The Drosophila member of the vestigial-like gene family (vestigial) is known primarily as a transcriptional activator that defines cell identity during Drosophila wing differentiation. We show that during embryo development Vestigial also has a role during specification of muscle–muscle attachments in ventral longitudinal muscles.
Abstract
The somatic muscles of Drosophila develop in a complex pattern that is repeated in each embryonic hemi-segment. During early development, progenitor cells fuse to form a syncytial muscle, which further differentiates via expression of muscle-specific factors that induce specific responses to external signals to regulate late-stage processes such as migration and attachment. Initial communication between somatic muscles and the epidermal tendon cells is critical for both of these processes. However, later establishment of attachments between longitudinal muscles at the segmental borders is largely independent of the muscle–epidermal attachment signals, and relatively little is known about how this event is regulated. Using a combination of null mutations and a truncated version of Sd that binds Vg but not DNA, we show that Vestigial (Vg) is required in ventral longitudinal muscles to induce formation of stable intermuscular attachments. In several muscles, this activity may be independent of Sd. Furthermore, the cell-specific differentiation events induced by Vg in two cells fated to form attachments are coordinated by Drosophila epidermal growth factor signaling. Thus, Vg is a key factor to induce specific changes in ventral longitudinal muscles 1–4 identity and is required for these cells to be competent to form stable intermuscular attachments with each other.
INTRODUCTION
Embryonic muscles are first specified as founder cells within the embryonic mesoderm. The specification of diversity among muscle founder cells has been linked to differences in expression of a combination of transcription factors known as muscle identity genes, including slouch, apterous, ladybird, vestigial (vg), nautilus, and even-skipped (Baylies et al., 1998). Neighboring myoblasts are recruited to founder cells with corresponding patterns of expression of these factors and fuse with them. This initial formation of a syncytial muscle is followed by a precise series of events whereby each muscle migrates to a specific location, interacting with neighboring cells to form attachments allowing for coordinated movement.
During later stages of Drosophila melanogaster embryonic development, somatic muscles (SMs) organize into a complex pattern in each abdominal hemi-segment from A2 to A7 (see Figure 1, A and B; Bate, 1993). Formation and maintenance of this pattern requires both internal differentiation events and intercellular signaling to direct a precise pattern of migration, and attachments. After migration, SMs form two different types of attachments: to epidermal cells (tendon cells) and intermuscular adhesions (diagrammed in Figure 1C). Ultrastructural analysis reveals intermuscular attachments contain extensive extracellular matrix consisting of fuzzy electron-dense fibers, whereas muscle–epidermis attachments contain only a thin line of extracellular electron-dense material (Prokop et al., 1998). Muscle-tendon cell interactions guide the initial stages of migration and attachment (Becker et al., 1997; Yarnitzky et al., 1997). Similar to its role in axon pathfinding (Kidd et al., 1999), the guidance protein Slit is secreted from tendon cell precursors at the segment borders and the corresponding receptor Robo is found on the surface of ventral longitudinal (VL) muscles. In muscles, Slit has a bifunctional role, repelling myotubes during early development, but attracting them later. In slit mutant embryos, VL muscles aberrantly cross the midline due to the lack of a repellent Slit source along the midline. If slit is expressed only in midline cells, VL muscles stop crossing the midline but fail to reach their normal attachment sites due to the lack of an attractive Slit source at the segment borders (Kramer et al., 2001).
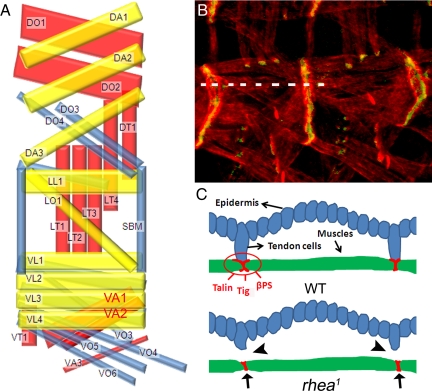
Figure 1.
(A) Schematic representation of the SMs in each abdominal hemi-segment A2–A7 of the developing embryo (lateral view with anterior left and dorsal up) by using the nomenclature of Bate (1993). Inner, middle, and outer muscle layers are shown in yellow, blue, and red, respectively (Bate, 1993). Dorsal oblique (DO), DA, dorsal transverse (Swan et al., 2004), LL, LO, LT (Cluzel et al., 2005), SBM, VL (Lundstrom et al., 2004), VA, VT, and VO. VA1 and VA2 are highlighted in red. (B) Muscle–muscle and muscle–tendon cell junctions in wild-type embryos visualized by staining developing muscle cells with actin (Tadokoro et al., 2003) and βPS integrin (green). (C) Diagrams showing a cross-sectional view along the broken line in B. The adhesion proteins (talin, βPS, and Tig, etc.) all concentrate at the end of SMs and are involved in forming stable muscle–muscle or muscle–tendon cell adhesions in wild-type embryos. In rhea1 mutant embryos, the muscle–tendon cell connections are broken (arrowheads), but the muscle–muscle connections remain (arrows).
Developing myotubes also secrete Vein, a ligand for the Drosophila epidermal growth factor receptor (DER), which activates the Ras pathway in the tendon cells, leading to the final differentiation of tendon cells through elevating expression of stripe (sr) (Yarnitzky et al., 1997; Nabel-Rosen et al., 1999). Sr, in turn, induces expression of the secreted protein thrombospondin (Tsp), which is required for building stable integrin-mediated junctions by binding the αPS2βPS (PS2) integrin receptor (Subramanian et al., 2007). In sr mutant embryos, myotubes fail to make attachments with epidermis, losing their elongated morphology and becoming rounded in appearance (Volk and VijayRaghavan, 1994; Frommer et al., 1996).
Formation of the junctions between muscles or muscle and tendon cells is largely mediated by integrins. Integrins are heterodimeric single-pass transmembrane receptors that mediate attachment to the extracellular matrix (ECM) (Hynes, 2002). The two major Drosophila integrins PS1 (αPS1βPS) and PS2 (αPS2βPS) have a complementary pattern of expression with PS2 concentrated at the ends of SMs and PS1 accumulating on the tendon cells (Brown, 1993; Brown et al., 2000). PS1 cannot substitute for PS2 at the muscle attachments and vice versa (Martin-Bermudo et al., 1997). The integrin-mediated myotendinous adhesions are established between muscles and tendon cells (see Figure 1, B and C; Brown et al., 2000). The process of muscle specification seems to be independent of actual formation of the adhesions because absence of one or more of the adhesion proteins, including PS integrins, does not affect initial specification, fusion, and attachment of SMs. Rather, these muscles detach and round up upon first contraction due to the lack of strong adhesion.
Several lives of evidence have established that formation of muscle–muscle adhesions is a distinct process from that involved in specification of muscle–tendon attachments. Muscle–tendon cell signaling is blocked by mutation of both engrailed and invected. In these embryos, Tiggrin (Tig) and βPS remain localized at the end of muscles in contact with each other. This suggests that initial localization of the ECM component Tig at the segment borders is independent of tendon cells and integrin but requires muscle–muscle contact (Martin-Bermudo and Brown, 2000). Mutations in rhea (encoding Talin) cause the specific disruption of the muscle tendon cell attachments but not muscle–muscle attachments (see Figure 1C) (Prout et al., 1997). Conversely, mutations in tiggrin (tig) lead to weak muscle–muscle attachments, but muscle tendon cell attachments are not affected significantly (Bunch et al., 1998). Notably, there seems to be significant redundancy in this process because embryos with mutations in either gene do not manifest a severe muscle detachment phenotype. However, in embryos with both tig and rhea mutations, SMs detach and round up due to the disruption in both types of attachments (Martin-Bermudo and Brown, 2000). Thus, embryos homozygous for rhea mutations are an excellent sensitized genetic background for studying the role of factors that influence establishment of intermuscular attachment.
Vg was first identified as a key “selector” gene that specifies wing identity during Drosophila development (Williams et al., 1991). Vg does not have a DNA binding domain but contains two domains important for gene activation (MacKay et al., 2003) and thus must partner with additional proteins that bring it to the DNA. In ectodermal cells of the wing imaginal disk, Vg interacts with Scalloped (Campbell et al., 1991), which has a conserved DNA binding domain and a well characterized Vg interaction domain (Campbell et al., 1991; Halder et al., 1998; Simmonds et al., 1998). These two proteins form a wing-specific transcription factor complex that directs wing development in any ectodermal cell type where it is expressed (Halder et al., 1998; Simmonds et al., 1998). This aspect of vg function is well known as many mutations in vg have been recovered that eliminate all adult wing formation but are otherwise viable. However, there are strong hypomorphic and dominant vg alleles that have phenotypes affecting other tissues. During pupal development, vg has been shown to be a muscle identity gene for specific flight muscles (Sudarsan et al., 2001). For these muscle cell-specific activities, Vg seems to require interaction with Dmef2, a key myogenic gene required for specification and subsequent differentiation of all muscles (Black and Olson, 1998; Deng et al., 2009).
To further clarify the role of Vg during embryonic muscle development, we performed both loss-of-vg-function and gain-of-vg-function analyses. Our results revealed a role of Vg in the establishment of stable intermuscular myotendinous junctions. Furthermore, we show DER signaling may mediate the intermuscular communication and muscles expressing Vg become competent to respond to this communication by building a stable intermuscular junctions.
MATERIALS AND METHODS
Drosophila Strains
The rhea1, robo1, and slit2 mutations and w1118 used as the untransformed reference strain were obtained from the Bloomington Stock Center (Department of Biology, Indiana University, Bloomington, IN); SD3L (Campbell et al., 1991), vgnull (Bernard et al., 2003), and dgripex36 (Swan et al., 2004) have been described previously. Ectopic transgene expression was performed using the Gal4-upstream activation sequence (UAS) system (Brand and Perrimon, 1993) using the following lines: Dmef2-Gal4 (Ranganayakulu et al., 1998), twi-Gal4 (Greig and Akam, 1993), SD-Gal4 (Roy et al., 1997), C23-Gal4, and Ap-Gal4 (from Bloomington Stock Center), UAS-robo (Kramer et al., 2001), UAS-vg (Deng et al., 2009), UAS-DN-egfr (Yarnitzky et al., 1997), UAS-λ-egfr (Queenan et al., 1997), UAS-SDΔTEA (Garg et al., 2007), and UAS-lacZ (Bloomington Stock Center).
Immunohistochemistry and Microscopy
Embryos were formaldehyde fixed (Hughes and Krause, 1999), and the following primary antibodies were used at the indicated dilutions: mouse anti-FLAG (1:1000; Sigma-Aldrich, St. Louis, MO), rat anti-hemagglutinin (HA) (1:200; Roche Diagnostics, Indianapolis, IN), rat anti-myosin (1:500; Abcam, Cambridge, MA); mouse anti-βPS-integrin (1:500; developed by Danny Brower and obtained from the Developmental Studies Hybridoma Bank, Department of Biological Sciences, The University of Iowa, Iowa City, IA), mouse anti-β-Gal (1:500; Promega, Madison, WI), anti-muscle myosin heavy chain monoclonal antibody FMM5 (1:10; from D. Kiehart, Duke University, Durham, NC), rabbit anti-Vg (Williams et al., 1991); rat anti-thrombospondin (Subramanian et al., 2007); mouse anti-talin (Brown et al., 2002), rabbit anti-PINCH (Clark et al., 2003), and rabbit anti-Kon (Schnorrer et al., 2007). Donkey Alexa488-, Alexa568-, Alexa594-, and Alexa647-conjugated secondary antibodies were used (1:4000; Invitrogen, Carlsbad, CA). Muscle actin was stained using Alexa546-conjugated phalloidin (1:25; Invitrogen). Images were obtained with a Zeiss LSM510 or UltraVIEW ERS confocal microscope (PerkinElmer-Cetus, Norwalk, CT) and assembled using Photoshop (version CS; Adobe Systems, San Jose, CA).
Tiggrin Antibody Production
A cDNA fragment encoding the C-terminal 270 amino acids including the RGD (Arg-Gly-Asp) domain of Tig (Fogerty et al., 1994) was cloned into pDEST17 bacterial expression vector (Invitrogen), expressed in Escherichia coli [BL21(DE3); Stratagene, La Jolla, CA], and purified using nickel-nitrilotriacetic acid according to the manufacturer's protocol (QIAGEN, Valencia, CA). Purified fusion protein was injected into rabbits (Pocono Rabbit Farm and Laboratory, Canadensis, PA). Specificity of the rabbit polyclonal serum was determined by testing it against purified Tig and fixed Drosophila embryos, confirming the localization pattern was the same as published for Tig previously (Fogerty et al., 1994).
RESULTS
vgnull But Not SD3L Mutant Embryos Show Muscle Detachment in a rhea1 Background
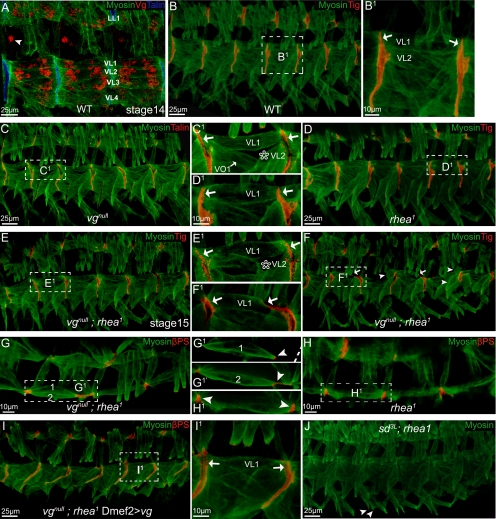
Antibody staining of wild-type embryos showed that vg is expressed at relatively high levels in muscles making both intermuscular and muscle–tendon cell attachments at the segment border (Figure 2A). To determine whether there is a significant role for Vg regulation of the migration or attachment function of these late-stage embryonic SMs; we examined the muscle phenotypes of embryos homozygous for the vgnull mutation. Some flies with a homozygous vgnull genotype do survive to adulthood, but they are invariably unable to produce progeny and have significant defects in the adult musculature (Bernard et al., 2006). In homozygous vgnull embryos, VL2 muscles were absent in at least 30% of segments (Figure 2, B–C1). This muscle-loss phenotype seems to be VL2 cell specific because VL1 muscles were present in all segments (Figure 2C and Supplemental Figure S2, C and D). However, the loss of Vg does not seem to block initial formation of adhesions between two differentiating muscles, because similar to stage 16 wild-type embryos (Figure 2, B–B1), in homozygous vgnull, pairs of VL muscles formed tight adhesions between their corresponding VL muscle in the next segment (Figure 2, C–C1).
Figure 2.
SMs were detached in vgnull; rhea1 embryos but not in SD3L; rhea1 embryos. Embryos (stage 16 or a specified stage) are shown as lateral views, with dorsal up, and anterior to the left. Staining is color coded and indicated on each panel. B1–I1 are the close-ups of the framed area in B–I. (A) vg is expressed in muscle LL1 and VL1–4. The arrowhead points to a neuronal cell also expressing vg. Compared with wild-type embryos (B), vgnull (C), or rhea1 single mutation (D), or vgnull; rhea1 double mutant embryos in early stages (before stage 15; E), all produced a muscle pattern similar to wild-type embryos, except that a vgnull mutation caused muscle VL2 to be missing in ∼30% of segments (star in C1 and E1). Notice VL muscles (e.g., VL1) all formed tight adhesions between each other (arrows in B1–E1). (F–F1) By late stage 16 when muscles start to contract, muscle VL1 or other VL muscles detached from the attachment sites only in the vgnull; rhea1 double mutant embryos (arrows in F1). Arrows in F indicate detaching muscles and arrowheads indicate detached muscles. (G–H1) Overview of the vgnull; rhea1 double mutant embryos (stage 16; G–G1′) compared with rhea1 embryos (H–H1). Both VL1 and VL2 are retracting from their normal attachment sites (arrowhead in G1–H1). G1 and G1′ are two different confocal sections, and the broken line in G1 indicates the segment border. (I–I1) The muscle detachment phenotype of vgnull; rhea1 embryos can be rescued by expression of Vg via Dmef2-GAL4. Notice VL1 muscles built tight adhesions between each other (arrows in I1). (J) SD3L; rhea1 embryos did not have a muscle detachment phenotype. Some muscles do not develop well (VO4–6; arrowheads) in these embryos, but this mirrors the phenotype seen in SD3L single mutants.
To further dissect the requirement for Vg during formation of adhesions between SMs, we paired mutations in vg with those in rhea (talin deficient) that would make small changes in attachments induced by loss of vg more apparent. In rhea1 mutant embryos, junction formation between pairs of VL muscles seems normal (Figure 2, D–D1). At early stages (15), the VL muscles showed no significant migration or attachment defects in vgnull; rhea1 double mutant embryos (Figure 2, E–E1). However, at later stages (16+) several muscles, most prevalently VL1 can be seen detaching from their normal location (Figure 2, F1–H1). This muscle detachment phenotype seems to be due solely to lack of Vg activity because it can be rescued by expression of wild-type Vg in muscle cells (Figure 2, I–I1). Because SD is the known binding partner for Vg function during wing development (Simmonds et al., 1998), the muscle phenotype of SD3L; rhea1double mutants also was examined. Mutants homozygous for a loss-of-function SD3L allele (Campbell et al., 1991; Srivastava et al., 2004) and rhea1 produce only a mild SM phenotype described previously (Deng et al., 2009). Otherwise, they are indistinguishable from rhea1 mutants (Figure 2J).
Expressing a Form of SD That Can Bind Vg, But Not DNA, Causes a Phenotype Similar to tig Null Mutants
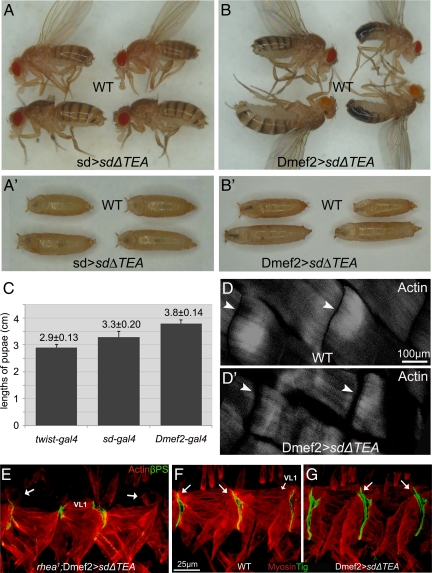
In cells of the wing imaginal disk, Vg must form a complex with SD to localize to the nucleus and bind chromatin via a conserved TEA binding domain within SD (Halder et al., 1998; Simmonds et al., 1998). Previously, a form of SD that removes the TEA DNA binding domain (SDΔTEA) has been shown to bind Vg, but because the resulting complex cannot bind DNA it acts to inhibit Vg activity in imaginal discs (Garg et al., 2007). During experiments to confirm that tissue-specific expression of a UAS-SDΔTEA transgene can specifically inhibit the gene activation function(s) of Vg in the wing disk, we noted that expression of SDΔTEA by SD-GAL4 also produced elongated pupae and adults (Figure 3, A and C). A database search produced only one other mutation that produces elongated body, muscle spacing and semilethality phenotypes, a null mutation in tig, required for intermuscular junction formation (Bunch et al., 1998). This phenotype also was notable because SD-GAL4 would lead to only weak activation of SDΔTEA in tissues such as embryonic SMs from stage13 to stage16 (Deng et al., 2009). Given the similarity of interfering with Vg function via SDΔTEA and tig mutations, we tested the effect of SDΔTEA in muscle cells by using a driver that is expressed at much higher levels (Dmef2-GAL4) (Figure 3B). This combination was semilethal, with 78.7% of pupae (n = 619) failing to eclose. The larvae hatched from embryos overexpressing SDΔTEA via Dmef2-GAL4 had obvious gaps between the dorsal acute (DA)1 muscles that were larger than wild type (Figure 3, D–D′), suggesting the elongated body type was caused by muscle defects. However, the requirement for Vg function was stage specific because expression of SDΔTEA in muscle progenitor cells at earlier embryo developmental stages (7–11) by using twist-GAL4 (Greig and Akam, 1993) did not lead to elongated adults and significant lethality (Figure 3C). Finally, to ensure that the phenotype we observed was caused by inhibition of Vg through formation of a nonfunctional Vg/SDΔTEA complex and not through overexpression of SD, we overexpressed a form of SD that cannot bind Vg (SDΔVID). This combination produced flies with no detectable defects in developing muscles.
Figure 3.
Tissue-specific expression of SDΔTEA interferes with Vg function and produced elongated larvae and adults. (A) Expression of SDΔTEA via SD-GAL4 (SD>SDΔTEA) in the wing disk caused loss of the adult wing by interfering with Vg. However, we also noted that the pupae (A′) and adult flies were elongated compared with wild-type (WT) siblings. (B) This phenotype was caused by interfering with Vg in the muscle cells because these effects were seen in pupae (B′) and adults when UAS-SDΔTEA was expressed exclusively in muscle cells via Dmef2-GAL4 (Dmef2>SDΔTEA). (C) Quantification of pupal length in animals overexpressing SDΔTEA by using the indicated GAL4 drivers (mean ± SD; n = 23). The pupal length of twist-GAL4>SDΔTEA animals was not statistically different from wild type. (D–D′) Larvae expressing SDΔTEA in the muscles (Dmef2-GAL4) (D′) had a larger gap (arrowhead) between DA1 muscles than wild type (D). (E) Expression of SDΔTEA in developing muscle cells in embryos that are homozygous for the rhea1 mutation produced a muscle detachment phenotype in which the majority of the VL1 cells became rounded (arrows). (F) Tig protein localizes to the tips of muscles forming junctions including VL1 (arrows). (G) Embryos expressing SDΔTEA in muscles show the same pattern of Tig localization (arrows). In all panels, ventral muscles in two or three segments are shown in embryos (stage 16) presented as lateral views, with dorsal up, and anterior to left.
VL Muscle Migration and Initial Adhesion Occurs Normally in the Absence of Vg
The progressive muscle detachment phenotype we observed when Vg activity is reduced could be caused by several events, including improper muscle specification, a failure of muscles to migrate to the attachment site, or select the appropriate target site, or the inability to form a strong connection that can resist the force of muscle contraction at later stages. To determine whether Vg was required for initial establishment and maintenance of the VL cell lineage, we used a marker that would be activated solely in the VL1 muscle and would persist during later development (5053-GAL4 and UAS-lacZ) (Schnorrer et al., 2007). The VL1 muscle is initially specified correctly in all segments in both vgnull and wild-type embryos (Supplemental Figure S2, C and D). Similarly, development of tendon cells was not affected in vgnull; rhea1 double mutants (Supplemental Figure S2, A and B).
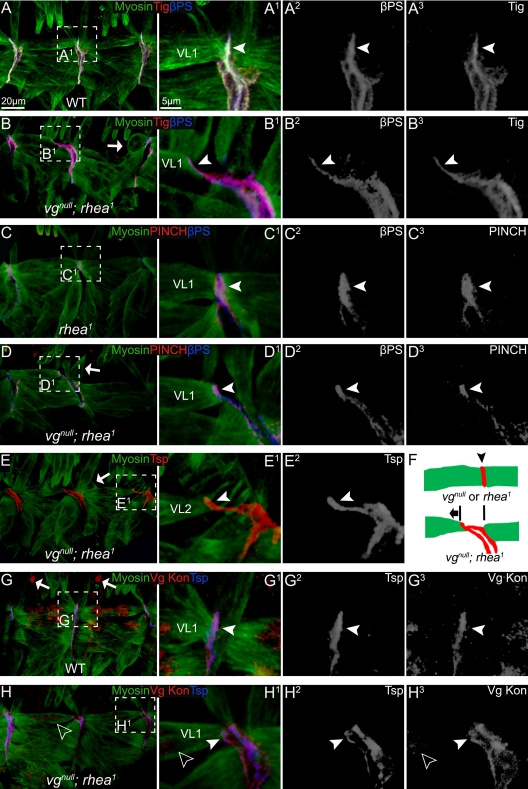
Because early specification of VL1 was unaffected when Vg is absent, we next assayed for changes in formation of junctions between two muscles at the segment border. There are three known major components of muscle–muscle junctions (Brown et al., 2000): 1) PS2 integrin; 2) ECM containing PS2 integrin ligands: Tig (Fogerty et al., 1994) and Tsp (Subramanian et al., 2007); and 3) talin and its associated proteins, including PINCH (Clark et al., 2003). Together, they link PS2 integrin to the muscle myofiber, forming a tight adhesion junction or integrin complex holding muscles together (Figure 4, A–A3). In the vgnull; rhea1 double mutant embryos, βPS and Tig (Figure 4, B–B3), PINCH and βPS (Figure 4, D–D3), and Tsp (Figure 4, E–E2), were all concentrated at the ends of detaching VL1 muscles and connected to myofibers, similar to wild type (Figure 4, A–A3 and G–G3) or rhea1 single mutant embryos (Figure 4, C–C3). Thus, the affinity of PS2 integrin for its ligands did not seem to be affected and the integrin complex was still largely intact in muscles of the double mutant embryos, before detachment.
Figure 4.
The muscle detachment phenotype observed in vgnull; rhea1 embryos was not due to lack of localization of integrin or its known ligands, nor to an obvious muscle migration defect. (A) In wild-type embryos, βPS and Tig can be seen localized normally at the junctions between two VL muscles (arrowhead). (B) In vgnull; rhea1 double mutant embryos, the VL muscles were either detaching (arrowheads) or were already detached (arrows). However, βPS and Tig remain concentrated at muscle termini and followed the detaching muscles (arrowheads). (C) In rhea1 mutant embryos, the adhesion proteins PINCH and βPS formed tight junctions between VL muscles (arrowheads). (D) Similar to βPS and Tig, in detaching muscles in vgnull; rhea1 embryos, PINCH and βPS remain concentrated at muscle termini and followed the detaching muscles (arrowheads). Many muscles seemed to be detaching from the posterior border of each segment. (E) In the vgnull; rhea1 embryos, Tsp shows the same localization to the end of detaching muscles as PINCH, βPS, and Tig. (F) A diagram of the localization of adhesion proteins (red; arrowhead) in vgnull or rhea1 mutant embryos and the direction (anterior, arrow) in which VL muscles are moving after they detach. (G) In wild-type embryos, Kon, the major migration guidance protein for VL muscles, normally found at the end of muscle cells (arrowhead). (H) In vgnull; rhea1 embryos, some residual (maternally supplied) Vg protein can still be seen in VL1 muscle (empty arrowheads). These muscles still had a detachment phenotype, but Kon is localized properly (arrowhead). A1–H1 are the close-ups of the framed area in A–H. A2–H2 and A3–H3 show each confocal channel separately.
Because the major adhesion proteins were being localized correctly at the end of VL1 muscles in vgnull; rhea1 mutant embryos, we examined the process of VL1 migration. The transmembrane protein Kon-tiki (Kon) is localized to the tips of VL muscles and is required for formation of filopodia and proper migration of developing muscles (Estrada et al., 2007; Schnorrer et al., 2007). The expression and localization of Kon in the vgnull; rhea1 double mutants was indistinguishable from those of wild-type embryos (Figure 4, G–H3). Second, the direction of VL1 muscle migration was unaffected. VL1 muscles normally migrate from the posterior border of each segment to the anterior border and then attach to both (Schnorrer et al., 2007). If muscles in embryos with vgnull; rhea1 mutations migrated inappropriately, then they would fail to attach to the anterior border and round up at the posterior side of each segment. However, in the double mutants, more than half of the VL1 muscles detached from the posterior borders remaining attached to the anterior (Figure 4, D–F), indicating they already reached to their attachment sites.
Ectopic Expression of Vg Induces Abnormal Muscle–Muscle Attachments
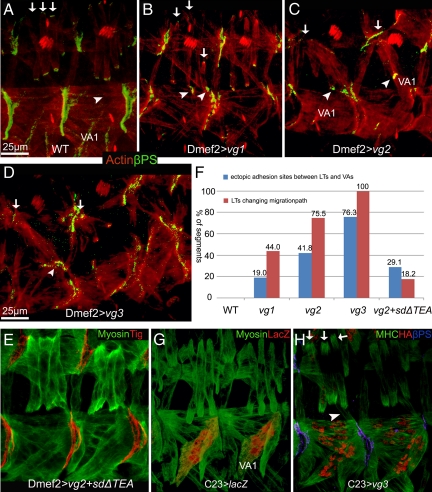
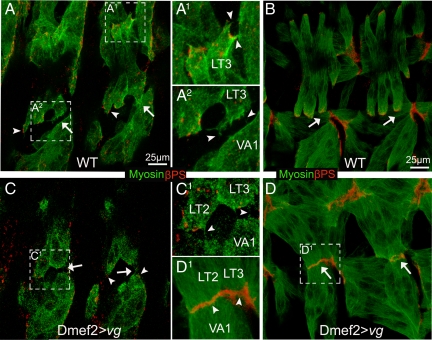
The phenotype associated with vgnull mutants strongly suggested that Vg expression induces cell-specific changes in VL muscles for them to be competent to form intermuscular attachments at the segment border. If this hypothesis is correct, then Vg should be able to induce additional attachments when expressed ectopically in muscles where it is not normally found at high levels. The lateral transverse (LT)1–4 muscles migrate vertically within each segment and normally make only muscle tendon cell attachments in wild-type embryos (Figure 5A). Ectopic-expression of Vg within the LT muscles caused them to migrate toward the segment borders and attach there, producing muscle–muscle attachments between LT and VA muscles, where they normally do not occur (Figure 5, B–D). Notably, this ectopic expression does not seem to alter the initial muscle identities of LT2, LT4, and VA2 (Supplemental Figure S2, E and F). The ectopic muscle–muscle adhesions induced by ectopic expression of Vg in the LT muscles seemed to be functional, because muscle VA1 was often located away from its normal position due to tension produced by abnormal attachment to the LT muscles (Figure 5C). We confirmed that this phenotype was Vg specific by expressing both vg and SDΔTEA. This combination showed fewer ectopic muscle attachments (24.5%, compared with 81.8% when Vg is expressed alone; Figure 5, E and F). Similarly, we saw a correlation between the severity of the ectopic LT muscle attachment phenotype and increasing the expression of ectopic Vg (Figure 5F). Finally, ectopic expression of Vg in both cells is required to induce them to form ectopic attachments, because expression of Vg in only a single muscle group failed to build ectopic adhesion sites between LTs and VAs (Figure 5, G and H). To further test this idea, expression of UAS-vg was also driven through apterous (ap)-GAL4 that is present only in muscle VA2 and LT1–4 starting at stage 14 when all SMs have been properly specified (Supplemental Figure S4, A–A′). This late stage expression of Vg still produced ectopic adhesions, but only between VA2 and LT muscles, not between VA1 and LT muscles although VA1 is closer to LT muscles than VA2 (Supplemental Figure S4, B–B′).
Figure 5.
Ectopic expression of Vg in the developing embryonic SMs produced ectopic intermuscular attachments. The transgenic lines vg1, vg2, and vg3 express relatively higher levels of Vg, respectively, as verified by Western blotting. (A) In wild-type embryos, LT1–4 muscles that stain brightly with muscle-specific actin (red; arrows) are seen passing left to right over the VA1 muscle and form βPS-mediated attachments (green) at intrasegmental sites. Normally, no adhesions form where the LT and VA muscles are adjacent (arrowhead). (B–D) Ectopic expression of progressively higher levels of Vg in all muscles via Dmef2-GAL4. (B) Ectopic expression of relatively lower levels of Vg (vg1) in SMs caused the LT muscles to abnormally form attachments at the segment borders. (C) Expression of relatively higher levels of Vg (vg2) cause the formation of abnormal attachments at the segment borders (arrows). Furthermore, ectopic muscle–muscle attachments were observed between LT and VA muscles (arrowheads). In some cases, muscle VA1 was observed deviating from its original position (arrowheads). (D) This number of abnormal and ectopic attachments becomes even more severe when a transgene (vg3) expressing relatively highest levels of Vg is used. (E) Quantification of the percentage of segments having LTs with abnormal migration (red columns) or ectopic adhesion sites between LT and VA muscle cells (blue columns) for each indicated overexpression line (n = 110). (F) Ectopic expression of Vg with SDΔTEA led to a partial rescue of the phenotype caused by ectopic expression of Vg from the UAS-vg2 transgene. (G) The C23-GAL4 line induces expression at high levels in VA1 but relatively low expression in LTs as detected by an UAS-lacZ reporter. (H) Ectopic attachments are not formed when Vg is present at relatively high levels in VA1 cells.
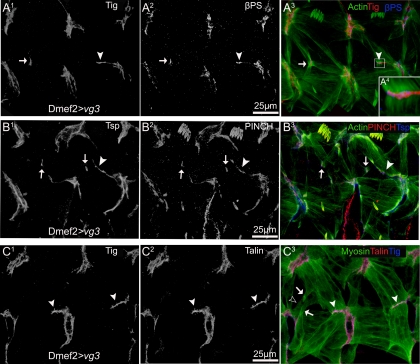
Ectopic adhesion sites were also induced between ventral transverse (VT)1 and LTs (Figure 6A3) or between segment border muscle (SBM) and lateral oblique (LO)1 (Figure 6B3; see Figure 1 for legend showing muscle identities) by higher levels of ectopic Vg expression. Although expression of Vg clearly induced formation of extra attachments between muscles, it is possible that these attachments were not functional. Assembly of intermuscular junctions can be perturbed at several steps. A chimeric mimic of activated integrins can recruit talin in embryonic muscles but not other integrin-associated proteins such as PINCH (Tanentzapf et al., 2006). Similarly, in mammalian cells, activation of high-affinity αVβ3 integrin produced ectopic integrin clusters that recruit talin but not other integrin-associated protein (Cluzel et al., 2005). We determined whether each of these integrin-associated components (Tig, Tsp, talin, and PINCH) was recruited to the additional adhesion sites associated by ectopic/overexpression of Vg (Figure 6, A1–C3). More importantly, the internal muscle myosin–actin fiber was connected to the ectopic integrin clusters (Figure 6A4). This suggests that a functional intermuscular attachment was induced in cells that migrate abnormally due to ectopic Vg.
Figure 6.
Muscle attachments induced by ectopic Vg include βPS integrin and its associated cytoplasmic linker proteins, PINCH and talin. (A) Ectopic expression of Vg by Dmef2-GAL4 caused additional attachments to form between muscles stained with muscle-specific actin (green). These ectopic attachments (arrowheads) contained Tig (red; A1), an extracellular ligand for PS2 integrin (blue; A2). Ectopic muscle attachments were also produced between muscle cells other than LTs and VAs, which also contained Tig (arrows). Individual myofibers were linked to the new adhesion sites through integrin complexes (A4 is a close-up of the boxed area in A3). (B) These ectopic attachments also contain Tsp (blue; B1) and PINCH (red; B2). (C) Talin is also localized to the ectopic muscle attachments (red; C2). Note the processes emerged from the lateral surface of muscle LTs (arrows in C3) and VT1 (empty arrowhead in C3).
Vg Is Required for the Ectopic Attachments Formed between Ventral Midline-Crossing Muscles in slit2 Mutants
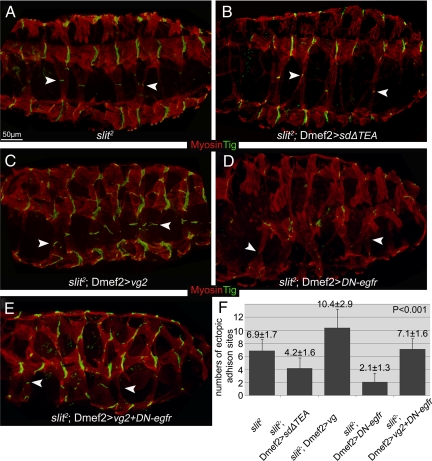
Although expression of Vg can induce the formation of muscle–muscle junctions, the contribution of signals from the surrounding tendon cells may be influencing this effect. Therefore, we examined the effect of blocking Vg function in slit2 mutant embryos, where the VL muscles cross dorsally over the CNS meeting those from the other side to form ectopic muscle attachments along the ventral midline (Figure 7A). This allows us to test the effect of loss of Vg on muscle–muscle attachments independently, because there are no tendon cells within this region (Vorbruggen and Jackle, 1997). Blocking Vg function via SDΔTEA in VL muscle cells that abnormally migrate along the midline led to fewer and smaller muscle–muscle adhesions (Figure 7, B and F). Conversely, increasing the expression of Vg produced more and larger adhesion sites (Figure 7, C and F).
Figure 7.
Altered levels of Vg function regulate ectopic intermuscular attachments independently of signaling from tendon cells in VL muscle cells, and DER mediated cell–cell communication is required for the production of intermuscular attachments between Vg expressing cells in slit2 mutant. (A) In slit2 mutant embryos, VL muscle cells migrate dorsally over the CNS from the lateral sides of the embryo meeting near the midline to form Tig marked muscle–muscle adhesions (arrowheads) in a region of the embryo devoid of tendon cells. (B) Interference with Vg function by expression of SDΔTEA in these slit2 mutant embryos led to fewer and smaller of these midline-located adhesion sites (arrowheads). (C) Overexpression of Vg in slit2 mutant embryos produced more and larger adhesion sites (arrowheads) in the abnormally positioned VL muscles. (D) Expression of a dominant-negative form of DER (DN-egfr) in slit2 mutant embryos strongly reduces the overall size and number of these muscle-muscle adhesion sites (arrowheads in D). (E) The size and number of these ectopic adhesion sites (arrowheads in E) in slit2 mutants that coexpress DN-egfr as well as Vg are reduced when compare with expressing Vg alone (compare E with C) but increased when compared with expressing DN-egfr alone (compare E with D). (F) Quantification of the number of VL cell adhesion sites formed in slit2 mutant embryos with varying levels of Vg activity and/or changing of DER activity (mean ± SD; n ≥ 15).
Embryonic Muscles Expressing Vg Require DER Signaling to Form Attachments
As a transcriptional activating “selector gene,” the role of Vg is assumed to be induction of cell-specific changes in gene expression (Halder et al., 1998; Simmonds et al., 1998). However, because we observed that Vg expression is required in both cells forming a muscle–muscle adhesion, this would suggest additional coordination via cell–cell communication. The DER is ubiquitously expressed within the mesoderm (Zak et al., 1990). Notably, one DER ligand, Vein, is enriched at the segment borders where intermuscular junctions are formed (Yarnitzky et al., 1997), making it the prime candidate for coordination of muscle–muscle adhesions in this region. Therefore, we tested whether DER signaling is required for Vg-mediated establishment of intermuscular attachments. Mesodermal expression of dominant-negative DER (DN-egfr; Buff et al., 1998) did not affect the specification of VL muscles that were crossing the midline, although the specification of muscle lateral longitudinal (LL)1 and ventral oblique (VO)4–6 were affected, as described previously (Supplemental Figure S2, G and H; Buff et al., 1998). Increasing Vg expression in the SMs of slit2 mutants greatly enhanced the adhesion level between the midline-crossing VL muscles (Figure 7C), whereas reducing the activity of DER signaling decreased the size and/or number of adhesion sites caused by ectopic Vg (Figure 7, E and F). Mesodermal expression of DN-egfr alone in slit2 mutants greatly decreased the size and number of the ectopic intermuscular adhesion sites along the midline (Figure 7, D and F). To remove the possibility that expression of DN-egfr via Dmef2-GAL4 might affect the specification of muscle cells, ap-GAL4 was used to drive expression of Vg as well as DN-egfr in late-stage muscle cells (Supplemental Figure S4, A–A′). ap-GAL4 produced a weak expression of Vg in VA2 and LT muscles, which led to production of ectopic adhesions between theses muscles in ∼7% segments (Supplemental Figure S4, B–B′). However, expression of DN-egfr made these ectopic adhesions disappear (Supplemental Figure S4, C–C′).
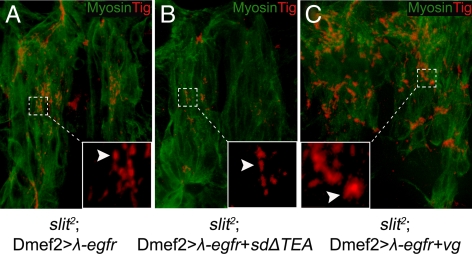
Mesodermal expression of constitutively active λ-egfr (Queenan et al., 1997) in slit2 mutants produced many small adhesion sites between midline-crossing VL muscles (Figure 8A). This ectopic attachment phenotype was shown to be Vg specific because interfering with Vg function by coexpression of SDΔTEA resulted in fewer of these adhesion sites (Figure 8B), whereas these adhesion sites become much bigger with increased Vg expression (Figure 8C).
Figure 8.
Changes in muscle–muscle adhesion caused by expression of a constitutively active DER (λ-egfr) are sensitive to the presence of Vg. (A) Many small adhesion sites were formed between the midline-crossing muscles in slit2 mutant embryos (arrowhead) when λ-egfr is expressed in developing muscle cells (Dmef2>λ-egfr) (arrowhead, inset). (B) Expression of λ-egfr in developing muscle cells where Vg function was inhibited by SDΔTEA produced fewer of these ectopic adhesions (arrowhead; inset). (C) Relatively more and larger ectopic adhesions were formed when embryonic muscle cells were overexpressing both λ-egfr and Vg (arrowhead, inset). Insets are close-ups of the area framed by the dotted lines.
Vg Induces Ectopic Adhesion between SM Cells That Make Contact with Each Other through Filopodial Extensions
Both loss of Vg and tig function produce a unique elongated body phenotype associated with defects in the embryonic musculature (Figure 3). One of the predicted roles of Tig is to induce formation of filopodia, and this may be required for muscle migration (Bunch et al., 1998). We noted that migrating SM cells seeking attachment targets extend filopodia at their leading edges. LTs muscle extend filopodia to the segment border and filopodia from migrating SM cells can be observed contacting each other between developing LT and ventral acute (VA) muscles (Figure 9, A1–A2). We did not observe filopodial contact between LT, VL, or VL and VA muscles. Correspondingly, ectopic adhesion sites were produced between LTs and VAs but not between LTs and VLs or between VLs and VAs when ectopically expressing Vg (Figure 9, B and D). Additional filopodia or integrin localization was not observed at the leading edge of muscles expressing elevated levels of Vg (Figure 9, C–C1). Thus, Vg induced formation of muscle-muscle adhesions requires close filopodial contact between migrating muscles.
Figure 9.
Filopodial contact between muscles forming junctions are not affected by changes in Vg expression. (A) In wild-type embryos (stage 14), the leading edge of LT muscles produce filopodia that contact with corresponding filopodia protruding from the lateral edge of VAs or other muscles (arrows in A and arrowheads in A1–A2). A1 and A2 are magnified photos of the framed areas in A. βPS integrin accumulates at the leading edge of the myotube (arrowhead). (B) At developmental stage 16, LTs normally find their attachment sites and form stable adhesion inside each segment. There are no connections between LTs and VAs (arrows in B). (C) Muscles in embryos that were expressing Vg ectopically (stage 14) produced similar number of filopodia compared with wild type (arrows in C and arrowheads in C1). Also, βPS integrin accumulated at the leading edge of muscles (arrowheads in C) in the same way as wild-type muscles. (D) At stage 16, LT muscle cells have formed stable attachments with muscle VAs (arrows in D and arrowheads in D1).
DISCUSSION
When two migrating somatic muscles come into close contact, there must be a cell-intrinsic mechanism to determine whether to build a stable adhesion junction. This would require coordinate regulation of this activity in each type of muscle to prevent inappropriate adhesions. We have shown that the transcriptional activator Vg is a key factor regulating this event in embryonic VL1–4 muscles. Although expression of Vg in muscle cells makes them competent to form intermuscular junctions, this process requires DER signaling to coordinate formation of attachments (Figures 7 and 8). Finally, this process is associated with contact between filopodia from each of the cells expressing Vg (Figures 5, 6, and 9).
We used three independent methods to test the requirement for Vg: null mutations, interfering with Vg function by using SDΔTEA, and ectopic Vg expression. When Vg function was blocked, adhesion between VL muscles was disrupted. This effect was enhanced in a rhea1 mutant background, whereas formation of intermuscular adhesions was reduced in slit2 mutants. Conversely, adhesion between VL muscles in slit2 mutants was enhanced when increasing vg expression in the muscles that normally express vg. It seems that formation of muscle–muscle attachments is directly related to the relative level of Vg in both cells as ectopic attachments failed to form if vg is expressed only in a single muscle (Figure 5, G and H, and Supplemental Figure S4, B–B′).
Blocking Vg function by using a vgnull mutation or overexpressing SDΔTEA produced similar phenotypes (Figure 2 and Supplemental Figure S1). The one paradoxical difference between these methods was that the surviving pupae and adult vgnull mutants were less elongated compared with those overexpressing SDΔTEA (Figure 2). The SDΔTEA transgene may block the functions of Vg-containing transcription factor complexes that do not normally include SD, which would explain the more severe phenotype. Our data support this conclusion, because vgnull; rhea1 double mutants have an identical phenotype to that caused by expression of SDΔTEA in rhea1 mutants (Supplemental Figure S1). We can confirm that Vg function is blocked specifically when SDΔTEA is expressed in SMs, because overexpressing SDΔTEA via Dmef2-GAL4 was able to significantly rescue the LT muscle rerouting phenotype caused by overexpression of Vg, whereas expression of a transgene that deleted only the Vg interaction domain produced a wild-type phenotype.
Ectopic expression of Vg in LT muscles redirects their migration to the segmental borders (Figure 5). This phenotype is similar to that caused by ectopic expression of Robo or Grip (Kramer et al., 2001; Swan et al., 2004). Slit-Robo signaling provides an important external cue to guide Robo-expressing muscles such as VL1–4 to the segment border (Kramer et al., 2001). The postsynaptic density 95/disc-large/zona occludens domain protein Grip also plays an important role in the migration of VL muscles (Swan et al., 2004). However, the aberrant muscle migration phenotype caused by ectopic Vg is independent of Slit-Robo signaling or Grip (Supplemental Figure S3). Rather, our results suggest that Vg induces cell competence to form attachments. Thus, in muscle expressing ectopic Vg, formation of extra attachments may induce abnormal migration.
The mechanical connections of muscle–muscle attachments are thought to be primarily mediated by integrin and its associated adhesion proteins (Brown et al., 2000). However, there must be a corresponding cellular regulation that determines whether it is appropriate for two muscles coming into contact form specific types of attachments. Examination of the proteins representative of the three major components of the integrin complex showed that they were all present at the termini of VL muscles in vgnull; rhea1 mutant embryos (Figure 4), suggesting that the integrin complex was established properly. Thus, the role of Vg is clearly not during initial establishment of the junctions. However, muscular junctions are relatively dynamic and may require cellular coordination to maintain their structure. The affinity of integrin to its ligands can change under different conditions and talin binding to the integrin β integrin cytoplasmic tail represents the final common step in integrin activation (Tadokoro et al., 2003). When integrin affinity to ECM is low due to the loss of interaction of talin with the β subunit cytoplasmic domains, the diffusible protein Tig does not colocalize with integrin at the end of detaching muscles (Tanentzapf and Brown, 2006), However, this phenotype is not what we observed in vgnull; rhea1 double mutants (Figure 4). Hyperactive PS2 integrins can be made when the cytoplasmic domain of αPS2 subunit is deleted, and mesodermal expression of this mutant integrin is able to produce ectopic intermuscular attachment (Martin-Bermudo et al., 1998). Even so, the phenotype produced by hyperactive PS2 integrins is far milder than what we observed when ectopic sites are induced between VA and LT muscles where Vg is over expressed. Therefore, our results suggest a role for Vg during the establishment of intermuscular attachment that is permissive rather than directly altering the affinity of integrin within the junctions.
We have shown previously that vg has a role in the specification of embryonic muscle VL2 together with Dmef2 (Deng et al., 2009). However, in vgnull mutant embryos, examination of molecular markers unique to the VL1 muscle (i.e., Kon) showed no apparent change in identity compared with wild type. It retained a VL1 identity and migrated to the correct location, making appropriate initial intermuscular attachments in most segments (Supplemental Figure S2, C and D). However, these same VL1 muscles detached from each other in vgnull; rhea1 double mutants. It would seem that vg is required to make a subset of muscles competent to establish intermuscular attachments. However, the cell-intrinsic role of Vg must be paired with a differential response to cell–cell communication. In cultured fibroblasts, epidermal growth factor receptor (EGFR) signaling was shown to play a role in the establishment of mature focal adhesions (Ridley and Hall, 1992). Knocking down EGFR signaling induces fast turnover of focal adhesions and produced small focal adhesions (FAs), suggesting EGFR is involved in focal adhesion stabilization (Winograd-Katz et al., 2009). FAs are integrin-mediated structures closely related to the myotendinous junctions formed by skeletal muscle cells (Turner et al., 1991). Although the establishment of muscle–muscle attachments is a complex process and the mechanism behind this process is not clear (Delon and Brown, 2007; Harburger and Calderwood, 2009). We observed that the relative level Vg activity directly affected the number and size of the intermuscular adhesion sites induced by ectopic λ-egfr (Figure 8). Thus, Vg might be responding to external signaling to induce as yet uncharacterized muscle-specific genes that regulate turnover of intermuscular attachment and its stabilization. Alternatively, Vg may induce expression of genes that are required for specific morphological changes in a subset of migrating muscles such as filopodia at the leading edge (Figure 9), which may be required for making initial contacts with neighboring cells to determine whether a muscle–muscle junction is to be formed.
The later stages of muscle migration and attachment are remarkably similar in both Drosophila and vertebrates (Schnorrer and Dickson, 2004). Our finding of a role for Vg in embryo SM development seems to make Drosophila Vg more analogous to the related Vestigial-like (Vgl) proteins in mammals (Maeda et al., 2002; Chen et al., 2004). Among them, Vestigial-like 2 (Vgl-2) is expressed in skeletal muscle and is able to augment myoD-induced myosin heavy chain expression in 10T1/2 cells (Maeda et al., 2002). In addition to the known roles in adult wing and flight muscle development, our results reveal a novel cell-autonomous role for Vg in somatic muscle development. Two muscle cells expressing Vg communicate via DER signaling to coordinate production of intermuscular attachments.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. J. Sigrist (Freie Universität, Berlin) for the dgripex36 line and Drs. Sarah Hughes, Gary Eitzen and Thomas Simmen and members of the Simmonds laboratory for critical reading of the manuscript. A.J.S. is an Alberta Heritage Foundation for Medical Research Senior Scholar. This work was supported by operating grants to the Simmonds laboratory from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada. J.B.B. was supported by a discovery grant from the National Sciences Engineering Research Council of Canada.
Abbreviations used:
- DER
Drosophila epidermal growth factor
- DA
dorsal acute
- LL
lateral longitudinal
- LO
lateral oblique
- LT
lateral transverse
- SBM
segment border muscle
- SM
somatic muscle
- VA
ventral acute
- VL
ventral longitudinal
- VO
ventral oblique
- VT
ventral transverse.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-04-0364) on August 4, 2010.
REFERENCES
- Bate M. The Development of Drosophila melanogaster, II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. The mesoderm and its derivatives; pp. 1013–1090. [Google Scholar]
- Baylies M. K., Bate M., Ruiz Gomez M. Myogenesis: a view from Drosophila. Cell. 1998;93:921–927. doi: 10.1016/s0092-8674(00)81198-8. [DOI] [PubMed] [Google Scholar]
- Becker S., Pasca G., Strumpf D., Min L., Volk T. Reciprocal signaling between Drosophila epidermal muscle attachment cells and their corresponding muscles. Development. 1997;124:2615–2622. doi: 10.1242/dev.124.13.2615. [DOI] [PubMed] [Google Scholar]
- Bernard F., Dutriaux A., Silber J., Lalouette A. Notch pathway repression by vestigial is required to promote indirect flight muscle differentiation in Drosophila melanogaster. Dev. Biol. 2006;295:164–177. doi: 10.1016/j.ydbio.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Bernard F., Lalouette A., Gullaud M., Jeantet A. Y., Cossard R., Zider A., Ferveur J. F., Silber J. Control of apterous by vestigial drives indirect flight muscle development in Drosophila. Dev. Biol. 2003;260:391–403. doi: 10.1016/s0012-1606(03)00255-0. [DOI] [PubMed] [Google Scholar]
- Black B. L., Olson E. N. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brown N. H. Integrins hold Drosophila together. Bioessays. 1993;15:383–390. doi: 10.1002/bies.950150604. [DOI] [PubMed] [Google Scholar]
- Brown N. H., Gregory S. L., Martin-Bermudo M. D. Integrins as mediators of morphogenesis in Drosophila. Dev. Biol. 2000;223:1–16. doi: 10.1006/dbio.2000.9711. [DOI] [PubMed] [Google Scholar]
- Brown N. H., Gregory S. L., Rickoll W. L., Fessler L. I., Prout M., White R. A., Fristrom J. W. Talin is essential for integrin function in Drosophila. Dev. Cell. 2002;3:569–579. doi: 10.1016/s1534-5807(02)00290-3. [DOI] [PubMed] [Google Scholar]
- Buff E., Carmena A., Gisselbrecht S., Jimenez F., Michelson A. M. Signalling by the Drosophila epidermal growth factor receptor is required for the specification and diversification of embryonic muscle progenitors. Development. 1998;125:2075–2086. doi: 10.1242/dev.125.11.2075. [DOI] [PubMed] [Google Scholar]
- Bunch T. A., Graner M. W., Fessler L. I., Fessler J. H., Schneider K. D., Kerschen A., Choy L. P., Burgess B. W., Brower D. L. The PS2 integrin ligand tiggrin is required for proper muscle function in Drosophila. Development. 1998;125:1679–1689. doi: 10.1242/dev.125.9.1679. [DOI] [PubMed] [Google Scholar]
- Campbell S. D., Duttaroy A., Katzen A. L., Chovnick A. Cloning and characterization of the scalloped region of Drosophila melanogaster. Genetics. 1991;127:367–380. doi: 10.1093/genetics/127.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. H., Mullett S. J., Stewart A. F. Vgl-4, a novel member of the vestigial-like family of transcription cofactors, regulates alpha1-adrenergic activation of gene expression in cardiac myocytes. The Journal of biological chemistry. 2004;279:30800–30806. doi: 10.1074/jbc.M400154200. [DOI] [PubMed] [Google Scholar]
- Clark K. A., McGrail M., Beckerle M. C. Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development. 2003;130:2611–2621. doi: 10.1242/dev.00492. [DOI] [PubMed] [Google Scholar]
- Cluzel C., Saltel F., Lussi J., Paulhe F., Imhof B. A., Wehrle-Haller B. The mechanisms and dynamics of (alpha)v(beta)3 integrin clustering in living cells. J. Cell Biol. 2005;171:383–392. doi: 10.1083/jcb.200503017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delon I., Brown N. H. Integrins and the actin cytoskeleton. Curr. Opin. Cell Biol. 2007;19:43–50. doi: 10.1016/j.ceb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Deng H., Hughes S. C., Bell J. B., Simmonds A. J. Alternative requirements for Vestigial, Scalloped, and Dmef2 during muscle differentiation in Drosophila melanogaster. Mol. Biol. Cell. 2009;20:256–269. doi: 10.1091/mbc.E08-03-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada B., Gisselbrecht S. S., Michelson A. M. The transmembrane protein Perdido interacts with Grip and integrins to mediate myotube projection and attachment in the Drosophila embryo. Development. 2007;134:4469–4478. doi: 10.1242/dev.014027. [DOI] [PubMed] [Google Scholar]
- Fogerty F. J., Fessler L. I., Bunch T. A., Yaron Y., Parker C. G., Nelson R. E., Brower D. L., Gullberg D., Fessler J. H. Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for Drosophila alpha PS2 beta PS integrins. Development. 1994;120:1747–1758. doi: 10.1242/dev.120.7.1747. [DOI] [PubMed] [Google Scholar]
- Frommer G., Vorbruggen G., Pasca G., Jackle H., Volk T. Epidermal egr-like zinc finger protein of Drosophila participates in myotube guidance. EMBO J. 1996;15:1642–1649. [PMC free article] [PubMed] [Google Scholar]
- Garg A., Srivastava A., Davis M. M., O'Keefe S. L., Chow L., Bell J. B. Antagonizing scalloped with a novel vestigial construct reveals an important role for scalloped in Drosophila melanogaster leg, eye and optic lobe development. Genetics. 2007;175:659–669. doi: 10.1534/genetics.106.063966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig S., Akam M. Homeotic genes autonomously specify one aspect of pattern in the Drosophila mesoderm. Nature. 1993;362:630–632. doi: 10.1038/362630a0. [DOI] [PubMed] [Google Scholar]
- Halder G., Polaczyk P., Kraus M. E., Hudson A., Kim J., Laughon A., Carroll S. The Vestigial and Scalloped proteins act together to directly regulate wing-specific gene expression in Drosophila. Genes Dev. 1998;12:3900–3909. doi: 10.1101/gad.12.24.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger D. S., Calderwood D. A. Integrin signalling at a glance. J. Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. C., Krause H. M. Single and double FISH protocols for Drosophila. Methods Mol. Biol. 1999;122:93–101. doi: 10.1385/1-59259-722-x:93. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Kidd T., Bland K. S., Goodman C. S. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Kramer S. G., Kidd T., Simpson J. H., Goodman C. S. Switching repulsion to attraction: changing responses to slit during transition in mesoderm migration. Science. 2001;292:737–740. doi: 10.1126/science.1058766. [DOI] [PubMed] [Google Scholar]
- Lundstrom A., Gallio M., Englund C., Steneberg P., Hemphala J., Aspenstrom P., Keleman K., Falileeva L., Dickson B. J., Samakovlis C. Vilse, a conserved Rac/Cdc42 GAP mediating Robo repulsion in tracheal cells and axons. Genes Dev. 2004;18:2161–2171. doi: 10.1101/gad.310204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay J. O., Soanes K. H., Srivastava A., Simmonds A., Brook W. J., Bell J. B. An in vivo analysis of the vestigial gene in Drosophila melanogaster defines the domains required for Vg function. Genetics. 2003;163:1365–1373. doi: 10.1093/genetics/163.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Chapman D. L., Stewart A. F. Mammalian vestigial-like 2, a cofactor of TEF-1 and MEF2 transcription factors that promotes skeletal muscle differentiation. J. Biol. Chem. 2002;277:48889–48898. doi: 10.1074/jbc.M206858200. [DOI] [PubMed] [Google Scholar]
- Martin-Bermudo M. D., Brown N. H. The localized assembly of extracellular matrix integrin ligands requires cell-cell contact. J. Cell Sci. 2000;113:3715–3723. doi: 10.1242/jcs.113.21.3715. [DOI] [PubMed] [Google Scholar]
- Martin-Bermudo M. D., Dunin-Borkowski O. M., Brown N. H. Specificity of PS integrin function during embryogenesis resides in the alpha subunit extracellular domain. EMBO J. 1997;16:4184–4193. doi: 10.1093/emboj/16.14.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Bermudo M. D., Dunin-Borkowski O. M., Brown N. H. Modulation of integrin activity is vital for morphogenesis. J. Cell Biol. 1998;141:1073–1081. doi: 10.1083/jcb.141.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel-Rosen H., Dorevitch N., Reuveny A., Volk T. The balance between two isoforms of the Drosophila RNA-binding protein how controls tendon cell differentiation. Mol. Cell. 1999;4:573–584. doi: 10.1016/s1097-2765(00)80208-7. [DOI] [PubMed] [Google Scholar]
- Prokop A., Martin-Bermudo M. D., Bate M., Brown N. H. Absence of PS integrins or laminin A affects extracellular adhesion, but not intracellular assembly, of hemiadherens and neuromuscular junctions in Drosophila embryos. Dev. Biol. 1998;196:58–76. doi: 10.1006/dbio.1997.8830. [DOI] [PubMed] [Google Scholar]
- Prout M., Damania Z., Soong J., Fristrom D., Fristrom J. W. Autosomal mutations affecting adhesion between wing surfaces in Drosophila melanogaster. Genetics. 1997;146:275–285. doi: 10.1093/genetics/146.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenan A. M., Ghabrial A., Schupbach T. Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development. 1997;124:3871–3880. doi: 10.1242/dev.124.19.3871. [DOI] [PubMed] [Google Scholar]
- Ranganayakulu G., Elliott D. A., Harvey R. P., Olson E. N. Divergent roles for NK-2 class homeobox genes in cardiogenesis in flies and mice. Development. 1998;125:3037–3048. doi: 10.1242/dev.125.16.3037. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Roy S., Shashidhara L. S., VijayRaghavan K. Muscles in the Drosophila second thoracic segment are patterned independently of autonomous homeotic gene function. Curr. Biol. 1997;7:222–227. doi: 10.1016/s0960-9822(06)00117-5. [DOI] [PubMed] [Google Scholar]
- Rushton E., Drysdale R., Abmayr S. M., Michelson A. M., Bate M. Mutations in a novel gene, myoblast city, provide evidence in support of the founder cell hypothesis for Drosophila muscle development. Development. 1995;121:1979–1988. doi: 10.1242/dev.121.7.1979. [DOI] [PubMed] [Google Scholar]
- Schnorrer F., Dickson B. J. Muscle building; mechanisms of myotube guidance and attachment site selection. Dev. Cell. 2004;7:9–20. doi: 10.1016/j.devcel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Schnorrer F., Kalchhauser I., Dickson B. J. The transmembrane protein Kon-tiki couples to Dgrip to mediate myotube targeting in Drosophila. Dev. Cell. 2007;12:751–766. doi: 10.1016/j.devcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Simmonds A. J., Liu X., Soanes K. H., Krause H. M., Irvine K. D., Bell J. B. Molecular interactions between Vestigial and Scalloped promote wing formation in Drosophila. Genes Dev. 1998;12:3815–3820. doi: 10.1101/gad.12.24.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Simmonds A. J., Garg A., Fossheim L., Campbell S. D., Bell J. B. Molecular and functional analysis of scalloped recessive lethal alleles in Drosophila melanogaster. Genetics. 2004;166:1833–1843. doi: 10.1534/genetics.166.4.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Wayburn B., Bunch T., Volk T. Thrombospondin-mediated adhesion is essential for the formation of the myotendinous junction in Drosophila. Development. 2007;134:1269–1278. doi: 10.1242/dev.000406. [DOI] [PubMed] [Google Scholar]
- Sudarsan V., Anant S., Guptan P., VijayRaghavan K., Skaer H. Myoblast diversification and ectodermal signaling in Drosophila. Dev. Cell. 2001;1:829–839. doi: 10.1016/s1534-5807(01)00089-2. [DOI] [PubMed] [Google Scholar]
- Swan L. E., Wichmann C., Prange U., Schmid A., Schmidt M., Schwarz T., Ponimaskin E., Madeo F., Vorbruggen G., Sigrist S. J. A glutamate receptor-interacting protein homolog organizes muscle guidance in Drosophila. Genes Dev. 2004;18:223–237. doi: 10.1101/gad.287604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro S., Shattil S. J., Eto K., Tai V., Liddington R. C., de Pereda J. M., Ginsberg M. H., Calderwood D. A. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- Tanentzapf G., Brown N. H. An interaction between integrin and the talin FERM domain mediates integrin activation but not linkage to the cytoskeleton. Nat. Cell Biol. 2006;8:601–606. doi: 10.1038/ncb1411. [DOI] [PubMed] [Google Scholar]
- Tanentzapf G., Martin-Bermudo M. D., Hicks M. S., Brown N. H. Multiple factors contribute to integrin-talin interactions in vivo. J. Cell Sci. 2006;119:1632–1644. doi: 10.1242/jcs.02859. [DOI] [PubMed] [Google Scholar]
- Turner C. E., Kramarcy N., Sealock R., Burridge K. Localization of paxillin, a focal adhesion protein, to smooth muscle dense plaques, and the myotendinous and neuromuscular junctions of skeletal muscle. Exp. Cell Res. 1991;192:651–655. doi: 10.1016/0014-4827(91)90090-h. [DOI] [PubMed] [Google Scholar]
- Volk T., VijayRaghavan K. A central role for epidermal segment border cells in the induction of muscle patterning in the Drosophila embryo. Development. 1994;120:59–70. doi: 10.1242/dev.120.1.59. [DOI] [PubMed] [Google Scholar]
- Vorbruggen G., Jackle H. Epidermal muscle attachment site-specific target gene expression and interference with myotube guidance in response to ectopic stripe expression in the developing Drosophila epidermis. Proc. Natl. Acad. Sci. USA. 1997;94:8606–8611. doi: 10.1073/pnas.94.16.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A., Bell J. B., Carroll S. B. Control of Drosophila wing and haltere development by the nuclear vestigial gene product. Genes Dev. 1991;5:2481–2495. doi: 10.1101/gad.5.12b.2481. [DOI] [PubMed] [Google Scholar]
- Winograd-Katz S. E., Itzkovitz S., Kam Z., Geiger B. Multiparametric analysis of focal adhesion formation by RNAi-mediated gene knockdown. J. Cell Biol. 2009;186:423–436. doi: 10.1083/jcb.200901105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnitzky T., Min L., Volk T. The Drosophila neuregulin homolog Vein mediates inductive interactions between myotubes and their epidermal attachment cells. Genes Dev. 1997;11:2691–2700. doi: 10.1101/gad.11.20.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak N. B., Wides R. J., Schejter E. D., Raz E., Shilo B. Z. Localization of the DER/flb protein in embryos: implications on the faint little ball lethal phenotype. Development. 1990;109:865–874. doi: 10.1242/dev.109.4.865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.