Abstract
Neonatal Chlamydia trachomatis pneumonia has been associated with respiratory sequelae in later life. We recently established a mouse model of neonatal pulmonary Chlamydia muridaum infection and found an important contribution of IFN-γ to protective immunity. In this study, we further characterized the role of Th1-type cytokines; IL-12, IFN-γ, and IFN-γ signaling using mice genetically deficient in IL-12, IFN-γ, or IFN-γ receptor 1. All 3 knockout (KO) mice challenged intranasally with C. muridarum 1 day after birth exhibited 100% mortality by day 17 post-challenge whereas wild-type (WT) animals survived the monitoring period of 1 month. The KO mice exhibited greater lung bacterial burdens and enhanced dissemination to the liver, compared to WT animals. The inflammatory cellular infiltration in C. muridarum-challenged KO animals was significantly reduced in the lungs, but markedly enhanced in the livers of the KO mice compared to similarly challenged WT mice. It was also found that a deficiency in IL-12 or IFN-γ resulted in correspondingly reduced IFN-γ or IL-12 production, respectively, suggesting an intricate interdependence in the induction of these cytokines. Collectively, these results suggest that the IL-12/IFN-γ axis induces pulmonary cellular infiltration, induces bacterial clearance from the lung, reduces dissemination to other organs, and promotes the survival of the host during neonatal pulmonary chlamydial infection.
Introduction
Chlamydia trachomatis, a gram-negative obligate intracellular bacterium acquired perinatally by human neonates, causes pulmonary infection (Beem and Saxon 1977) manifesting as acute broncho- and/or interstitial pneumonia (Darville 2005). Efficacious antimicrobial therapy has been shown to be effective in treatment of the acute pneumonia (Beem and others 1979). However, several reports have suggested an association between abnormal respiratory function in children and a history of neonatal C. trachomatis pneumonia (Weiss and others 1986). This underscores a need to better understand the pathogenesis of neonatal pulmonary chlamydial infection to uncover the mechanisms of development of subsequent respiratory sequelae.
We recently developed a mouse model of neonatal pulmonary chlamydial infection and showed that mice genetically deficient in IFN-γ production (IFN-γ−/− mice), but not the corresponding WT animals, succumb to infection following challenge with 100 IFU of Chlamydia muridarum (Jupelli and others 2008). The importance of IFN-γ in clearance of chlamydial infections in the adult mouse has been suggested previously by several reports (Williams and others 1988; Zhong and others 1989). IFN-γ was shown initially to reduce growth of C. trachomatis in vitro (Byrne and others 1988) and IFN-γ−/− mice were shown to display suboptimal clearance of C. muridarum from the lung (Wang and others 1999) and genital tract (Cotter and others 1997), and increased dissemination to other organs, compared to WT animals. However, the extent and underlying mechanisms of clearance mediated by IFN-γ during chlamydial infection are less well understood. Recent reports have suggested that Chlamydia utilizes host-specific IFN-γ evasion mechanisms for survival (Nelson and others 2005). Specifically, C. trachomatis can utilize indole to synthesize tryptophan to abrogate the IFN-γ-induced tryptophan depletion and growth restriction in human cells (Roshick and others 2006). C. muridarum also has been shown to significantly overcome IFN-γ-induced growth restriction in mouse cells (Roshick and others 2006), although mechanisms other than tryptophan depletion may be involved in the mouse (Nelson and others 2005). Moreover, initial clearance of genital C. muridarum infection in the mouse was comparable in the presence or absence of endogenous IFN-γ production (Perry and others 1997) suggesting that mechanisms other than IFN-γ may be instrumental in chlamydial clearance. To this end, impaired clearance of C. muridarum during the first 2 weeks after genital challenge was observed in interleukin-12 (IL-12)-depleted mice (Perry and others 1997). IL-12 is an inducer of IFN-γ production (Kobayashi and others 1989) and is crucial for the development of Th1-type cellular responses (Trinchieri and others 1992). Collectively, IL-12 and IFN-γ are 2 Th1-type cytokines with pleiotropic effects, inducing the activation of various cell types and production of several cytokines (Pearlman and others 1997; Dixon and others 2000), and thus may be important in bacterial clearance and protection against neonatal pulmonary chlamydial infection and subsequent respiratory sequelae.
In this study, we further evaluated the role of Th1-type immune responses, including IL-12 and IFN-γ in influencing disease progression following neonatal pulmonary chlamydial challenge. We used mice genetically deficient in the production of: (a) IL-12 (disrupted p35 subunit gene; IL-12p35−/− mice), (b) IFN-γ (disrupted IFN-γ gene, IFN-γ−/− mice), or (c) mice incapable of responding to IFN-γ (disrupted IFN-γ receptor 1 gene; IFN-γR−/− mice). We found that IL-12 and IFN-γ, acting in an interdependent fashion, induce inflammatory cellular recruitment into the lungs, reduce dissemination of the bacterium to other organs, and promote survival of the host following neonatal pulmonary chlamydial challenge.
Materials and Methods
Bacteria
C. muridarum was grown in HeLa cell monolayers and purified as described previously (Zhong and others 1990; Murthy and others 2004). In brief, chlamydial elementary bodies (EBs) were harvested by lysing the infected HeLa cells using a sonicator (Fisher, Pittsburgh, PA) and the EBs were purified on renograffin gradients. Titered aliquots of bacteria were stored at −70°C in sucrose phosphate glutamine (SPG) buffer until further use.
Mice
All mice were purchased from Jackson Laboratory (Bar Harbor, ME), housed, and bred at the University of Texas at San Antonio Animal Care Facility. Six-to-eight-week-old BALB/c and BALB/c IFN-γ deficient (IFN-γ−/− mice; backcrossed to BALB/c for 6 generations), C57BL/6 and C57BL/6 IL-12p35 deficient (IL-12p35−/− mice; backcrossed to C57BL/6 for 11 generations), and C57BL/6 IFN-γ receptor 1 deficient (IFN-γR−/− mice; backcrossed to C57BL/6 for 10 generations). Mice were provided food and water ad libitum, and 1-day-old pups were used for all experiments. Animal care and experimental procedures were performed in compliance with the Institutional Animal Care and Use Committee (IACUC) guidelines.
Intranasal chlamydial challenge
We utilized a neonatal mouse model of pulmonary chlamydial infection, previously established in our laboratory (Jupelli and others 2008) for these studies. In brief, groups of 1-day-old BALB/c, IFN-γ−/−, C57BL/6, IFN-γR−/−, or IL-12p35−/− mice were challenged intranasally (i.n.) with 100 IFU (Jupelli and others 2008) of C. muridarum in 5 μL of sterile PBS, while mock-challenged mice received sterile PBS alone. All mice were monitored daily for survival. Given that knockout (KO)-infected animals demonstrated significant morbidity at day 10 and beyond, animals were euthanized at days 4, 7, and 9 for various analyses.
Chlamydial burden in tissues
Following neonatal bacterial challenge, the lungs and livers were collected at indicated time periods. The tissues were homogenized, centrifuged (250g for 5 min), and the supernatants incubated for 24 h with HeLa cell monolayers grown on culture coverslips in 24-well plates, followed by staining for chlamydial inclusions as described previously (Murthy and others 2006). In brief, the infected HeLa cells were fixed with 2% paraformaldehyde, permeabilized with 1% saponin, and blocked with 10% FBS for 1 h to prevent nonspecific binding. The cells were then incubated with polyclonal rabbit anti-Chlamydia genus-specific Ab (Murthy and others 2006) for 1 h, and then incubated for an additional 1 h with goat anti-rabbit Ig conjugated to FITC (Sigma Aldrich, St. Louis, MO) plus Hoechst nuclear stain. The coverslips were then mounted onto microscope slides using Fluorsave reagent (Calbiochem, La Jolla, CA). Chlamydial inclusions were enumerated using a Zeiss Axioskop 2 Plus research microscope at 40× magnification and expressed as mean ± SD per group.
Hematoxylin and eosin (H&E) staining
Tissue sections were stained using hematoxylin and eosin (H&E) as described previously (Murthy and others 2004). Following i.n. challenge of 1-day-old mice with C. muridarum or PBS (mock), lungs and livers were collected at indicated time intervals, as described previously (Murthy and others 2004). In brief, the lungs were intratracheally instilled with 10% neutral formalin and fixed overnight in 10% neutral formalin. Livers were collected and also fixed overnight in 10% neutral formalin. The fixed lungs and livers were embedded in paraffin and serial horizontal sections (5-μm thick) were prepared and mounted onto silane-coated slides (VWR International, West Chester, PA). The tissue sections were stained with H&E, visualized using a Zeiss Axioskop 2 Plus research microscope, and images acquired using an Axiocam digital camera (Zeiss, Thornwood, NY). A scoring scheme previously established by our lab (Jupelli and others 2008) was used to score pulmonary pathology. In brief, 0, no inflammation; 1, dispersed cellular infiltration; 2, rim of peribronchiolar infiltration; 3, 1–2 foci (≤200 μm) of peribronchiolar infiltration; 4, >2 foci (≤200 μm) of peribronchiolar infiltration; 5, 1–2 foci (≥200 μm) of peribronchiolar infiltration; 6, >2 foci (≥200 μm) of peribronchiolar infiltration; 7, one partially consolidated lobe of the lung; 8, one fully consolidated lobe of the lung; 9, >1 partially consolidated lobe of the lung; 10, >1 fully consolidated lobe of the lung. A score of 2 was added if luminal cellular plugs were detected in >2 bronchioles. Similarly, livers were scored in a blinded fashion with images acquired at 25× magnification using a cumulative scoring scheme as follows: an individual score of 1 was given for the presence of inflammation at either of the subcapsular, periportal, sinusoidal, lobular, or central venule regions and a score of 2 was given for the presence of necrotic areas in the liver. The score reported is the sum of all these values.
Cytokine responses in lung and liver
One-day-old C. muridarum or PBS (mock)-challenged mice were euthanized at the indicated time periods and the lungs and liver were removed and cytokine profiles measured as described previously (Murthy and others 2006, 2007). In brief, the collected tissues were homogenized in sterile SPG buffer with protease inhibitors, centrifuged (250g for 5 min), normalized, and analyzed by ELISA for induction of IL-1β, IL-12, TNF-α, or IFN-γ using BD OptEIA kits according to the manufacturer's (BD Pharmingen, San Diego, CA) instructions. The limits of detection of the ELISA's performed were 31.25, 31.25, 15.63, or 31.25 pg/mL for IL-1β, TNF-α, IL-12, and IFN-γ, respectively.
Statistics
Sigma Stat (Systat Software Inc., San Jose, CA) was used to perform all tests of significance. The Kaplan–Meier test was used to determine differences between groups in mean time to death. Student's t-test was used for comparisons between 2 groups. Differences were considered statistically significant if P values were <0.05. All data are representative of at least 2–3 independent experiments and each experiment was analyzed independently.
Results
Susceptibility of neonatal IFN-γ−/−, IFN-γR−/−, and IL-12p35−/− mice to pulmonary chlamydial infection
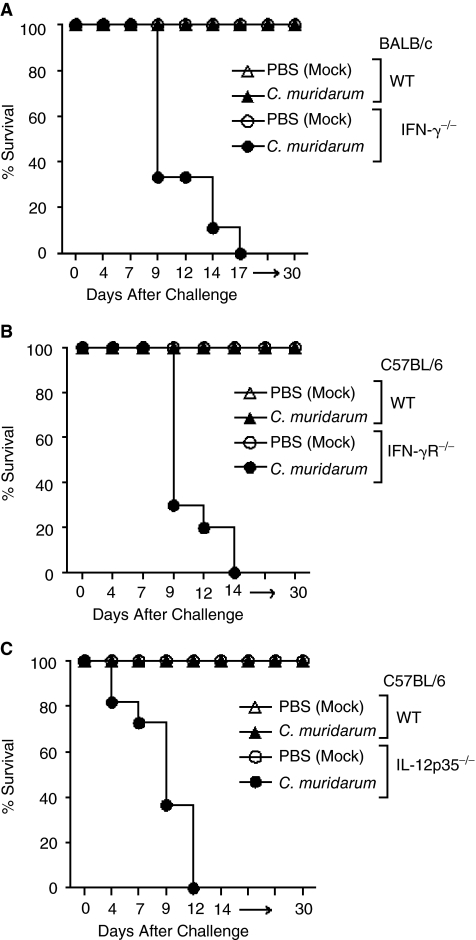
IFN-γ−/−, IFN-γR−/−, and IL-12p35−/− and the corresponding WT mice were challenged on day 1 after birth with 100 IFU of C. muridarum and monitored daily for survival. As shown in Figure 1A, some (66%) IFN-γ−/− mice (n = 9) exhibited mortality as early as day 9, and all (100%) infected mice succumbed to the infection by day 17 post-challenge. Greater susceptibility also was observed in challenged IFN-γR−/− (n = 10) and IL-12p35−/− (n = 9) animals with mortality as early as day 10 in 70% of IFN-γR−/− and day 7 in 33% of IL-12p35−/− mice. All (100%) IFN-γR−/− and IL-12p35−/− mice succumbed to the infection by days 14 and 12 post-challenge, respectively (Fig. 1B and 1C). All (100%) of similarly challenged WT (IFN-γ+/+, IFN-γR+/+, and IL-12p35+/+) mice survived the challenge dose throughout the 30-day monitoring period of the experiment. These results demonstrate the important roles of IL-12, IFN-γ, and IFN-γ signaling in protection against neonatal pulmonary chlamydial infection.
FIG. 1.
Susceptibility of neonatal IFN-γ−/−, IFN-γR−/−, and IL-12p35−/− mice following i.n. C. muridarum challenge. Groups of 1-day-old IFN-γ−/− (n = 9) or IFN-γR−/− (n = 10) or IL-12p35−/− (n = 9) mice were challenged i.n. with 100 IFU of C. muridarum or treated with PBS (mock) and were monitored for survival. (A) IFN-γ−/− and IFN-γ+/+ animals. (B) IFN-γR−/− and IFN-γR+/+ animals. (C) IL-12p35−/− and IL-12p35+/+ animals. *P ≤ 0.05 (Kaplan–Meier test). All results are representative of 2 independent experiments.
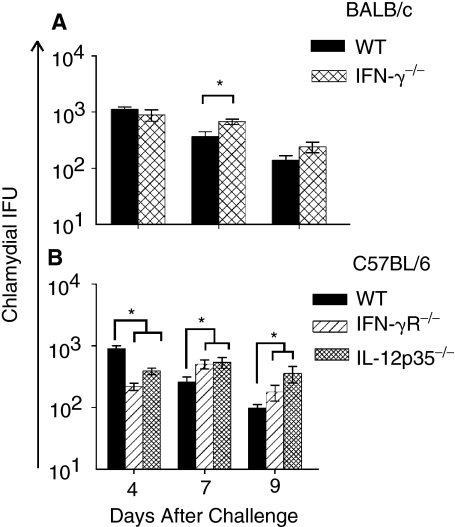
The lung bacterial burdens in the challenged KO and corresponding WT mice (n = 4) also were measured at the indicated time periods. As shown in Figure 2A, C. muridarum-challenged IFN-γ−/− mice exhibited significantly greater bacterial burden in the lungs on day 7 compared to similarly challenged WT BALB/c mice. Significantly greater lung bacterial burdens also were observed with C. muridarum-challenged IFN-γR−/− and IL-12p35−/− mice from day 7 through day 9 post-challenge compared to WT C57BL/6 mice (Fig. 2B). These results demonstrate an important role for IL-12, IFN-γ, and IFN-γ signaling in controlling lung chlamydial burden.
FIG. 2.
Chlamydial burden in the lungs of neonatal IFN-γ−/−, IFN-γR−/−, and IL-12p35−/− mice following i.n. C. muridarum challenge. Groups (n = 4) of 1-day-old IFN-γ−/− or IFN-γR−/− or IL-12p35−/− mice were challenged i.n. with 100 IFU of C. muridarum or treated with PBS (mock). The tissues were harvested at the indicated days after challenge and chlamydial burden was quantitated. (A) BALB/c IFN-γ+/+ and IFN-γ−/− mice. *P ≤ 0.05 (Student's t-test). (B) C57BL/6, IFN-γR−/−, and IL-12p35−/− animals. *P ≤ 0.05 between C. muridarum-challenged C57BL/6 and IFN-γR−/− or C57BL/6 and IL-12p35−/− mice (Student's t-test). The results are expressed as the mean ± SD of chlamydial IFU per group and are representative of 2 independent experiments.
The role of IL-12 and IFN-γ in inflammatory cellular infiltration into the lung
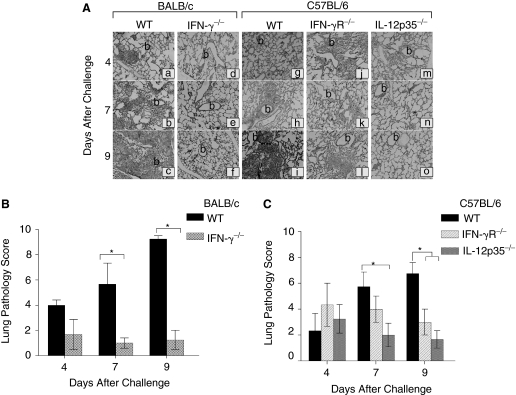
Given that leukocytes play an important role in control of chlamydial infection (Barteneva and others 1996), the inflammatory cellular recruitment was evaluated in H&E stained lung sections from C. muridarum-infected IFN-γ−/−, IFN-γR−/−, IL-12p35−/−, and corresponding WT mice at the indicated time periods (Fig. 3). The H&E-stained lung sections from C. muridarum-infected WT BALB/c (Fig. 3Aa–c) or C57BL/6 (Fig. 3Ag–i) mice displayed diffuse cellular infiltration with few inflammatory cells dispersed along the alveolar/peribronchiolar region on day 4 post-challenge (Fig. 3Aa and g). By day 7 (Fig. 3Ab and h), both the WT mice displayed bilateral peribronchiolar and perivascular inflammatory cellular infiltrates that were predominantly composed of polymorphonuclear leukocytes (PMNs) (observed at 100× magnification). The inflammatory responses in these mice progressively increased with partial consolidation of one or more lobes of the lung by day 9 (Fig. 3Ac and i). Lung sections from challenged IFN-γ−/− (Fig. 3Ad–f) or IL-12p35−/− (Fig. 3Am–o) mice exhibited minimal cellular recruitment throughout the monitored period of challenge compared to corresponding WT animals. The lung sections from these challenged KO mice resembled those of mock (PBS)-treated mice (data not shown). IFN-γR−/− mice exhibited comparable cellular infiltration to WT mice on day 4 (Fig. 3Aj) and day 7 (Fig. 3Ak), but significant reductions by day 9 after challenge (Fig. 3Al).
FIG. 3.
Pulmonary inflammatory cellular infiltration in neonatal IFN-γ−/−, IFN-γR−/−, and IL-12p35−/− mice following i.n. C. muridarum challenge. (A) Groups (n = 3–4) of 1-day-old BALB/c (a–c); IFN-γ−/− (d–f); or C57BL/6 (g–i); or IFN-γR−/− (j–l); or IL-12p35−/− (m–o) mice were challenged i.n. with 100 IFU of C. muridarum. The mice were euthanized on the indicated days after challenge. The lungs were removed, embedded in paraffin, and serial horizontal sections prepared and stained with H&E. Total magnification 25× (b—bronchiole). The lungs were further scored quantitatively for pulmonary pathology. (B) IFN-γ−/− and wild-type (WT); (C) IFN-γR−/−, IL-12p35−/−, and WT control mice. Results are expressed as the score of individual mice and the mean ± SD of the scores in each group. Results are representative of 2 independent experiments. *P ≤ 0.05 between C. muridarum-challenged BALB/c and IFN-γ−/− mice, C57BL/6 and IFN-γR−/−, or C57BL/6 and IL-12p35−/− mice.
The lung sections from infected WT, IFN-γ−/−, IFN-γR−/−, and IL-12p35−/− mice were scored on a quantitative cellular infiltration scale at different time intervals in a blinded fashion as described (Jupelli and others 2008). As shown in Figure 3B, WT BALB/c and C57BL/6 (Fig. 3C) mice exhibited a progressively increasing trend in pulmonary inflammation with highest scores on day 9 post-challenge. However, the lung sections from IL-12p35−/− and IFN-γ−/− mice demonstrated significantly less pulmonary cellular recruitment on days 7 and 9 post-challenge compared to corresponding WT animals. Inflammatory cellular infiltration in IFN-γR−/− mice was similar on days 4 and 7, but significantly reduced by day 9 post-challenge, when compared to WT mice. Collectively these results demonstrate that IL-12, IFN-γ, and IFN-γ signaling are important for cellular recruitment into the lungs of the Chlamydia-infected neonatal mice.
Bacterial burden in livers of IFN-γ−/−, IFN-γR−/−, and IL-12p35−/− mice following i.n. chlamydial challenge
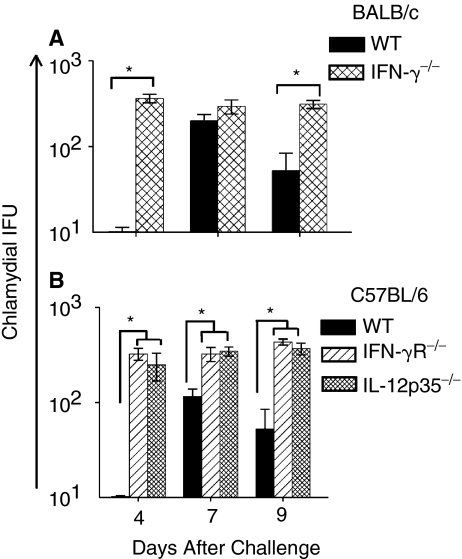
The dissemination of Chlamydia to secondary organs in the absence of endogenous IFN-γ production (Cotter and others 1997; Jupelli and others 2008) has been described previously. Therefore, we examined the chlamydial burden in livers of the different groups of animals at the indicated time periods. As shown in Figure 4A, C. muridarum-challenged IFN-γ−/− mice exhibited significantly greater chlamydial burden in the liver on day 9 compared to WT BALB/c mice. Significantly greater bacterial burdens also were observed in the livers of C. muridarum-challenged IFN-γR−/− and IL-12p35−/− mice compared to challenged WT C57BL/6 mice (Fig. 4B). Together, these results indicate significantly increased dissemination of Chlamydia to the liver in the KO animals, supporting a role for IL-12, IFN-γ, and IFN-γ signaling in control of chlamydial dissemination.
FIG. 4.
Chlamydial burden in the livers of neonatal IFN-γ−/−, IFN-γR−/−, and IL-12p35−/− mice following i.n. C. muridarum challenge. Groups (n = 3–4) of 1-day-old IFN-γ−/−, IFN-γR−/−, and IL-12p35−/− mice were challenged i.n. with 100 IFU of C. muridarum or treated with PBS (mock). The tissues were harvested at the indicated days after challenge and chlamydial burden was quantitated. (A) BALB/c and IFN-γ−/− animals. *P ≤ 0.05 between C. muridarum-challenged BALB/c and IFN-γ−/− animals. (B) C57BL/6 and IFN-γR−/− or IL-12p35−/− animals. *P ≤ 0.05 between C. muridarum-challenged C57BL/6 and IFN-γR−/−, or between C57BL/6 and IL-12p35−/− mice. The results are expressed as the mean ± SD of chlamydial IFU per group and are representative of 2 independent experiments.
The role of IL-12 and IFN-γ in inflammatory cellular infiltration into the liver
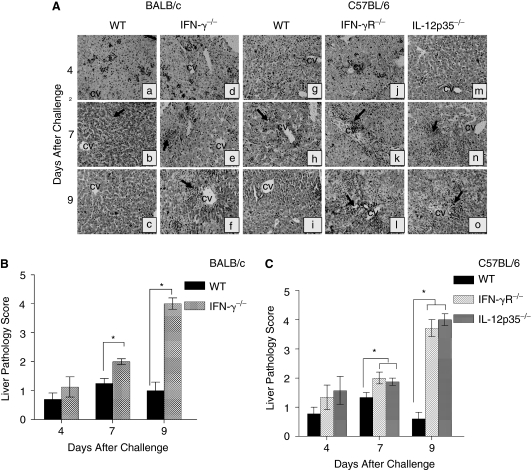
Given the paucity of inflammatory responses in the KO mouse lungs, and the greater dissemination of Chlamydia into livers, we evaluated the inflammatory cellular infiltration into the liver (Fig. 5A). As shown in Figure 5A, the H&E-stained liver sections from C. muridarum-infected WT BALB/c and C57BL/6 mice exhibited minimal inflammatory responses throughout the liver on day 4 post-challenge. By day 7 (Fig. 5Ab and h), both the challenged WT mice demonstrated minimal cellular recruitment with scattered foci of inflammatory responses along sinusoidal and periportal regions of the liver, predominantly composed of PMNs (observed at 100× magnification). However, unlike the lung, the inflammatory responses in the liver of these challenged WT mice decreased by day 9 post-challenge (Fig. 5Ac and i), and resembled that of mock (PBS)-treated mice (data not shown). In contrast, liver sections from challenged IFN-γ−/−, IFN-γR−/−, and IL-12p35−/− mice displayed progressively increasing cellular inflammatory infiltration, with few inflammatory foci on day 4 and considerable numbers on day 7 around the lobular and periportal regions, with minimal inflammation along the subcapsular regions (Fig. 5Ae, k, and n). The inflammatory cells at this stage were composed largely of PMNs and some mononuclear cells (observed at 100× magnification). On day 9, inflammatory abscesses and necrosis in periportal areas and hepatic parenchyma were apparent, along with cell debris around the central venules (Fig. 5Af, 1, and o).
FIG. 5.
Liver pathology of neonatal IFN-γ−/−, IFN-γR−/−, and IL-12p35−/− mice following i.n. C. muridarum challenge. Groups (n = 3–4) of 1-day-old (A) BALB/c (a–c); or IFN-γ−/− (d–f); or C57BL/6 (g–i); or IFN-γR−/− (j–l); or IL-12p35−/− (m–o) mice were challenged i.n. with 100 IFU of C. muridarum. The mice were euthanized on the indicated days after challenge. The livers were removed, embedded in paraffin, serial horizontal sections cut and stained with H&E. Total magnification 25×. Arrows point toward foci of inflammation. The livers were further scored quantitatively for pathology: (B) IFN-γ−/− and WT; (C) IFN-γR−/−, IL-12p35−/−, and wild-type (WT) animals. Results are expressed as the mean ± SD of the scores in each group. *P ≤ 0.05 between C. muridarum-challenged BALB/c and IFN-γ−/− mice, C57BL/6 and IFN-γR−/−, or C57BL/6 and IL-12p35−/− mice. Results are representative of 2 independent experiments.
Liver sections from challenged WT and KO mice also were scored in a blinded fashion using a quantitative scale at different time periods. WT BALB/c (Fig. 5B) and C57BL/6 (Fig. 5C) exhibited significantly less inflammation compared to challenged KO animals that displayed a progressively increasing trend of inflammatory cellular infiltration, with the highest score on day 9 post-challenge. Together, these results reveal enhanced cellular recruitment into the livers of Chlamydia-challenged neonatal mice in the absence of IL-12, IFN-γ, and IFN-γ signaling.
The induction of IL-12 and IFN-γ following neonatal pulmonary C. muridarum challenge
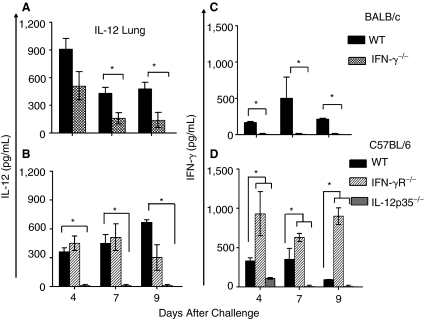
Since IL-12 and IFN-γ have been reported to be important in recruitment of inflammatory cells to sites of infection (Pearlman and others 1997; Sun and others 2007), we evaluated the induction of the 2 cytokines in the lungs and livers following neonatal pulmonary chlamydial challenge. As shown in Figure 6A–D, both WT BALB/c and C57BL/6 mice showed a significant induction of both IL-12 and IFN-γ throughout the course of the infection. Similar analyses of IL-12 or IFN-γ induction in the lungs of IFN-γ−/− or IL-12p35−/− mice revealed abrogation of both cytokines compared to the corresponding WT animals. As shown in Figure 6A, IFN-γ−/− mice exhibited significantly less induction of IL-12 and IFN-γ (Fig. 6C) compared to the corresponding WT animals. There also was significantly reduced induction of both IL-12 (Fig. 6B) and IFN-γ (Fig. 6D) in IL-12p35−/− mice at the corresponding time intervals. IFN-γR−/− mice exhibited comparable IL-12 induction at early time points, but significantly reduced levels on day 9 compared to WT mice. IFN-γ levels were significantly elevated throughout the course of the infection in these IFN-γR−/− mice compared to the corresponding WT animals, as reported previously (Geng and others 2000). These results suggest an interdependence of IL-12 and IFN-γ production on the induction of each other during neonatal chlamydial infection. Moreover, IFN-γ signaling appears to be required for sustained IL-12 production, and for negative feedback regulation of IFN-γ production. The trends of IL-12 and IFN-γ production observed in the lungs also were reflective of the trends in the liver after neonatal chlamydial challenge (data not shown).
FIG. 6.
Induction of IL-12 or IFN-γ in the lungs of neonatal IFN-γ−/−, or IFN-γR−/− and IL-12p35−/− mice following i.n. C. muridarum challenge. Groups (n = 3–4) of 1-day-old (A) BALB/c or IFN-γ−/− mice, (B) C57BL/6, IFN-γR−/−, and IL-12p35−/− mice challenged i.n. with 100 IFU of C. muridarum. The lungs were collected at the indicated time periods and induction of IL-12 (A and B); or IFN-γ (C and D) were quantitated by ELISA. Results are expressed as mean ± SD from each animal group. *P ≤ 0.05 between C. muridarum-challenged BALB/c and IFN-γ−/− mice, C57BL/6 and IFN-γR−/−, or C57BL/6 and IL-12p35−/− mice. All results are representative of 2 independent experiments.
Discussion
In this study, we examined the role of IL-12 and IFN-γ in neonatal pulmonary chlamydial infection. All 3 KO (IL-12−/−, IFN-γ−/−, and IFN-γR−/−) mice, when challenged i.n. with Chlamydia exhibited 100% mortality by day 17 post-challenge, with greater bacterial burdens in the lungs and dissemination to the liver compared to challenged WT animals. However, challenged KO mice exhibited significantly less pulmonary inflammatory cellular infiltration compared to challenged WT animals. In contrast to these observations in the lung, the livers of all the KO mice exhibited significantly greater levels of inflammatory responses than WT animals. Taken together, our results suggest that IL-12 and IFN-γ play an important role in recruitment of inflammatory cells and containment of the infection within the lung, and bacterial clearance, thus promoting survival of the neonatal host.
Our results corroborate previous reports suggesting an important contribution of these Th1-type cytokines to pulmonary cellular infiltration and chlamydial clearance (Wang and others 1999; Jupelli and others 2008). Perry and others (1997) previously reported the contributions of IL-12 and IFN-γ in C. muridarum infection of the adult mouse genital tract and suggested that the organism is largely resistant to the effects of mouse IFN-γ and that early chlamydial clearance occurs in an IL-12-dependent, IFN-γ-independent fashion (Perry and others 1997). We found significant impairment of chlamydial clearance in the KOs compared to WT mice, but did not find significant differences between IFN-γ−/−, IFN-γR−/−, and IL-12−/− mice. Additionally, IFN-γ production in IL-12−/− mice, and IL-12 production in IFN-γ−/− mice, was reduced in C. muridarum-challenged lungs, when compared to corresponding WT animals. IL-12 production in IFN-γR−/− mice was comparable to WT mice at early time periods, but significantly reduced at day 9 following neonatal C. muridarum challenge. The significantly elevated levels of IFN-γ production in IFN-γR−/− mice corroborates previous reports demonstrating the elevated production of this cytokine in the absence of a negative feedback regulation loop via the IFN-γR (Swihart and others 1995; Dai and others 1997). Collectively, these results suggest an intricate interdependence in the induction of these cytokines and an important role for the IL-12/IFN-γ axis in neonatal pulmonary chlamydial clearance.
Additionally, our results are also broadly comparable to those of Geng and others (2000) demonstrating the contribution of IL-12 and IFN-γ to pulmonary cellular recruitment following Chlamydia pneumoniae challenge in adult mice. Geng and others also suggested that IL-12, more than IFN-γ, contributes to cellular infiltration. They utilized IL-12-depleting monoclonal antibodies and B6/129 IFN-γR−/− mice and found that levels of IL-12 production (by in situ immunohistochemistry) and cellular infiltration in the IFN-γR−/− mice were comparable to that in WT 129 mice (Geng and others 2000). We found comparable IL-12 production (cytokine ELISA) and cellular infiltration scores in C57BL/6 IFN-γR−/− and WT mice at early time points, but significant reductions in both parameters in the IFN-γR−/− mice on day 9 after challenge. Such reductions in IL-12 production at later time points may be due to a requirement of signaling via the IFN-γ receptor to sustain IL-12 induction in the lungs (Trinchieri 1995). Thus IL-12 may contribute directly to cellular infiltration, whereas IFN-γ and downstream signaling via the IFN-γR may act indirectly via the maintenance of IL-12 production in the lung.
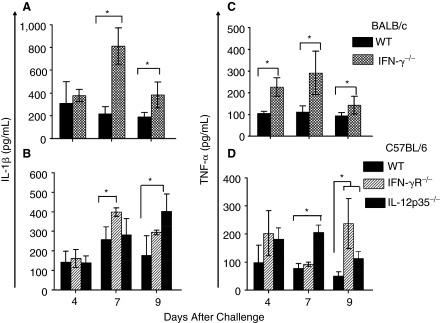
The site of infection also may play an important role in the contribution of these cytokines. Specifically, cellular infiltration into the lungs of C. muridarum-challenged KO mice was significantly reduced, but that into the liver was enhanced, while chlamydial burdens within both organs were significantly greater than in WT mice. Therefore, the effects of these cytokines may be organ-specific. To this end, IL-12 has been shown to enhance the expression of the chemotactic molecule VCAM (Meyts and others 2006), which is important for pulmonary cellular recruitment (Chin and others 1997). However, the liver has been shown to constitutively express ICAM that allows cellular infiltration independent of IL-12-mediated up-regulation of VCAM expression (Myers and others 1998). In fact, the enhanced bacterial dissemination to the liver in the KO mice may result in significantly enhanced cellular recruitment and production of inflammatory cytokines leading to death of the animals. To this end, we have found significantly enhanced levels of the acute phase proinflammatory cytokines TNF-α and IL-1β in the livers of C. muridarum-challenged KO compared to WT mice (Fig. 7). Specifically, livers of IFN-γ−/− mice showed significantly (P ≤ 0.05) greater induction of IL-1β and TNF-α (Fig. 7A and C) than similarly challenged WT mice on days 7 and 9 after challenge. In IL-12p35−/− mice, there was significantly greater induction of TNF-α on days 7 and 9, and IL-1β on day 9 following challenge compared to wild-type (WT) mice. In IFN-γR−/− mice, IL-1β was significantly elevated on day 7, and TNF-α on day 9 after challenge compared to WT animals (Fig. 7D).
FIG. 7.
Acute phase cytokine response in the livers of neonatal IFN-γ−/−, IFN-γR−/−, and IL-12p35−/− mice after i.n. C. muridarum challenge. Groups (n = 3–4) of 1-day-old (A) BALB/c and IFN-γ−/− and (B) C57BL/6, IFN-γR−/−, and IL-12p35−/− mice were challenged i.n. with 100 IFU of C. muridarum. The livers were collected at the indicated time periods and the induction of IL-1β (A and B); or TNF-α (C and D) were quantitated by ELISA. Results are expressed as mean ± SD from each animal group. *P ≤ 0.05 between C. muridarum-challenged BALB/c and IFN-γ−/−, C57BL/6 and IFN-γR−/−, or C57BL/6 and IL-12p35−/− mice. All results are representative of 2 independent experiments.
In summary, Th1-type cytokines, IL-12 and IFN-γ, are crucial for induction of cellular recruitment into the lungs, containment and clearance of chlamydial infection, and survival of the host during pulmonary C. muridarum infection in neonatal mice. The results from this study further underscore the ability of neonatal mice to induce effective Th1-type responses and the importance of such responses for protection of the host against bacterial infections.
Acknowledgments
We thank Ms. Heather Ray and Bharat K. Chaganty (University of Texas San Antonio) for technical assistance. This work is supported by National Institutes of Health Grants No. 1 R01 AI074860.
Author Disclosure Statement
The authors have no financial conflicts of interest.
References
- Barteneva N. Theodor I. Peterson EM. de la Maza LM. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect Immun. 1996;64(11):4830–4833. doi: 10.1128/iai.64.11.4830-4833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beem MO. Saxon EM. Respiratory-tract colonization and a distinctive pneumonia syndrome in infants infected with Chlamydia trachomatis. N Engl J Med. 1977;296(6):306–310. doi: 10.1056/NEJM197702102960604. [DOI] [PubMed] [Google Scholar]
- Beem MO. Saxon E. Tipple MA. Treatment of chlamydial pneumonia of infancy. Pediatrics. 1979;63(2):198–203. [PubMed] [Google Scholar]
- Byrne GI. Grubbs B. Marshall TJ. Schachter J. Williams DM. Gamma interferon-mediated cytotoxicity related to murine Chlamydia trachomatis infection. Infect Immun. 1988;56(8):2023–2027. doi: 10.1128/iai.56.8.2023-2027.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin JE. Hatfield CA. Winterrowd GE. Brashler JR. Vonderfecht SL. Fidler SF. Griffin RL. Kolbasa KP. Krzesicki RF. Sly LM. Staite ND. Richards IM. Airway recruitment of leukocytes in mice is dependent on alpha4-integrins and vascular cell adhesion molecule-1. Am J Physiol. 1997;272(2 Pt 1):L219–L229. doi: 10.1152/ajplung.1997.272.2.L219. [DOI] [PubMed] [Google Scholar]
- Cotter TW. Ramsey KH. Miranpuri GS. Poulsen CE. Byrne GI. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65(6):2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai WJ. Bartens W. Köhler G. Hufnagel M. Kopf M. Brombacher F. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J Immunol. 1997;158(11):5297–5304. [PubMed] [Google Scholar]
- Darville T. Chlamydia trachomatis infections in neonates and young children. Semin Pediatr Infect Dis. 2005;16(4):235–244. doi: 10.1053/j.spid.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Dixon AE. Mandac JB. Madtes DK. Martin PJ. Clark JG. Chemokine expression in Th1 cell-induced lung injury: prominence of IFN-gamma-inducible chemokines. Am J Physiol Lung Cell Mol Physiol. 2000;279(3):L592–L599. doi: 10.1152/ajplung.2000.279.3.L592. [DOI] [PubMed] [Google Scholar]
- Geng Y. Berencsi K. Gyulai Z. Valyi-Nagy T. Gonczol E. Trinchieri G. Roles of interleukin-12 and gamma interferon in murine Chlamydia pneumoniae infection. Infect Immun. 2000;68(4):2245–2253. doi: 10.1128/iai.68.4.2245-2253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupelli M. Guentzel MN. Meier PA. Zhong G. Murthy AK. Arulanandam BP. Endogenous IFN-gamma production is induced and required for protective immunity against pulmonary chlamydial infection in neonatal mice. J Immunol. 2008;180(6):4148–4155. doi: 10.4049/jimmunol.180.6.4148. [DOI] [PubMed] [Google Scholar]
- Kobayashi M. Fitz L. Ryan M. Hewick RM. Clark SC. Chan S. Loudon R. Sherman F. Perussia B. Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyts I. Hellings PW. Hens G. Vanaudenaerde BM. Verbinnen B. Heremans H. Matthys P. Bullens DM. Overbergh L. Mathieu C. De Boeck K. Ceuppens JL. IL-12 contributes to allergen-induced airway inflammation in experimental asthma. J Immunol. 2006;177(9):6460–6470. doi: 10.4049/jimmunol.177.9.6460. [DOI] [PubMed] [Google Scholar]
- Murthy AK. Chambers JP. Meier PA. Zhong G. Arulanandam BP. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infect Immunity. 2007;75:666–676. doi: 10.1128/IAI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy AK. Cong Y. Murphey C. Guentzel MN. Forsthuber TG. Zhong G. Arulanandam BP. Chlamydial protease-like activity factor induces protective immunity against genital chlamydial infection in transgenic mice that express the human HLA-DR4 allele. Infect Immun. 2006;74(12):6722–6729. doi: 10.1128/IAI.01119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy AK. Sharma J. Coalson JJ. Zhong G. Arulanandam BP. Chlamydia trachomatis pulmonary infection induces greater inflammatory pathology in immunoglobulin A deficient mice. Cell Immunol. 2004;230(1):56–64. doi: 10.1016/j.cellimm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Myers KJ. Eppihimer MJ. Hall L. Wolitzky B. Interleukin-12-induced adhesion molecule expression in murine liver. Am J Pathol. 1998;152(2):457–468. [PMC free article] [PubMed] [Google Scholar]
- Nelson DE. Virok DP. Wood H. Roshick C. Johnson RM. Whitmire WM. Crane DD. Steele-Mortimer O. Kari L. McClarty G. Caldwell HD. Chlamydial IFN-gamma immune evasion is linked to host infection tropism. Proc Natl Acad Sci USA. 2005;102(30):10658–10663. doi: 10.1073/pnas.0504198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman E. Lass JH. Bardenstein DS. Diaconu E. Hazlett FE., Jr. Albright J. Higgins AW. Kazura JW. IL-12 exacerbates helminth-mediated corneal pathology by augmenting inflammatory cell recruitment and chemokine expression. J Immunol. 1997;158(2):827–833. [PubMed] [Google Scholar]
- Perry LL. Feilzer K. Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158(7):3344–3352. [PubMed] [Google Scholar]
- Roshick C. Wood H. Caldwell HD. McClarty G. Comparison of gamma interferon-mediated antichlamydial defense mechanisms in human and mouse cells. Infect Immun. 2006;74(1):225–238. doi: 10.1128/IAI.74.1.225-238.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K. Salmon SL. Lotz SA. Metzger DW. Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect Immun. 2007;75(3):1196–1202. doi: 10.1128/IAI.01403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swihart K. Fruth U. Messmer N. Hug K. Behin R. Huang S. Del Giudice G. Aguet M. Louis JA. Mice from a genetically resistant background lacking the interferon gamma receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. J Exp Med. 1995;181(3):961–971. doi: 10.1084/jem.181.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and interferon-gamma. Do they always go together? Am J Pathol. 1995;147(6):1534–1538. [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Wysocka M. D'Andrea A. Rengaraju M. Aste-Amezaga M. Kubin M. Valiante NM. Chehimi J. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res. 1992;4(4):355–368. doi: 10.1016/0955-2235(92)90016-b. [DOI] [PubMed] [Google Scholar]
- Wang S. Fan Y. Brunham RC. Yang X. IFN-gamma knockout mice show Th2-associated delayed-type hypersensitivity and the inflammatory cells fail to localize and control chlamydial infection. Eur J Immunol. 1999;29(11):3782–3792. doi: 10.1002/(SICI)1521-4141(199911)29:11<3782::AID-IMMU3782>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Weiss SG. Newcomb RW. Beem MO. Pulmonary assessment of children after chlamydial pneumonia of infancy. J Pediatr. 1986;108(5 Pt 1):659–664. doi: 10.1016/s0022-3476(86)81037-x. [DOI] [PubMed] [Google Scholar]
- Williams DM. Byrne GI. Grubbs B. Marshal TJ. Schachter J. Role in vivo for gamma interferon in control of pneumonia caused by Chlamydia trachomatis in mice. Infect Immunity. 1988;56:3004–3006. doi: 10.1128/iai.56.11.3004-3006.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong GM. Peterson EM. Czarniecki CW. Schreiber RD. de la Maza LM. Role of endogenous gamma interferon in host defense against Chlamydia trachomatis infections. Infect Immun. 1989;57(1):152–157. doi: 10.1128/iai.57.1.152-157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong GM. Reid RE. Brunham RC. Mapping antigenic sites on the major outer membrane protein of Chlamydia trachomatis with synthetic peptides. Infect Immun. 1990;58(5):1450–1455. doi: 10.1128/iai.58.5.1450-1455.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]