed that the neurons are GlyT2-EGFP by merging the images taken with EGFP and rhodamine filters (C2–C4). Scale bar, 15 μm. IO, Inferior olive; SP5, spinal trigeminal nucleus; XIIN, hypoglossal nucleus.
We measured soma size of preBötC NK1R-ir (n = 24) and EGFP-labeled (n = 45) neurons from three preparations. Somas of EGFP-labeled neurons were smaller than those of NK1R-ir neurons (soma diameters: EGFP-labeled 13.6 ± 0.5 μm; NK1R-ir 16.3 ± 0.6 μm; p < 0.001).
Discussion
Our principal result is that in the preBötC of neonatal rodents, there is a population of pacemaker neurons that are glycinergic and inspiratory-modulated.
Are the GlyT2-EGFP neurons exclusively glycinergic?
Studies of the anatomy of GlyT2-EGFP mice using immunohistochemistry against glycine or GlyT2 showed that >90% of EGFP fluorescent neurons are glycine-ir (in soma and dendrites) and GlyT2-ir (in axon terminals) (Zeilhofer et al., 2005).
Precursors of GABAergic interneurons in the molecular layer of the cerebellum transiently express GlyT2 during development (Simat et al., 2007). This suggests that, at least in cerebellum, GlyT2 is a marker of immature GABAergic neurons. While Cl− currents are outward in some neurons at early stages of development, activation of glycinergic and GABAA/B receptors in the mouse respiratory network in vitro in all stages of postnatal development hyperpolarizes inspiratory neurons (Ramirez et al., 1996; Zhang et al., 1999), confirming the inhibitory nature of glycinergic and GABAergic neurons. Moreover, glycine elicits IPSPs in preBötC neurons (Shao and Feldman, 1997). We are unaware of experimental evidence suggesting a dual excitatory/inhibitory neuronal phenotype, such as the glutamatergic/GABAergic phenotype expressed by developing hippocampal granule cells (Gutiérrez and Heinemann, 2006). While glycine modulates excitability by acting as coagonist of NMDA receptor, NMDA receptors are not required for generation of respiratory rhythm or motor output (Morgado-Valle and Feldman, 2007) in standard in vitro conditions in the medullary slice.
Here we established the presence of pacemaker properties in EGFP-labeled inspiratory preBötC neurons in GlyT2-EGFP mice. Given the lack of studies of preBötC neurons in GlyT2-EGFP mice that suggest otherwise, we refer to EGFP-labeled neurons in these mice as “glycinergic” with presumptive inhibitory function.
The presence of inspiratory-modulated glycinergic, presumably inhibitory, pacemaker neurons in the preBötC constitutes a novel subpopulation of preBötC neurons that challenges the current models of respiratory rhythm generation where only excitatory pacemakers are stipulated [e.g., Butera et al. (1999a,b), Smith et al. (2000), Del Negro et al. (2001), and Rybak et al. (2003, 2008)]. This stipulation is based on previous recordings of pacemaker neurons in the preBötC, where the nature of neurotransmission (excitatory or inhibitory) was assumed but not determined. The glycinergic pacemakers we identified cannot directly generate inspiratory rhythm in these models, because they would inhibit activity during the inspiratory phase. Recently identified excitatory pacemakers in the preBötC also appear inconsistent with these models insofar as these neurons may not possess pacemaker properties under normal conditions (St-John et al., 2009).
Glycinergic neurons are not NK1R-ir
In the preBötC, glycinergic and NK1R-ir neurons appear to be two distinct, non-overlapping populations, as we found colocalization of NK1R-ir in ≤1% of EGFP-labeled preBötC neurons. This lack of overlap follows from previous work. mRNA encoding GlyT2 is detected only in ∼1% NK1R-ir neurons (Wang et al., 2001). Furthermore, in the ventral respiratory group (VRG) at least 77 ± 9% of NK1R-ir neurons are excitatory, as they contain mRNA that encodes the VGluT2, a reliable marker of glutamatergic neurons (Guyenet et al., 2002).
Can we estimate what fraction of preBötC neurons are GlyT2-EGFP pacemakers?
The rat preBötC contains ∼300 NK1R-ir neurons that represent ∼10% of all preBötC neurons (Gray et al., 1999; Wang et al., 2001). In our experience ∼60% of neurons that we record in active slices from neonatal rodents are inspiratory modulated. Based on our cell counts in the mice studied here, we estimate that ∼20% of all preBötC neurons were EGFP labeled, making GlyT2-EGFP pacemaker neurons ∼2% of all preBötC neurons and ∼3% of preBötC inspiratory neurons (see Table 1). The estimate of the prevalence of pacemaker neurons in randomly recorded preBötC inspiratory neurons (with the presumption of no sampling bias, see below) ranges from 5% (Del Negro et al., 2002) to 25% (Peña et al., 2004). Based on our data and estimates, GlyT2-EGFP pacemaker neurons represent ∼3% of preBötC inspiratory neurons, making them up to ∼50% of preBötC pacemaker neurons in our experimental conditions or as low as ∼10% in different conditions (Peña et al., 2004). Conversely, from ∼50% up to ∼90% of preBötC inspiratory pacemaker neurons may be excitatory.
Table 1.
Estimated numbers of preBötC neurons in various categories
| Neurons | Estimated |
Recorded |
||||||
|---|---|---|---|---|---|---|---|---|
| # Total | % Total | # | % of GlyT2 | # | % GlyT2 inspiratory | # | % Ectopic bursters | |
| PreBötC | 3000 | 100 | ||||||
| NK1R+ | 300 | 10 | ||||||
| GlyT2+ | 600 | 20 | 115 | 100 | ||||
| GlyT2+ noninspiratory | 348 | 12 | 67 | 58 | ||||
| GlyT2+ inspiratory | 252 | 8 | 48 | 42 | 48 | 100 | ||
| GlyT2+ inspiratory ectopic burster | 93 | 3 | 18 | 37 | 18 | 100 | ||
| GlyT2+ inspiratory ectopic burster pacemaker | 57 | 2 | 11 | 61 | ||||
PreBötC, NK1R+, and GlyT2+ were estimated from histological counts. Other estimated numbers/percentages were extrapolated from recordings of neurons of various types.
A recent blind-patch study in perfused in situ preparations of juvenile (P14–P21) and neonatal (P6–P8) rats identified four preBötC intrinsic pacemaker neurons (St-John et al., 2009); three were positive for VGluT2, suggesting that they were excitatory. The remaining VGluT2-positive pacemakers (15/19) were located caudal to the preBötC in a region that does not appear obligatory for rhythmogenesis (Smith et al., 1991). Many of these VGluT2-positive neurons exhibited pacemaker properties only after blocking Cl−-mediated inhibition, elevating local K+, or by applying sodium cyanide, so whether they are pacemakers during normal breathing, or contribute to the breathing rhythm, remains unresolved.
From our limited sample, GlyT2-EGFP preBötC pacemaker neurons express both INaP and ICAN. Whether GlyT2-EGFP preBötC pacemaker neurons have different biophysical, synaptic, or network properties from those of excitatory preBötC neurons also remains unresolved.
Neurotransmitter phenotype of preBötC pacemaker neurons
The hypothesis that pacemaker neurons play an obligatory role in respiratory rhythmogenesis in vitro arose from the observation that inhibition is not essential for generation of inspiratory rhythm (Feldman and Smith, 1989). The presence of inspiratory-modulated preBötC pacemaker neurons is well documented (Smith et al., 1991; Johnson et al., 1994; Thoby-Brisson and Ramirez, 2001; Del Negro et al., 2002; Peña et al., 2004), but in none of these papers (cf. St-John et al., 2009) was the transmitter phenotype determined. In the authors' interpretations of these various papers, and in models that cite them as evidence of the presence of pacemaker neurons [e.g., Butera et al. (1999a,b), Smith et al. (2000), Del Negro et al. (2001), and Rybak et al. (2003, 2008)], they are universally presumed to be excitatory. However, there is no a priori reason that pacemaker neurons need to be excitatory to either generate or modulate rhythm.
Models of respiratory rhythm in vitro recognize that inhibitory interactions are not obligatory as rhythm persists when synaptic inhibition is blocked. Nonetheless, such interactions could play a role in rhythmogenesis under normal conditions in more intact preparations, even in vivo, and certainly could affect patterning of respiratory output, even in slices. While inhibitory pacemaker neurons are obligatory for rhythmogenesis in other systems, such as in the generation of the pyloric rhythm in invertebrates (Cardi and Nagy, 1994; Mamiya and Nadim, 2004), we propose that the principal role of glycinergic preBötC pacemaker neurons is in modulation/stabilization of respiratory rhythm.
Neuron size and sampling bias
By virtue of exploiting visualized recording, we were limited to recording relatively superficial neurons in the slice, up to ∼120 μm deep. However, patch-clamping methodologies used in several studies of preBötC pacemaker neurons can have a sampling bias toward neurons with larger somas with strong inspiratory drive. In the present sample, size was not an explicit or implicit criterion. There also is sampling bias in studies using blind patch recording (Johnson et al., 1994; Thoby-Brisson and Ramirez, 2001; Peña et al., 2004; St-John et al., 2009) and extracellular recording (Del Negro et al., 2001), which can record neurons much deeper in the slice but require detecting strong, likely somatic, electrical signals. This increases the probability of recording from larger somas, reducing the probability of recording smaller neurons that, according to our observations, are more likely to be glycinergic.
Establishing the role of any preBötC neuronal class, including pacemakers, in respiratory rhythm generation requires determination of their neurotransmitter phenotype. A basic requirement for validation of models that stipulate that excitatory pacemaker neurons are essential for rhythm generation in vitro is that this is actually the case. The demonstration of preBötC glycinergic pacemaker neurons suggests that this remains to be done.
Footnotes
This research was supported by National Institutes of Health Grants R01 HL-040959 and F32-HL087589. C.M.-V. is a Parker B. Francis fellow in Pulmonary Research (Francis Family Foundation, Kansas City, MO). We thank Dr. S. du Lac for providing GlyT2-EGFP mice, which are derived from Dr. Zeilhofer's GlyT2-EGFP lines; Dr. M. Bagnall for sharing preliminary data and helpful discussions throughout; and M. Fuentes and G. Li for technical assistance.
References
- Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Büsselberg D, Bischoff AM, Paton JF, Richter DW. Reorganisation of respiratory network activity after loss of glycinergic inhibition. Pflugers Arch. 2001;441:444–449. doi: 10.1007/s004240000453. [DOI] [PubMed] [Google Scholar]
- Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. I. Bursting pacemaker neurons. J Neurophysiol. 1999a;82:382–397. doi: 10.1152/jn.1999.82.1.382. [DOI] [PubMed] [Google Scholar]
- Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. II. Populations of coupled pacemaker neurons. J Neurophysiol. 1999b;82:398–415. doi: 10.1152/jn.1999.82.1.398. [DOI] [PubMed] [Google Scholar]
- Cardi P, Nagy F. A rhythmic modulatory gating system in the stomatogastric nervous system of Homarus gammarus. III. Rhythmic control of the pyloric CPG. J Neurophysiol. 1994;71:2503–2516. doi: 10.1152/jn.1994.71.6.2503. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. NMDA as well as non-NMDA receptors mediate the neurotransmission of inspiratory drive to phrenic motoneurons in the adult rat. Brain Res. 1996;715:104–112. doi: 10.1016/0006-8993(95)01565-5. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Johnson SM, Butera RJ, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. III. Experimental tests of model predictions. J Neurophysiol. 2001;86:59–74. doi: 10.1152/jn.2001.86.1.59. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Feldman JL. Respiratory rhythm: an emergent network property? Neuron. 2002;34:821–830. doi: 10.1016/s0896-6273(02)00712-2. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Kam K, Hayes JA, Feldman JL. Asymmetric control of inspiratory and expiratory phases by excitability in the respiratory network of neonatal mice in vitro. J Physiol. 2009;587:1217–1231. doi: 10.1113/jphysiol.2008.164079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Smith JC. Cellular mechanisms underlying modulation of breathing pattern in mammals. Ann N Y Acad Sci. 1989;563:114–130. doi: 10.1111/j.1749-6632.1989.tb42194.x. [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol. 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez R, Heinemann U. Co-existence of GABA and Glu in the hippocampal granule cells: implications for epilepsy. Curr Top Med Chem. 2006;6:975–978. doi: 10.2174/156802606777323692. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci. 2002;22:3806–3816. doi: 10.1523/JNEUROSCI.22-09-03806.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Funk GD, Feldman JL. Pacemaker behavior of respiratory neurons in medullary slices from neonatal rat. J Neurophysiol. 1994;72:2598–2608. doi: 10.1152/jn.1994.72.6.2598. [DOI] [PubMed] [Google Scholar]
- Mamiya A, Nadim F. Dynamic interaction of oscillatory neurons coupled with reciprocally inhibitory synapses acts to stabilize the rhythm period. J Neurosci. 2004;24:5140–5150. doi: 10.1523/JNEUROSCI.0482-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado-Valle C, Feldman JL. NMDA receptors in preBötzinger complex neurons can drive respiratory rhythm independent of AMPA receptors. J Physiol. 2007;582:359–368. doi: 10.1113/jphysiol.2007.130617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Inhibitory synaptic inputs to the respiratory rhythm generator in the medulla isolated from newborn rats. Pflugers Arch. 1990;417:425–432. doi: 10.1007/BF00370663. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Adachi T, Ren J, Funk GD, Greer JJ. Fluorescent tagging of rhythmically active respiratory neurons within the pre-Bötzinger complex of rat medullary slice preparations. J Neurosci. 2005;25:2591–2596. doi: 10.1523/JNEUROSCI.4930-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Purvis LK, Smith JC, Koizumi H, Butera RJ. Intrinsic bursters increase the robustness of rhythm generation in an excitatory network. J Neurophysiol. 2007;97:1515–1526. doi: 10.1152/jn.00908.2006. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJ, Richter DW. Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. J Physiol. 1996;491:799–812. doi: 10.1113/jphysiol.1996.sp021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Champagnat J, Denavit-Saubié M. Electroresponsive properties and membrane potential trajectories of three types of inspiratory neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1996;75:795–810. doi: 10.1152/jn.1996.75.2.795. [DOI] [PubMed] [Google Scholar]
- Ren J, Greer JJ. Modulation of respiratory rhythmogenesis by chloride-mediated conductances during the perinatal period. J Neurosci. 2006;26:3721–3730. doi: 10.1523/JNEUROSCI.0026-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 2001;24:464–472. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon BY, Ma Y, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, St-John WM, Paton JF, Pierrefiche O. Endogenous rhythm generation in the pre-Bötzinger complex and ionic currents: modelling and in vitro studies. Eur J Neurosci. 2003;18:239–257. doi: 10.1046/j.1460-9568.2003.02739.x. [DOI] [PubMed] [Google Scholar]
- Rybak IA, O'Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol. 2008;100:1770–1799. doi: 10.1152/jn.90416.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Bötzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Simat M, Ambrosetti L, Lardi-Studler B, Fritschy JM. GABAergic synaptogenesis marks the onset of differentiation of basket and stellate cells in mouse cerebellum. Eur J Neurosci. 2007;26:2239–2256. doi: 10.1111/j.1460-9568.2007.05846.x. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Butera RJ, Koshiya N, Del Negro C, Wilson CG, Johnson SM. Respiratory rhythm generation in neonatal and adult mammals: the hybrid pacemaker-network model. Respir Physiol. 2000;122:131–147. doi: 10.1016/s0034-5687(00)00155-9. [DOI] [PubMed] [Google Scholar]
- St-John WM, Stornetta RL, Guyenet PG, Paton JF. Location and properties of respiratory neurones with putative intrinsic bursting properties in the rat in situ. J Physiol. 2009;587:3175–3188. doi: 10.1113/jphysiol.2009.170308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC, Guyenet PG. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Bötzinger complex. J Comp Neurol. 2003;455:499–512. doi: 10.1002/cne.10504. [DOI] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez JM. Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J Neurophysiol. 2001;86:104–112. doi: 10.1152/jn.2001.86.1.104. [DOI] [PubMed] [Google Scholar]
- Wang H, Stornetta RL, Rosin DL, Guyenet PG. Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. J Comp Neurol. 2001;434:128–146. doi: 10.1002/cne.1169. [DOI] [PubMed] [Google Scholar]
- Winter SM, Fresemann J, Schnell C, Oku Y, Hirrlinger J, Hülsmann S. Glycinergic interneurons are functionally integrated into the inspiratory network of mouse medullary slices. Pflugers Arch. 2009;458:459–469. doi: 10.1007/s00424-009-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU, Studler B, Arabadzisz D, Schweizer C, Ahmadi S, Layh B, Bösl MR, Fritschy JM. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J Comp Neurol. 2005;482:123–141. doi: 10.1002/cne.20349. [DOI] [PubMed] [Google Scholar]
- Zhang W, Elsen F, Barnbrock A, Richter DW. Postnatal development of GABAB receptor-mediated modulation of voltage-activated Ca2+ currents in mouse brain-stem neurons. Eur J Neurosci. 1999;11:2332–2342. doi: 10.1046/j.1460-9568.1999.00655.x. [DOI] [PubMed] [Google Scholar]