Abstract
Human embryonic stem cell (ESC)–derived neural cells are a potential cell source for neural tissue regeneration. Understanding the biochemical and biophysical regulation of neural differentiation and axon growth will help us develop cell therapies and bioactive scaffolds. We demonstrated that basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) had different effects on human ESC differentiation into neural cells. EGF was more effective in inducing expression of neuron and glial markers and cell extensions. In addition to biochemical cues, poly(l-lactic acid) scaffolds with aligned nanofibers increased axon growth from ESC-derived neural cells, demonstrating the significant effects of biophysical guidance at nanoscale. To combine the biochemical and biophysical cues, bFGF and EGF were either adsorbed or bound to heparin on nanofibrous scaffolds. EGF, but not bFGF, was effectively adsorbed onto nanofibers. However, adsorbed EGF and bFGF did not effectively enhance axon growth. In contrast, immobilization of bFGF or EGF onto nanofibers using heparin as the adapter molecule significantly promoted axon growth. This study elucidated the effect of bFGF and EGF in neural differentiation and axon growth, and demonstrated a method to immobilize active bFGF and EGF onto aligned nanofibers to promote neural tissue regeneration.
Introduction
Damage to the central nervous system in the adult brain or spinal cord cannot heal spontaneously due to the incapability of neuron cell replication. Axons in the adult peripheral nervous system can regenerate to a certain extent; however, functional recovery is often incomplete. Therefore, expandable sources of neural cells and optimal scaffolds that can facilitate neural cell differentiation and axon growth are needed for effective neural tissue engineering.
The pluripotency and self-renewal capability of human embryonic stem cells (ESCs)1 make these cells an ideal source to derive neural cells for tissue engineering applications.2 To harness the therapeutic potential of human ESCs, we need to establish methods for directed cell differentiation and cell delivery. There is evidence that biochemical factors can promote stem cell differentiation into various stages and types of neural cells3,4 such as neural progenitor cells (NPCs),5,6 mature neurons,7 dopaminergic neurons,8 and glial cells.9 However, the exact roles of various growth factors in neural differentiation remain to be elucidated to improve the efficiency of cell differentiation. Among the studies on neural differentiation, basic fibroblast growth factor (bFGF) is known to be an important growth factor that increases neurite extensions in PC12 cells,10 increases neurogenesis from human NPCs,11 and stimulates neural cell differentiation.4 bFGF is also used to maintain the undifferentiated human ESCs.12 These results suggest that the effects of growth factors depend on the cell type, differentiation stage, and other cofactors. Similar to bFGF, epidermal growth factor (EGF) has been used to differentiate and maintain NPCs.4 Early studies in rat13 and mouse14 models have shown that sustained EGF treatment increases NPC differentiation into the glial lineage, and more recent studies with human NPCs show an increase in radial glial differentiation.9 In this study, we directly compared the effects of bFGF and EGF on gene expression of neural markers during human ESC differentiation, and determined their effects on axon growth.
In addition to soluble growth factors, the extracellular matrix (ECM) also affects cell differentiation and growth. Cell–ECM interactions are important in neural development.15 For example, matrix protein such as laminin is often used as a substrate for neural differentiation and culture, and has been shown to increase neurite extensions,16 as well as promote ESC differentiation into the neural lineage.17 Further, there is evidence that biophysical property of ECM such as topography can regulate neural differentiation and axon growth. For example, nanofibrous scaffold induces neural gene expression in mesenchymal stem cells.18 Micro- and nanofibers guide axon growth from dorsal root ganglion tissue, which can be further enhanced by laminin coating and immobilized bFGF.19,20 Here, we used neural cells derived from human ESCs to evaluate the effectiveness of growth factors bFGF and EGF, in combination with aligned nanofibers, in promoting axon growth from ESC-derived neural cells.
Materials and Methods
ESC culture and ESC differentiation into neural cells
Human ESC line H9 (Wicell, Madison, WI)21 was cultured as previously described.22 To derive neural cells from ESCs, ESCs were detached with the treatment of 1 mg/mL collagenase and 0.5 mg/mL dispase (Invitrogen, Carlsbad, CA) in knockout Dulbecco's modified Eagle's medium/F12 for 20 min. The colonies were gently resuspended in B27 medium (Dulbecco's modified Eagle's medium/F12 +2% B27 supplement, 1% L-glutamine, and 1% nonessential amino acid [NEAA]; Invitrogen) and grown for 5 days in nonadhesive 6-well culture dishes (Nunc, Rochester, NY) to form embryoid bodies (EBs). EBs were then plated onto tissue culture dishes coated with laminin (1 mg/mL; Invitrogen) and polyornithine (0.1 mg/mL; Sigma, St. Louis, MO), and grown for 2–3 days in B27 medium supplemented with 20 ng/mL bFGF (Peprotech, Rocky Hill, NJ) to allow rosette formation. After rosettes formed, the rosettes were manually collected and used in experiments.
Immunofluorescent staining and microscopy
Samples were fixed and stained as described previously.22 Primary antibodies against the following proteins were used: nestin (NES; goat; Santa Cruz Biotechnology, Santa Cruz, CA), class III β-tubulin (TUBB3; rabbit; Sigma), glial fibrillary acidic protein (GFAP; guinea pig; Advanced Immunochemical, Long Beach, CA), bFGF (rabbit; Santa Cruz Biotechnology), and EGF (rabbit; Chemicon).
Phase-contrast images were taken with a Nikon TE300 microscope. Fluorescent images were collected using either a fluorescence microscope (Nikon or Zeiss microscope) or a Leica TCS SL confocal microscopy system. Images were collected with same settings for software and hardware.
RNA isolation and quantitative polymerase chain reaction
RNA was extracted and quantitative polymerase chain reaction was run as previously described.23 The primers used are listed in Table 1. Relative expression of genes in each sample was normalized with 18S rRNA level in the same sample.
Table 1.
Primers Used for Quantitative Reverse Transcription-Polymerase Chain Reaction
| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
|---|---|---|
| GFAP | GAAAGAGATCCGCACGCAGTA | GCAAACTTGGAGCGGTACCA |
| KLF4 | TCCTTCCTGCCCGATCAG | GGCATGAGCTCTTGGTAATGG |
| c-Myc | GGACCCGCTTCTCTGAAAGG | GAGGCTGCTGGTTTTCCACTA |
| Nanog | TGCCTCACACGGAGACTGTC | TGCTATTCTTCGGCCAGTTG |
| NEF3 | CGTCATTTGCGCGAATACC | TCACCCTCCAGGAGTTTTCTGTA |
| NES | ACTCCCGGCTGCAAACAC | AGCTTGGGGTCCTGAAAGCT |
| Oct-4 | CGACCATCTGCCGCTTTGAG | CCCCCTGTCCCCCATTCCTA |
| SOX2 | CACTGCCCCTCTCACACATG | CCCATTTCCCTCGTTTTTCTT |
| TH | TGTCCACGCTGTACTGGTTCAC | CGGCACCATAGGCCTTCA |
| TUBB3 | GGGCCAAGTTCTGGGAAGTC | CGAGTCGCCCACGTAGTTG |
GFAP, glial fibrillary acidic protein; NEF3, neurofilament 3; NES, nestin; TH, tyrosine hydroxylase; TUBB3, class III β-tubulin.
Fabrication of nanofibrous scaffolds
Biodegradable poly(l-lactic acid) (PLLA; 1.09 dL/g inherent viscosity; Lactel Absorbable Polymers, Pelham, AL) was used to fabricate nanofibrous scaffolds by electrospinning as described previously.20 The PLLA solution (18% w/v in hexafluoro-isopropanol [HFIP]) was delivered by a programmable pump to a grounded collecting drum in a high electric field, resulting in nanofibrous membranes. The alignment of the nanofibers was produced during the electrospinning process. For random nanofibers, the collecting drum rotated slowly. For aligned nanofibers, the drum spun at ∼800 rpm while fibers were collected. Nanofibrous scaffolds were ∼100 μm in thickness. Scanning electron microscopy was used to observe the organization and alignment of nanofibers.
Heparin conjugation to nanofibers
Heparin was covalently crosslinked to the surfaces of PLLA nanofibers. First, the density of reactive carboxylic groups on the PLLA nanofibers was increased by briefly treating the scaffolds with 0.01 N NaOH (Sigma). Heparin (Sigma) molecules were covalently attached to nanofibers using crosslinkers 1-ethyl-3-(3-imethylaminopropyl) carbodiimide hydrochloride and N-hydroxysulfosuccinimide (Pierce Biotechnology, Rockford, IL). Any remaining reactive sites were blocked by incubating the samples in 10% w/v glycine in phosphate-buffered saline. Then, bFGF (100 ng/cm2; Peprotech) or EGF (100 ng/cm2; R&D Systems, Minneapolis, MN) was incubated with the nanofibrous scaffold to allow for their binding to heparin and immobilization on the surface of nanofibers, followed by polyornithine (0.1 mg/mL; Sigma) and laminin (10 μg/cm2; Invitrogen) in phosphate-buffered saline. For passive adsorption, the same concentration of bFGF or EGF was incubated with NaOH-treated scaffolds, and then treated with polyornithine and laminin as described.
Immunofluorescent staining was used to observe growth factor attachment to nanofibers. The difference in the amount of growth factor on the nanofibers was quantified by measuring the mean gray value of grayscale images using Image J.
Statistical analysis
Each experiment was repeated independently for at least three times. Quantitative polymerase chain reaction data were analyzed by normalizing values from individual experimental samples with corresponding controls. The normalized ratios were then pooled from at least three independent experiments. Since the normalization resulted in a constant value of “1” for all control samples, statistical significance was determined using a log-transformed one-sample t-test. All other quantitative data were analyzed using Holm's t-test. Asterisk (*) indicates statistical significance (p < 0.05). Bar graphs are presented as mean ± standard error. For the measurement of neurite length, at least 50 neurites were measured for each group.
Results
ESC differentiation into neural cells
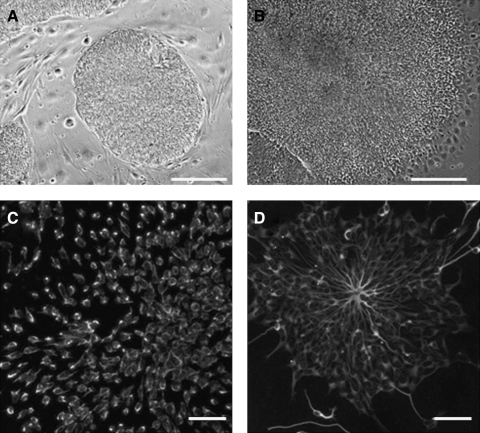
Previous studies have shown that laminin and bFGF promote differentiation of human ESCs into neural lineages.4 Here, we used a similar protocol to differentiate ESCs into neural cells. Figure 1 shows the cells at different stages of differentiation. Undifferentiated ESCs (Fig. 1A) were first cultured in suspension as EBs, plated onto laminin/polyornithine-coated dishes to form rosettes (Fig. 1B), and manually detached and expanded in suspension. After the expansion in suspension, NES-positive cells were found in the rosettes (Fig. 1C). With further differentiation, many cell clusters were stained positive for neural cell marker TUBB3 (Fig. 1D).
FIG. 1.
Derivation of neural cell from ESCs. (A) Undifferentiated H9 ESCs on mouse embryonic fibroblast feeders. (B) ESC-derived rosettes growing on laminin surfaces for 2 days. (C, D) After 10 days of culture, immunofluorescence staining shows neural cell phenotype, expressing (C) nestin and (D) TUBB3. Scale bars = 200 μm. ESC, embryonic stem cell; TUBB3, class III β-tubulin.
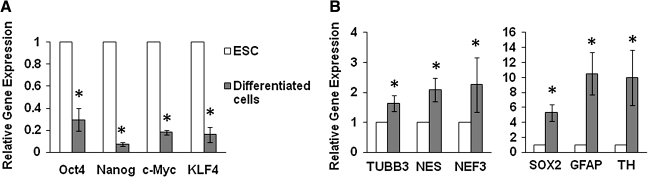
Gene expression analysis showed that ESC markers (Oct-4 and Nanog) and genes involved in ESC growth (c-Myc and KLF4) were significantly decreased after 14 days of culture (Fig. 2A), indicating a loss of ESC phenotype and the differentiation of ESCs. In contrast, neural progenitor markers (NES and SOX2) and neural cell markers (neurofilament 3 [NEF3], TUBB3, GFAP, and tyrosine hydroxylase) all increased (Fig. 2B), indicating successful neural differentiation.
FIG. 2.
Gene expression of human ESC-derived neural cells (after 14 days of culture) compared to undifferentiated ESC controls. (A) Significant decrease of Oct-4, Nanog, c-Myc, and KLF4 upon ESC differentiation. (B) Significant increase of neural markers TUBB3, NES, NEF3, GFAP, SOX2, and TH. *p < 0.05 (three experiments) when compared with ESCs (control) using log-transformed one-sample t-test. TUBB3, βIII-tubulin; NES, nestin; NEF3, neurofilament 3; GFAP, glial fibrillary acidic protein; TH, tyrosine hydroxylase.
Soluble growth factor EGF and bFGF had different effects on expression of neural markers
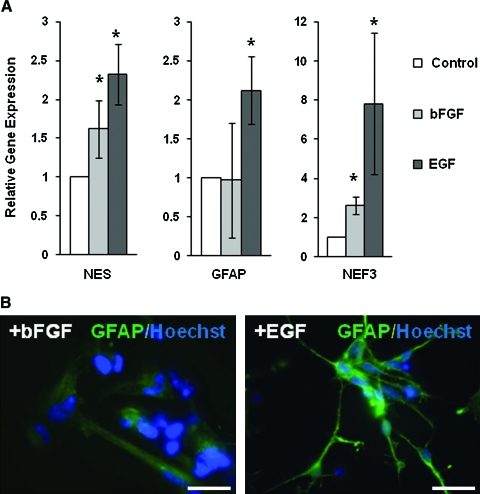
To determine the effects of EGF and bFGF on neural differentiation, rosettes were cultured on laminin-coated surfaces for 2 weeks in the absence or presence of EGF or bFGF. As shown in Figure 3A, gene expression of neural progenitor marker NES and glial cell marker GFAP was significantly increased by EGF treatment compared to controls (without growth factor). Neuronal marker NEF3 was also increased by EGF treatment in addition to glial marker. In contrast, bFGF increased the expression of NES and NEF3 to a lesser extent and did not increase expression of glial marker GFAP. The morphology of the cells treated with soluble bFGF was also different, with the EGF-treated cells displaying more cell extensions as in mature Schwann cells and oligodendrocytes (Fig. 3B). In addition, neuron cells in both growth factor treatment groups stained positive for GFAP, although EGF-treated cells had significantly stronger staining.
FIG. 3.
Effects of bFGF or EGF on gene and protein expression in ESC-derived neural cells. ESC-derived neural cells were cultured for 2 weeks in B27 medium supplemented with or without either 20 ng/mL of bFGF or 20 ng/mL of EGF. (A) Significant increase in NES and NEF3 expression with both GF treatments, but significant GFAP increase in EGF treatment only. (B) Immunofluorescent staining of neural cells shows positive staining for GFAP with GF treatments, but stronger staining and more cell extensions with EGF. Scale bars indicate 200 μm. *p < 0.05 (at least three experiments) when compared with the control samples (in B27 medium without EGF or bFGF) using log-transformed one-sample t-test. bFGF, basic fibroblast growth factor; EGF, epidermal growth factor; GF, growth factor. Color images available online at www.liebertonline.com/ten.
Aligned nanofibers enhanced axon growth
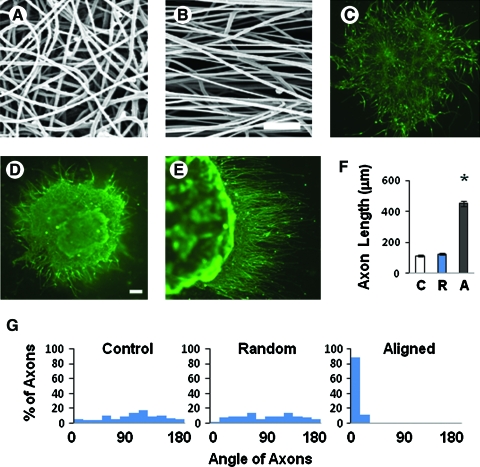
Axon growth is an important process during neural tissue regeneration. Previously, we had shown that aligned nanofibers increase axon outgrowth from dorsal root ganglion tissues.20 Here, we examined the effects of aligned nanofibers on axon growth from ESC-derived neural cells. Random (Fig. 4A) or aligned (Fig. 4B) PLLA nanofiber scaffolds were fabricated and coated with laminin/polyornithine. Rosettes were seeded onto the nanofibrous scaffolds for 1 week, and neural differentiation occurred on the nanofibrous scaffolds as on culture dishes. However, axons on culture dishes (Fig. 4C) and random nanofibers (Fig. 4D) extended randomly, but on aligned nanofibers axons grew and aligned in the direction of the nanofibers (Fig. 4E). Further, aligned nanofibers significantly increased axon length (Fig. 4F) in addition to guiding the direction of axon growth (Fig. 4G).
FIG. 4.
Effects of nanofibers on axon growth from ESC-derived neural cells. Rosettes were grown for a week on culture dish, random fibers, and aligned fibers coated with laminin and polyornithine. (A, B) Scanning electron microscopy images of scaffolds with either (A) random or (B) aligned nanofibers. (C–E) Immunofluorescence staining of TUBB3 in neural cells on (C) culture dish (control), (D) random nanofibers, and (E) aligned nanofibers (horizontal direction) after 2 weeks of culture in B27 medium. (F) Quantification of axon extension on culture dish, random nanofibers, and aligned nanofibers. *p < 0.05 compared to the samples on random nanofibers. At least 50 axons in each group were measured. Scale bars = 50 μm. C, control; R, random nanofibers; A, aligned fibers. (G) Quantification of the angles of axons on culture dish, random nanofibers, and aligned nanofibers. The angles of aligned nanofiber were about zero degree. Color images available online at www.liebertonline.com/ten.
Immobilization of growth factors on nanofibers further increased axon growth
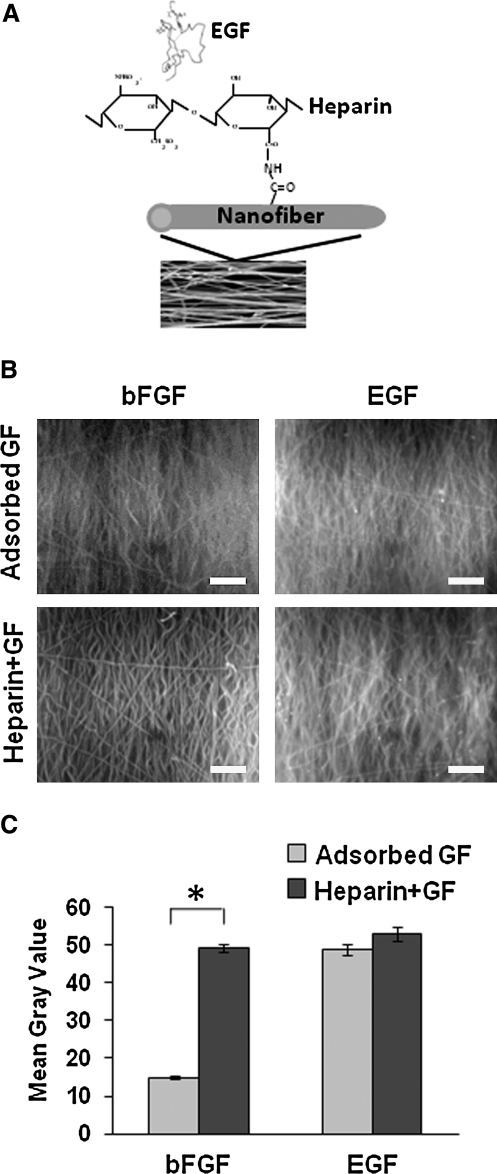
To combine the biochemical (bFGF and EGF) and biophysical (aligned nanofibers) factors and make it deliverable for in vivo therapies, we developed methods to immobilize bFGF and EGF. Heparin functionalization has been shown to be an effective way to attach bFGF to nanofibers and preserve its activity.20 Here, we used heparin as an adaptor molecule to attach bFGF and EGF to nanofibers (Fig. 5A). As a comparison, bFGF and EGF were also adsorbed onto nanofibers without heparin functionalization. The immobilization of growth factors was observed using immunofluorescence staining for bFGF or EGF. As shown in Figure 5B, both bFGF and EGF adsorbed onto PLLA nanofibers, but bFGF adsorption had much lower efficiency. In addition, bFGF and EGF were successfully immobilized onto nanofibers functionalized by heparin. The mean gray value of the nanofiber brightness was measured at randomly selected areas on the immunofluorescently stained nanofibers to quantify the relative amount of growth factors immobilized on nanofibers (Fig. 5C) and showed the same trend.
FIG. 5.
Characterization of immobilized GF nanofibers. (A) Immobilization of GFs (EGF is shown) on poly(l-lactic acid) nanofibers using covalently attached heparin as a linker molecule. (B) Immunofluorescent staining of adsorbed or immobilized bFGF and EGF on nanofibers. Top row shows passive adsorption of bFGF and EGF onto NaOH-treated nanofibers. Bottom row shows immobilized GF on conjugated heparin. (C) Quantification of GF intensity as a mean gray value. *p < 0.05 (10 samples from three experiments). Scale bars = 200 μm.
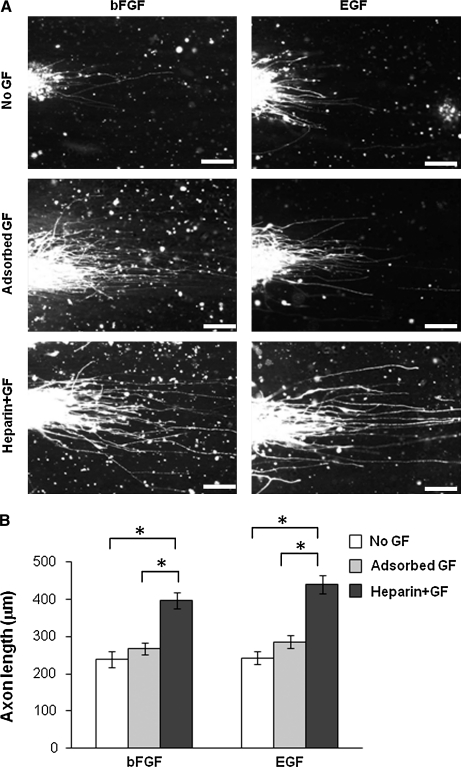
To determine the effects of immobilized bFGF and EGF on axon growth, rosettes were seeded onto nanofibers and cultured for 3 weeks. Immunofluorescent staining did not show significant changes in axon outgrowth from rosettes if bFGF and EGF were adsorbed onto nanofibers (Fig. 6). Conjugation of heparin to nanofibers did not enhance axon growth either (data not shown). However, immobilization of bFGF and EGF onto nanofibers functionalized with heparin promoted axon growth more dramatically, indicating that the bioactivity of bFGF and EGF was preserved through heparin binding but not by adsorption.
FIG. 6.
Effects of immobilized GFs on axon growth on nanofibers. ESCs were used to make rosettes, and rosettes were cultured on nanofibers for 2 weeks. (A) Staining of TUBB3 shows the axon extensions from cell clusters. Scale bars = 100 μm. (B) Quantification of axon extension on nanofibers with GFs bound to conjugated heparin. *p < 0.05 (at least 50 axons were measured in each group).
Discussion
The process of neural repair is complex, and there are needs for cell- and scaffold-based therapies that can address issues with regenerating lost tissue and reconnecting undamaged areas. In this study, neural cell differentiation and axon growth were enhanced using biochemical factors (bFGF and EGF) and topographical cues of nanofibrous scaffolds.
For cell therapy applications, pluripotent stem cells such as ESCs and induced pluripotent stem cells can provide unlimited cell sources, and the development of a practical method for deriving and enriching specific populations of neural cells is desirable. Here we showed that soluble EGF and bFGF had different effects on ESC differentiation into neural cells and that immobilized EGF and bFGF could enhance axon growth. The results from this study can help us enrich specific neural cell types from ESCs and develop bioactive scaffolds for neural regeneration. For example, Schwann cells and oligodendrocytes can be more effectively derived from ESCs and used to treat spinal cord injuries, peripheral nerve injuries, and demyelinating diseases. EGF and bFGF can be used to coat scaffold biomaterials to promote axon regeneration in peripheral nerves and spinal cord.
Biocompatible and biodegradable nanofibrous scaffolds are useful for neural tissue repair and growth. Quantification of axon extension showed a significant increase on aligned nanofibers versus random nanofibers, demonstrating that the importance of biophysical factors at the nanoscale. Thus, aligned nanofibers can be used to fabricate scaffolds to guide and accelerate axon growth during nerve repair. In addition, biochemical factors such as EGF and bFGF can synergize with biophysical guidance to further enhance neural tissue regeneration. Creating bioactive nanofibers with specific growth factors can restrict the bioactivity to the implantation site, increase the efficacy of drug delivery, and avoid side effects on other tissues.
bFGF and EGF were not effective in inducing axon growth if they were directly adsorbed onto PLLA nanofibers. Presumably, passive adsorption results in heterogeneous protein conformation and orientation, which could compromise the activity of immobilized proteins. The conjugation of heparin onto nanofibers not only allows the binding of bFGF and EGF to heparin as in native matrix, but also can help preserve the activity and increase the half-life of bFGF and EGF. Since nanofibrous scaffolds have much larger surface areas than scaffolds with smooth surfaces, it will increase the loading capacity of bioactive factors. In addition, the nanofibrous structure facilitates the interactions of receptors on the surface of axons with immobilized growth factors when axons grow along the nanofibers. The combination of nanofibers and immobilized neurotrophic factors will have tremendous potential for neural tissue engineering applications.
Acknowledgments
The authors would like to thank Wenqian Shen for assistance with protocol development. This work was supported in part by a grant from the TATRC, a grant from Department of Defense, and a College of Engineering Research fund at Berkeley.
Disclosure Statement
No competing financial interests exist.
References
- 1.Keller G. Snodgrass H.R. Human embryonic stem cells: the future is now. Nat Med. 1999;5:151. doi: 10.1038/5512. [DOI] [PubMed] [Google Scholar]
- 2.Zeng X. Rao M.S. Human embryonic stem cells: long term stability, absence of senescence and a potential cell source for neural replacement. Neuroscience. 2007;145:1348. doi: 10.1016/j.neuroscience.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Sujoy K. Dhara S.L.S. Neural differentiation of human embryonic stem cells. J Cell Biochem. 2008;105:633. doi: 10.1002/jcb.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson P. Stice S. Development and differentiation of neural rosettes derived from human embryonic stem cells. Stem Cell Rev. 2006;2:67. doi: 10.1007/s12015-006-0011-1. [DOI] [PubMed] [Google Scholar]
- 5.Reubinoff B.E. Itsykson P. Turetsky T. Pera M.F. Reinhartz E. Itzik A. Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 6.Sunghoi Hong U.J.K. Isacson O. Kim K.-S. Neural precursors derived from human embryonic stem cells maintain long-term proliferation without losing the potential to differentiate into all three neural lineages, including dopaminergic neurons. J Neurochem. 2008;104:316. doi: 10.1111/j.1471-4159.2007.04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter M.K. Inokuma M.S. Denham J. Mujtaba T. Chiu C.-P. Rao M.S. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- 8.Park S. Lee K.S. Lee Y.J. Shin H.A. Cho H.Y. Wang K.C. Kim Y.S. Lee H.T. Chung K.S. Kim E.Y. Lim J. Generation of dopaminergic neurons in vitro from human embryonic stem cells treated with neurotrophic factors. Neurosci Lett. 2004;359:99. doi: 10.1016/j.neulet.2004.01.073. [DOI] [PubMed] [Google Scholar]
- 9.Nelson A.D. Suzuki M. Svendsen C.N. A High concentration of epidermal growth factor increases the growth and survival of neurogenic radial glial cells within human neurosphere cultures. Stem Cells. 2008;26:348. doi: 10.1634/stemcells.2007-0299. [DOI] [PubMed] [Google Scholar]
- 10.Rydel R. Greene L. Acidic and basic fibroblast growth factors promote stable neurite outgrowth and neuronal differentiation in cultures of PC12 cells. J Neurosci. 1987;7:3639. doi: 10.1523/JNEUROSCI.07-11-03639.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson A.D. Svendsen C.N. Low concentrations of extracellular FGF-2 are sufficient but not essential for neurogenesis from human neural progenitor cells. Mol Cell Neurosci. 2006;33:29. doi: 10.1016/j.mcn.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Xu R.-H. Peck R.M. Li D.S. Feng X. Ludwig T. Thomson J.A. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn H.G. Winkler J. Kempermann G. Thal L.J. Gage F.H. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds B. Tetzlaff W. Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Letourneau P. Condic M. Snow D. Interactions of developing neurons with the extracellular matrix. J Neurosci. 1994;14:915. doi: 10.1523/JNEUROSCI.14-03-00915.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manthorpe M. Engvall E. Ruoslahti E. Longo F. Davis G. Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983;97:1882. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma W. Tavakoli T. Derby E. Serebryakova Y. Rao M. Mattson M. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yim E.K. Pang S.W. Leong K.W. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res. 2007;313:1820. doi: 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rangappa N. Romero A. Nelson K.D. Eberhart R.C. Smith G.M. Laminin-coated poly(L-lactide) filaments induce robust neurite growth while providing directional orientation. J Biomed Mater Res. 2000;51:625. doi: 10.1002/1097-4636(20000915)51:4<625::aid-jbm10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Patel S. Kurpinski K. Quigley R. Gao H. Hsiao B.S. Poo M.M. Li S. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7:2122. doi: 10.1021/nl071182z. [DOI] [PubMed] [Google Scholar]
- 21.Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S. Jones J. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 22.Lam H. Patel S. Wong J. Chu J. Lau A. Li S. Localized decrease of beta-catenin contributes to the differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2008;372:601. doi: 10.1016/j.bbrc.2008.05.116. [DOI] [PubMed] [Google Scholar]
- 23.Kurpinski K. Chu J. Hashi C. Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci U S A. 2006;103:16095. doi: 10.1073/pnas.0604182103. [DOI] [PMC free article] [PubMed] [Google Scholar]