Abstract
Background
Active tuberculosis (TB) must be excluded before initiating isoniazid preventive therapy (IPT) in HIV-infected persons, but currently used screening strategies suffer from poor sensitivity and specificity and high patient attrition rates. Liquid TB culture is now recommended for the detection of Mycobacterium tuberculosis in TB suspects. This study compared the efficacy, effectiveness and speed of the microscopic-observation drug-susceptibility (MODS) assay with currently used strategies for tuberculosis screening prior to IPT in HIV-infected persons.
Methods
471 HIV-infected IPT candidates at three hospitals in Lima, Peru, were enrolled into a prospective comparison of tuberculosis screening strategies, including laboratory, clinical and radiographic assessments.
Results
Of 435 patients who provided two sputum samples, M. tuberculosis was detected in 27 (6.2%) by MODS, 22 (5.1%) by Lowenstein-Jensen culture and 7 (1.6%) by smear. Of patients with any positive microbiological test, a MODS culture was positive in 96% by 14 days and 100% by 21 days. MODS simultaneously detected multidrug-resistant tuberculosis in two patients. Screening strategies involving combinations of clinical assessment, chest radiograph and sputum smear were less effective than two liquid TB cultures in accurately diagnosing and excluding tuberculosis (p<0.01). Screening strategies that included non-culture tests had poor sensitivity and specificity.
Conclusions
MODS identified, and reliably excluded, cases of pulmonary tuberculosis more accurately than other screening strategies, while providing results significantly faster than Lowenstein-Jensen culture. The streamlining of TB rule-out through the use of liquid culture-based strategies could help facilitate the massive upscaling of IPT required to reduce HIV and TB morbidity and mortality.
Keywords: tuberculosis diagnosis, preventive therapy, screening, HIV
Introduction
Tuberculosis (TB) is the most common serious opportunistic disease and a leading cause of death in HIV-infected persons. An important source of new TB cases is the reactivation of latent tuberculosis infection (LTBI). This contribution has mushroomed in regions of high HIV prevalence because HIV greatly increases the risk for progression of LTBI to active TB disease [1]. This risk can be reduced by isoniazid preventive therapy (IPT), with benefits for both the individual [2, 3], and, by averting incident TB, public health [4, 5]. Effectiveness has been demonstrated in many populations [2, 6-9], though not all [10, 11], and it is generally accepted that the lifetime risk of reactivation TB can be reduced to 4% or less [12, 13].
Isoniazid alone is appropriate treatment for LTBI but inadvertent isoniazid monotherapy for active TB is ineffective and leads to drug resistance. Therefore, the pathway to commencing IPT must reliably exclude active TB. Surprisingly, international consensus guidelines on how this should be achieved are lacking. Challenges [3, 14] include procedural factors (the requirement for multiple investigations involving several visits leads to patient attrition of 20% [15, 16]) and the poor sensitivity of conventional tests (clinical history [17], sputum smear and chest radiography [16, 18, 19]) for detection of HIV-associated TB in which cough may be unproductive and chest radiograph normal. This bottleneck to successfully ruling out active TB is a significant obstacle to scale-up of IPT.
The microscopic-observation drug-susceptibility (MODS) assay is a low-cost, liquid culture tool for TB culture and direct drug susceptibility testing (DST) developed for resource-limited settings. Diverse studies of MODS [20-22] have demonstrated high specificity and sensitivity for diagnosis of TB and multidrug-resistant (MDR) TB in symptomatic patients. Importantly for TB rule-out, time to definitively negative culture with MODS is only 21 days; 99% of positive cultures are positive by 14 days.
These characteristics suggest that MODS might be a useful tool for the pre-IPT TB screening of HIV-infected patients. We hypothesized that the high sensitivity of MODS would negate the need for other investigations, and the rapid turnaround time would mitigate patient loss to follow-up and thus facilitate a greater flow of patients onto IPT.
The Peruvian national TB rule-out strategy prior to IPT involves clinical evaluation by a physician, chest radiography and analysis of two sputum samples by Ziehl-Neelsen smear and solid media TB culture. In this study we investigated how the culture of one or two sputum samples by MODS alone would compare to the Peruvian and other TB screening strategies in terms of sensitivity, specificity, delay to diagnosis and cost. The intended outcome was the delivery to policymakers of robust comparative data to enable rational decision-making about resource allocation for TB rule-out.
Methods
Study Setting and Population
HIV-infected candidates for IPT were consecutively recruited at the start of the TB rule-out work-up at three public hospitals in Lima, Peru, from February through December 2007. Exclusion criteria were age under 15 years, current treatment with anti-tuberculosis agents, any IPT in the previous 12 months and inability to give written informed consent. Patients were not paid for participation but travel costs were fully reimbursed.
Field Procedures
All patients underwent clinical evaluation by a physician to screen for pulmonary and extrapulmonary TB. Physicians referred to the study only those patients for whom the purpose of TB rule-out was the initiation of IPT. Though no enrolled patient was strongly suspected of having active TB, physicians were asked to assess the risk (none, low, moderate or high) of active TB relative to other IPT candidates, based on clinical history, symptoms and CD4 count. For subsequent analyses, “none” or “low” were grouped as “lower risk”; “moderate” or “high” were grouped as “higher risk.” After providing informed consent and interview to record demographic, clinical and socioeconomic data, patients were asked to provide two sputum samples (on different days) for the study in addition to the two samples required by the hospital laboratory for routine work-up.
Chest radiographs were interpreted by a single investigator blinded to patient details, who was required to respond “yes” or “no” to the question, “Are there any findings suggestive of active TB for which further testing would be recommended prior to IPT?”
In Peru no confirmation of LTBI (by tuberculin skin test [TST] or other means) is sought prior to IPT in HIV-infected persons. However, a positive TST is used to identify IPT candidates in other settings such as southern Africa [23], and was therefore included in this study. TST (5 IUs PPD) was performed according to standard guidelines - transverse induration of ≥5 mm after 48-72 hours was considered positive. TST was not undertaken if the patient declined or reported having had a TST in the previous six months. Automated CD4 cell count was undertaken unless performed within the six months prior to enrollment, in which case this value was used.
Dedicated study personnel who rotated through all three study sites were trained collectively in data and specimen acquisition. Weekly team meetings ensured standardization of practices across the sites.
Laboratory Procedures
Sputum samples were maintained at 4°C until same or next-day transport to the study laboratory, where they were digested and decontaminated by the standard N-acetyl-L-cysteine-NaOH-sodium citrate method [24]. An aliquot was used for auramine smear microscopy, the remainder for parallel Lowenstein-Jensen (LJ) and MODS cultures, performed by three experienced laboratory technologists who were blinded to patient details and the results of other tests. After inoculation LJ slants were incubated at 37°C and examined twice weekly from day 7 through day 60 [24]. Contaminated slants were discarded and repeat decontamination and culture were undertaken using a stored portion of the original sample.
MODS Culture
Five samples were each cultured in one 4-well column of a 24-well plate; a middle column served as a negative control. Each well contained 900 μL of decontaminated sputum, Middlebrook 7H9 broth, OADC and PANTA (full SOP available at www.modsperu.org). Plates were incubated at 37°C and examined daily (weekdays only) from days 5 to 15, then on alternate days to day 25, under an inverted microscope at 40× and 100× magnification. To minimize cross-contamination and occupational exposure, plates were sealed inside plastic ziploc bags and subsequently examined within the bag. If ≥ 2 colony-forming units of characteristic morphology [25-27] were detected in each of the two drug-free wells, the sample was considered positive. Growth in a drug-containing well indicated resistance to that drug. If there was no evidence of growth by day 25, the culture was considered negative [20]. Fungal or bacterial contamination was recognized by rapid overgrowth or clouding; if detected, the stored portion of the original sample was decontaminated and cultured.
Definitions
A TB-positive patient was defined as one for whom M. tuberculosis was detected by at least one microbiological test (auramine stain, Lowenstein-Jensen culture or MODS). Indeterminate cultures were those that were persistently contaminated after repeat decontamination and culture. Patients with 2 smear and culture-negative sputum samples were considered TB-negative.
Estimated Initial Costs of Screening Packages
Costs of the screening packages (combinations of screening tests) were estimated by including wholesale prices of test reagents including shipping, pro-rata costs of local medical personnel time and patient costs (median of transportation cost, personal lost income and family lost income) as derived from a questionnaire. Costs for repeat culture due to contamination were also included. Downstream costs, overhead and capital costs were not included. All costs were converted to US$2007 and based on current market costs for Lima public hospitals.
Data Analysis
Data were analyzed utilizing Stata 9. To compare the equivalence of screening packages, McNemar's test was used with a significance level of α<0.05. Because some patients did not complete all strategies, due to either lack of follow-up or indeterminate results, separate tables of sensitivity and specificity were derived to reflect the results from an intent-to-screen perspective and from the sub-group for whom all test results were available. Characteristics of TB-confirmed and TB-excluded patients were compared using two-sided t tests and chi-square tests with a significance level of α<0.05.
Ethical Review
Study protocol and consent and assent forms were approved by the ethics committees of Universidad Peruana Cayetano Heredia, Hospital Nacional Dos de Mayo, Hospital Nacional Hipólito Unanue and Hospital Nacional General Arzobispo Loayza.
Results
Patients and Samples
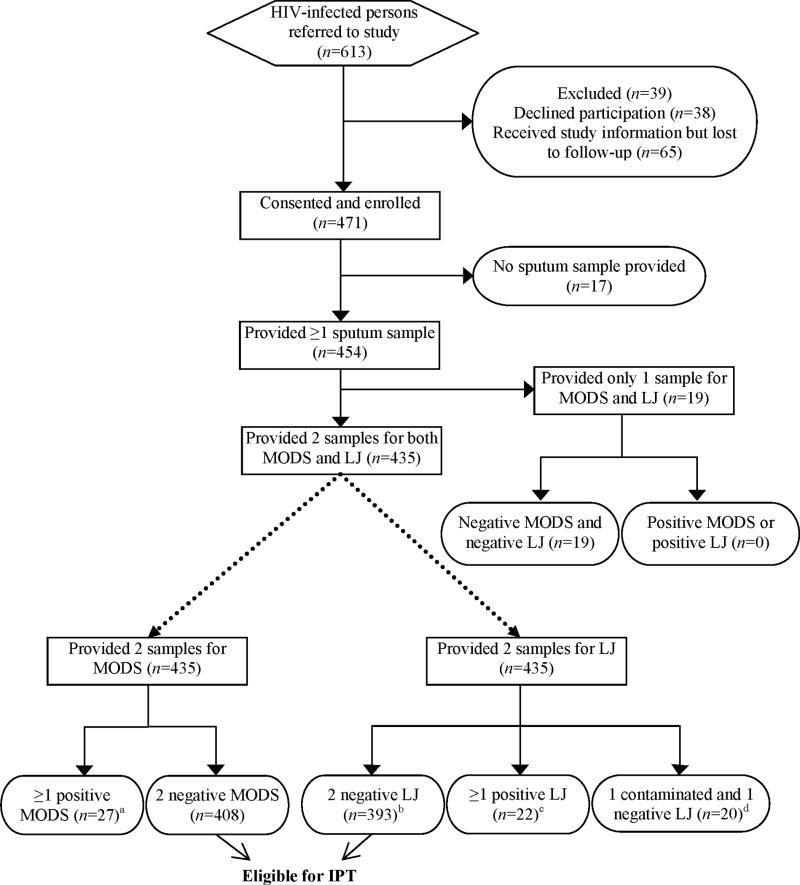
The characteristics of the population studied are shown in Table 1, and the flow chart for sputum testing in Figure 1. Of 454 patients who provided at least one sputum sample, 129 (28%) reported taking highly active anti-retroviral therapy at the time of enrollment. Nineteen patients provided only one sample for the study; their culture results were included only when the sample was used as the unit of analysis. Of 889 sputum samples, 44 (4.9%) were culture-positive for M. tuberculosis by at least one method, of which 13 (30% of culture-positive samples) were auramine-positive. By participant, 27 (6.2%) had at least 1 positive culture; 7 (26% of culture-positive patients) had a positive auramine smear microscopy result. No smear-positive sample was culture-negative.
Table 1.
Participant characteristics
| Patients with no demonstrated TBa (n=408) |
Patients with active TBb (n=27) |
p-valuec | |
|---|---|---|---|
| Age in years, mean ± SD | 34.1 ± 10.7 | 34.7 ± 10.3 | 0.78 |
| Women | 158 (39%) | 4 (15%) | 0.01 |
| Previous TBd | 60 (15%) | 2 (7%) | 0.29 |
| Previous isoniazid preventive therapye | 58 (14%) | 4 (15%) | 0.94 |
| Recent TB contactd | 125 (31%) | 8 (30%) | 0.91 |
| Positive tuberculin skin test (≥5 mm)f | 116 (32%) | 13 (59%) | 0.008 |
| CD4 cells/μL, mean ± SDg | 246 ± 190 | 164 ± 96 | 0.06 |
| Two or more symptoms (cough, fever, weight loss, night sweats) for ≥ 14 daysh,i | 60 (15%) | 8 (30%) | 0.08 |
Data are presented as number (percentage), unless otherwise noted. Nineteen patients who provided less than two sputum samples are excluded. Five patients were hospitalized at the time of enrollment; the remainder were ambulatory.
Defined as having only negative microbiologic tests.
Defined as having at least one positive microbiologic test.
Chi-square for categorical variables, t-test for continuous variables.
Self-reported, one non-response.
Self-reported, two non-responses.
368 TB-negative patients and 22 TB-positive patients returned for reading of a tuberculin skin test placed at the time of enrollment.
375 TB-negative patients and 20 TB-positive patients had a documented CD4 count within six months of study enrollment.
Self-reported by 404 TB-negative patients and 27 TB-positive patients.
The positive predictive value (PPV) of having two or more of these symptoms for ≥14 days was 11.8%, the negative predictive value (NPV) was 94.8%, and the positive likelihood ratio (LR) was 1.7; for those with CD4<200, PPV was 10.0% (p=0.75), while for those with CD4≥200, PPV was 4.5% (p=0.62). The PPV of having any of these symptoms for any period of time was 8.0%, the NPV was 97.7%, and the positive LR was 3.5. Three patients with active TB were asymptomatic. Because of the low PPVs, and because symptoms do not figure in Peruvian screening guidelines, no further evaluations of symptoms as a screening tool were undertaken.
Figure 1.
Patient flow chart for sputum testing by MODS and by Lowenstein-Jensen culture (LJ)
a15 patients had two positive MODS cultures. Twelve patients had one positive and one negative MODS culture; of these, three patients had no positive LJ culture or auramine stain.
bThree of these LJ-negative patients had at least one positive MODS culture.
c10 patients had two positive LJ cultures, 10 had one positive and one negative culture and two had one positive and one contaminated culture. All 22 of these patients had at least one positive MODS culture.
dTwo of these patients had at least one positive MODS culture.
eDemographic data was available for 131 of the 178 patients referred to the study but ultimately not part of the inclusion group: 37.4% were women (p=0.97 when compared with participants), and mean age ± standard deviation was 36.7 ± 10.8 (p=0.02). CD4 count was available for 101 of these patients: mean ± standard deviation was 261 ± 170 (p=0.31).
Table 2 shows comparative performances of culture methods. Sensitivities (and 95% CI) of MODS and LJ by sample were 95% (85-99%) and 73% (57-85%), respectively (p=0.02, McNemar's test); and by patient were 100% (87-100%) and 81% (62-94%), respectively (p=0.07, McNemar's test).
Table 2.
Comparison of MODS and Lowenstein-Jensen culture results
| Lowenstein-Jensen culture result | |||||
|---|---|---|---|---|---|
| Per sample | Per patientc | ||||
| TB | Not TB | TB | Not TB | ||
| MODS | TB | 30 | 12 | 22 | 5 |
| result | Not TB | 2a | 845b | 0 | 408d |
For the 2 samples that were LJ-positive but MODS-negative, the other sample from each patient was MODS-positive; poor sample quality or division might explain the discrepancy.
Includes 24 samples that were contaminated (indeterminate) in Lowenstein-Jensen media.
Excludes 19 patients who provided only one sputum sample.
Includes 20 patients who had one contaminated (indeterminate) and one negative culture in Lowenstein-Jensen media.
Contamination
Sixty-five (7.3%) samples were initially contaminated in MODS; repeat decontamination yielded 64 definitively negative and one positive culture. In LJ, 192 (22%) samples were initially contaminated (versus MODS, p<0.001); repeat decontamination yielded 166 negative, four positive and 22 persistently contaminated and thus indeterminate cultures (2.5% of all samples) (Figure 1).
Added Value of Second Culture
Of 27 patients with at least one positive MODS culture, nine (33%) had only a positive second sample; three (11%) had only a positive first sample. Of 22 patients with at least one positive LJ culture, seven (32%) had only a positive second sample; five (23%) had only a positive first sample.
Time to Culture Result, Including Delays Due To Contamination and Re-culture
Median time to culture-positivity by MODS and LJ was 8 days (interquartile range 7-10 days) and 26 days (21-33 days), respectively (p<0.001, Wilcoxon test). Median time to definitive culture-negativity by MODS and LJ was 28 days (26-31 days) and 63 days (61-66 days), respectively (p<0.001, Wilcoxon test). All TB-positive patients had a positive MODS culture within 21 days; 96% within 14 days.
Direct Drug-Susceptibility Testing
Simultaneous MODS DST revealed that six of the 27 patients with culture-positive TB had strains resistant to isoniazid only, and two had strains resistant to both isoniazid and rifampin (multidrug-resistant).
Clinical Examination, Chest Radiography and Tuberculin Skin Testing
Physician-judged clinical evaluation assigned 394 patients as at lower risk and 60 patients as at higher risk of active TB, of which 15 (3.8%) and 12 (20%) respectively had culture-positive TB. The presence or absence of two or more constitutional symptoms did not usefully differentiate between those with and without active TB (Table 1). Although clinical outcomes were not a primary objective of this study, follow-up vital status data was available for 228 baseline TB-negative patients representing 109,026 patient-days (297.12 patient-years); the remainder were lost to follow-up. Fourteen incident cases of TB were identified; mean time to diagnosis following negative screen was 298 days (median 178.5) and 79% (11/14) occurred more than three months after screening.
The sensitivity of chest radiography for culture-positive TB in the 437 patients who completed x-ray was 56% (95% CI 35-75%). Culture-positive TB was demonstrated in 11 of 356 patients (3.1%) whose radiograph showed no evidence of active TB, and in 14 of 81 patients (17%) whose radiographic findings suggested further work-up was needed before initiating IPT.
TST was performed in 445 patients, of whom 391 (88%) returned for reading in 2-3 days; TST was positive in 130 (33% of returners).
Comparison of MODS and Other Tests for TB Rule-Out
Performance characteristics of single tests and multi-test screening packages and their associated costs are presented in Table 3. Comparative times to correct assignment as TB-positive or TB-negative are plotted in Figure 2.
Table 3.
Tuberculosis screening package performance comparison (ordered by proportion of patients correctly assigned)
| Completed packagea (n varies) | Intent-to-screenb (n=435) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Package | Patients correctly assignedc (n=435) | Sensitivity of tests % | Specificity of tests % | Positive predictive value of package | Negative predictive value of package | Failed to complete packaged (n) | TB-positive cases missed (n) | US$2007 per person intended-to-screene | ||
| More patients correctly assigned than no screening |  |
2 MODS | 435 (100%) | 27/27 (100%) | 408/408 (100%) | 27/27 (100%) | 408/408 (100%) | 19 | 0 | $13.62 |
| 1 MODS | 426 (97.9%) | 18/27 (67%) | 408/408 (100%) | 18/18 (100%) | 408/417 (97.8%) | 0 | 9 | $7.31 | ||
| 2 Smears | 415 (95.4%) | 7/27 (26%) | 408/408 (100%) | 7/7 (100%) | 408/428 (95.3%) | 19 | 20 | $5.57 | ||
| 1 LJ | 414 (95.2%) | 15/25 (60%) | 399/399 (100%) | 15/15 (100%) | 408/420 (97.1%) | 11 | 12 | $6.39 | ||
| 2 LJ | 412 (94.7%) | 22/25 (88%) | 390/390 (100%) | 22/22 (100%) | 408/413 (98.8%) | 39 | 5 | $11.77 | ||
| No Screening (all would get IPT) | 408 (93.8%) | 0/27 (0%) | 408/408 (100%) | n/a | 408/435 (93.8%) | 0 | 27 | $0.00 | ||
| Fewer patients correctly assigned than no screening |  |
Clinical Exam + 2 MODS | 393 (90.3%) | 27/27 (100%) | 366/408 (89.7%) | 27/69 (39.1%) | 366/366 (100%) | 19 | 0 | $14.14 |
| Clinical Exam + 2 Smears | 380 (87.4%) | 14/27 (52%) | 366/408 (89.7%) | 14/56 (25%) | 366/379 (96.6%) | 19 | 13 | $6.09 | ||
| Clinical Exam | 378 (86.9%) | 12/27 (44%) | 366/408 (89.7%) | 12/54 (22%) | 366/381 (96.1%) | 0 | 15 | $1.52 | ||
| Clinical Exam + 2 Smears + 2 LJ | 371 (85.3%) | 23/25 (92%) | 348/390 (89.2%) | 23/65 (35%) | 366/370 (98.9%) | 39 | 4 | $14.86 | ||
| CXR | 351 (80.7%) | 14/25 (56%) | 337/399 (84.5%) | 14/76 (18%) | 346/359 (96.4%) | 17 | 13 | $9.17 | ||
| Southern Africa Algorithmf (430 total patients) | 350 (81.4%) | 11/21 (52%) | 338/358 (94.4%) | 12/33 (36%) | 382/397 (96.2%) | 87 | 15 | $17.03 | ||
| CXR + Clinical Exam | 330 (75.9%) | 18/25 (72%) | 310/399 (77.7%) | 20/111 (18%) | 317/324 (97.8%) | 17 | 7 | $9.70 | ||
| CXR + Clinical Exam + 2 Smears | 330 (75.9%) | 18/25 (72%) | 310/399 (77.7%) | 20/111 (18%) | 317/324 (97.8%) | 30 | 7 | $13.26 | ||
| CXR + Clinical Exam + 2 Smears + 2 LJ | 320 (73.6%) | 22/23 (96%) | 295/381 (77.4%) | 25/116 (22%) | 317/319 (99.4%) | 50 | 2 | $22.03 | ||
TB-positive cases were defined as patients having any positive microbiologic test. Patients who provided fewer than two sputum samples did not have an adequate culture reference standard and thus were excluded from the table. Positive clinical exam=higher risk of active TB, according to referring clinician. Positive chest radiograph (CXR)=findings consistent with active TB for which further testing would be recommended prior to isoniazid preventive therapy. Southern Africa algorithm27=preliminary screening by tuberculin skin test (TST); then, for only those with positive TST: clinical exam, chest radiograph, two smears, and two LJ.
Excludes patients who did not undergo one or more components of the package (e.g. CXR), or who had one contaminated (indeterminate) and one negative LJ; denominators vary according to completion rate of the package. This is the “as screened” analysis which provides efficacy data for completed packages.
Within a package, a positive result for any component was considered to be a positive result for the package. This “intention-to-screen” analysis describes overall performance in all those who entered into screening, and incorporates the effect of loss-to-follow-up.
Indicates those correctly assigned as TB-positive or TB-negative by the package, with the reference standard being any positive microbiologic test. Patients who failed to complete all components of a package were considered not correctly assigned, unless at least one component test was positive in the context of a positive culture result.
This is the only column that includes the 19 patients who provided only one sputum sample; these patients were considered to have failed to complete all packages involving two sputum samples.
This value was attained by summing the cost of diagnosis and patient costs from Supplemental Tables 1 and 2, then dividing the result by the 435 patients presented in Table 3. The median of the patient costs, including transport, was used.
Five patients did not undergo TST and thus were excluded from analysis of this package.
Figure 2.
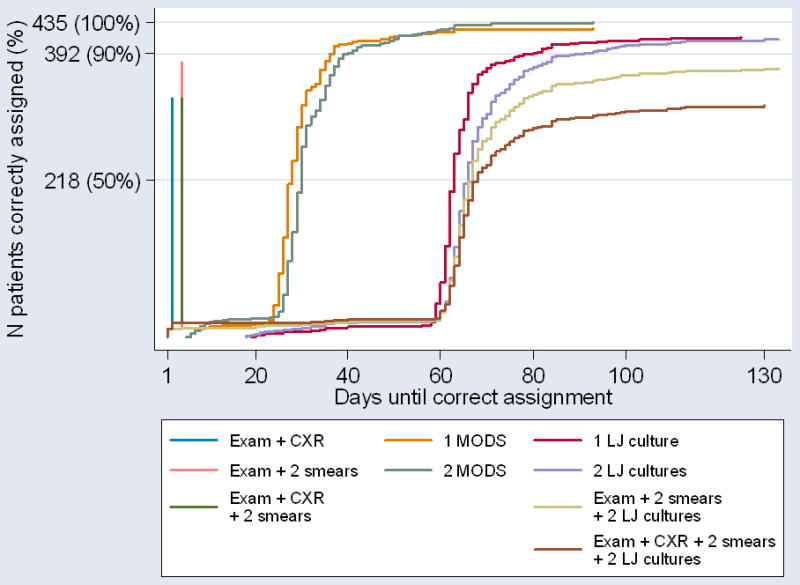
Time to correct assignment of patients by the various screening packages. This graph represents the time until the 435 patients who provided two sputum samples would have been correctly assigned as TB-positive or TB-negative by the various screening packages. Each package is represented by a distinct line, with the maximum height on the y-axis signifying the number of patients correctly assigned by that package. A patient was considered “correctly assigned” if and when 1) any component of the screening package was positive and any microbiologic test result was positive or 2) all components of the screening package were negative and all microbiologic test results were negative. Patients who failed to complete all components of a package were considered “not correctly assigned,” unless at least one component test was positive in the context of a positive microbiologic test result. Packages delivering the best performance in correct patient assignment reach furthest up the y axis, whilst the speed with which results are available is reflected on the x axis. An ideal package would be represented by a straight vertical line which ended in the top left hand corner. Re-culturing times for contaminated samples are included. Exam=clinical exam by referring physician. CXR=chest radiograph. LJ=Lowenstein-Jensen.
Costs of Initial Diagnostic Packages
The costs per person intended to screen are presented in Table 3. Specific costs and assumptions are in Supplemental Tables 1 and 2.
Discussion
The enormous disconnect between international policy recommendations of IPT for HIV-infected persons and the implementation of this proven intervention (reaching <0.08% of eligible people) owes much to the cumbersome nature of current TB rule-out strategies and uncertainty about optimal approaches. This study demonstrates that culture-based approaches are the only way to deliver optimum TB screening. MODS detected M. tuberculosis with greater sensitivity and speed, and ruled out TB more quickly and with fewer indeterminate culture results, than LJ, consistent with previous data [20, 28].
The strengths of this study derive from its prospective, real-world design, carried out at multiple sites. All tests were intended for all patients, and were interpreted by staff blinded to other results. Previous studies of TB screening strategies have used combinations of reported symptoms as a proxy for clinical assessment [29, 30]. Here, referring clinicians directly assessed the global likelihood of active TB in each patient, similar to an approach previously described for TB suspects [31]. This more accurately represents the real-world situation wherein a clinician decides based on overall assessment rather than a formalized score whether a patient should continue on the path to IPT.
IPT candidates have a lower prevalence of TB than the groups previously studied with MODS, who were largely active TB suspects [20-22]. Nevertheless, 6.2% of the patients in this study were found to have culture-positive TB. If initiated on IPT, these patients are at risk for developing drug-resistant TB [32]. Twenty of 27 culture-positive patients were smear-negative and would have been administered IPT according to practices in which treatment decisions are made before obtaining culture results.
Wider use of DST features in WHO STAG-TB recommendations [33]. MODS provides rapid, direct DST; results for the two patients with multidrug-resistant TB were available in six and nine days.
We previously demonstrated limited incremental value of a second MODS culture in TB suspects and hospitalized, HIV-infected persons [20]. We predicted a similar finding in the present study, in a population with relatively low suspicion of TB. However, 50% more cases were detected with a second culture. This likely reflects the pauci-bacillary nature of TB in HIV-infected persons with minimal symptoms, and the quality of sputum obtained. The increased yield of second sputum samples might be due to recruitment and follow-up practices: second samples were more likely than first samples to be provided in the early morning. Nevertheless, a single liquid culture still correctly assigned more people as TB-positive or TB-negative than any screening package that did not include MODS.
Deciding upon the most important parameter when comparing screening packages is not straightforward. Specificity indicates how well non-cases are identified but gives no information about cases misclassified as non-cases (having no screening at all and giving all candidates IPT has a specificity of 100%). Negative predictive value describes how much confidence to put in a non-case result but is an unstable parameter modified by local prevalence of cases. To capture the overall performance, we compared screening packages by determining the number of patients who would have been correctly assigned to IPT or active TB treatment. Interestingly, all currently recommended screening packages would have correctly assigned fewer patients than having no screening at all (i.e. assigning all patients referred to the study as TB-negative); only the laboratory-based strategies correctly assigned more patients than no testing at all
As previously described [16, 18], chest radiography appears to be a particularly costly and ineffective tool; low sensitivity for detecting culture-positive cases indicates that a negative radiograph is not sufficient grounds to start IPT. These data suggest, perhaps controversially, that chest radiography could be dropped from TB rule-out strategies in settings where only pauci-symptomatic patients enter the algorithm.
Attrition rates – assessed by how many patients failed to undergo chest radiography, or provide a second sputum sample – were low. Attrition is likely to be higher in non-study settings where patients assume transportation and test costs and follow-up supervision is less rigorous.
The 19 patients who did not provide a second sputum sample were excluded from analyses of strategies because all had a single negative culture, insufficient for definitive classification as TB-negative. However, these would be considered failures of the two MODS strategy.
We defined active TB as any positive microbiological result. As the primary purpose was TB rule-out, positive results from any test with proven high specificity, including those under evaluation for this new indication (such as MODS) cannot be ignored, particularly when the test is known to be more sensitive than existing reference standards [20-22]. High specificity and infrequent cross-contamination of MODS have been previously demonstrated, which somewhat attenuates the threat of incorporation bias [20].
Of 14 baseline TB-negative patients subsequently diagnosed with TB, only three occurred within three months of negative screen. Though IPT initiation and monitoring were beyond the scope of the study, it was determined that many patients had not initiated IPT, or had not been adherent. While the 11 later diagnoses almost certainly represent either newly acquired disease or reactivation of pre-existing LTBI in patients truly disease-free at baseline, it is challenging to determine whether this also applies to the three early cases or if their disease represents progression of previously undetected active TB, and thus a false-negative screen.
The high rate of initially-contaminated Lowenstein-Jensen cultures is not easily explained; when the same sputum decontaminate is used for both LJ and MODS, as here, contamination in LJ usually only slightly exceeds MODS. Nevertheless, after repeat decontamination only 2.5% of all samples yielded an indeterminate LJ culture, so although turnaround times were prolonged the detection performance of LJ was not significantly compromised.
An important consideration is the assumed validity of our definition of non-TB (negative microbiologic tests for two sputum samples). Obvious weaknesses are that poor sputum sample quality can compromise smear and culture performance and sputum testing cannot detect isolated extrapulmonary TB. False-positive MODS cultures are extremely unusual [28], so specificity and positive predictive value are high.
IPT has the potential to save millions of lives and contribute importantly to TB control in HIV high-burden regions. TB rule-out is a major bottleneck to implementation. Strategies to streamline and expedite this process could get millions of eligible HIV+ patients onto the IPT they require. Increasing the proportion of eligible patients that are screened from the current <0.1% to just 10% could avert over one million new cases of TB.
Based on the results of this study, we propose that liquid culture of two sputum samples alone can be used as an effective screening strategy for pulmonary TB prior to IPT in HIV-infected persons. The worldwide movement towards greater use of liquid culture and TB laboratory capacity building in resource-limited settings provides an opportunity for roll-out that could translate this research into large-scale practice. Clinical history and examination can provide additional information about the risk of extrapulmonary TB but they are not a useful filter for pulmonary TB screening. Future studies can address the utility of improved sputum collection, perhaps after instruction [34], as a means to improve the sensitivity of a single culture in this population.
Supplementary Material
Acknowledgments
We thank all study participants and the following: Beatriz Castro, Fanny García, Virginia Huancaré, Miriam Huayta, Ruth Limascca, Sonia López, Jeny Rodríguez, Eleana Sánchez, Esther Soto, Rosa Yataco and Christian Solis for field assistance and data collection and verification; Paula Maguina for administrative support; Don Juanito; Sara Benites for database management; Pilar Navarro, Gianina Luna, William Roldán, Mirko Diaz and Jesús Rojas for assistance with laboratory studies; Yuri García, MD, Jaime Soria, MD, Leonel Martínez, MD, Eduardo Matos, MD, Lisset García, MD, and Miriam Calderón, MD, for patient referral; and Aldo Vivar, MD, Jorge Arévalo, MD, Victor Chavez, MD, and Carlton Evans, MD, PhD, for protocol review and guidance.
This work was supported by Wellcome Trust Career Development Fellowship 078067/Z/05 (DAJM), National Institutes of Health/Fogarty Global Research Training Grant 3D43 TW006581-04S1 (RHG) and National Institutes of Health/Fogarty International Clinical Research Scholar Awards (KPR and MFB). DAJM and JSF are grateful for support from the NIHR Biomedical Research Centre funding scheme.
Footnotes
Contributors: KPR and DAJM had full access to all the data in the study and had final responsibility for the decision to submit for publication. KPR, RHG, JSF and DAJM were involved in study concept and design and supervising study implementation. KPR was the main author. DAJM, MFB, RHG and JSF were involved in manuscript preparation. MFB assisted with data analysis and interpretation. JC supervised laboratory testing. MÑ, ET, GC, ES, CR, LS, JV, YV and CB recruited and examined participants.
Conflict of Interest Statement: We declare that we have no conflict of interest.
References
- 1.Antonucci G, Girardi E, Raviglione MC, Ippolito G. Risk factors for tuberculosis in HIV-infected persons. A prospective cohort study. The Gruppo Italiano di Studio Tubercolosi e AIDS (GISTA) JAMA. 1995;274:143–8. doi: 10.1001/jama.274.2.143. [DOI] [PubMed] [Google Scholar]
- 2.Moreno S, Miralles P, Diaz MD, et al. Isoniazid preventive therapy in human immunodeficiency virus-infected persons. Long-term effect on development of tuberculosis and survival. Arch Intern Med. 1997;157:1729–34. [PubMed] [Google Scholar]
- 3.Foster S, Godfrey-Faussett P, Porter J. Modelling the economic benefits of tuberculosis preventive therapy for people with HIV: the example of Zambia. AIDS. 1997;11:919–25. doi: 10.1097/00002030-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Bell JC, Rose DN, Sacks HS. Tuberculosis preventive therapy for HIV-infected people in sub-Saharan Africa is cost-effective. AIDS. 1999;13:1549–56. doi: 10.1097/00002030-199908200-00016. [DOI] [PubMed] [Google Scholar]
- 5.Godfrey-Faussett P, Ayles H. Can we control tuberculosis in high HIV prevalence settings? Tuberculosis (Edinb) 2003;83:68–76. doi: 10.1016/s1472-9792(02)00083-5. [DOI] [PubMed] [Google Scholar]
- 6.Mwinga A, Hosp M, Godfrey-Faussett P, et al. Twice weekly tuberculosis preventive therapy in HIV infection in Zambia. AIDS. 1998;12:2447–57. doi: 10.1097/00002030-199818000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Quigley MA, Mwinga A, Hosp M, et al. Long-term effect of preventive therapy for tuberculosis in a cohort of HIV-infected Zambian adults. AIDS. 2001;15:215–22. doi: 10.1097/00002030-200101260-00011. [DOI] [PubMed] [Google Scholar]
- 8.Grant AD, Charalambous S, Fielding KL, et al. Effect of routine isoniazid preventive therapy on tuberculosis incidence among HIV-infected men in South Africa: a novel randomized incremental recruitment study. JAMA. 2005;293:2719–25. doi: 10.1001/jama.293.22.2719. [DOI] [PubMed] [Google Scholar]
- 9.Bucher HC, Griffith LE, Guyatt GH, et al. Isoniazid prophylaxis for tuberculosis in HIV infection: a meta-analysis of randomized controlled trials. AIDS. 1999;13:501–7. doi: 10.1097/00002030-199903110-00009. [DOI] [PubMed] [Google Scholar]
- 10.Hawken MP, Meme HK, Elliott LC, et al. Isoniazid preventive therapy for tuberculosis in HIV-1-infected adults: results of a randomized controlled trial. AIDS. 1997;11:875–82. doi: 10.1097/00002030-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Mohammed A, Myer L, Ehrlich R, Wood R, Cilliers F, Maartens G. Randomised controlled trial of isoniazid preventive therapy in South African adults with advanced HIV disease. Int J Tuberc Lung Dis. 2007;11:1114–20. [PubMed] [Google Scholar]
- 12.Woldehanna S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2004:CD000171. doi: 10.1002/14651858.CD000171.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald DW, Morse MM, Pape JW, Johnson WD., Jr Active tuberculosis in individuals infected with human immunodeficiency virus after isoniazid prophylaxis. Clin Infect Dis. 2000;31:1495–7. doi: 10.1086/317485. [DOI] [PubMed] [Google Scholar]
- 14.Hawken MP, Muhindi DW. Tuberculosis preventive therapy in HIV-infected persons: feasibility issues in developing countries. Int J Tuberc Lung Dis. 1999;3:646–50. [PubMed] [Google Scholar]
- 15.Zachariah R, Spielmann MP, Harries AD, et al. Passive versus active tuberculosis case finding and isoniazid preventive therapy among household contacts in a rural district of Malawi. Int J Tuberc Lung Dis. 2003;7:1033–9. [PubMed] [Google Scholar]
- 16.Mosimaneotsile B, Talbot EA, Moeti TL, et al. Value of chest radiography in a tuberculosis prevention programme for HIV-infected people, Botswana. Lancet. 2003;362:1551–2. doi: 10.1016/s0140-6736(03)14745-9. [DOI] [PubMed] [Google Scholar]
- 17.Hawken M, Nganga L, Meme H, Chakaya J, Porter J. Is cough alone adequate to screen HIV-positive persons for tuberculosis preventive therapy in developing countries? Int J Tuberc Lung Dis. 1999;3:540–1. [PubMed] [Google Scholar]
- 18.Ho TT, Wong KH, Lee SS. Low yield of chest radiography in screening for active pulmonary tuberculosis in HIV-infected patients in Hong Kong. Int J STD AIDS. 1999;10:409–12. [PubMed] [Google Scholar]
- 19.Palmieri F, Girardi E, Pellicelli AM, et al. Pulmonary tuberculosis in HIV-infected patients presenting with normal chest radiograph and negative sputum smear. Infection. 2002;30:68–74. doi: 10.1007/s15010-002-2062-9. [DOI] [PubMed] [Google Scholar]
- 20.Moore DA, Evans CAW, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355:1539–50. doi: 10.1056/NEJMoa055524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arias M, Mello FC, Pavon A, et al. Clinical evaluation of the microscopic-observation drug-susceptibility assay for detection of tuberculosis. Clin Infect Dis. 2007;44:674–80. doi: 10.1086/511639. [DOI] [PubMed] [Google Scholar]
- 22.Shiferaw G, Woldeamanuel Y, Gebeyehu M, Girmachew F, Demessie D, Lemma E. Evaluation of microscopic observation drug susceptibility assay for detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol. 2007;45:1093–7. doi: 10.1128/JCM.01949-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guidelines for tuberculosis preventive therapy in HIV infection. Consensus guidelines from a workshop, 29-30 October 1999. HIV Clinicians Society of Southern Africa. S Afr Med J. 2000;90:592–4. [PubMed] [Google Scholar]
- 24.Laboratory services in TB control Parts I to III. Geneva: World Health Organization; 1998. [Google Scholar]
- 25.Caviedes L, Lee TS, Gilman RH, et al. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. The Tuberculosis Working Group in Peru. J Clin Microbiol. 2000;38:1203–8. doi: 10.1128/jcm.38.3.1203-1208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore DA, Mendoza D, Gilman RH, et al. Microscopic observation drug susceptibility assay, a rapid, reliable diagnostic test for multidrug-resistant tuberculosis suitable for use in resource-poor settings. J Clin Microbiol. 2004;42:4432–7. doi: 10.1128/JCM.42.10.4432-4437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park WG, Bishai WR, Chaisson RE, Dorman SE. Performance of the microscopic observation drug susceptibility assay in drug susceptibility testing for Mycobacterium tuberculosis. J Clin Microbiol. 2002;40:4750–2. doi: 10.1128/JCM.40.12.4750-4752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore DA, Caviedes L, Gilman RH, et al. Infrequent MODS TB culture cross-contamination in a high-burden resource-poor setting. Diagn Microbiol Infect Dis. 2006;56:35–43. doi: 10.1016/j.diagmicrobio.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Day JH, Charalambous S, Fielding KL, Hayes RJ, Churchyard GJ, Grant AD. Screening for tuberculosis prior to isoniazid preventive therapy among HIV-infected gold miners in South Africa. Int J Tuberc Lung Dis. 2006;10:523–9. [PubMed] [Google Scholar]
- 30.Mohammed A, Ehrlich R, Wood R, Cilliers F, Maartens G. Screening for tuberculosis in adults with advanced HIV infection prior to preventive therapy. Int J Tuberc Lung Dis. 2004;8:792–5. [PubMed] [Google Scholar]
- 31.Catanzaro A, Perry S, Clarridge JE, et al. The role of clinical suspicion in evaluating a new diagnostic test for active tuberculosis: results of a multicenter prospective trial. JAMA. 2000;283:639–45. doi: 10.1001/jama.283.5.639. [DOI] [PubMed] [Google Scholar]
- 32.Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerg Infect Dis. 2006;12:744–51. doi: 10.3201/eid1205.050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strategic & Technical Advisory Group for Tuberculosis (STAG-TB) Seventh Meeting: Report on Conclusions and Recommendations. Geneva: World Health Organization; 2007. [Google Scholar]
- 34.Khan MS, Dar O, Sismanidis C, Shah K, Godfrey-Faussett P. Improvement of tuberculosis case detection and reduction of discrepancies between men and women by simple sputum-submission instructions: a pragmatic randomised controlled trial. Lancet. 2007;369:1955–60. doi: 10.1016/S0140-6736(07)60916-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.