Abstract
Mesenchymal stem cells/marrow stromal cells (MSC) are adult multipotent cells that can augment tissue repair. We previously demonstrated that culturing MSC in hypoxic conditions causes upregulation of the hepatocyte growth factor (HGF) receptor c-Met, allowing them to respond more robustly to HGF. MSC preconditioned in hypoxic environments contributed to restoration of blood flow after an ischemic injury more rapidly than MSC cultured in normoxic conditions. We now investigated the specific role of HGF/c-Met signaling in MSC function. An shRNA-mediated knockdown (KD) of c-Met in MSC did not alter their phenotypic profile, proliferation, or viability in vitro. However, we determined that while HGF/c-Met signaling does not play a role in the adipogenic differentiation of the cells, the disruption of this signaling pathway inhibited the ability of MSC to differentiate into the osteogenic and chondrogenic lineages. We next assessed the impact of c-Met KD on human MSC function in a xenogeneic hindlimb ischemia injury model. A 70% KD of c-Met in MSC resulted in a significant decrease in their capacity to regenerate blood flow to the ischemic limb, as compared to the MSC transduced with control shRNA. MSC with only a 60% KD of c-Met exhibited an intermediate capacity to restore blood flow, suggesting that MSC function is sensitive to the dosage of c-Met signaling. The current study highlights the significance of HGF/c-Met signaling in the capacity of MSC to restore blood flow after an ischemic injury and in their ability to differentiate into the osteogenic and chondrogenic lineages.
Introduction
Mesenchymal stem cells/marrow stromal cells (MSC) are adult multipotent cells with defined characteristics1 that are most commonly isolated from the bone marrow, but are also present in other adult tissues. MSC differentiate into multiple tissue-specific cells in vitro and in vivo, including chondrogenic, osteogenic, adipogenic, neural, and cardiac lineages.2–4 An intriguing and widely explored area of research has been focused on using MSC as a cell therapeutic agent, promoting repair after tissue damage. In several injury models such as cardiac infarction and hindlimb ischemia, MSC have been shown to improve tissue regeneration and blood flow recovery.5,6 Although the exact mechanism of how MSC mediate tissue repair is still unknown, the current evidence suggests that their ability to secrete trophic factors is important for their regenerative properties.7
Hepatocyte growth factor (HGF) is a pleiotropic cytokine that has been described to be activated upon tissue injury such as cardiac infarction, liver damage, and skeletal muscle injury.5,8,9 In the adult tissues, HGF serves as a potent mitogen and organotrophic factor, promoting tissue regeneration. It is also an angiogenic factor stimulating endothelial cell proliferation.10,11 Additionally, HGF stimulates cell scattering and directional migration in vitro, and possibly acts as a trophic factor in vivo.12,13 HGF plays an important role in tissue repair. During regeneration, expression of HGF from the damaged tissue is upregulated, and is highest at 48 h.14,15 The role of HGF in repair has been well documented in the liver,16,17 heart,18,19 and muscle.20
An HGF receptor c-Met is expressed on hematopoietic progenitors that respond to HGF21–29 as well as cells that are capable of regenerating liver and muscle. c-Met is expressed on immature human marrow-derived hematopoietic stem/progenitor cells with the phenotype CD34+CD33− and CD34+CD38−, but not on more mature progenitors with the phenotype CD34+CD33+ and CD34+CD38+.28 Recently, it has been reported that MSC express both HGF and its receptor c-Met.13 As predicted, HGF acts as a motogenic stimulus for MSC, increasing their scattering, as shown by the scratch test assay, as well as directional migration/chemotaxis toward an HGF gradient in a transwell assay. Low concentrations of HGF demonstrate a mitogenic effect on MSC, enhancing their proliferation rate.30 Surprisingly, high levels of HGF have a negative effect on MSC proliferation in vitro.12,13 In several in vivo injury models, MSC overexpressing HGF have been shown to improve cardiac tissue regeneration after myocardial infarction injury faster than nontransduced MSC,31 whereas HGF knockdown (KD) in MSC rendered them unable to improve revascularization in the hindlimb ischemia injury model.32 Altogether, these results suggest that HGF does play a key role in ischemic tissue repair.
The tissues and cells of multicellular organisms have an ability to sense low oxygen availability, to ensure an adequate oxygen supply to all tissues. The cellular response to hypoxia is important for tissue survival, and plays a role in cancer and other ischemic diseases. For instance, hypoxia activates multiple proteins that promote the angiogenesis and glucose transport pathways to improve survival in the affected cells.5,33–37 Hypoxic stimulation has been previously shown to activate the HGF/c-Met signaling pathway. c-Met, the receptor for HGF, is rapidly internalized and degraded after ligand binding.38,39 There is evidence suggesting that hypoxia enhances not only expression of c-Met but also its protein stability.40
In our previous studies, we demonstrated that the HGF receptor c-Met is upregulated in MSC that are cultured at 1%–3% oxygen, whereas these cells are then able to mediate tissue repair more robustly at an early time point than MSC cultured at 21% oxygen.5 In the current report, we examine the effect of c-Met KD on the characteristics, differentiation potential, and function of human MSC. First, we assessed the in vitro characteristics of KD MSC, including their cell surface marker profile, colony-forming efficiency, and differentiation potential, and then we determined whether HGF/c-Met signaling is important in the in vivo tissue repair capacity of MSC in a hindlimb ischemia injury model.
Materials and Methods
Cell culture
MSC were cultured from normal human donor marrow aspirates, as we have previously described.41,42 All studies were done in accordance with university regulatory committees. Aspirates were filtered through 70 μm filters (BD Falcon Ref 352350) and the filters were subsequently flushed with the MSC medium (Iscove's Modified dulbecco's modified Eagle's medium [DME], 15% fetal bovine serum, 15% horse serum, 10−6 M hydrocortisone, 10−4 M 2-Mercaptoethanol, and 2 mM L-glutamine), to recover bony spicules, from which MSC were isolated as described.43 Cultures were kept under 80% confluence at all times. All experiments were done with cells passaged 3–9 times.
shRNA transduction
shRNA constructs targeting c-Met and scrambled control were purchased from Sigma (cat# NM_000245, SHC002). Lentiviral vectors expressing these constructs were generated using pHR’8.2deltaR packaging plasmid and pCMV-VSV-G envelope plasmid at 8:1 ratio and transfected into 293T cells in the presence of Fugene (Roche Applied Science; cat. no. 04709691001). MSC were treated with viral supernatant and 10 μg/mL protamine sulfate (Sigma; cat. no. P3369) for 4 h, and after 48 h selected with 1 μg/mL puromycin (Sigma; cat. no. P7255).
Western blot analysis
c-Met KD was verified for each batch of shRNA vector-transduced cells using Western blotting. After washing with cold phosphate-buffered saline (PBS), lysis buffer (1% Triton ×100, 150 mM NaCl, 50 mM HEPES, 1 mM Na3VO4, and protease inhibitor cocktail [Complete Mini; Roche; cat. no. 11836153001]) were directly applied to MSC on tissue culture plates. Western blotting was performed as previously described.44 Protein bands were quantified using Image J software (Rasband, WS; Image J, National Institutes of Health, http://rsb.info.nih.gov/ij/, 1997–2007).
Flow cytometry
Cells that had been released by cell dissociation buffer (Gibco; cat. no. 13151-014) were phenotyped using monoclonal antibodies and a BD FACScan flow cytometer. Antibodies for CD34, CD45, CD90, and CD73 were purchased from BD Pharmingen (cat. nos. 55824, 34796, 555595, and 550257, respectively) and CD105 was purchased from R&D Systems (10971A).
Cell cycle assay
Cells were collected and fixed with ice-cold 90% ethanol, added drop wise while vortexing. The fixation reaction was allowed to go 1–24 h, while the cells were kept at 4°C. Cells were then collected by centrifugation and resuspended in PBS containing 0.1% Triton ×100 and 20 μg/mL RNAse and incubated for 30 min at 37°C. Propidium Iodide at a final concentration of 50 μg/mL was added and cells were analyzed by flow cytometry.
Cell viability
MSC were harvested and stained with Annexin V and 7AAD according to the manufacturer's instructions (Apoptosis detection kit; BD Biosciences). The cells were analyzed using a FACScan flow cytometer (Becton Dickinson).
Colony forming units–fibroblast assay
MSC were plated at a density of 52.6 cells/cm2 in six-well tissue-culture-coated plates in the MSC medium. Fourteen days after plating the cells were fixed with cold methanol for 5 min and stained with Giemsa stain (Sigma; cat. no. GS500). The number of colonies was counted using a phase-contrast microscope (Nikon TMS).
Differentiation assays
The differentiation assays were performed according to manufacturer's instructions (Lonza). At the end of differentiation process, the cell monolayers were fixed with cold methanol for 5 min and air-dried. The adipogenic cultures were stained with Oil Red O (Sigma; cat. no. O0625) as previously described.45 The osteogenic cultures were stained with Alizarin Red S (Sigma; cat. no. A5533), 2% Alizarin Red S, pH 4.2 solution for 5 min, washed with water, and air-dried. The chondrogenic pellets were either frozen, cryosectioned, and stained with Alcian Blue (Rowley Biochemical; cat. no. E-325) (n = 1 pellet per treatment), or fixed in 10% formalin, paraffinized, sectioned, and stained with Safranin O. The pictures were taken with an upright Olympus BX41 microscope and Olympus DP20 camera (Olympus Imaging America Inc.), or Nikon Eclipse TE300 inverted microscope (Nikon) and Magnafire camera model S99802 (Optronics).
Hypoxia
The hypoxic condition was generated using a hypoxia chamber (Stem Cell Technologies; cat. no. 27310) according to the manufacturer's instructions. Briefly, the cultures were enclosed in the chamber and flushed with a mixture of gasses (95% N2 and 5% CO2) for 3 min. At the end of the flushing period, the chamber was closed to prevent free flow of exogenous air into the chamber. The final level of hypoxia was 1%–3%, as specified by the manufacturer. The cells were incubated in hypoxic conditions for 21.5 to 28 h before transplantation.
Animal surgery and MSC injection
All animal procedures were approved by the Animal Studies Committee at Washington University in St. Louis. Under anesthesia, NOD/SCID mice were subjected to unilateral hindlimb ischemia surgeries as described.46,47 The mice were shaved and prepped; then, the right femoral artery and vein were exposed and dissected from the femoral nerve; the proximal portion of the femoral artery and vein was ligated with 6–0 silk sutures. The distal portion of the saphenous artery and vein and the remaining collateral arterial and venous side branches were ligated and completely excised from the hindlimb. The overlying skin was closed using Nexaband veterinary glue (Abbott Animal Health). Anti-CD122 antibody, to reduce murine NK activity, was injected intraperitoneally at a concentration of 200 μg/mouse right after the surgery. MSC (106 cells per mouse) were injected intramuscularly into the injured area in 100 μL of PBS in three different sites 1 day after the surgery.
Laser Doppler perfusion imaging
Blood perfusion was monitored by laser Doppler imaging (MoorLDI-2; Moor Instruments). Animals were anesthetized and placed on a heating block at 37°C before scanning to minimize temperature variations. The laser Doppler images were analyzed by averaging the perfusion, expressed as the relative unit of flux over the surface of both ischemic and nonischemic foot, as determined by the Doppler imaging. The final data were expressed as the flux ratio of ischemic and nonischemic foot. This provides a healthy contralateral positive control in each image, to minimize light and temperature variations.
Statistics
One-way analysis of variance (ANOVA) was used to analyze the statistical significance in all in vitro experiments. The significance of the in vivo hindlimb ischemia functional studies was examined using two-way ANOVA analysis. A p-value <0.05 was considered to be significant throughout all studies.
Results
shRNA KD of c-Met in MSC
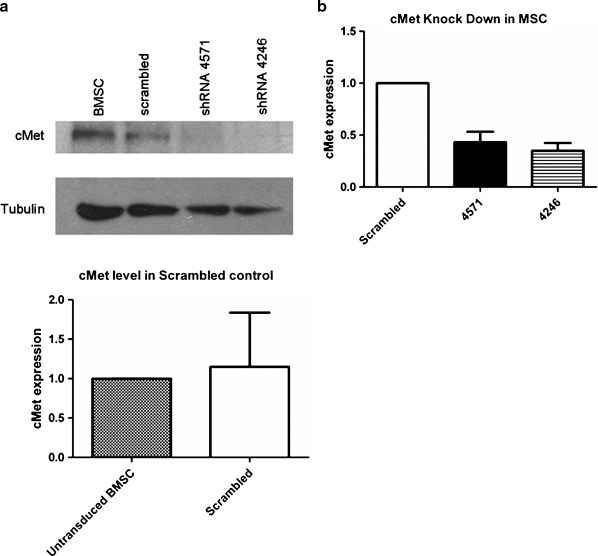
To study the importance of HGF/c-Met signaling for MSC function, we used shRNA constructs to generate a KD of c-Met in MSC. Two out of five constructs tested resulted in 60%–70% KD of the protein, as confirmed by Western blotting, whereas the level of c-Met protein in MSC SCR was unaffected (Fig. 1a). The average KD level of c-Met protein from multiple transduction experiments was quantified by densitometry and c-Met semi-quantification was normalized by tubulin expression. There was no significant difference in the level of reduction in protein levels between the two vectors (p > 0.05, Fig. 1b), whereas both were significantly decreased as compared to the scrambled control (p < 0.05, n = 9).
FIG. 1.
Western blot analysis of shRNA-mediated c-Met KD. (a) MSC were transduced with lentivirus expressing shRNA constructs targeting HGF-receptor c-Met or scrambled control. The level of c-Met KD was assessed after the puromycin selection using Western blot analysis. Both vectors 4246 and 4571 resulted in observable KD, whereas the scrambled control did not affect the c-Met protein levels on MSC. (b) The KD of c-Met was verified after every transduction, and the level of KD was semi-quantitated using densitometry analysis (Image J). C-Met semi-quantification was normalized by tubulin expression. There was no significant difference in the level of reduction in protein levels between the two vectors (p > 0.05), while both were significantly decreased as compared to the scrambled control (p < 0.05). The data are representative of nine transductions of primary MSC with the scrambled, 4246, and 4571 vectors. MSC, mesenchymal stem cells/marrow stromal cells; HGF, hepatocyte growth factor; KD, knockdown; BMSC, bone-marrow-derived MSC.
The KD of c-Met in MSC does not alter expression of phenotypic cell surface markers
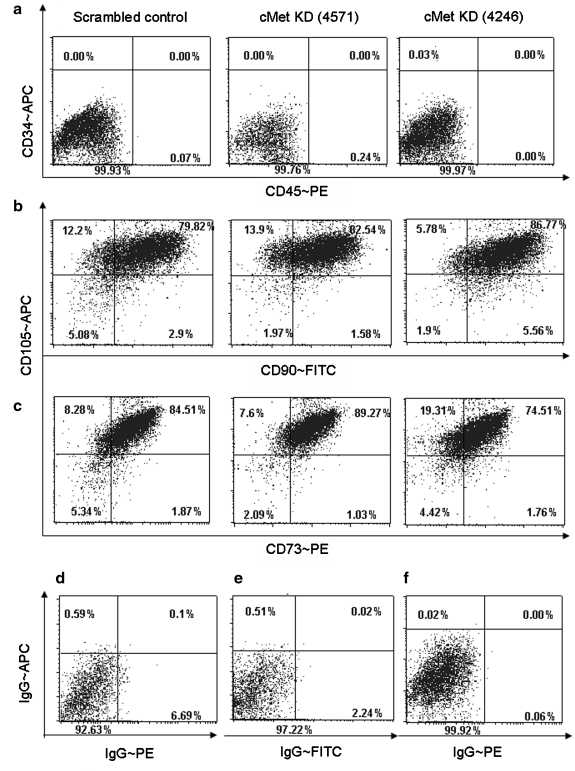
In the following experiments, we investigated whether c-Met KD MSC can maintain their in vitro characteristics. Although there are no exclusive cell surface markers for MSC, the previously established minimal criteria require MSC to express CD73, CD90, and CD105 on their cell surface, as well as to lack CD34 and CD45 markers.1 The flow cytometry analysis of the KD MSC and the MSC transduced with scrambled control shRNA (SCR) MSC showed that both groups of cells did present equivalent levels of the appropriate cell surface markers (Fig. 2), suggesting that neither the process of transduction nor the lack of c-Met signaling disrupted the cell surface phenotype of the MSC. The average numbers of cells expressing CD73, 90, and 105 with the standard deviations are summarized in Table 1.
FIG. 2.
Marker profile of KD MSC. The cell surface marker profile of KD versus scrambled shRNA MSC (KD MSC and SCR MSC, respectively) was analyzed using flow cytometry analysis. There was no difference between KD MSC and SCR MSC in their cell surface marker expression. Consistent with MSC characteristics, MSC lacked hematopoietic markers CD34 and CD45 (a) and expressed MSC markers CD105 and CD90 (b) and CD73 (c). The gates were set based on the analysis of cells stained with IgG controls, as shown in (d), (e), and (f), respectively. The images are representative of three separate experiments. The summary of the flow cytometry data (% of cells expressing the marker ± standard deviation) is shown in Table 1. SCR, scrambled control shRNA.
Table 1.
Summary of Marker Profile of Knockdown Mesenchymal Stem Cells/Marrow Stromal Cells
| CD90 | CD73 | CD105 | |
|---|---|---|---|
| c-Met KD (4246) | 95.06 ± 3.5 | 78.35 ± 18.37 | 92.31 ± 1.95 |
| c-Met KD (4571) | 88.25 ± 6.9 | 90.39 ± 2.91 | 98.5 ± 1.97 |
| Scrambled control | 82.43 ± 14.47 | 84.44 ± 8.03 | 88.5 ± 7.86 |
MSC marker cell surface expression was analyzed using flow cytometry, and the data from three separate experiments are summarized as a percentage of cells that expressed a marker ± standard deviation.
MSC, mesenchymal stem cells/marrow stromal cells; KD, knockdown.
The KD of c-Met in MSC has no apparent effect on cell cycle, survival, or colony-forming efficiency
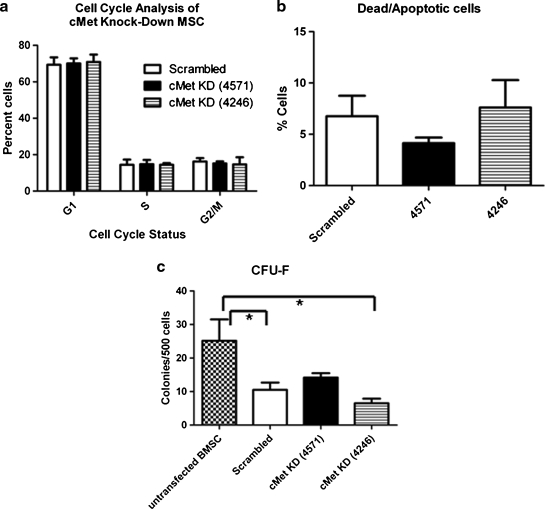
HGF/c-Met signaling has been previously implicated to have a mitogenic function.10,11 To determine whether c-Met KD affects MSC proliferation, the cell cycle status of MSC was analyzed by flow cytometry. Figure 3a summarizes the data and shows that there was no difference between KD MSC and SCR MSC in the percentage of cells in the different phases of the cell cycle. Additionally, the proportion of cycling cells in this experiment was similar to that of the nontransduced MSC, as shown in previous studies.5
FIG. 3.
c-Met KD does not alter MSC cell cycle, survival, or colony-forming efficiency. (a) Cell cycle: KD MSC or SCR MSC were permeabilized, stained with propidium iodide and their cell cycle status was assessed based on their DNA content. The bar graph summarizes the results of three separate experiments, showing that there was no significant difference in proliferation between KD MSC and SCR MSC. (b) Survival: KD or SCR MSC were collected, stained with Annexin V and 7AAD, and analyzed by flow cytometry. The bar graph summarizes the proportion of dead and apoptotic cells from three separate experiments, and demonstrates no significant differences. (c) CFU-F assay: MSC were plated at low density (500 cells/well of six-well plate) and the colonies were counted after 14 days of culture. The bar graph summarizes the data from two separate experiments (n = 6 for each treatment), showing that although there was a significant difference between transduced and untransduced MSC (*), there was no significant difference between KD MSC and SCR MSC as assessed using one-way ANOVA. CFU-F, colony forming unit-fibroblast; ANOVA, analysis of variance.
We next investigated the effect of c-Met KD on MSC survival. The results of flow cytometry analyses demonstrate that there was an equivalent fraction of dead and apoptotic cells in KD MSC and SCR MSC, suggesting that 62%–70% KD of c-Met did not alter the viability of MSC (Fig. 3b).
To further characterize KD MSC in vitro, we examined their colony-forming efficiency, which is a measure of the proliferative potential of the MSC. The colony forming unit–fibroblast (CFU-F) assay has been shown to be predictive of differentiation potential, as well as to indicate the self-renewal capacity of the MSC.48 Data analyzed using one-way ANOVA and the Bonferroni postanalysis showed significant differences in untransduced versus scrambled (p < 0.05) and untransduced versus 4246 MSC (p < 0.05) (Fig. 3c). Altogether, these data suggest that transduction by shRNA vectors did cause a reduction in CFU-F formation, but that, as compared to the scrambled control, interference with c-Met did not significantly affect the proliferative capacity of MSC.
HGF/c-Met signaling is required for osteogenic and chondrogenic, but not adipogenic, differentiation of MSC
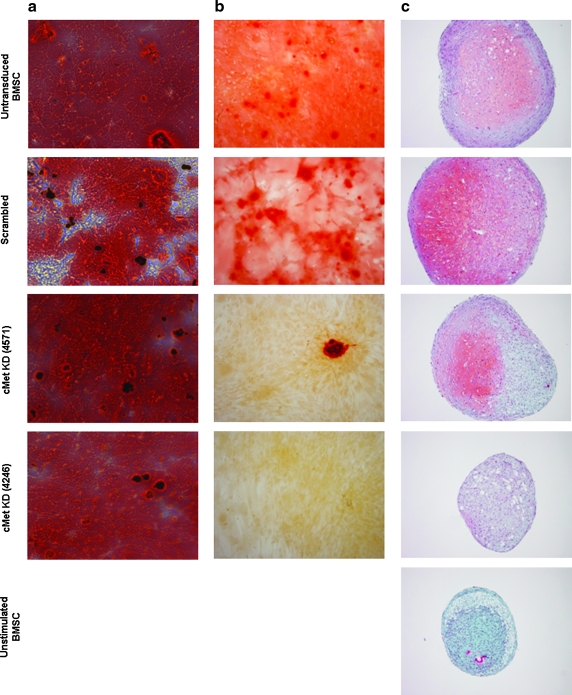
An important indicator of the stem cell potential of MSC is their capacity to differentiate in vitro into multiple lineages. We next tested the ability of KD MSC to differentiate into the adipogenic, osteogenic, and chondrogenic lineages. Notably, SCR MSC demonstrated a differentiation potential that was equivalent to unmaninpulated MSC in all three differentiation pathways (Fig. 4). Further, Oil red O staining revealed that KD MSC could differentiate into adipocytes to the same degree as SCR MSC, indicating that HGF/c-Met signaling is not necessary for this differentiation pathway (Fig. 4a). The osteogenic and chondrogenic differentiation, however, was decreased in KD MSC as compared to SCR MSC (Fig. 4b, c). Notably, 4571 shRNA construct, which resulted in 60% c-Met KD in MSC, exhibited an intermediate phenotype in both chondrogenic and osteogenic differentiation assays, as 4571 KD MSC could produce a low level of glycosaminoglycan staining and calcium deposits, respectively. The 4246 shRNA construct, which resulted in 70% c-Met KD in MSC, caused a more pronounced reduction in both chondrogenic and osteogenic differentiation of MSC. In addition to the lack of Safranin O staining, the chondrogenic pellets from 4246 MSC were also smaller in size and in general comparable to the pellet of MSC that had not been stimulated with chondrogenic differentiation factors (Fig. 4c). Interestingly, this result is consistent with the colony-forming efficiency of MSC transduced with this clone, as well as with the level of c-Met protein KD, which was higher for this construct. Nonetheless, the fact that both KD vectors did decrease MSC differentiation potential suggests that HGF/c-Met signaling is necessary for both osteogenic and chondrogenic differentiation processes.
FIG. 4.
HGF/c-Met signaling alters the osteogenic and chondrogenic differentiation capacity of MSC, but not their adipogenic differentiation potential. MSC were cultured in the commercial differentiation medium to stimulate differentiation along adipogenic, osteogenic, and chondrogenic lineages. (a) Adipogenic cultures were stained with Oil Red O, showing no differences between c-Met KD MSC and SCR MSC in their adipogenic differentiation capacity. (b) Alizarin Red S staining of calcium deposits revealed a decrease of osteogenic differentiation in KD MSC compared to SCR MSC and untransduced MSC. (c) Safranin O staining of chondrogenic pellets demonstrated a decrease in chondrogenic differentiation potential in KD MSC compared to SCR MSC and untransduced MSC. Notably, the level of differentiation of SCR MSC in all three differentiation assays was comparable to the untransduced MSC. Shown are representative images of 5–7 replicates of osteogenic and adipogenic cultures performed in three separate experiments. The chondrogenic assays were performed as three independent replicates.
MSC-mediated blood flow recovery after hindlimb ischemia
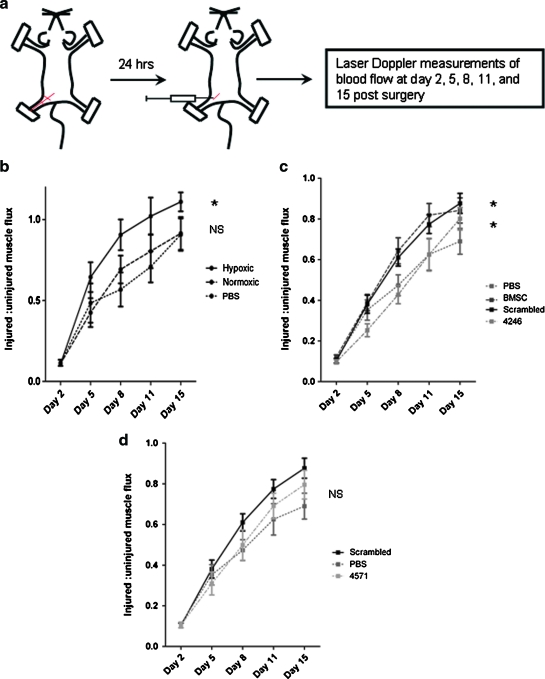
We next examined whether HGF/c-Met signaling is important for the tissue repair potential of MSC in vivo, using a murine xenotransplantation hindlimb ischemia injury model. In previous studies, we have demonstrated that human bone-marrow-derived MSC can significantly improve blood flow recovery in the ischemic hindlimb in NOD/SCID/Beta 2 microglobulin null (B2M−/−) immunodeficient mice. Additionally, we have shown that culturing MSC at 1%–3% oxygen before transplantation resulted in a more robust blood flow recovery at an early time point.5 In the current experiments, we first investigated whether this finding is reproducible in NOD/SCID mice, which had been injected with anti CD122 antibody to reduce rejection of human cells, as previously described.49 Approximately 24 h after the hindlimb ischemia surgery, animals were injected intramuscularly with 1 million human bone-marrow-derived MSC that had been cultured at 1%–3% oxygen or 21% oxygen (Fig. 5a). The blood flow recovery to the ischemic limb was followed by Laser Doppler perfusion imaging. The results revealed that mice injected with hypoxia-preconditioned MSC recovered significantly better than the saline control group. There was, however, no significant difference between mice injected with MSC cultured at 21% oxygen and the saline controls (Fig. 5b). Therefore, the MSC used in subsequent experiments were always preconditioned in a hypoxic environment before injection.
FIG. 5.
The effect of HGF/c-Met signaling on MSC tissue repair capacity in a hindlimb ischemia injury model. (a) Hindlimb ischemia surgeries were performed on immunodeficient NOD/SCID mice, followed by intraperitoneal injection of anti-CD122 antibody. Approximately 24 h after the surgery, the mice were injected intramuscularly with 106 human BMSC that had been cultured at 3% oxygen or 21% oxygen. The blood flow recovery to the ischemic limb was followed by laser Doppler perfusion imaging for 2 weeks after the surgery. (b) The laser Doppler perfusion imaging results demonstrate that the mice injected with hypoxia-preconditioned MSC recovered significantly better than the saline controls (two-way ANOVA), whereas there was no significant difference between the control group and the group injected with MSC cultured in 21% oxygen (two-way ANOVA). On the basis of these results, the MSC used in our next hindlimb ischemia experiment were all preconditioned at 1%–3% oxygen before the transplantation. (c) Mice injected with SCR MSC were able to improve blood flow recovery after hindlimb ischemia to the same degree as nontransduced MSC; both SCR- and nontransduced MSC restored blood flow significantly faster than the saline controls (two-way ANOVA). The blood flow recovery in mice transplanted with 4246 KD MSC (70% KD of c-Met) was significantly slower than that in the SCR MSC group (two-way ANOVA). (d) The blood flow recovery of animals treated with 4571 KD MSC (60% c-Met KD) was not significantly different from those treated with SCR MSC. The summarized data are based on seven (b, hypoxic and PBS groups) and eight (b, normoxic) animals per group. The graphs shown in (c) and (d) belong to the same experiment and are only separated to improve clarity. Therefore, these data can be directly compared to each other, and the SCR MSC and PBS curves are identical in both graphs. The data are representative of 12 (4246), 14 (untransduced MSC and SCR MSC), and 15 (PBS and 4571) animals per group. *Signifies p < 0.05 compared with saline-treated mice. NS, not significant. PBS, phosphate-buffered saline. Color images available online at www.liebertonline.com/ten.
We next assessed the ability of c-Met KD MSC to mediate blood flow regeneration after a hindlimb ischemia injury. Using the same experimental design as described above (Fig. 5a), we showed that SCR MSC had a tissue repair capacity comparable to that of nontransduced MSC, and that they significantly improved the regeneration of blood flow to the ischemic limb compared to the saline control group (Fig. 5c). Further, the comparison between SCR MSC and KD MSC revealed that 4246 KD MSC, which had c-Met expression reduced by 70%, were not able to improve regeneration after the hindlimb ischemia injury, as the mice injected with these cells recovered significantly more slowly than the SCR MSC group and to a similar degree as the saline controls. The 4571 KD MSC, however, which had less reduction in c-Met levels, exhibited an intermediate phenotype. Their regeneration ability was not significantly different from that of SCR MSC (Fig. 5d). These data therefore demonstrate that 60% KD of c-Met did not cause a significant defect in MSC-mediated blood flow recovery in the hindlimb ischemia injury model, whereas 70% KD of c-Met ablated the potential of MSC to mediate repair in this model.
Discussion
MSC are known to secrete a variety of cytokines and growth factors that have both paracrine and autocrine activities. The predominant mechanism of tissue repair and regeneration by MSC appears to be that they home or lodge in hypoxic and/or inflamed areas and release factors that hasten endogenous repair. These secreted bioactive factors suppress the local immune system, enhance angiogenesis, strengthen small collateral vessels, inhibit fibrosis and apoptosis, and stimulate recruitment, retention, mitosis, and differentiation of endogenous stem cells. These effects, which are referred to as “trophic effects,” are distinct from the direct differentiation of stem cells into the tissue to be regenerated. It is important to understand the signaling pathways involved in stem cell recruitment to hypoxic or damaged regions, to develop improved models for enhancing tissue repair using adult stem cells.
We have previously reported that pretreatment of MSC under hypoxic conditions increased the levels and extent of activation of c-Met,50 which could enhance HGF-mediated chemotactic recruitment to sites of tissue damage. This could also enhance the survival of MSC upon arrival at the damaged site, through increasing the levels of phosphorylation of the pro-survival protein AKT. Phosphorylation of Akt on Ser-473, which we had shown to be enhanced in hypoxic preconditioned MSC,50 stimulates its activity and plays a major role in the suppression of anoikis,51 or “death by detachment.” This is especially important for MSC, which begin to undergo apoptosis within hours after their integrin detachment from the substrate. AKT has been used to modify MSC for injection to circumvent this problem. Gnecchi et al. showed that, in a mouse model, a conditioned medium from AKT-transduced MSC had the same protective effect as the injected cells in acute myocardial infarction.52 These data, and others, suggest that a paracrine effect from the MSC is more important than direct differentiation or fusion. Our previous data showed that preculture of the cells in hypoxia before injection naturally increased AKT activity, without artificial overexpression, which could be tumorigenic.
In the current study, we investigated the importance of HGF/c-Met signaling in MSC and specifically its influence on the in vitro and in vivo characteristics of these cells. We show that two different shRNA vectors were able to KD the level of c-Met protein in MSC to 30% and 40% of expression of scrambled control-transduced cells. Further, we show that this signaling pathway did not affect MSCs' cell surface marker profile, their cell cycle progression, or their survival. Upon examining the differentiation potential of c-Met KD MSC, we discovered that although KD MSC were able to differentiate into the adipogenic lineage, the decrease in HGF/c-Met signaling caused a defect in their chondrogenic and osteogenic differentiation potential. Although HGF has been implicated to promote osteogenic differentiation of MSC,30 to our knowledge this is the first report showing a defect in MSCs' osteogenic and chondrogenic differentiation capacity after a genetic KD of this signaling pathway.
In additional in vivo studies, we next demonstrated that although 60% KD of c-Met had only an intermediate effect on the tissue repair capacity of MSC, a 70% KD of this receptor caused a significant decrease in their ability to promote blood flow regeneration after a hindlimb ischemia injury. The 60% KD of c-Met in MSC also displayed an intermediate phenotype in osteogenic and chondrogenic differentiation assays, when compared to the 70% KD.
MSC were initially identified as fibroblast-like cells resident to the bone marrow, which can form tissue-culture-adherent colonies in vitro.53 After their ability to reconstitute a bone marrow microenvironment in vivo was discovered,54–56 many researchers have tried to characterize these cells and their in vitro properties. Although no exclusive cell surface marker for MSC has been identified, a minimal set of markers that they lack or express has been established.1 Additionally, a CFU-F assay was established as a measure of MSC colony-forming efficiency to reflect their self-renewal capacity. In this study, we show that neither MSC marker profile nor their colony-forming efficiency is altered after a c-Met KD. As HGF/c-Met signaling was expected to alter the MSC function rather than their phenotype, it is not surprising that KD MSC still express the key characteristic markers. It is, however, somewhat unexpected that KD MSC colony-forming efficiency is comparable to that of SCR MSC, especially in the light of the fact that HGF is a known mitogenic factor. A low concentration of HGF has been previously shown to increase the MSC proliferation rate,30 while high HGF concentrations are known to be inhibitory to MSC growth.12,13 We predicted that MSC production of HGF in addition to that present in the serum would result in low levels of HGF in the tissue culture medium, to which KD MSC would not be able to respond. The cell cycle analysis and the CFU-F assay of KD MSC, however, showed no significant differences when compared to the SCR MSC, suggesting that 60%–70% KD of c-Met is not sufficient to alter MSC proliferation and self-renewal. These results are in contrast to previously published data, which demonstrated a diminished growth of MSC treated with anti c-Met antibody.30 It is, nevertheless, possible that a higher level of c-Met KD is necessary to see an effect in these assays.
Another important characteristic that defines MSC is their in vitro differentiation potential. MSC have been shown to be able to differentiate into multiple lineages, but the three best defined are adipogenic, chondrogenic, and osteogenic. In this study, we demonstrate that MSC expressing decreased levels of c-Met were able to differentiate into adipocytes; however, these cells exhibited a defect in their osteogenic and chondrogenic differentiation. Previous studies have shown that while HGF supplementation alone did not induce osteogenic differentiation, HGF in concert with vitamin D3 was able to induce bone matrix mineralization in culture.30 Our results confirm the observation that HGF signaling is important for osteogenic differentiation, demonstrating that the loss of this signaling pathway leads to a decreased formation of bone nodules. Additionally, we make a novel observation that the HGF/c-Met axis is also necessary for chondrogenic differentiation, as the KD MSC were not able to produce extracellular matrix characteristic for cartilage. Although signaling pathways involved in MSC fate determination are complicated and are still not well understood, a number of them such as insulin-like growth factor, fibroblast growth factor, and transforming growth factor-β have been implicated to be involved in processes leading to both chondrogenesis and osteogenesis.57,58 Our results prompt further investigation into the possibility that HGF/c-Met signaling interacts with some of these pathways, which could explain why disrupted signaling along this axis does not affect the adipogenic differentiation, but plays a significant role in osteogenic and chondrogenic differentiation.
The major interest in MSC research has been due to their tissue repair potential. Our group and others have previously demonstrated that MSC are able to mediate blood flow regeneration in the hindlimb ischemia injury model.5,7,59,60 In this report, we show that a 70% KD of the c-Met receptor on MSC disrupted their capacity to improve recovery after an ischemic injury, whereas a 60% KD of c-Met exhibited an intermediate effect, not statistically significant from the blood flow recovery stimulated by SCR MSC. HGF has been previously implicated to be effective in improving blood flow in several animal models of angiogenesis as well as in clinical trials.5,61–63 In a myocardial infarction injury model, MSC overexpressing HGF have been shown to improve cardiac tissue regeneration faster than nontransduced MSC, suggesting that HGF does play a key role in ischemic tissue repair.31 Additionally, multiple in vitro studies revealed that MSC support endothelial cell survival, proliferation, and tube formation, and that this in vitro effect is dependent on the release of endogenous HGF and vascular endothelial growth factor.59 In agreement with these results, HGF KD in MSC rendered the cells unable to improve blood flow in the hindlimb ischemia injury model.32 Altogether, these results suggest that HGF is an important growth factor that significantly contributes to ischemic injury regeneration, and that the ability of MSC to mediate tissue repair is partially due to their release of this cytokine. The data presented in the current study confirm the importance of HGF/c-Met signaling for the ability of MSC to mediate tissue repair. Our data further suggest that not only does HGF act as a trophic factor on the endogenous cells in the injury area, but also the c-Met signaling is necessary for the MSC to respond properly to the injury signals and thus to mediate blood flow recovery in ischemic tissues. In our study the 60% KD of c-Met did not recapitulate the phenotype of the 70% c-Met KD. Several lines of evidence suggest that the level of c-Met expression is important. The 60% KD cells have, for example, showed an intermediate phenotype in the chondrogenic and osteogenic differentiation assays, as well as significantly higher colony-forming efficiency than the 70% KD MSC. This suggests that MSC are sensitive to local gradients and microenvironmental bioavailability of HGF to activate c-Met, like many growth factor/receptor pairs.
In this report, we have uncovered the importance of the HGF/c-Met signaling pathway in the osteogenic and chondrogenic potential of human MSC, as well as in their capacity for tissue repair. We assessed the capacity of MSC with c-Met reduced to variable levels by shRNA to produce bone mineralized nodules and cartilage-specific extracellular matrix as endpoints to test their osteogenic and chondrogenic differentiation, respectively. In future studies, it would be interesting to investigate whether these MSC are arrested at a particular stage of differentiation, and whether HGF/c-Met signaling interacts with other signaling pathways that regulate chondrogenic and osteogenic cell determination. We also determined the importance of c-Met signaling in the capacity of human MSC to increase blood flow in a hindlimb ischemia model. The understanding of how MSC mediate tissue repair and whether the c-Met KD affects their cytokine secretory profile would be an intriguing future direction that could contribute to improving the clinical treatment of ischemic diseases.
Acknowledgments
This work was supported by the National Institutes of Health (NIH), National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK #2R01DK61848 and 2R01DK53041 [J.N.]), and National Heart, Lung, and Blood Institute (NHLBI #RO1HL073256 [J.N.]). We would like to thank the California Institute for Regenerative Medicine (CIRM) for funding a continuation of the work to study MSC producing siRNA (CIRM TR1-01257: [Nolta]). I.R. performed the research. All authors contributed to experimental design. D.L. and J.N. analyzed the data. I.R. and J.N. wrote the article, and D.L. edited it before submission.
Author Contributions
Ivana Rosova: concept and design, collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of the manuscript.
Dan Link: concept and design, financial support, data analysis and interpretation, and final approval of the manuscript.
Jan Nolta: concept and design, data analysis and interpretation, financial support, manuscript writing, and final approval of the manuscript.
Disclosure Statement
No competing financial interests exist.
References
- 1.Horwitz E.M. Le Blanc K. Dominici M. Mueller I. Slaper-Cortenbach I. Marini F.C. Deans R.J. Krause D.S. Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Makino S. Fukuda K. Miyoshi S. Konishi F. Kodama H. Pan J. Sano M. Takahashi T. Hori S. Abe H. Hata J. Umezawa A. Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez-Ramos J. Song S. Cardozo-Pelaez F. Hazzi C. Stedeford T. Willing A. Freeman T.B. Saporta S. Janssen W. Patel N. Cooper D.R. Sanberg P.R. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 5.Rosova I. Dao M. Capoccia B. Link D. Nolta J.A. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laflamme M.A. Murry C.E. Regenerating the heart. Nat Biotechnol. 2005;23:845. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 7.Kinnaird T. Stabile E. Burnett M.S. Lee C.W. Barr S. Fuchs S. Epstein S.E. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 8.Sato T. Fujieda H. Murao S. Sato H. Takeuchi T. Ohtsuki Y. Sequential changes of hepatocyte growth factor in the serum and enhanced c-Met expression in the myocardium in acute myocardial infarction. Jpn Circ J. 1999;63:906. doi: 10.1253/jcj.63.906. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T. Nawa K. Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- 10.Maroun C.R. Naujokas M.A. Holgado-Madruga M. Wong A.J. Park M. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol. 2000;20:8513. doi: 10.1128/mcb.20.22.8513-8525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware L.B. Matthay M.A. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol. 2002;282:L924. doi: 10.1152/ajplung.00439.2001. [DOI] [PubMed] [Google Scholar]
- 12.Neuss S. Becher E. Woltje M. Tietze L. Jahnen-Dechent W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells. 2004;22:405. doi: 10.1634/stemcells.22-3-405. [DOI] [PubMed] [Google Scholar]
- 13.Forte G. Minieri M. Cossa P. Antenucci D. Sala M. Gnocchi V. Fiaccavento R. Carotenuto F. De Vito P. Baldini P.M. Prat M. Di Nardo P. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24:23. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T. Nishizawa T. Hagiya M. Seki T. Shimonishi M. Sugimura A. Tashiro K. Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 15.Sonnenberg E. Meyer D. Weidner K.M. Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993;123:223. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noji S. Tashiro K. Koyama E. Nohno T. Ohyama K. Taniguchi S. Nakamura T. Expression of hepatocyte growth factor gene in endothelial and Kupffer cells of damaged rat livers, as revealed by in situ hybridization. Biochem Biophys Res Commun. 1990;173:42. doi: 10.1016/s0006-291x(05)81018-6. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto J. Gene therapy for liver cirrhosis. J Gastroenterol Hepatol. 2000;(15 Suppl):D33. doi: 10.1046/j.1440-1746.2000.02146.x. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda S. Goto Y. Baba T. Satoh T. Sumida H. Miyazaki S. Nonogi H. Enhanced secretion of cardiac hepatocyte growth factor from an infarct region is associated with less severe ventricular enlargement and improved cardiac function. J Am Coll Cardiol. 2000;36:115. doi: 10.1016/s0735-1097(00)00675-6. [DOI] [PubMed] [Google Scholar]
- 19.Sato T. Fujieda H. Murao S. Sato H. Takeuchi T. Ohtsuki Y. Sequential changes of hepatocyte growth factor in the serum and enhanced c-Met expression in the myocardium in acute myocardial infarction. Jpn Circ J. 1999;63:906. doi: 10.1253/jcj.63.906. [DOI] [PubMed] [Google Scholar]
- 20.Sheehan S.M. Tatsumi R. Temm-Grove C.J. Allen R.E. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve. 2000;23:239. doi: 10.1002/(sici)1097-4598(200002)23:2<239::aid-mus15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Mizuno K. Higuchi O. Ihle J.N. Nakamura T. Hepatocyte growth factor stimulates growth of hematopoietic progenitor cells. Biochem Biophys Res Commun. 1993;194:178. doi: 10.1006/bbrc.1993.1801. [DOI] [PubMed] [Google Scholar]
- 22.Galimi F. Bagnara G.P. Bonsi L. Cottone E. Follenzi A. Simeone A. Comoglio P.M. Hepatocyte growth factor induces proliferation and differentiation of multipotent and erythroid hemopoietic progenitors. J Cell Biol. 1994;127:1743. doi: 10.1083/jcb.127.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishino T. Hisha H. Nishino N. Adachi M. Ikehara S. Hepatocyte growth factor as a hematopoietic regulator. Blood. 1995;85:3093. [PubMed] [Google Scholar]
- 24.Ikehara S. Role of hepatocyte growth factor in hemopoiesis. Leuk Lymphoma. 1996;23:297. doi: 10.3109/10428199609054832. [DOI] [PubMed] [Google Scholar]
- 25.Ratajczak M.Z. Marlicz W. Ratajczak J. Wasik M. Machalinski B. Carter A. Gewirtz A.M. Effect of hepatocyte growth factor on early human haemopoietic cell development. Br J Haematol. 1997;99:228. doi: 10.1046/j.1365-2141.1997.3563170.x. [DOI] [PubMed] [Google Scholar]
- 26.Yu C.Z. Hisha H. Li Y. Lian Z. Nishino T. Toki J. Adachi Y. Inaba M. Fan T.X. Jin T. Iguchi T. Sogo S. Hosaka N. Song T.H. Xing J. Ikehara S. Stimulatory effects of hepatocyte growth factor on hemopoiesis of SCF/c-kit system-deficient mice. Stem Cells. 1998;16:66. doi: 10.1002/stem.160066. [DOI] [PubMed] [Google Scholar]
- 27.Iguchi T. Sogo S. Hisha H. Taketani S. Adachi Y. Miyazaki R. Ogata H. Masuda S. Sasaki R. Ito M. Fukuhara S. Ikehara S. HGF activates signal transduction from EPO receptor on human cord blood CD34+/CD45+ cells. Stem Cells. 1999;17:82. doi: 10.1002/stem.170082. [DOI] [PubMed] [Google Scholar]
- 28.Weimar I.S. Miranda N. Muller E.J. Hekman A. Kerst J.M. de Gast G.C. Gerritsen W.R. Hepatocyte growth factor/scatter factor (HGF/SF) is produced by human bone marrow stromal cells and promotes proliferation, adhesion and survival of human hematopoietic progenitor cells (CD34+) Exp Hematol. 1998;26:885. [PubMed] [Google Scholar]
- 29.Goff J.P. Shields D.S. Petersen B.E. Zajac V.F. Michalopoulos G.K. Greenberger J.S. Synergistic effects of hepatocyte growth factor on human cord blood CD34+ progenitor cells are the result of c-met receptor expression. Stem Cells. 1996;14:592. doi: 10.1002/stem.140592. [DOI] [PubMed] [Google Scholar]
- 30.D'Ippolito G. Schiller P.C. Perez-stable C. Balkan W. Roos B.A. Howard G.A. Cooperative actions of hepatocyte growth factor and 1,25-dihydroxyvitamin D3 in osteoblastic differentiation of human vertebral bone marrow stromal cells. Bone. 2002;31:269. doi: 10.1016/s8756-3282(02)00820-7. [DOI] [PubMed] [Google Scholar]
- 31.Duan H.F. Wu C.T. Wu D.L. Lu Y. Liu H.J. Ha X.Q. Zhang Q.W. Wang H. Jia X.X. Wang L.S. Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther. 2003;8:467. doi: 10.1016/s1525-0016(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 32.Cai L. Johnstone B.H. Cook T.G. Liang Z. Traktuev D. Cornetta K. Ingram D.A. Rosen E.D. March K.L. Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells. 2007;25:3234. doi: 10.1634/stemcells.2007-0388. [DOI] [PubMed] [Google Scholar]
- 33.Damert A. Ikeda E. Risau W. Activator-protein-1 binding potentiates the hypoxia-induciblefactor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem J. 1997;327(Pt 2):419. doi: 10.1042/bj3270419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loike J.D. Cao L. Brett J. Ogawa S. Silverstein S.C. Stern D. Hypoxia induces glucose transporter expression in endothelial cells. Am J Physiol. 1992;263:C326. doi: 10.1152/ajpcell.1992.263.2.C326. [DOI] [PubMed] [Google Scholar]
- 35.Hu X. Yu S.P. Fraser J.L. Lu Z. Ogle M.E. Wang J.A. Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 36.Liu X.B. Jiang J. Gui C. Hu X.Y. Xiang M.X. Wang J.A. Angiopoietin-1 protects mesenchymal stem cells against serum deprivation and hypoxia-induced apoptosis through the PI3K/Akt pathway. Acta Pharmacol Sin. 2008;29:815. doi: 10.1111/j.1745-7254.2008.00811.x. [DOI] [PubMed] [Google Scholar]
- 37.Won Kim H. Haider H.K. Jiang S. Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284:33161. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li N. Hill K.S. Elferink L.A. Analysis of receptor tyrosine kinase internalization using flow cytometry. Methods Mol Biol (Clifton, NJ) 2008;457:305. doi: 10.1007/978-1-59745-261-8_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naka D. Shimomura T. Yoshiyama Y. Sato M. Sato M. Ishii T. Hara H. Internalization and degradation of hepatocyte growth factor in hepatocytes with down-regulation of the receptor/c-Met. FEBS Lett. 1993;329:147. doi: 10.1016/0014-5793(93)80212-d. [DOI] [PubMed] [Google Scholar]
- 40.Pennacchietti S. Michieli P. Galluzzo M. Mazzone M. Giordano S. Comoglio P.M. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 41.Dao M.A. Pepper K.A. Nolta J.A. Long-term cytokine production from engineered primary human stromal cells influences human hematopoiesis in an in vivo xenograft model. Stem Cells. 1997;15:443. doi: 10.1002/stem.150443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nolta J.A. Hanley M.B. Kohn D.B. Sustained human hematopoiesis in immunodeficient mice by cotransplantation of marrow stroma expressing human interleukin-3: analysis of gene transduction of long-lived progenitors. Blood. 1994;83:3041. [PubMed] [Google Scholar]
- 43.Meyerrose T. Rosova I. Dao M. Herrbrich P. Bauer G. Nolta J. Establishment and Transduction of Primary Human Sromal/Mesenchymal Stem Cell Monolayers. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2006. [Google Scholar]
- 44.Dao M.A. Nolta J.A. Cytokine and integrin stimulation synergize to promote higher levels of GATA-2, c-myb, and CD34 protein in primary human hematopoietic progenitors from bone marrow. Blood. 2007;109:2373. doi: 10.1182/blood-2006-05-026039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rich J.T. Rosova I. Nolta J.A. Myckatyn T.M. Sandell L.J. McAlinden A. Upregulation of Runx2 and Osterix during in vitro chondrogenesis of human adipose-derived stromal cells. Biochem Biophys Res Commun. 2008;372:230. doi: 10.1016/j.bbrc.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Capoccia B.J. Robson D.L. Levac K.D. Maxwell D.J. Hohm S.A. Neelamkavil M.J. Bell G.I. Xenocostas A. Link D.C. Piwnica-Worms D. Nolta J.A. Hess D.A. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113:5340. doi: 10.1182/blood-2008-04-154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou P. Hohm S. Capoccia B. Wirthlin L. Hess D. Link D. Nolta J. Immunodeficient mouse models to study human stem cell-mediated tissue repair. Methods Mol Biol. 2008;430:213. doi: 10.1007/978-1-59745-182-6_15. [DOI] [PubMed] [Google Scholar]
- 48.Digirolamo C.M. Stokes D. Colter D. Phinney D.G. Class R. Prockop D.J. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 49.McKenzie J.L. Gan O.I. Doedens M. Dick J.E. Human short-term repopulating stem cells are efficiently detected following intrafemoral transplantation into NOD/SCID recipients depleted of CD122+ cells. Blood. 2005;106:1259. doi: 10.1182/blood-2005-03-1081. [DOI] [PubMed] [Google Scholar]
- 50.Rosova I. Dao M. Capoccia B. Link D. Nolta J.A. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;8:2173. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Attwell S. Roskelley C. Dedhar S. The integrin-linked kinase (ILK) suppresses anoikis. Oncogene. 2000;19:3811. doi: 10.1038/sj.onc.1203711. [DOI] [PubMed] [Google Scholar]
- 52.Gnecchi M. He H. Liang O.D. Melo L.G. Morello F. Mu H. Noiseux N. Zhang L. Pratt R.E. Ingwall J.S. Dzau V.J. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 53.Friedenstein A.J. Chailakhjan R.K. Lalykina K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 54.Friedenstein A.J. Chailakhyan R.K. Latsinik N.V. Panasyuk A.F. Keiliss-Borok I.V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Bianco P. Gehron Robey P. Marrow stromal stem cells. J Clin Invest. 2000;105:1663. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sacchetti B. Funari A. Michienzi S. Di Cesare S. Piersanti S. Saggio I. Tagliafico E. Ferrari S. Robey P.G. Riminucci M. Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 57.Ng F. Boucher S. Koh S. Sastry K.S. Chase L. Lakshmipathy U. Choong C. Yang Z. Vemuri M.C. Rao M.S. Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 58.Watson P. Lazowski D. Han V. Fraher L. Steer B. Hodsman A. Parathyroid hormone restores bone mass and enhances osteoblast insulin-like growth factor I gene expression in ovariectomized rats. Bone. 1995;16:357. doi: 10.1016/8756-3282(94)00051-4. [DOI] [PubMed] [Google Scholar]
- 59.Nakagami H. Maeda K. Morishita R. Iguchi S. Nishikawa T. Takami Y. Kikuchi Y. Saito Y. Tamai K. Ogihara T. Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 60.Kim S.W. Han H. Chae G.T. Lee S.H. Bo S. Yoon J.H. Lee Y.S. Lee K.S. Park H.K. Kang K.S. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger's disease and ischemic limb disease animal model. Stem Cells. 2006;24:1620. doi: 10.1634/stemcells.2005-0365. [DOI] [PubMed] [Google Scholar]
- 61.Morishita R. Aoki M. Hashiya N. Makino H. Yamasaki K. Azuma J. Sawa Y. Matsuda H. Kaneda Y. Ogihara T. Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease. Hypertension. 2004;44:203. doi: 10.1161/01.HYP.0000136394.08900.ed. [DOI] [PubMed] [Google Scholar]
- 62.Morishita R. Nakamura S. Hayashi S. Taniyama Y. Moriguchi A. Nagano T. Taiji M. Noguchi H. Takeshita S. Matsumoto K. Nakamura T. Higaki J. Ogihara T. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension. 1999;33:1379. doi: 10.1161/01.hyp.33.6.1379. [DOI] [PubMed] [Google Scholar]
- 63.Hayashi S. Morishita R. Nakamura S. Yamamoto K. Moriguchi A. Nagano T. Taiji M. Noguchi H. Matsumoto K. Nakamura T. Higaki J. Ogihara T. Potential role of hepatocyte growth factor, a novel angiogenic growth factor, in peripheral arterial disease: downregulation of HGF in response to hypoxia in vascular cells. Circulation. 1999;100:II301. doi: 10.1161/circ.100.suppl_2.Ii-301. [DOI] [PubMed] [Google Scholar]