Summary
To gain insight into the mechanisms by which host cells detect cytosolic invasion by intracellular pathogens, a genetic screen was performed to identify Listeria monocytogenes mutants that induced altered levels of host cell death. A mutation in lmo2473 resulted in hyper-stimulation of host cell death and IL-1β secretion (pyroptosis) following bacteriolysis in the macrophage cytosol. In addition, strains engineered to lyse in the cytosol by expression of both bacteriophage holin and lysin or induced to lyse by treatment with ampicillin stimulated pyroptosis. Pyroptosis was independent of the Nlrp3 and Nlrc4 receptors, but dependent on ASC and AIM2. Importantly, wild type L. monocytogenes were also found to lyse, albeit at low levels, and trigger AIM2-dependent pyroptosis. Since AIM2 is activated by DNA, these data suggested that pyroptosis is triggered by bacterial DNA released during lysis.
Introduction
Intracellular pathogens have evolved to survive and replicate within the protected environment of their host cells, concealed from many innate and adaptive immune responses. One host strategy to limit microbial replication is programmed cell death, thereby eliminating the intracellular niche of the pathogen (Martinon et al., 2009; Roulston et al., 1999). Many intracellular pathogens have evolved mechanisms to counter these responses by either avoiding detection or by actively inhibiting programmed cell death pathways (Roulston et al., 1999; Stehlik and Dorfleutner, 2007). The mechanisms leading to activation and/or avoidance of programmed cell death, from both the host and pathogen perspective, remain incompletely defined.
One cell death pathway resulting from microbial infection is pyroptosis, a proinflammatory cell death triggered upon activation of an inflammasome complex. Multiple inflammasomes have been described and each responds to unique stimuli (Martinon et al., 2009). Inflammasomes are composed of a receptor, often a nod-like receptor (NLR), coupled to caspase-1, either directly or through the adaptor molecule ASC. Ligand recognition by a NLR leads to the autocatalysis of the zymogen pro- caspase-1 (Martinon et al., 2009). Active caspase-1 can lead to cell death and cleavage of pro-IL-1β and pro-IL-18 into their active, secreted forms (Mariathasan and Monack, 2007).
Listeria monocytogenes, the causative agent of listeriosis, is a Gram-positive facultative intracellular pathogen which grows rapidly within host cells while largely avoiding induction of host cell death (Barsig and Kaufmann, 1997). Upon internalization, L. monocytogenes escapes from the primary phagosome through the activity of a cholesterol-dependent cytolysin, Listeriolysin O (hly) (Portnoy et al., 1988). Indeed, L. monocytogenes mutants that induce host cell death due to misregulation of Listeriolysin O are severely attenuated (Glomski et al., 2003). Recently, L. monocytogenes infection has been found to trigger pyroptosis, although the magnitude of this response and which NLR(s) recognize L. monocytogenes have been controversial. The adaptor molecule ASC is a central component required for L. monocytogenes-induced pyroptosis (Franchi et al., 2007; Mariathasan et al., 2006; Ozoren et al., 2006). Nlrp3 and Nlrc4 have been reported to detect L. monocytogenes infection to varying degrees (Mariathasan et al., 2006; Meixenberger et al., 2009; Warren et al., 2008), while other groups find no role for Nlrp3 (Franchi et al., 2007). L. monocytogenes flagellin has a minor role in inflammasome activation; however, the dominant ligand(s) remains unknown (Warren et al., 2008).
We performed a forward genetic screen to identify L. monocytogenes mutants that resulted in altered induction of host cell death. The mutant with the most robust phenotype was identified as a transposon insertion in lmo2473. Deletion of lmo2473 resulted in mutant bacteria that hyper-induced pyroptosis. Bacterial cell lysis caused either by the loss of lmo2473, expression of bacteriophage holin and lysin or treatment with antibiotics led to inflammasome-mediated cell death and IL-1β release that was dependent on the adaptor molecule ASC. We found that detection of DNA released during bacteriolysis in the cytosol was a natural mechanism of inflammasome activation by wild type L. monocytogenes and that the receptor AIM2 was essential for this process. We propose that ASC-dependent inflammasome responses to other cytosolic pathogens similarly proceed through activation of an AIM2-dependent inflammasome due to bacteriolysis.
Results
Identification of L. monocytogenes mutants that hyper-induce pyroptosis
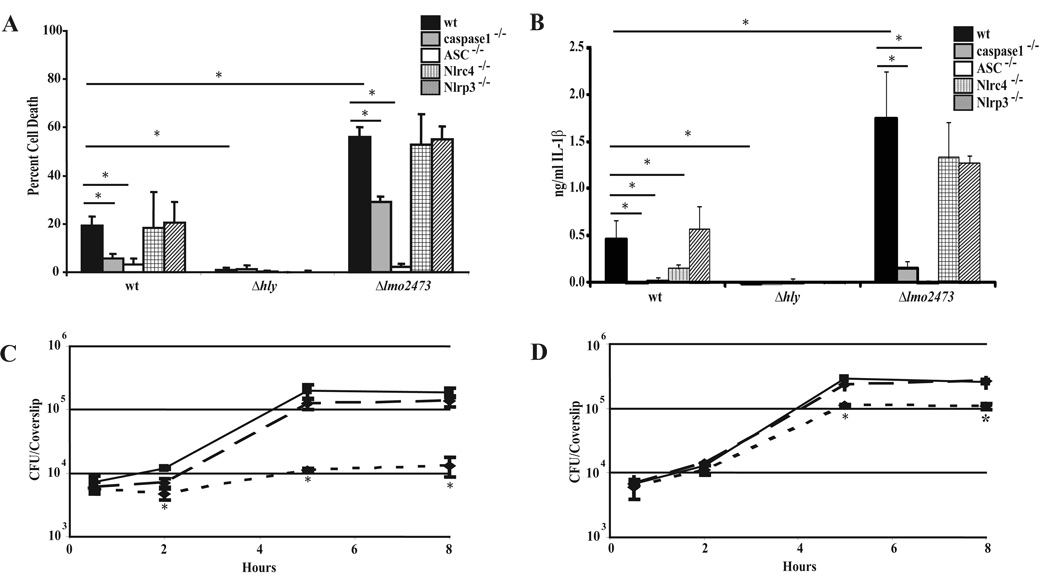
Upon infection of macrophages, L. monocytogenes activates a modest level of host cell death. To gain insight into how L. monocytogenes interacts with cell death pathways, we screened a bacterial Himar1 transposon library for mutants that showed increased activation of host cell death. Following infection, cell death was indirectly measured by the amount of macrophage lactate dehydrogenase released into the supernatant. The mutant that caused the most cytotoxicity harbored a transposon insertion in lmo2473 (data not shown) and in-frame deletion of lmo2473 resulted in a similar increase in host cell death (Fig. 1A). Cell death was dependent on caspase-1 and accompanied by IL-1β release, indicating that Δlmo2473 mutants were inducing pyroptosis (Fig. 1A, B). Both cell death and IL-1β secretion returned to wild type levels following complementation of lmo2473 (data not shown). Access to the cytosol was required for induction of pyroptosis as neither Δhly nor ΔhlyΔlmo2473 double mutants were cytotoxic (Fig. 1A, B and Fig. S1A, B). Furthermore, caspase-1 was cleaved into its active subunits following infection with either wild type L. monocytogenes or Δlmo2473 mutants but not Δhly mutants (Fig. S2).
Figure 1.
Induction of pyroptosis by L. monocytogenes. Cell death (A) and IL-1β (B) were measured following a 6 hour infection at an MOI of 5 with the indicated strains in wild type, caspase-1−/−, ASC−/−, Nlrp3−/− or Nlrc4−/− bone marrow-derived macrophages. Data are presented as the average of at least 3 independent experiments and the error bars represent the standard deviation of the mean. Intracellular growth of wild type L. monocytogenes (solid line), Δlmo2473 mutant (dotted line) and Δlmo2473 mutant complement (dashed line) was quantified in wild type C57BL/6. *indicates these values are statistically different with a p value <0.05 using a one-way ANOVA followed by a post-hoc LSD analysis (C) or caspase-1−/− bone marrow-derived macrophages (D). Growth curves are representative of at least three independent experiments. *indicates these values are statistically different with a p value <0.05 using a Students’ t-test
To address which host inflammasome components were required to activate caspase-1, we infected Nlrc4-, Nlrp3- or ASC-deficient macrophages and found that cell death and IL-1β secretion induced by both wild type and Δlmo2473 were independent of Nlrp3 (Fig. 1A, B). In agreement with previous observations, IL-1β secretion was partially dependent on Nlrc4 (Fig. 1B). Nlrc4- and Nlrp3-deficent macrophages responded as expected to known stimuli: Salmonella typhimurium and purified Listerolysin O, respectively (Fig. S3A, B). Also consistent with previous reports, wild type and mutant-induced IL-1β secretion and cell death were fully dependent on ASC (Fig. 1A,B) (Franchi et al., 2007; Mariathasan et al., 2006; Ozoren et al., 2006; Warren et al., 2008).
Cytotoxic strains of L. monocytogenes are severely attenuated (Glomski et al, 2003), therefore, we hypothesized that hyper-induction of pyroptosis by Δlmo2473 would negatively affect its virulence. Although growth in broth culture was unaffected (data not shown), Δlmo2473 mutants were severely defective for intracellular growth in macrophages compared to wild type bacteria (Fig. 1C). The growth defect was largely rescued (~90%) in caspase-1-deficient macrophages that are unable to undergo pyroptosis (Fig. 1D). These data indicated that Δlmo2473 mutants hyper-activated caspase-1/ASC-dependent pyroptosis compared to the low level activation by wild type bacteria, subsequently resulting in a severe intracellular growth defect.
Wild type and Δlmo2473 L. monocytogenes lyse in the macrophage cytosol
lmo2473 encodes a protein of unknown function that is conserved in many Gram positive and Gram negative bacteria. Bacillus subtilis mutants lacking yvcK, a homologue of lmo2473, have defects in cell wall biosynthesis leading to aberrant cell morphology and eventual bacteriolysis in broth culture (Gorke et al., 2005). Although the intracellular growth of Δlmo2473 mutants was mostly rescued in the absence of caspase-1 (~90%), this mutant still showed a measurable defect. In addition, we observed irregular cell morphology of Δlmo2473 mutants in the cytosol of infected macrophages (data not shown). Taken together these observations suggested that Δlmo2473 mutants may lyse in the host cytosol.
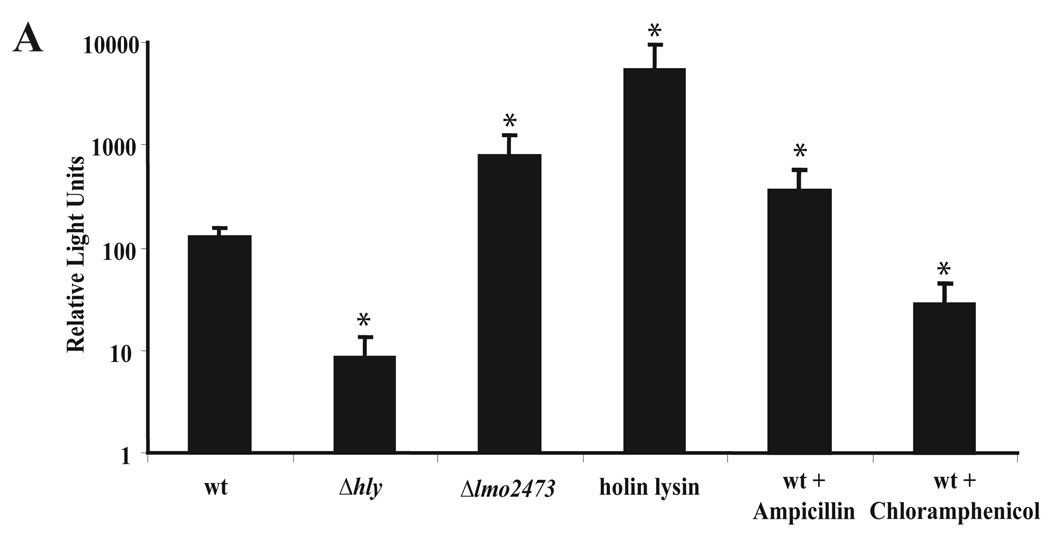
To test whether Δlmo2473 lyses in the host cytosol, we developed a reporter system to indirectly measure bacteriolysis. A luciferase reporter plasmid was constructed that encoded firefly luciferase under control of a cytomegalovirus (CMV) promoter. Lysis of cytosolic bacteria results in release of the reporter plasmid into the host cell and subsequent expression of luciferase from the CMV promoter. As a control for bacteriolysis we designed a L. monocytogenes strain expressing PSA bacteriophage holin and lysin from the actA promoter. Luciferase production by macrophages infected with the holin/lysin control strain was ~100-fold higher than that produced in macrophages infected with wild type L. monocytogenes (Fig. 2). Macrophages infected with Δlmo2473 mutants expressed ~5-10-fold more luciferase than those infected with wild type L. monocytogenes. Furthermore, intracellular wild type L. monocytogenes treated with ampicillin, a β-lactam antibiotic, but not with chloramphenicol, a protein synthesis inhibitor, resulted in bacteriolysis and increased production of luciferase from infected macrophages. Importantly there was a statistically significant increase in lysis of wild type bacteria compared to bacteria trapped in the vacuole (Δhly). These data indicated that there was a reproducible, but low level of lysis of wild type bacteria in the host cell cytoplasm, however, Δlmo2473 mutants, the holin/lysin-expressing strain, and β-lactam-treated bacteria lyse at a higher frequency than wild type bacteria.
Figure 2.
Delivery of plasmid DNA by L. monocytogenes strains. Lysis was measured by delivery and expression of luciferase from the luciferase reporter plasmid, pBHE573, in IFNAR−/− bone marrow-derived macrophages following a 6 hour infection at an MOI of 5. Luciferase expression is represented by relative luminescence units. Data are presented as the average of at least 3 independent experiments and the error bars represent the standard deviation of the mean. *indicates these values are statistically different with a p value <0.05 using a Students’ t-test
Bacteriolysis triggers inflammasome activation
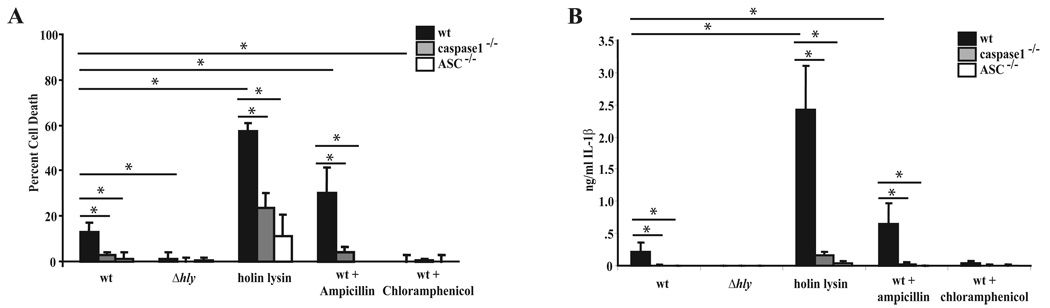
Since Δlmo2473 mutants lysed in the cytosol of host macrophages and hyper-induced pyroptosis, we hypothesized that bacteriolysis triggers pyroptosis. Therefore, to test this hypothesis, we measured host cell death and IL-1β secretion from macrophages infected with the holin/lysin control strain or ampicillin-treated bacteria. Similar to macrophages infected with Δlmo2473 mutants, cells infected with the holin/lysine-expressing strain or bacteria treated with ampicillin underwent pyroptosis and secreted IL-1β at a higher level than wild type infected cells (Fig. 3A, B). Conversely, chloramphenicol treatment following infection resulted in decreased cell death and IL-1β secretion. Bacterial access to the cytosol was still required for antibiotic stimulated host cell death as Δhly mutants treated with ampicillin did not stimulate pyroptosis (Fig. S1C, D). Similar to pyroptosis stimulated by wild type L. monocytogenes, inflammasome activation induced by the holin/lysin-expressing strain or ampicillin-treated bacteria was predominantly dependent on caspase-1 and ASC. This data indicated that bacteriolysis in the cytosol, either by expression of autolysins or treatment with β-lactam antibiotics, results in caspase-1/ASC-dependent pyroptosis.
Figure 3.
Pyroptosis and IL-1β release induced by L. monocytogenes that lyse. Cell death (A) and IL-1β (B) release were measured following a 6 hour infection of wild type, caspase-1−/− or ASC−/− bone marrow-derived macrophages at an MOI of 5 with the indicated strains. Data are presented as the average of at least 3 independent experiments and the error bars represent the standard deviation of the mean. *indicates these values are statistically different with a p value <0.05 using a one-way ANOVA followed by a post-hoc LSD analysis
AIM2 recognition of DNA released by bacteriolysis leads to pyroptosis
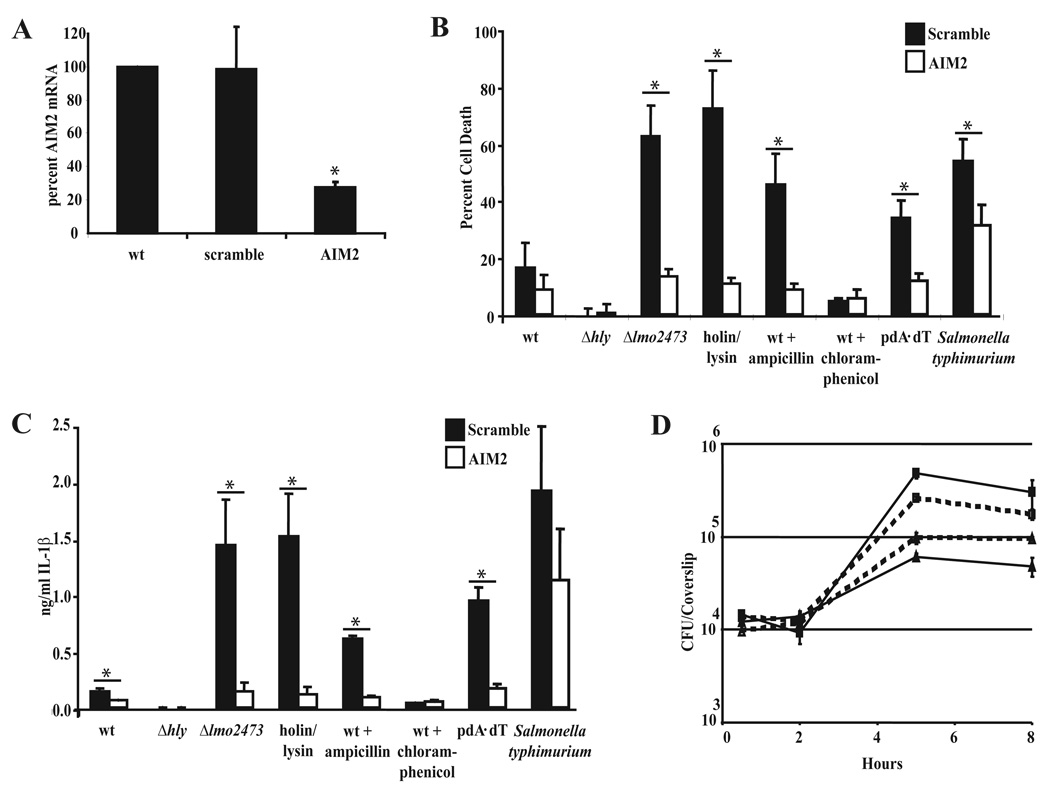
Lysis of intracellular bacteria could result in the release of many bacterial components into the host cytosol, including DNA. AIM2 was recently identified as a cytosolic DNA receptor that stimulates ASC-dependent inflammasome formation (Burckstummer et al., 2009; Fernandes-Alnemri et al., 2009; Hornung et al., 2009). We hypothesized that DNA released due to bacteriolysis in the cytosol of host macrophages would be recognized by AIM2 and induce pyroptosis. To test this hypothesis we developed stable shRNA-mediated AIM2 knockdowns in C57BL/6 immortalized macrophages. Lentiviral-based shRNA knockdown resulted in >75% knockdown of AIM2 mRNA in stably transduced immortalized macrophages compared to scramble shRNA transduced cells (Fig. 4A). Immortalized macrophages transduced with a scrambled shRNA responded similarly to wild type bone marrow-derived macrophages with respect to LDH release and IL-β secretion following infection with L. monocytogenes (Fig. 1A, B and 4C, D). Similar to the response to DNA (pdA-dT), knockdown of AIM2 resulted in significant decreases in both cell death and IL-1β secretion following infection with Δlmo2473 mutants, the holin/lysin-expressing strain, and ampicillin-treated bacteria (Fig. 4B, C). Meanwhile, pyroptosis in response to Salmonella typhimurium, a known Nlrc4 activator, was largely independent of AIM2. Importantly, even the low level of pyroptosis induced by wild type L. monocytogenes was partially AIM2-dependent. Consistent with this observation, cleavage of caspase-1 to the active p10 subunit was diminished in the AIM2-knockdown macrophages (Fig. S4A). Purified L. monocytogenes genomic DNA was also capable of inducing cell death and IL-1β when transfected into the cytosol of wild type macrophages (Fig. S4B, C). In addition, knockdown of AIM2 resulted in a partial rescue of the intracellular growth defect of Δlmo2473 (~50%) (Fig. 4D). Together this data indicated that bacteriolysis of L. monocytogenes in the cytoplasm released DNA that was sensed by AIM2 and triggered inflammasome activation.
Figure 4.
Knock-down of AIM2 abrogates inflammasome activation in response to L. monocytogenes. (A) The percentage of AIM2 mRNA following shRNA knockdown in immortalized macrophages was measured by qRT-PCR. mRNA analysis represents the average of 3 independent experiments and error bars represent the standard deviation of the mean. Cell death (B) and IL-1β (C) were measured following a 6 hour infection at an MOI of 5 of Scramble or AIM2 knockdown immortalized macrophages with the indicated strains. Data are presented as the average of 3 independent experiments and the error bars represent the standard deviation of the mean. (D) Representative intracellular growth of wild type L. monocytogenes (squares) or Δlmo2473 (triangles) in Scramble (solid lines) or AIM2 knockdown (dotted lines) immortalized macrophages. *indicates these values are statistically different with a p value <0.05 using a Students’ t-test.
Discussion
The results of this study show that L. monocytogenes strains that lyse in the host cytosol induced cell death and IL-1β secretion. Inflammasome activation was largely independent of Nlrp3 and Nlrc4, but was primarily dependent on the recently described DNA receptor, AIM2. These results suggested that DNA released during bacteriolysis is a ligand for inflammasome activation during infection with L. monocytogenes. This is the first report that AIM2 detects bacterial infection and may represent a conserved mechanism for the detection of intracellular bacterial pathogens.
Intracellular pathogens, by definition, require live host cells to support replication; accordingly, L. monocytogenes has evolved multiple mechanisms to minimize host cell death. Maintenance of this protected niche is tightly regulated and is central to virulence. Indeed, L. monocytogenes mutants that kill their host cells are severely attenuated in mice. For example, the pore-forming toxin Listeriolysin O (LLO), which facilitates bacterial escape from the phagosome, is under transcriptional, translational, and post-translational control that compartmentalize its activity to an acidic phagosome to minimize damage to the host plasma membrane (Glomski et al., 2002; Schnupf et al., 2007). L. monocytogenes mutants that fail to properly compartmentalize LLO activity induce non-inflammasome-mediated host cell death and are avirulent (Glomski et al., 2003).
Additionally, flagellin, a known activator of pyroptotic cell death (Lightfield et al., 2008; Molofsky et al., 2006; Ren et al., 2006), is tightly controlled during L. monocytogenes infection. During growth at 37°C, transcription of flaA, the gene encoding flagellin, is repressed through the activity of the transcriptional repressor MogR (Shen and Higgins, 2006). Deletion of mogR results in significant attenuation of virulence. Previous reports have demonstrated a role for L. monocytogenes flagellin in induction of pyroptosis, particularly when it is misregulated by deletion of the flagellar adaptor molecule FlgK (Warren et al., 2008). The wild type L. monocytogenes used in this and in Warren et al both contain a mutation in MogR that makes control of flagellin expression less stringent (Grundling et al., 2004), potentially explaining a partial role for Nlrc4 in response to wild type bacteria. The intricate regulation of LLO and flagellin demonstrate how L. monocytogenes has evolved to avoid induction of host cell death and maintain its intracellular niche.
The results of this study are consistent with the premise that L. monocytogenes causes very low levels of pyroptosis and that lack of host cell death is an essential aspect of L. monocytogenes pathogenesis. Indeed, L. monocytogenes strains engineered to hyper-activate the inflammasome by ectopically secreting flagellin are highly attenuated (Sauer, JD., Burke, T., Hanson, B., Lauer, P. and Portnoy D.A., unpublished data). Other groups, however, have reported much higher levels of inflammasome-activation by wild type L. monocytogenes and some have observed a role for Nlrp3. We do not observe a significant role for Nlrp3, even in L. monocytogenes strains that activate high levels of inflammasome activation. However, we and others have found that purified LLO added to the outside of cells induces Nlrp3-mediated IL-1β secretion (Fig. S3B, (Meixenberger et al., 2009)). Therefore it is possible that some of the discrepancies in the literature can be explained by the level of extracellular LLO.
The transposon mutant from our screen that caused the most cytotoxicity had an insertion in lmo2473. This gene encodes a protein of unknown function and resides in an operon with a predicted NADH dehydrogenase (lmo2471) and two additional genes encoding proteins of unknown function (lmo2474 and lmo2472). The architecture of the operon is unique to Listeria species, although homologues of lmo2474-lmo2472 are frequently found together in other organisms. YvcK in Bacillus subtilis is a close homologue of Lmo2473 with 46% sequence identity. B. subtilis ΔyvcK mutants were defective for growth with Kreb’s cycle intermediates as a sole carbon source (Gorke et al., 2005). Growth on Kreb’s cycle intermediates resulted in phenotypes indicative of cell wall biosynthesis defects including loss of optical density and abnormal cell morphology. Although L. monocytogenes is unable to utilize Kreb’s cycle intermediates as primary carbon sources (Trivett and Meyer, 1971), our observation that lmo2473 mutants lyse in the cytosol indicates that similar to the role of YvcK in B. subtilis, Lmo2473 may have a role in maintenance of the cell wall. Future studies of the Δlmo2473 mutant will help elucidate the role of this gene during wild type L. monocytogenes growth.
Identification of lmo2473 and the observation that Δlmo2473 mutants appeared to have cell wall defects led to the hypothesis that bacteriolysis was responsible for hyper-induction of the inflammasome. To directly evaluate the role of bacteriolysis in triggering pyroptosis, we constructed a strain designed to lyse and deliver DNA to the cytosol. The holin/lysin-expressing strain resulted in high levels of inflammasome activation. This observation, coupled with the low levels of pyroptosis stimulated by wild type bacteria, lead us to the conclusion that cytosolic wild type L. monocytogenes undergo very little bacteriolysis. Increased autolysis, resulting in pyroptosis, can result from genetic mutation, antibiotic stress or a number of other environmental conditions.
Treatment of intracellular bacteria with β-lactam antibiotics resulted in bacteriolysis and induction of AIM2-dependent pyroptosis, while chloramphenicol treatment did not. Ampicillin, a cell wall synthesis inhibitor, promotes autolysis, however, chloramphenicol is a bacteriostatic protein synthesis inhibitor and therefore does not result in bacteriolysis. Clinically, listeriosis is treated with ampicillin, often in combination with an aminoglycoside antibiotic (gentamicin) (Lorber, 1997). It is possible that ampicillin is a powerful a ntibiotic in vivo because in addition to targeting bacterial cell wall synthesis, β-lactam activity may increase bacteriolysis leading to induction of pyroptosis. Rationally designing antibiotics that not only target centrally important bacterial processes but also help stimulate host innate immune processes may be an attractive approach for the development of novel antimicrobials.
Similar to previous reports with Francisella, we observed some level of host cell death that was independent of caspase-1 but dependent on the inflammasome adaptor ASC (Fig. 1A, 3A)(Henry et al., 2007). It is possible that some inflammasome components intersect with caspase-1-independent cell death pathways in the cell. A recent report demonstrated that bacteriolysis of Shigella flexneri triggered caspase-3-dependent apoptosis in epithelial cells; however, in these cells bacteriolysis of L. monocytogenes did not stimulate any caspase activation (Tattoli et al., 2008). The function of AIM2 in different cell types remains incompletely understood, but it is clear that a variety of cells can recognize and respond to bacteriolysis in the cytosol. Furthermore, inflammasome components may interact with multiple cell death pathways independent of caspase-1.
In the future it will be important to test other cytosolic pathogens, such as Francisella tularensis, to determine if they are also recognized by AIM2 and if bacteriolysis is a conserved pattern of pathogenesis. Future studies to identify the role of AIM2 during acute infection and the development of acquired immunity will further highlight the role of the inflammasome in innate immunity to intracellular bacterial pathogens.
Methods
Bacterial strains and cell culture
Wild type 10403S L. monocytogenes and isogenic mutants were grown in Brain Heart Infusion media at 30°C overnight without shaking to stationary phase (OD600 – 1.3–8 1.6) for macrophage infections. Deletion of lmo2473 was performed by splice overlap extension and introduced into L. monocytogenes by allelic exchange (Camilli et al., 1993). Bone marrow-derived macrophages were prepared and frozen from femurs of 6–8 week old female mice as previously described (Jones and Portnoy, 1994). All knockout mice in this study were in the C57BL/6 genetic background. C57BL/6 mice were from The Jackson Lab (Bar Harbor, ME), IFNAR−/− mice were previously described (Leber et al., 2008), caspase 1−/− and Nlrc4−/− mice were from Dr. Russell Vance (University of California, Berkeley, CA), Nlrp3−/− and ASC−/− femurs were from Dr. Vishva Dixit (Genentech Inc., South San Francisco, CA).
Macrophage infections and treatments
Macrophages were pretreated for 12–16 hours with 100 ng/ml Pam3CSK4 (Invivogen, San Diego, CA) prior to infection. 5×105 bone marrow-derived macrophages were infected with L. monocytogenes at a multiplicity of infection (MOI) of 5 bacteria per cell in 24-well plates for 30 minutes. At 30 minutes post infection media was removed and replaced with media containing 50 µg/ml gentamicin and 100 ng/ml Pam3CSK4. Six hours post infection, supernatants were collected and analyzed for LDH release and IL-1β secretion. Where indicated, infected macrophages were treated with 1 µg/ml ampicillin or 100 µg/ml chloramphenicol at 2 hours post infection.
For infections with Salmonella typhimurium LT2, overnight cultures were grown in Luria Bertani (LB) broth at 37°C with shaking. Prior to infection of macrophages, cultures were diluted 1:100 into LB broth and grown for 3 hours at 37°C with shaking. Infections were then performed similar to L. monocytogenes infections described above resulting in an MOI of ~5 bacteria per cell. Visual inspection of the infected macrophages reveals that this MOI results in greater than 90% of the macrophages being infected with one or more bacteria.
Poly (dA-dT) was purchased from Sigma-Aldrich and transfected into cells using Lipofectamine2000 (Invitrogen, Grand Island, NY) at a concentration of 500 ng of Poly (dA-dT) per well. Transfections were carried according to the manufacturers protocol for a total of 6 hours at which point samples were collected for LDH and IL-1β analysis.
Lactate Dehydrogenase release and Interleukin-1β ELISA
To measure LDH release, 60 µL infection supernatant was added to 60 µL of LDH detection reagent, as previously described (Decker and Lohmann-Matthes, 1988) in triplicate in 96-well plates. Absorbance was read on a SpectraMax 340 spectrophotometer (Molecular Devices) at wavelength 490nm and lysis values were calculated as a percentage of cells lysed with 1% TritonX-100.
IL-1β secretion was determined using mouse IL-1 beta ELISA Ready-SET-Go according to the manufacturer’s instructions (eBioscience, San Diego, CA).
Intracellular growth curves
2×106 macrophages were pretreated with 100 ng/ml Pam3CSK4 (Invivogen, San Diego, CA) overnight, infected with bacteria at a multiplicity of infection of one bacterium per cell and replication was quantified as previously described (Portnoy et al., 1988).
Holin/lysin and luciferase reporter construction
The actA promoter was amplified from 10403S genomic DNA and cloned into the MCS of a derivative of the site-specific integration vector pPL (Lauer et al., 2002). The holin-lysin gene cassette was then amplified from PSA genomic DNA (a gift from Richard Calendar, University of California, Berkeley) and cloned downstream of the actA promoter.
The luciferase reporter plasmid was constructed in a stepwise manner. The RP4 oriT was cloned into the plasmid pAM401 (Wirth et al., 1986) resulting in the plasmid pAM401oriT allowing for direct conjugation from E. coli into L. monocytogenes. The modified firefly luciferase gene (luc+) was digested from pGL3-Control (Promega, Madison, WI) and ligated into pAM401oriT. The CMV enhancer, immediate early promoter and chimeric intron were subcloned from pRL-CMV (Promega) and ligated into the pAM401oriT-luc plasmid, resulting in the luciferase reporter plasmid pBHE573.
Luciferase reporter delivery system
5×105 non-stimulated bone marrow-derived IFNAR−/− macrophages per well of 24-well plates were infected with L. monocytogenes carrying the luciferase-expressing reporter plasmid, pBHE573, at a multiplicity of infection of 5 bacteria per cell for one hour. At one hour post infection, media was removed and replaced with media containing 50 µg/ml gentamicin. Six hours post infection, supernatants were removed and cells were lysed with TNT Lysis Buffer (20 mM Tris, mM NaCl, 1% triton, pH 8.0). Cell lysates were transferred to opaque 96-well plates and luciferase reagent added as previously described (McWhirter et al., 2009). Luciferase activity was measured by luminometer (VICTOR, PerkinElmer).
AIM2 knockdown
AIM2 shRNA knockdown vectors were a kind gift from Dr. Katherine Fitzgerald and immortalized C57Bl/6 macrophages were a gift from Dr. Russell Vance. Lentiviral-mediated knockdowns were performed using the pLKO.1 system as previously described (Stewart et al., 2003) in immortalized C57Bl/6 bone marrow-derived macrophages (Blasi et al., 1985).
Macrophage gene expression by qRT-PCR
RNA was purified from 2×106 immortalized macrophages using the RNaqueous kit(Ambion, Austin, TX). RNA was then DNase treated, processed and analyzed as previously described (Leber et al., 2008).
Statistical analysis
Statistical Analysis was performed using Analyse-It (Leeds, UK). Students’ t-test or one way ANOVA followed by a post-hoc LSD test was performed as indicated. * represent p-values of <.05.
Supplementary Material
Acknowledgments
We would like to thank Dr. Russell Vance, Dr. Denise Monack, Dr. Lee Shaughnessy and Jonathan Jones for helpful discussions and critical review of the manuscript. This work was supported by National Institutes of Health Grant PO1 A1063302 (D.A.P.), National Institutes of Health Grant AI27655 and American Cancer Society Postdoctoral Fellowship PF-07-066-01-LIB (JD.S.). Daniel A. Portnoy has a consulting relationship with and a financial interest in Aduro Biotech. Peter Lauer and Bill Hanson are employees of Aduro Biotech.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barsig J, Kaufmann SH. The mechanism of cell death in Listeria monocytogenes-infected murine macrophages is distinct from apoptosis. Infect Immun. 1997;65:4075–4081. doi: 10.1128/iai.65.10.4075-4081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E, Mathieson BJ, Varesio L, Cleveland JL, Borchert PA, Rapp UR. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature. 1985;318:667–670. doi: 10.1038/318667a0. [DOI] [PubMed] [Google Scholar]
- Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- Camilli A, Tilney LG, Portnoy DA. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods. 1988;115:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- Glomski IJ, Decatur AL, Portnoy DA. Listeria monocytogenes mutants that fail to compartmentalize listerolysin O activity are cytotoxic, avirulent, and unable to evade host extracellular defenses. Infect Immun. 2003;71:6754–6765. doi: 10.1128/IAI.71.12.6754-6765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J Cell Biol. 2002;156:1029–1038. doi: 10.1083/jcb.200201081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorke B, Foulquier E, Galinier A. YvcK of Bacillus subtilis is required for a normal cell shape and for growth on Krebs cycle intermediates and substrates of the pentose phosphate pathway. Microbiology. 2005;151:3777–3791. doi: 10.1099/mic.0.28172-0. [DOI] [PubMed] [Google Scholar]
- Grundling A, Burrack LS, Bouwer HG, Higgins DE. Listeria monocytogenes regulates flagellar motility gene expression through MogR, a transcriptional repressor required for virulence. Proc Natl Acad Sci U S A. 2004;101:12318–12323. doi: 10.1073/pnas.0404924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med. 2007;204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof H. An update on the medical management of listeriosis. Expert Opin Pharmacother. 2004;5:1727–1735. doi: 10.1517/14656566.5.8.1727. [DOI] [PubMed] [Google Scholar]
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Portnoy DA. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect Immun. 1994;62:5608–5613. doi: 10.1128/iai.62.12.5608-5613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer P, Chow MY, Loessner MJ, Portnoy DA, Calendar R. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J Bacteriol. 2002;184:4177–4186. doi: 10.1128/JB.184.15.4177-4186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber JH, Crimmins GT, Raghavan S, Meyer-Morse NP, Cox JS, Portnoy DA. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog. 2008;4:e6. doi: 10.1371/journal.ppat.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun YH, Cado D, Dietrich WF, et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9:1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber B. Listeriosis. Clin Infect Dis. 1997;24:1–9. doi: 10.1093/clinids/24.1.1. quiz 10-11. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- McWhirter SM, Barbalat R, Monroe KM, Fontana MF, Hyodo M, Joncker NT, Ishii KJ, Akira S, Colonna M, Chen ZJ, et al. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J Exp Med. 2009;206:1899–1911. doi: 10.1084/jem.20082874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meixenberger K, Pache F, Eitel J, Schmeck B, Hippenstiel S, Slevogt H, Guessan PN, Witzenrath M, Netea MG, Chakraborty T, et al. Listeria monocytogenes-Infected Human Peripheral Blood Mononuclear Cells Produce IL-1{beta}, Depending on Listeriolysin O and NLRP3. J Immunol. 2009 doi: 10.4049/jimmunol.0901346. [DOI] [PubMed] [Google Scholar]
- Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozoren N, Masumoto J, Franchi L, Kanneganti TD, Body-Malapel M, Erturk I, Jagirdar R, Zhu L, Inohara N, Bertin J, et al. Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J Immunol. 2006;176:4337–4342. doi: 10.4049/jimmunol.176.7.4337. [DOI] [PubMed] [Google Scholar]
- Portnoy DA, Jacks PS, Hinrichs DJ. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulston A, Marcellus RC, Branton PE. Viruses and apoptosis. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- Schnupf P, Zhou J, Varshavsky A, Portnoy DA. Listeriolysin O secreted by Listeria monocytogenes into the host cell cytosol is degraded by the N-end rule pathway. Infect Immun. 2007;75:5135–5147. doi: 10.1128/IAI.00164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen A, Higgins DE. The MogR transcriptional repressor regulates nonhierarchal expression of flagellar motility genes and virulence in Listeria monocytogenes. PLoS Pathog. 2006;2:e30. doi: 10.1371/journal.ppat.0020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik C, Dorfleutner A. COPs and POPs: modulators of inflammasome1 activity. J Immunol. 2007;179:7993–7998. doi: 10.4049/jimmunol.179.12.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. Rna. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp H, Starr MP. Bacteriolysis. Annu Rev Microbiol. 1965;19:79–104. doi: 10.1146/annurev.mi.19.100165.000455. [DOI] [PubMed] [Google Scholar]
- Tattoli I, Lembo-Fazio L, Nigro G, Carneiro LA, Ferraro E, Rossi G, Martino MC, de Stefano ME, Cecconi F, Girardin SE, et al. Intracellular bacteriolysis triggers a massive apoptotic cell death in Shigella-infected epithelial cells. Microbes Infect. 2008;10:1114–1123. doi: 10.1016/j.micinf.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Trivett TL, Meyer EA. Citrate cycle and related metabolism of Listeria1 monocytogenes. J Bacteriol. 1971;107:770–779. doi: 10.1128/jb.107.3.770-779.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol. 2008;180:7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth R, An FY, Clewell DB. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J Bacteriol. 1986;165:831–836. doi: 10.1128/jb.165.3.831-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.