Abstract
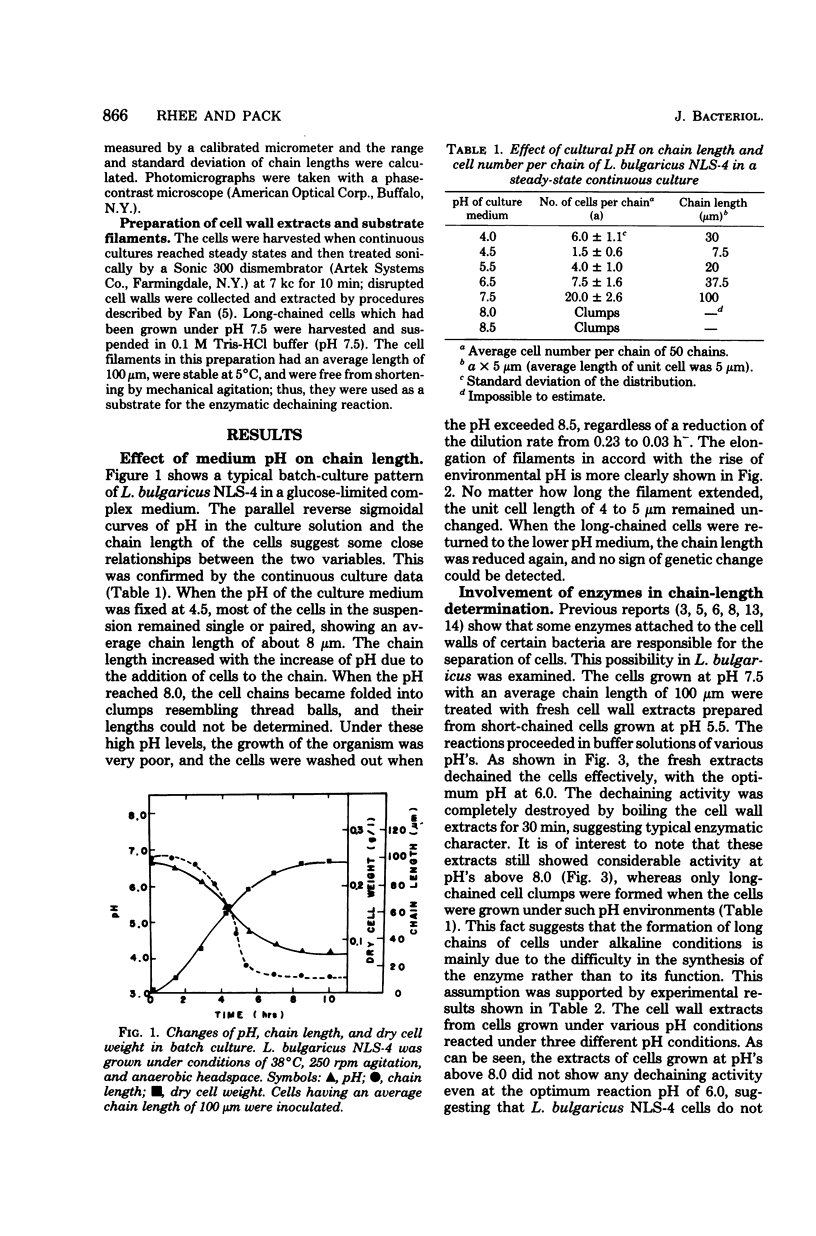
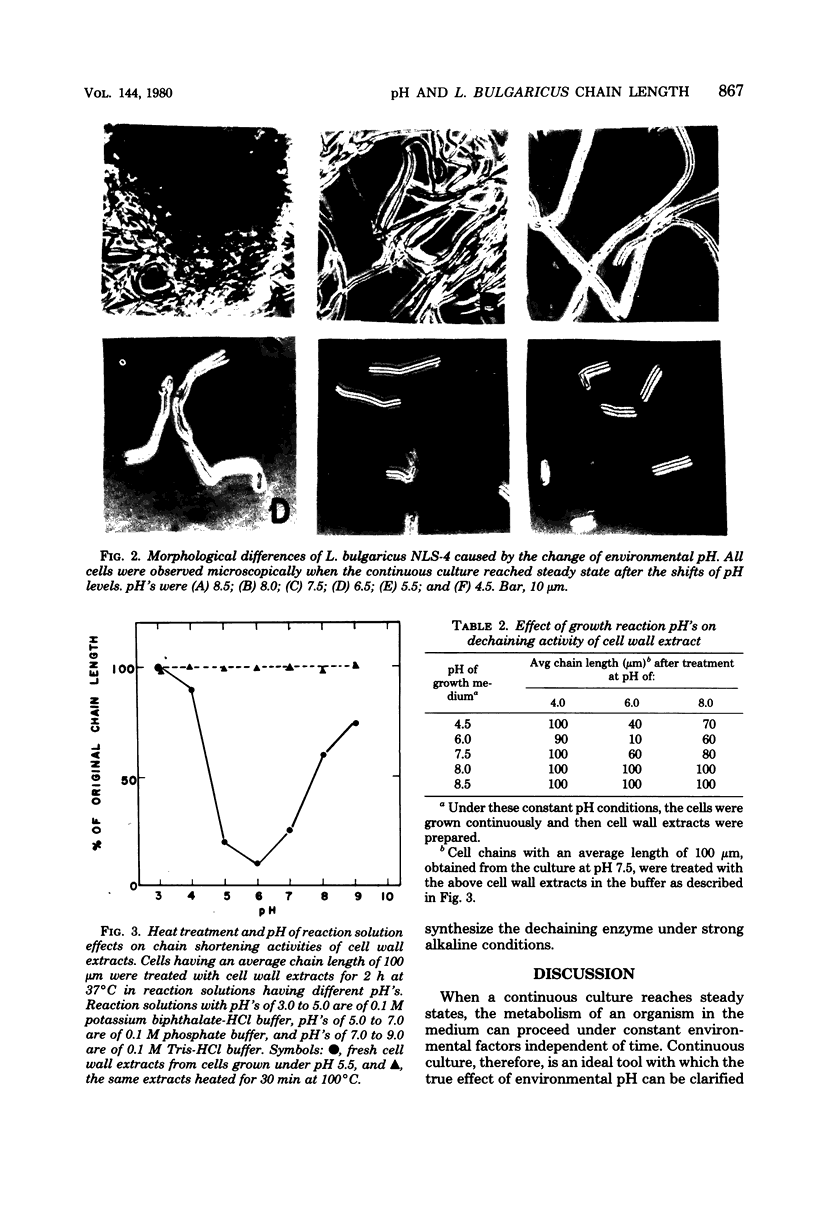
Culture medium pH was found to affect strongly the chain length of lactobacillus bulgaricus NLS-4 cells. The organism was cultured continuously in glucose-limited complex medium of different pH's with constant agitation at 250 rpm under anaerobic headspace. The cell chains increased their lengths with an increase in pH and yielded clumps of folded filaments at pH above 8.0. Involvement of an autolytic enzyme(s) in the separation of L. bulgaricus cells was confirmed, and the poor synthesis of this enzyme(s) under alkaline culture conditions could explain the pH-related filamentous growth of this organism.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chatterjee A. N., Mirelman D., Singer H. J., Park J. T. Properties of a novel pleiotropic bacteriophage-resistant mutant of Staphylococcus aureus H. J Bacteriol. 1969 Nov;100(2):846–853. doi: 10.1128/jb.100.2.846-853.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EKSTEDT R. D., STOLLERMAN G. H. Factors affecting the chain length of group A streptococci. I. Demonstration of a metabolically active chain-splitting system. J Exp Med. 1960 Oct 1;112:671–686. doi: 10.1084/jem.112.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Autolysin(s) of Bacillus subtilis as dechaining enzyme. J Bacteriol. 1970 Aug;103(2):494–499. doi: 10.1128/jb.103.2.494-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Cell wall binding properties of the Bacillus subtilis autolysin(s). J Bacteriol. 1970 Aug;103(2):488–493. doi: 10.1128/jb.103.2.488-493.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOMINSKI I., CAMERON J., WYLLIE G. Chaining and unchaining Streptococcus faecalis; a hypothesis of the mechanism of bacterial cell separation. Nature. 1958 May 24;181(4621):1477–1477. doi: 10.1038/1811477a0. [DOI] [PubMed] [Google Scholar]
- Rhee S. K., Pack M. Y. Effect of environmental pH on fermentation balance of Lactobacillus bulgaricus. J Bacteriol. 1980 Oct;144(1):217–221. doi: 10.1128/jb.144.1.217-221.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. BIOCHEMICAL ASPECTS OF COMPETENCE IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. II. AUTOLYTIC ENZYME ACTIVITY OF CELL WALLS. J Biol Chem. 1963 Sep;238:3126–3130. [PubMed] [Google Scholar]
- Young F. E. Autolytic enzyme associated with cell walls of Bacillus subtilis. J Biol Chem. 1966 Aug 10;241(15):3462–3467. [PubMed] [Google Scholar]
- de Vries W., Kapteijn W. M., van der Beek E. G., Stouthamer A. H. Molar growth yields and fermentation balances of Lactobacillus casei L3 in batch cultures and in continuous cultures. J Gen Microbiol. 1970 Nov;63(3):333–345. doi: 10.1099/00221287-63-3-333. [DOI] [PubMed] [Google Scholar]