The regulation of the V-ATPase, the proton pump mediating intraorganellar acidification, is still elusive. We find that excess of the neuroendocrine V-ATPase accessory subunit Ac45 reduces the intragranular pH and consequently disturbs prohormone convertase activation and prohormone processing. Thus, Ac45 represents the first V-ATPase regulator.
Abstract
The vacuolar (H+)-ATPase (V-ATPase) is an important proton pump, and multiple critical cell-biological processes depend on the proton gradient provided by the pump. Yet, the mechanism underlying the control of the V-ATPase is still elusive but has been hypothesized to involve an accessory subunit of the pump. Here we studied as a candidate V-ATPase regulator the neuroendocrine V-ATPase accessory subunit Ac45. We transgenically manipulated the expression levels of the Ac45 protein specifically in Xenopus intermediate pituitary melanotrope cells and analyzed in detail the functioning of the transgenic cells. We found in the transgenic melanotrope cells the following: i) significantly increased granular acidification; ii) reduced sensitivity for a V-ATPase-specific inhibitor; iii) enhanced early processing of proopiomelanocortin (POMC) by prohormone convertase PC1; iv) reduced, neutral pH–dependent cleavage of the PC2 chaperone 7B2; v) reduced 7B2-proPC2 dissociation and consequently reduced proPC2 maturation; vi) decreased levels of mature PC2 and consequently reduced late POMC processing. Together, our results show that the V-ATPase accessory subunit Ac45 represents the first regulator of the proton pump and controls V-ATPase-mediated granular acidification that is necessary for efficient prohormone processing.
INTRODUCTION
The vacuolar (H+)-ATPase (V-ATPase) is a proton pump that is crucial for a wide variety of biological processes, such as bone resorption by osteoclasts (Xu et al., 2007), maintenance of the acid-base balance by the renal alpha intercalated cells (Brown and Breton, 2000; Wagner et al., 2004), embryonic left-right patterning (Adams et al., 2006), and Wnt signaling during anterior-posterior patterning (Cruciat et al., 2010). Moreover, intracellular events such as membrane trafficking, receptor-mediated endocytosis, lysosomal hydrolysis, neurotransmitter uptake and release, and prohormone processing also highly depend on a low intraorganellar pH provided by the V-ATPase (Schoonderwoert and Martens, 2001; Nishi and Forgac, 2002; Paroutis et al., 2004).
Prohormone processing to peptide hormones occurs in the regulated secretory pathway via endoproteolytic cleavage at pairs of basic amino acid residues by the prohormone convertase 1/3 (hereafter referred to as PC1) and prohormone convertase 2 (PC2) (reviewed by Hook et al., 2008). The maturation of the proform of PC1 takes place in the ER (Zhou and Mains, 1994), whereas proPC2 maturation occurs later in the secretory pathway, namely in the more acidic trans-Golgi network (TGN) and secretory granules (Zhou and Mains, 1994; Muller et al., 1997). Furthermore, the regulation of proPC2 transport and maturation by its chaperone 7B2 (Barbero and Kitabgi, 1999; Mbikay et al., 2001) as well as the enzymatic activities of PC1 and PC2 are highly dependent on the local intraorganellar pH (Anderson and Orci, 1988; Tanaka et al., 1997; reviewed by Schoonderwoert and Martens, 2001). Thus, correct prohormone processing is critically relying on the supply of the proper pH to the various subcompartments of the regulated secretory pathway and therefore on the regulation of the V-ATPase.
At present, surprisingly little is known regarding the mechanism controlling the proton pump. A general mechanism of V-ATPase regulation may be represented by the coupling and uncoupling of its two main sectors (Beyenbach and Wieczorek, 2006), namely the cytoplasmic V1-sector responsible for energy delivery by ATP hydrolysis and the membrane-bound V0-sector that harbors the rotary mechanism to translocate protons across a membrane (reviewed by Jefferies et al., 2008). In the secretory pathway, the regulation and targeting of the pump has been hypothesized to be accomplished by an accessory subunit of the V-ATPase (Supek et al., 1994; Schoonderwoert and Martens, 2001; Jansen et al., 2008). The neuroendocrine-enriched type I transmembrane glycoprotein Ac45 is such a V-ATPase accessory subunit (Supek et al., 1994; Getlawi et al., 1996; Ludwig et al., 1998; reviewed by Xu et al., 2007). Interestingly, in Xenopus laevis intermediate pituitary melanotrope cells, Ac45 has been found to be coordinately expressed with the prohormone proopiomelanocortin (POMC) (Holthuis et al., 1995), suggesting an important role for this V-ATPase accessory subunit in the process of prohormone processing. Because this cell type can be activated in vivo to produce large amounts of POMC simply by placing the animal on a black background (Jenks et al., 1993; Holthuis et al., 1995), the neuroendocrine Xenopus melanotrope cells represent an interesting model to study secretory pathway processes.
To explore the regulation of the V-ATPase and the function of its accessory subunit Ac45, we combined the unique characteristics of the Xenopus melanotrope cell model with the genetic manipulation of melanotrope Ac45 expression. Because down-regulating gene expression is not possible in Xenopus (Dirks et al., 2003), we decided to apply melanotrope-specific Ac45 transgene overexpression. For the specific targeting of Ac45 transgene expression to the Xenopus melanotrope cells, we used a Xenopus POMC-gene promoter fragment (Jansen et al., 2002) and explored the effect of excess Ac45 on intragranular acidification and the V-ATPase–dependent process of POMC processing. We conclude that Ac45 is responsible for the targeting and regulation of the V-ATPase, thereby modulating prohormone processing in the regulated secretory pathway.
MATERIALS AND METHODS
Animals
Xenopus laevis were reared in the Xenopus facility of the Department of Molecular Animal Physiology (Central Animal Facility, Radboud University Nijmegen). For transgenesis experiments, adult female Xenopus laevis were directly obtained from South Africa (Africa Reptile Park, Muizenberg, South Africa). Experimental animals were adapted to a black background for at least three weeks with a light/dark cycle of 12 h. All animal experiments were carried out in accordance with the European Communities Council Directive 86/609/EEC for animal welfare and permits GGO 01-285 and RBD0166(H10) to generate and house transgenic Xenopus laevis.
Generation of Xenopus laevis Stably Transgenic for Ac45 Fused to GFP
In vivo, the 62-kDa Xenopus intact-Ac45 protein is proteolytically processed to ∼40-kDa cleaved-Ac45 (Holthuis et al., 1999; Schoonderwoert et al., 2002) that corresponds to the ∼45-kDa Ac45 protein originally isolated from secretory granules (Supek et al., 1994). We have previously shown that transgenically expressed intact-Ac45 accumulates in the endoplasmic reticulum (ER) and does not affect the regulated secretory pathway (Jansen et al., 2008). In the present study, we generated two independent transgenic Xenopus lines, #533 and #604, expressing an excess of cleaved-Ac45 under the control of a POMC gene promoter fragment (Jansen et al., 2002), using methods previously described (Jansen et al., 2008). In the melanotrope cells of the two transgenic lines, similar plasma membrane localizations of the green fluorescent protein (GFP)/cleaved-Ac45 transgene products were observed. Given its higher melanotrope Ac45 transgene expression level, line #533 was used for our detailed analyses.
Antibodies
Anti-Xenopus POMC (ST62, only recognizing the proform of POMC) (Berghs et al., 1997) was kindly provided by Dr. S. Tanaka (Shizuoka University, Japan), anti-rat PC1 2B6 (Vindrola and Lindberg, 1992) and anti-rat 7B2 LSU13 (Lee and Lindberg, 2008) by Dr. I. Lindberg (University of Maryland, Baltimore, MD), anti-mouse PC2 (Benjannet et al., 1992) by Dr. N. Seidah (IRCM, Canada), anti-Xenopus calnexin (Beggah and Geering, 1997) by Dr. K. Geering, University of Lausanne, Switzerland). The anti–α-MSH polyclonal antibody was described previously (van Zoest et al., 1989).
Cryosectioning and Immunohistochemistry
Brain-pituitary preparations were dissected from juvenile transgenic frogs and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4). After cryo protection in 10% sucrose-PBS, sagittal 20-μm cryo sections were mounted on poly-L-lysine–coated slides and dried for 2 h at 45°C. For immunohistochemistry, sections were rinsed for 30 min in 50-μM Tris-buffered saline (pH 7.6) containing 150 μM NaCl and 0.1% Triton X100 (TBS-TX). To prevent nonspecific binding, blocking was performed with 0.5% BSA in TBS-TX. Sections were incubated with anti-POMC (ST62, 1: 2000) antibodies for 16 h at 37°C in TBS-TX containing 0.5% BSA. After rinsing the slides with TBS-TX, a second antibody, Goat-anti-Rabbit-Alexa Fluor 568 (Molecular Probes, Eugene, Oregon, USA) at a dilution of 1:100, was applied and sections were incubated for 1 h at 37°C. After an additional washing step, the sections were mounted in Mowiol (Calbiochem, La Jolla, CA) containing 2.5% sodium azide and coverslipped. Immunofluorescence was viewed under a Leica DMRA fluorescence microscope.
Measurement of Intragranular Acidification
To study granular acidification, the 3-(2,4-dinitroanilo)-3′amino-N-methyldipropylamine (DAMP) method of Orci (Orci et al., 1986) was applied with slight modifications. After dissection, neurointermediate lobes (NILs) of wild-type and transgenic Xenopus were preincubated for 60 min in Ringer's/BSA. To test specificity of the procedure, control NILs were preincubated in Ringer's/BSA containing 1 μM bafilomycin A1 (Sigma-Aldrich, St. Louis, MO) and transferred to Ringer's/BSA containing 60 μM DAMP (Molecular Probes, Eugene, OR), incubated for 2 h at 22°C and fixed in Karnovsky's Fixative (2% paraformaldehyde, 2% glutaraldehyde in phosphate buffer pH 7.4). The tissue was rapidly frozen and immersed in acetone containing 0.5% uranyl acetate as fixing agent at –90°C. The temperature was raised stepwise to –45°C and the tissue was then infiltrated with Lowicryl HM20 (Aurion, Wageningen, The Netherlands). Thin sections were cut and mounted on one-hole nickel grids coated with a formvar film. For postembedding immunohistochemistry, ultrathin Lowicryl sections were washed for 10 min in PBS containing 50 mM glycine and for 10 min in PBS containing 0.5% BSA and 0.1% cold fish skin gelatin (PBG). For immunolabeling, sections were incubated overnight at 4°C in drops of PBG containing anti-dinitrophenol (DNP) antibodies (1:100, Invitrogen Carlsbad USA). Sections were washed for 20 min in PBG, incubated with protein A–labeled 10-nm gold markers, washed in PBS, and postfixed with 2.5% glutaraldehyde in PB for 5 min to minimize loss of gold label during the contrasting steps. After washing with distilled water, sections were contrasted in uranyl acetate and studied using a Jeol transmission electron microscopy (TEM) 1010 electron microscope. For quantification, gold particles in dense-core granules were counted and the surfaces of the granules were measured using the ImageJ free software package.
Metabolic Cell Labeling and Immunoprecipitations
For radioactive labeling of newly synthesized proteins, freshly isolated Xenopus NILs were preincubated for 10 min in Ringer's medium (112 mM NaCl, 2 mM KCl, 2 mM CaCl2, 15 mM HEPES pH 7.4, 2 mg/ml glucose 0.3 mg/ml BSA) containing 0.3 mg/ml BSA (Ringer's/BSA), then incubated in Ringer's/BSA containing 1.7 mCi/ml Tran35S label (MP Biomedicals) for indicated time periods and subsequently chased in 50 μl Ringer's/BSA supplemented with 0.5 mM L-methionine as described previously (Bouw et al., 2004; Strating et al., 2007; van Rosmalen and Martens, 2007; Strating and Martens, 2009). Chase incubations were in the absence or presence 0.25 μM and 0.5 μM bafilomycin A1 (Sigma). Lobes were lysed in 100 μl lysis buffer (50 mM HEPES pH 7.4, 140 mM NaCl, 0.1% Triton-X100, 1% Tween-20, supplemented with Complete protease inhibitor mix (Roche Diagnostics, Mannheim, Germany), and lysates and media were cleared by centrifugation (13,000g, 7 min) and directly analyzed by SDS-PAGE. The gel migration positions of 37-kDa POMC, 18-kDa POMC, the various PC2 forms, and the GFP-Ac45 protein corresponded to those previously observed (Braks and Martens, 1994; Holthuis et al., 1995; Jansen et al., 2008). POMC represents more than 80% of all newly synthesized melanotrope proteins (Holthuis et al., 1995) allowing its direct analysis (i.e., no need for immunoprecipitation). The amounts of newly synthesized 37-kDa POMC and 18-kDa POMC were quantified by a Phosphoimager (Bio-Rad), and the degree of POMC processing was determined by calculating the ratio of 18-kDa to 37-kDa POMC (n = 3), taking into account the number of methionine and cysteine residues in the proteins. The amount of secreted 18-kDa POMC was calculated relative to newly synthesized actin (n = 3) (van Rosmalen and Martens, 2007).
To keep PC2 in the proform during the native immunoprecipitation procedure, the pH of the lysis buffer was raised to pH 8.2. For direct 7B2 immunoprecipitation, the pH of the buffer was pH 7.4. Immune complexes were precipitated with protein-A Sepharose CL-4B (Amersham Biosciences, Uppsala, Sweden), analyzed by SDS-PAGE and visualized by fluorography. The immunoprecipitated pro- and mature forms of PC2 (n = 4), and pro- and mature forms of 7B2 (n = 4) were quantified by densitometric scanning of the autoradiographs and ratio calculations were performed taking into account the number of methionine and cysteine residues in the proteins.
Western Blot Analysis
Freshly dissected NILs were homogenized in 100 μl lysis buffer and 20% of the lysates was denatured, separated on SDS-PAGE and transferred to nitrocellulose or PVDF membrane. Following blocking, blots were incubated with anti-POMC (ST-62, 1:10.000), anti-PC2 (1:3000), anti-PC1 (1:1000), or anti-calnexin (1:10.000) rabbit antisera and with secondary peroxidase-conjugated Goat-anti-rabbit antibody followed by chemoluminescence. Signals were detected and quantified using a BioImaging system with Labworks 4.0 software (UVP BioImaging systems, Cambridge, UK). Calnexin was used as a reference because expression levels of this protein were unaffected by our transgenic manipulations (van Rosmalen and Martens, 2007 and our unpublished observations).
Superfusion and α-MSH Radioimmunoassay
NILs were isolated from wild-type and Ac45-transgenic Xenopus and transferred to Ringer's solution in superfusion chambers. The NILs were then superfused in Ringer's supplemented with 1 μg/ml ascorbic acid at a rate of 30 μl/min. 7.5-min fractions were collected and stored on ice. Fifty microliters of each fraction was used in an α-MSH RIA (van Zoest et al., 1989). In short, I125 labeled α-MSH (10,000 cpm/100 μl) was diluted in 90 ml VAT-buffer (0.02 M Veronal, 0.2 g/l sodium azide, 0.3% BSA, 100 IU/ml trasylol, pH 8.6) and rabbit α-MSH antiserum L9 (1:6000) (van Zoest et al., 1989) in 180 ml VAT buffer and pooled (RIAmix). Fifty microliters of each superfusion fraction was incubated with 450 μl RIAmix for 48 h at 4°C. An α-MSH standard dilution series (100 pg/μl to 0 pg/μl) was included. Reactions were stopped by adding 1 ml precipitation mix (30% polyethylene glycol, 4.8% chicken egg albumin), mixed, and pelleted (20 min, 4000 rpm, Jouan CR 4.11 with an E4 swingout rotor, 4°C). Radioactivity was measured using a 2 Clinigamma counter (LKB Wallac). Two independent experiments, n = 4 wt NILs, n = 4, Ac45-transgenic NILs, per experiment were performed. After the superfusion experiment, NILs were recovered, homogenized in lysis buffer, and the lysates were subjected to Western blot analysis with anti-calnexin antibodies to measure the relative amounts of melanotrope cells.
Statistics
Data are presented as means ± SEM. Statistical evaluation was performed using an unpaired Student's t test.
RESULTS
Generation of Transgenic Xenopus laevis Expressing Excess Ac45 Specifically in the Intermediate Pituitary Melanotrope Cells
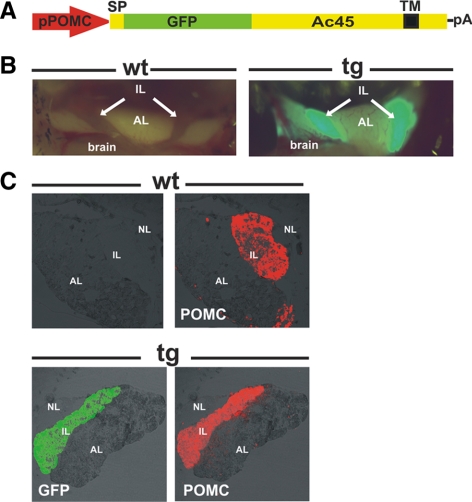
We performed stable Xenopus transgenesis using a 529-bp fragment of the POMC gene promoter (Figure 1A) to drive transgenic Ac45 expression specifically to the neuroendocrine melanotrope cells. The length of the POMC promoter fragment was such that transgene expression was observed only in the melanotrope cells and not in other POMC-expressing cell types such as the corticotrope cells in the anterior pituitary or hypothalamic neurons (Jansen et al., 2002). Independent transgenic Xenopus lines were established, expressing Ac45 fused to the GFP. Direct fluorescence microscopy on pituitaries of adult Ac45-transgenic animals clearly showed that the transgene expression was exclusively in the intermediate pituitary melanotrope cells (Figure 1B) and the specificity was confirmed by the colocalization of the transgene product with the main melanotrope secretory cargo protein POMC (Figure 1C).
Figure 1.
Generation of transgenic Xenopus laevis with expression of GFP-Ac45 specifically in the intermediate pituitary melanotrope cells. (A) Schematic representation of the linear transgene construct used for Xenopus transgenesis (pPOMC(A)2+-SP-GFP-Ac45) with the Xenopus POMC-A gene promoter fragment (pPOMC), Ac45 signal peptide (SP), the region encoding the GFP-Ac45 fusion protein, and the SV40 polyadenylation signal (pA). TM: transmembrane region. (B) Ventral view on the pituitary of a wild-type (wt) and GFP-Ac45 (#533) transgenic (tg) juvenile frogs. Fluorescence was detected in the intermediate lobe (IL) but not in the anterior lobe (AL) and the brain. (C) Sagittal cryosections of brain and pituitary from wild-type (wt) and Ac45-transgenic (tg) Xenopus. Transgene expression was detected by direct fluorescence and endogenous POMC expression by immunostaining with an anti-POMC antibody. NL, neural lobe.
The Ac45-transgene product was targeted to secretory granules and efficiently transported through the secretory pathway to the plasma membrane where it was colocalized with the endogenous V-ATPase (Jansen et al., 2008). Furthermore, the Ac45-transgenic cells were characterized by a higher abundance of immature secretory granules and harbored an increased Ca2+-dependent secretory efficiency (Jansen et al., 2008). To examine the effect of excess Ac45 on granular acidification and proprotein processing in the regulated secretory pathway, we analyzed melanotrope cells that expressed an ∼10 times excess of the Ac45 protein (line #533, Jansen et al., 2008).
Excess Ac45 Increases Granular Acidification
Because Ac45 is an accessory subunit of the V-ATPase and in view of the role of the V-ATPase in proton pumping (Schmidt and Moore, 1995; Schoonderwoert et al., 2000), we wondered about the effect of the excess of Ac45 on intragranular acidification.
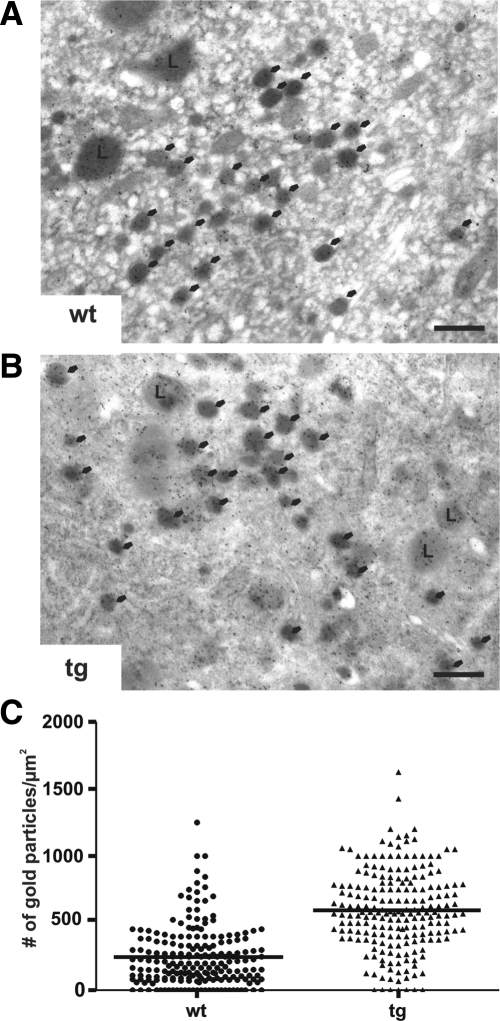
Therefore, we incubated wild-type and Ac45-transgenic NILs with the acidotrophic reagent DAMP and analyzed the granular accumulation of DAMP via immunogold electron microscopy with anti-DNP antibodies (Figure 2A). We found in the dense-core secretory granules of the Ac45-transgenic cells a clear increase in the number of gold particles compared with the number of gold particles in the wild-type granules (tg: 595 ± 21 particles/μm2, [n = 206 granules] versus wt: 248 ± 15 particles/μm2 [n = 205] p <0.001) (Figure 2B). No gold particles were found in granules of wild-type cells preincubated with a specific inhibitor of the proton pump (bafilomycin A1; data not shown), confirming that DAMP accumulation depended on a low intragranular pH. Based on the formula of Orci (Orci et al., 1986) the estimated average pH in the granules of wild-type cells was 5.2 and in granules of the Ac45-transgenic cells 4.8. No morphological differences were found between the granules of wild-type and Ac45-transgenic melanotrope cells. These results show that the increased Ac45 expression level in the transgenic melanotrope cells resulted in a decreased intragranular pH.
Figure 2.
Excess Ac45 causes enhanced intragranular acidification in transgenic melanotrope cells. (A) Immunogold labeling of ultrathin sections of DAMP-incubated wild-type (wt) or Ac45-transgenic (tg) Xenopus melanotrope cells with the anti-DNP antibody in combination with 10-nm protein-A gold. Gold labeling was predominantly found in the secretory granules (arrows) and lysosomes (L). Bars equal 200 nm. (B) Quantification of intragranular DAMP accumulation. The number of gold particles per μm2 found in 205 wt and 206 tg dense-core secretory granules of 20 randomly selected wt or tg melanotrope cells from three wt or tg frogs.
Excess Ac45 Affects the Sensitivity of the Melanotrope Cells for the V-ATPase–Specific Inhibitor Bafilomycin A1
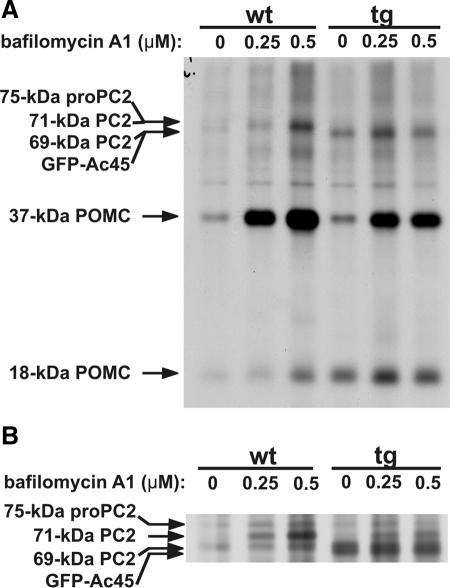
We then examined the V-ATPase system by establishing the effect of the V-ATPase-specific inhibitor bafilomycin A1 on processing of newly synthesized proproteins in the transgenic Xenopus melanotrope cells. In the early secretory pathway of Xenopus melanotrope cells, 37-kDa POMC is processed by PC1 into 18-kDa POMC, representing the N-terminal portion of the POMC molecule and containing the only N-glycosylation site in the POMC molecule (Martens et al., 1982; Ayoubi et al., 1990). We decided to use bafilomycin A1 because the action of this inhibitor toward the V-ATPase has been well characterized (Bowman and Bowman, 2002; Huss et al., 2002; Bowman et al., 2006). Interfering with neuroendocrine V-ATPase activity through its inhibition by bafilomycin A1 greatly reduced prohormone trafficking and processing and the secretion of peptide hormones via the regulated secretory pathway (Tanaka et al., 1997; Schoonderwoert et al., 2000). The 37-kDa POMC protein and the ∼75-kDa proform of PC2 clearly represent the majority (∼90%) of the newly synthesized protein pool in the Xenopus melanotrope cells (Holthuis et al., 1995) and their subsequent processing products have been previously characterized (Braks and Martens, 1994; Holthuis et al., 1995). We therefore focused on the processing of these two proteins.
In wild-type Xenopus melanotrope cells, bafilomycin A1 inhibited the proteolytic processing of newly synthesized 37-kDa POMC and of ∼75-kDa proPC2, resulting in an accumulation of 37-kDa POMC and the 71-kDa intermediate processing form of PC2 (Figure 3, A and B and Schoonderwoert et al., 2000). While in our biosynthetic labeling studies with wild-type cells and in the presence of bafilomycin A1 a clear accumulation of 37-kDa POMC and 71-kDa PC2 was indeed observed, in the drug-treated Ac45-transgenic cells relatively low amounts of the precursor proteins accumulated during the 180-min chase period (Figure 3, A and B). Together with our finding that excess Ac45 caused a significant increase in granular acidification, the results of these biosynthetic studies suggest that the granular V-ATPase activity in the transgenic melanotrope cells was indeed enhanced by the excess of Ac45.
Figure 3.
Proprotein processing in Ac45-transgenic melanotrope cells is less effectively inhibited by bafilomycin A1. (A and B) Neurointermediate lobes from wild-type (wt) and Ac45-transgenic (tg) animals were pulse labeled for 30 min and subsequently chased for 180 min in medium containing 0, 0.25, or 0.5 μM bafilomycine A1. Newly synthesized proteins were extracted from the lobes, directly analyzed on 15% SDS-PAGE to resolve the 37-kDa POMC and 18-kDa POMC products (A) and on 10% SDS-PAGE to resolve the various PC2 forms (B). Signals were visualized by autoradiography.
Excess Ac45 Affects the Steady-State Level of PC2 but not of PC1 or POMC
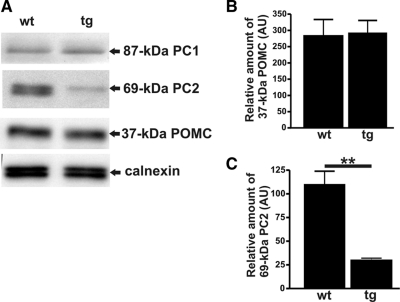
Having established that excess Ac45 affected the V-ATPase system, we then studied the steady-state levels of POMC and its processing enzymes PC1 and PC2. Quantitative Western blot analysis revealed no significant differences in the expression levels of POMC, PC1, as compared with the reference protein, the ER chaperone calnexin (Figure 4, A and B). Intriguingly, in the Ac45-transgenic NILs we found an ∼75% reduction of the steady state PC2 expression level compared with that in wild-type NILs (Figure 4, A and C). Apparently, the decrease in intragranular pH caused by the excess of Ac45 provided a change in microenvironment of the secretory pathway affecting the steady state expression level of mature PC2 but not of PC1 and POMC.
Figure 4.
Steady-state PC2 protein expression levels are lower in Ac45-transgenic melanotrope cells. (A) Western blot analysis of total proteins extracted from wild-type and Ac45-transgenic neurointermediates lobes of black-adapted Xenopus using various antibodies. (B–C) Steady-state protein levels of POMC (B), and PC2 (C) are presented in arbitrary units (AU) relative to the steady-state levels of calnexin.
Excess Ac45 Increases the Rate of the Early Endoproteolytic Cleavage of Newly Synthesized 37-kDa POMC to 18-kDa POMC
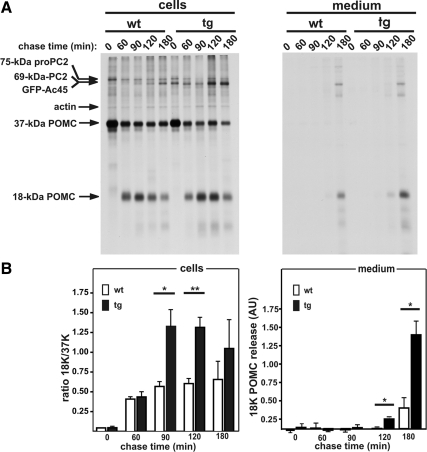
To examine the effect of the excess of Ac45 on the processing of newly synthesized 37-kDa POMC in the regulated secretory pathway, we performed metabolic cell labeling studies on wild-type and Ac45-transgenic NILs. In addition to the biosynthetically active melanotrope cells of the intermediate lobe, the Xenopus NIL consists of nerve terminals of hypothalamic origin (the neural lobe) and because the nerve terminals are biosynthetically inactive the radiolabeled proteins are synthesized by the melanotropes. Following a 30-min pulse incubation of wild-type NILs, clearly the most prominent newly synthesized product was 37-kDa POMC that during the subsequent chase periods was converted into 18-kDa POMC. The 18-kDa POMC product was subsequently secreted into the incubation medium (Figure 5A). After the 30-min pulse metabolic labeling of the Ac45-transgenic NILs, the biosynthesis of the newly synthesized proteins was similar to that in the wild-type NILs. However, during the chase periods the transgenic cells had converted clearly more 37-kDa POMC into 18-kDa POMC, resulting in an ∼3 times higher 18K/37K ratio (Figure 5B). Moreover, the release of 18-kDa POMC by the transgenic cells started already during the 120-min chase, whereas 18-kDa POMC secretion by the wild-type cells was detected only after 180 min of chase. After the 180-min chase period, the amount of 18-kDa POMC released into the incubation medium by the transgenic cells was ∼3 times higher than that released by wild-type cells (Figure 5B). These results suggest that excess Ac45 increased the rate of POMC processing in the secretory pathway.
Figure 5.
Excess Ac45 affects the processing of newly synthesized POMC in transgenic melanotrope cells. (A) Neurointermediate lobes (NILs) from wild-type (wt) and Ac45-transgenic (tg) animals were pulse labeled for 30 min and subsequently chased for the indicated time periods. 5% of the newly synthesized proteins extracted from the lobes and 20% of the proteins secreted into the incubation medium were directly resolved by 12.5% SDS-PAGE and visualized by autoradiography. The experiments were performed in triplicate, and a representative example is shown. (B) The processing rate of 37-kDa POMC to 18-kDa POMC and the secretion of 18-kDa POMC were quantified by densitometric scanning using a phosphoimager. For each chase period, the ratio of 18-kDa POMC to 37-kDa POMC was calculated for wild-type (wt, white bars) and transgenic (tg, black bars) NILs. The amount of secreted 18-kDa POMC was calculated relative to the levels of newly synthesized actin and is presented in AU. Note that the newly synthesized Ac45-transgene product comigrated with mature PC2 (Jansen et al., 2008). Shown are the means ± SEM (n = 3). Significant differences are indicated by *(p < 0.05) or **(p < 0.01).
Excess Ac45 Affects ProPC2 Maturation, 7B2 Cleavage, and ProPC2–7B2 Interaction
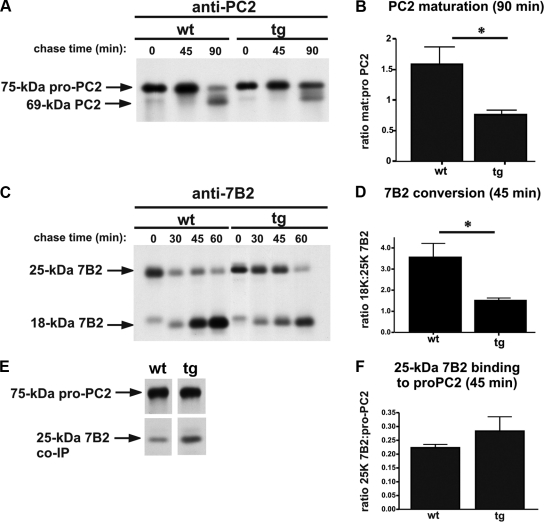
Biosynthetic labeling studies combined with anti-PC2 immunoprecipitations revealed that the newly synthesized expression levels of proPC2 did not differ between wild-type and Ac45-transgenic melanotrope cells (Figure 6A). After a 20-min pulse/45-min chase period, no proPC2 conversion was observed in both wild-type and the Ac45-transgenic cells. However, after a 90-min chase period, in the transgenic cells proPC2 conversion was clearly delayed compared with that in wild-type cells, resulting in an ∼2.5 times lower ratio of mature to proPC2 in the transgenic melanotropes (Figure 6, A and B).
Figure 6.
ProPC2 maturation and pro7B2 processing are delayed in Ac45-transgenic melanotrope cells. (A) Wild-type (wt) and Ac45-transgenic (tg) neurointermediate lobes (NILs) were pulse labeled for 20 min and subsequently chased for indicated time periods. After protein extraction, immunoprecipitation analyses were performed using an anti-PC2 antibody. (B) Quantification of PC2 maturation was calculated from the matPC2: proPC2 ratio. (C) Pulse-chase-labeled NIL proteins were immunoprecipitated using an anti-7B2 antibody. (D) Quantification of 7B2 processing was calculated from the 18-kDa 7B2:25-kDa 7B2 ratio. Shown are the means ± SEM (n = 4). Significant differences are indicated by *(p < 0.05). (E) Pulse-chase-labeled NIL proteins were extracted under native conditions, and proPC2 and 7B2 were coimmunoprecipitated under native conditions using an anti-PC2 antibody; the PC2 gel was exposed for one day and the 7B2 gel for 12 d. (F) Quantification of the amount of 25-kDa 7B2 binding to proPC2 was calculated from the 25-kDa 7B2: proPC2 ratio; the difference was not statistically significant (p = 0.3).
Transport and activation/maturation of PC2 depends on its neuroendocrine chaperone 7B2 (Mbikay et al., 2001). We therefore also compared the fate of 7B2 in wild-type and Ac45-transgenic melanotrope cells using metabolic cell labeling and immunoprecipitations for 7B2. The expression levels of newly synthesized 25-kDa 7B2 (pro7B2) were similar between wild-type and the transgenic cells (Figure 6C). In wild-type cells, newly synthesized 25-kDa 7B2 was gradually converted to its 18-kDa processed form (Figure 6C). However, in the Ac45-transgenic cells, 7B2 processing was delayed resulting in the sustained presence of the intact 7B2 25-kDa form during the subsequent chase periods (Figure 6C). Interestingly, after a 60-min chase period, also in the transgenic melanotrope cells most 25-kDa 7B2 was converted to its 18-kDa form.
Because the processing of 7B2 takes place in the Golgi compartment (Paquet et al., 1994), the delay in 7B2 processing was apparently caused in the early secretory pathway. We therefore focused on 7B2 processing and its binding to proPC2 during the first hour after the start of its synthesis. Indeed, after a 20-min pulse/45-min chase period, a significant reduction in 7B2 processing was observed in the Ac45-transgenic melanotrope cells (Figure 6, C and D). We then performed coimmunoprecipitation experiments under native conditions to study the proPC2/7B2 complex. Interestingly, the lower rate of 7B2 processing in the Ac45-transgenic cells (Figure 6, C and D) resulted in a slight increase of 25-kDa 7B2 coimmunoprecipitating with proPC2 after the 45-min chase period (Figure 6, E and F). We conclude that manipulation of the V-ATPase system in the regulated secretory pathway by excess Ac45 results in a slower degree of proPC2 maturation, a slower processing of 7B2, and a sustained proPC2-pro7B2 interaction in the secretory pathway.
Excess Ac45 Decreases the Secretion of α-MSH
The initial processing of 37-kDa POMC to 18-kDa POMC is an early, PC1-mediated endoproteolytic cleavage taking place in the TGN/immature secretory granules (Ayoubi et al., 1990; Berghs et al., 1997), and the rate of this processing event is increased in the Ac45-transgenic melanotrope cells (Figure 5). Subsequent processing events result in the generation of the main melanotrope hormone α-MSH and are mediated by PC2 in the later stages of the secretory pathway, namely in the acidic secretory granules (Berghs et al., 1997). Superfusion experiments combined with an α-MSH radioimmunoassay (van Zoest et al., 1989) revealed that α-MSH secretion was decreased ∼40% (Figure 7A); the number of transgenic and wild-type melanotrope cells superfused was similar (Figure 7B). Thus, excess Ac45 reduced the amount of α-MSH secreted, presumably as a consequence of the observed lower degree of proPC2 conversion to mature PC2 in the Ac45-transgenic cells.
Figure 7.
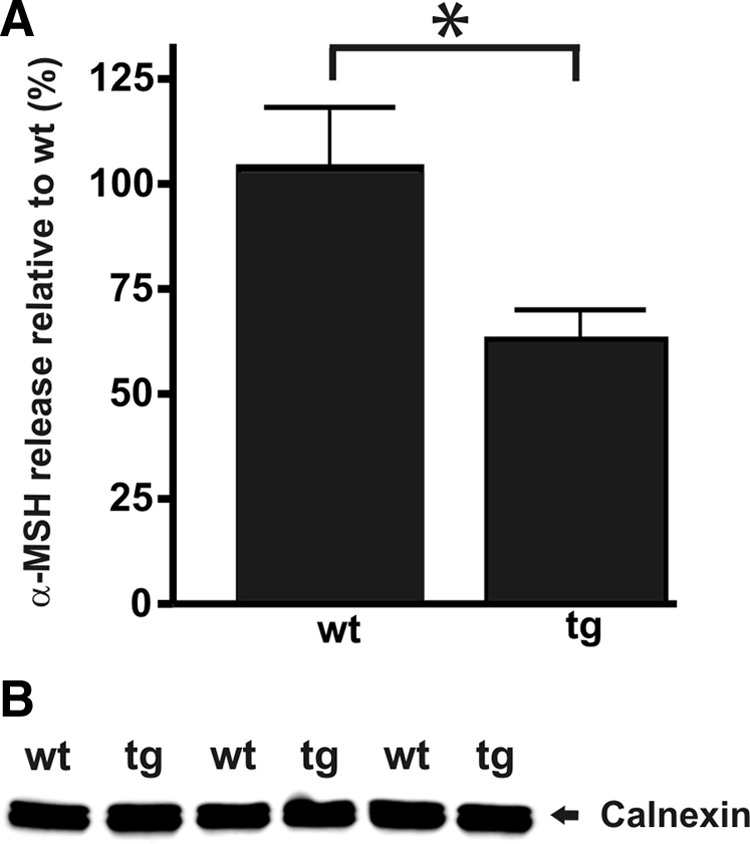
Ac45-transgenic melanotrope cells secrete less α-MSH than wild-type cells. (A) Wild-type (wt) and Ac45-transgenic (tg) neurointermediate lobes were isolated and directly superfused. To measure the amount of α-MSH released into the medium, fractions of the superfusion medium were collected and subjected to an α-MSH radioimmunoassay. The average α-MSH release during a ∼50-min period (7 fractions) was measured. The amount of α-MSH released was normalized for calnexin levels (see B) and the release of α-MSH by wild-type cells was set to 100%. Shown are the means ± SEM (n = 4). Significant difference is indicated by *(p < 0.05). (B) To estimate the number of wt and tg melanotrope cells, after superfusion the amount of the reference protein calnexin was determined by Western blot analysis.
DISCUSSION
To study the role of the V-ATPase accessory subunit Ac45 and the regulation of the V-ATPase pump, we generated transgenic Xenopus expressing the Ac45 protein specifically in the melanotrope cells and examined the effect of the excess of Ac45 on granular acidification and prohormone processing. Excess Ac45 increased intravesicular acidification because a clearly higher number of gold particles was found in vesicles of the Ac45-transgenic melanotrope cells (595 per μm2) than in the granules of wild-type Xenopus melanotrope cells (248 per μm2). The number of gold particles per μm2 found in the wild-type granules is in line with that detected in POMC-containing vesicles of mouse corticotrope AtT-20 cells (∼250 per μm2) (Tanaka et al., 1997). Thus, the intravesicular pH is comparable between these two neuroendocrine and POMC-producing cell types. Application of the formula of Orci (Orci et al., 1986) to estimate the pH in acidic subcellular organelles on the basis of the density of gold particles due to DAMP accumulation indeed revealed a substantial reduction (∼0.4 pH unit) in the average intragranular pH in the transgenic melanotrope cells when compared with that in granules of wild-type melanotropes.
The fact that in the transgenic cells the specific V-ATPase inhibitor bafilomycin A1 less effectively interfered with proPC2 maturation and POMC processing than in wild-type cells also points to an increased V-ATPase activity and thus a lower pH in the TGN/immature secretory granules. Interestingly, disruption of the gene encoding the proprotein cleavage enzyme furin in mouse pancreatic β-cells resulted in an impaired intragranular acidification in these cells (Louagie et al., 2008). Furin is thought to cleave the Ac45 protein into its functional form, and therefore Louagi et al. speculated that a lack of functional Ac45 may have caused the observed decrease in granular acidification (Louagie et al., 2008). Our results now provide direct evidence for an important role of Ac45 in granular acidification. Intriguingly, a proper intracellular pH is also crucial for correct early embryonic development (Allan et al., 2005; Adams et al., 2006; Liegeois et al., 2006; Nuckels et al., 2009; Cruciat et al., 2010) and may well explain the fact that our ablation of the Ac45 gene in the mouse led to early embryonic lethality (Schoonderwoert and Martens, 2002).
We recently found that in the Ac45-transgenic Xenopus melanotrope cells not only the plasma membrane morphology was affected, but also that vesicle biogenesis and the secretory efficiency were enhanced (Jansen et al., 2008). The present results show that excess Ac45 also causes a lower pH in subcompartments of the regulated secretory pathway, a situation that is beneficial for the formation of immature secretory granules (Chanat and Huttner, 1991; Taupenot et al., 2005). In addition, the more acidified secretory pathway subcompartments affected proprotein processing. Because only the steady-state level of mature PC2 and not that of mature PC1 was decreased, the pH in the ER, the site of proPC1 maturation (Zhou and Mains, 1994), likely remained unaffected.
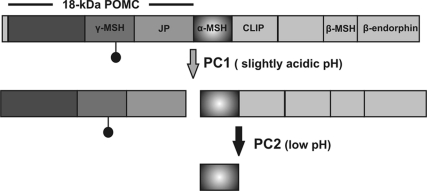
In biosynthetic pulse-chase studies on metabolically labeled melanotrope cells, we focused on the fate of newly synthesized POMC, the prohormone that represents ∼80% of all radiolabeled proteins produced in the melanotropes. The transgenic manipulation did not affect the level of newly synthesized 37-kDa POMC but caused a higher rate of processing of the prohormone to the 18-kDa POMC cleavage product and consequently more 18-kDa POMC was secreted by the transgenic than the wild-type cells. This first POMC cleavage event is thought to be accomplished by PC1 and occurs in the TGN/immature secretory granules (Berghs et al., 1997). Thus, the excess of Ac45 apparently provided an attractive microenvironment for proPC1 enzyme activation (which has a pH optimum of 6.0, Cameron et al., 2001) that led to efficient early POMC processing to 18-kDa POMC. In addition, or alternatively, the observed increased levels of intracellular and secreted 18-kDa POMC may have been caused by reduced PC2-mediated cleavage of this N-terminal portion of POMC to γ-MSH, due to the low levels of mature PC2. Likewise, reduced PC2-mediated processing of the C-terminal part of POMC may well have led to the significantly lower amounts of another form of MSH, namely of the main melanotrope bioactive peptide α-MSH (Figure 8).
Figure 8.
Processing of POMC by PC1 and PC2. Simplified version of the POMC processing scheme of Bicknell (Bicknell, 2008). Only the cleavage events pertinent to the present study are indicated. Filled dot, N-linked glycosylation site; MSH, melanophore-stimulating hormone; JP, joining peptide; CLIP, corticotropin-like intermediate lobe peptide.
Earlier studies have shown that the proteolytic automaturation and processing activity of the PC2 enzyme highly depend on a correct local pH (Lamango et al., 1999; Li et al., 2003). It is therefore not surprising that less amounts of mature PC2 were found in the Ac45-transgenic cells. The reduced PC2 cleavage activity led to less hormone-producing POMC processing events in the late secretory pathway. In the transgenic cells, the activities of other late-acting POMC processing enzymes may be affected as well, including the activity of the recently described cysteine protease cathepsin L (Funkelstein et al., 2008). Moreover, the time period for these late processing events is likely reduced due to the increased secretory efficiency of the transgenic cells (Jansen et al., 2008). Interestingly, in the furin−/− mouse pancreatic β-cells with a distorted granular pH the rate of proPC2 maturation was also decreased and consequently a lower degree of proinsulin II processing was observed (Louagie et al., 2008).
In the Ac45-transgenic melanotrope cells, the degree of proteolytic cleavage of 7B2, an event taking place in the TGN and thought to be executed by furin (Paquet et al., 1994), was clearly reduced, resulting in higher levels of intact 7B2 and prolonged 7B2-proPC2 binding. Because furin displays its highest activity at neutral pH (Hatsuzawa et al., 1992), it is likely that in the more acidic environment of the transgenic cells this proprotein cleavage enzyme is less active, leading to the sustained 7B2-proPC2 interaction and ultimately to the observed low levels of mature PC2.
In conclusion, our results show for the first time a central role for the Ac45 protein in controlling and assisting the V-ATPase, thereby greatly affecting granular acidification and subsequent proprotein processing in the regulated secretory pathway. Moreover, our results suggest that other V-ATPase accessory subunits may have an important role as well in cell- and cell organelle–specific targeting and regulation of the V-ATPase.
ACKNOWLEDGMENTS
The authors thank Peter Cruijsen and Tim Arentsen for technical assistance, Ron Engels for animal care, and Drs. Iris Lindberg (University of Maryland, Baltimore, MD), Nabil Seidah, Annik Prat (IRCM, Canada), and Käthi Geering (University of Lausanne, Switzerland) for antibodies. Microscopic imaging was performed at the Microscopic Imaging Centre (MIC) of the NCMLS.
Abbreviations used:
- DAMP
Acidotrophic reagent 3-(2,4-dinitroanilo)-3′amino-N-methyldipropylamine
- DNP
dinitrophenol
- GFP
green fluorescent protein
- α-MSH
α-melanophore stimulating hormone
- NIL
neurointermediate lobe
- PC
prohormone convertase
- POMC
proopiomelanocortin
- RIA
radioimmunoassay
- TGN
trans-Golgi network
- V-ATPase
vacuolar (H+)-ATPase.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-04-0274) on August 4, 2010.
REFERENCES
- Adams D. S., Robinson K. R., Fukumoto T., Yuan S., Albertson R. C., Yelick P., Kuo L., McSweeney M., Levin M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development. 2006;133:1657–1671. doi: 10.1242/dev.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan A. K., Du J., Davies S. A., Dow J. A. Genome-wide survey of V-ATPase genes in Drosophila reveals a conserved renal phenotype for lethal alleles. Physiol. Genomics. 2005;22:128–138. doi: 10.1152/physiolgenomics.00233.2004. [DOI] [PubMed] [Google Scholar]
- Anderson R. G., Orci L. A view of acidic intracellular compartments. J. Cell Biol. 1988;106:539–543. doi: 10.1083/jcb.106.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoubi T. A., van Duijnhoven H. L., van de Ven W. J., Jenks B. G., Roubos E. W., Martens G. J. The neuroendocrine polypeptide 7B2 is a precursor protein. J. Biol. Chem. 1990;265:15644–15647. [PubMed] [Google Scholar]
- Barbero P., Kitabgi P. Protein 7B2 is essential for the targeting and activation of PC2 into the regulated secretory pathway of rMTC 6–23 cells. Biochem. Biophys. Res. Commun. 1999;257:473–479. doi: 10.1006/bbrc.1999.0495. [DOI] [PubMed] [Google Scholar]
- Beggah A. T., Geering K. Alpha and beta subunits of Na,K-ATPase interact with BiP and calnexin. Ann. N.Y. Acad. Sci. 1997;834:537–539. doi: 10.1111/j.1749-6632.1997.tb52311.x. [DOI] [PubMed] [Google Scholar]
- Benjannet S., Reudelhuber T., Mercure C., Rondeau N., Chretien M., Seidah N. G. Proprotein conversion is determined by a multiplicity of factors including convertase processing, substrate specificity, and intracellular environment. Cell type-specific processing of human prorenin by the convertase PC1. J. Biol. Chem. 1992;267:11417–11423. [PubMed] [Google Scholar]
- Berghs C. A., Tanaka S., Van Strien F. J., Kurabuchi S., Roubos E. W. The secretory granule and pro-opiomelanocortin processing in Xenopus melanotrope cells during background adaptation. J. Histochem. Cytochem. 1997;45:1673–1682. doi: 10.1177/002215549704501211. [DOI] [PubMed] [Google Scholar]
- Beyenbach K. W., Wieczorek H. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J. Exp. Biol. 2006;209:577–589. doi: 10.1242/jeb.02014. [DOI] [PubMed] [Google Scholar]
- Bicknell A. B. The tissue-specific processing of pro-opiomelanocortin. J. Neuroendocrinol. 2008;20:692–699. doi: 10.1111/j.1365-2826.2008.01709.x. [DOI] [PubMed] [Google Scholar]
- Bouw G., Van Huizen R., Jansen E. J., Martens G. J. A cell-specific transgenic approach in Xenopus reveals the importance of a functional p24 system for a secretory cell. Mol. Biol. Cell. 2004;15:1244–1253. doi: 10.1091/mbc.E03-08-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Bowman E. J. Mutations in subunit C of the vacuolar ATPase confer resistance to bafilomycin and identify a conserved antibiotic binding site. J. Biol. Chem. 2002;277:3965–3972. doi: 10.1074/jbc.M109756200. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., McCall M. E., Baertsch R., Bowman E. J. A model for the proteolipid ring and bafilomycin/concanamycin-binding site in the vacuolar ATPase of Neurospora crassa. J. Biol. Chem. 2006;281:31885–31893. doi: 10.1074/jbc.M605532200. [DOI] [PubMed] [Google Scholar]
- Braks J. A., Martens G. J. 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway. Cell. 1994;78:263–273. doi: 10.1016/0092-8674(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Brown D., Breton S. H(+)V-ATPase-dependent luminal acidification in the kidney collecting duct and the epididymis/vas deferens: vesicle recycling and transcytotic pathways. J. Exp. Biol. 2000;203:137–145. doi: 10.1242/jeb.203.1.137. [DOI] [PubMed] [Google Scholar]
- Cameron A., Apletalina E., Lindberg I. The enzymology of prohormone convertases PC1 and PC2. In: Dalbey R. E., editor. The Enzymes. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Chanat E., Huttner W. B. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J. Cell Biol. 1991;115:1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciat C. M., Ohkawara B., Acebron S. P., Karaulanov E., Reinhard C., Ingelfinger D., Boutros M., Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459–463. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- Dirks R. P., Bouw G., van Huizen R., Jansen E. J., Martens G. J. Functional genomics in Xenopus laevis: Towards transgene-driven RNA interference and cell-specific transgene expression. Current Genomics. 2003;4:699–711. [Google Scholar]
- Funkelstein L., Toneff T., Mosier C., Hwang S. R., Beuschlein F., Lichtenauer U. D., Reinheckel T., Peters C., Hook V. Major role of cathepsin L for producing the peptide hormones ACTH, beta-endorphin, and alpha-MSH, illustrated by protease gene knockout and expression. J. Biol. Chem. 2008;283:35652–35659. doi: 10.1074/jbc.M709010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getlawi F., Laslop A., Schagger H., Ludwig J., Haywood J., Apps D. Chromaffin granule membrane glycoprotein IV is identical with Ac45, a membrane-integral subunit of the granule's H(+)-ATPase. Neurosci. Lett. 1996;219:13–16. doi: 10.1016/s0304-3940(96)13151-7. [DOI] [PubMed] [Google Scholar]
- Hatsuzawa K., Nagahama M., Takahashi S., Takada K., Murakami K., Nakayama K. Purification and characterization of furin, a Kex2-like processing endoprotease, produced in Chinese hamster ovary cells. J. Biol. Chem. 1992;267:16094–16099. [PubMed] [Google Scholar]
- Holthuis J. C., Jansen E. J., Schoonderwoert V. T., Burbach J. P., Martens G. J. Biosynthesis of the vacuolar H+-ATPase accessory subunit Ac45 in Xenopus pituitary. Eur. J. Biochem. 1999;262:484–491. doi: 10.1046/j.1432-1327.1999.00396.x. [DOI] [PubMed] [Google Scholar]
- Holthuis J. C., Jansen E. J., van Riel M. C., Martens G. J. Molecular probing of the secretory pathway in peptide hormone-producing cells. J. Cell Sci. 1995;108:3295–3305. doi: 10.1242/jcs.108.10.3295. [DOI] [PubMed] [Google Scholar]
- Hook V., Funkelstein L., Lu D., Bark S., Wegrzyn J., Hwang S. R. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu. Rev. Pharmacol. Toxicol. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss M., Ingenhorst G., Konig S., Gassel M., Drose S., Zeeck A., Altendorf K., Wieczorek H. Concanamycin A, the specific inhibitor of V-ATPases, binds to the V(o) subunit c. J. Biol. Chem. 2002;277:40544–40548. doi: 10.1074/jbc.M207345200. [DOI] [PubMed] [Google Scholar]
- Jansen E. J., Holling T. M., van Herp F., Martens G. J. Transgene-driven protein expression specific to the intermediate pituitary melanotrope cells of Xenopus laevis. FEBS Lett. 2002;516:201–207. doi: 10.1016/s0014-5793(02)02523-1. [DOI] [PubMed] [Google Scholar]
- Jansen E. J., Scheenen W. J., Hafmans T. G., Martens G. J. Accessory subunit Ac45 controls the V-ATPase in the regulated secretory pathway. Biochim. Biophys. Acta. 2008;1783:2301–2310. doi: 10.1016/j.bbamcr.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Jefferies K. C., Cipriano D. J., Forgac M. Function, structure and regulation of the vacuolar (H(+))-ATPases. Arch Biochem Biophys. 2008;476:33–42. doi: 10.1016/j.abb.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks B. G., Leenders H. J., Martens G.J.M., Roubos E. W. Adaptation Physiology: the functioning of pituitary melanotrope cells during background adaptation of the amphibian Xenopus laevis. Zool. Sci. 1993;10:1–11. [Google Scholar]
- Lamango N. S., Apletalina E., Liu J., Lindberg I. The proteolytic maturation of prohormone convertase 2 (PC2) is a pH-driven process. Arch. Biochem. Biophys. 1999;362:275–282. doi: 10.1006/abbi.1998.1033. [DOI] [PubMed] [Google Scholar]
- Lee S. N., Lindberg I. 7B2 prevents unfolding and aggregation of prohormone convertase 2. Endocrinology. 2008;149:4116–4127. doi: 10.1210/en.2008-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. L., Naqvi S., Shen X., Liu Y. J., Lindberg I., Friedman T. C. Prohormone convertase 2 enzymatic activity and its regulation in neuro-endocrine cells and tissues. Regul. Pept. 2003;110:197–205. doi: 10.1016/s0167-0115(02)00207-0. [DOI] [PubMed] [Google Scholar]
- Liegeois S., Benedetto A., Garnier J. M., Schwab Y., Labouesse M. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J. Cell Biol. 2006;173:949–961. doi: 10.1083/jcb.200511072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louagie E., Taylor N. A., Flamez D., Roebroek A. J., Bright N. A., Meulemans S., Quintens R., Herrera P. L., Schuit F., Van de Ven W. J., Creemers J. W. Role of furin in granular acidification in the endocrine pancreas: identification of the V-ATPase subunit Ac45 as a candidate substrate. Proc. Natl. Acad. Sci. USA. 2008;105:12319–12324. doi: 10.1073/pnas.0800340105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J., Kerscher S., Brandt U., Pfeiffer K., Getlawi F., Apps D. K., Schagger H. Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J. Biol. Chem. 1998;273:10939–10947. doi: 10.1074/jbc.273.18.10939. [DOI] [PubMed] [Google Scholar]
- Martens G. J., Jenks B. G., Van Overbeeke A. P. Biosynthesis of a gamma 3-melanotropin-like peptide in the pars intermedia of the amphibian pituitary gland. Eur. J. Biochem. 1982;126:23–28. doi: 10.1111/j.1432-1033.1982.tb06740.x. [DOI] [PubMed] [Google Scholar]
- Mbikay M., Seidah N. G., Chretien M. Neuroendocrine secretory protein 7B 2, structure, expression and functions. Biochem. J. 2001;357:329–342. doi: 10.1042/0264-6021:3570329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller L., Zhu X., Lindberg I. Mechanism of the facilitation of PC2 maturation by 7B 2, involvement in ProPC2 transport and activation but not folding. J. Cell Biol. 1997;139:625–638. doi: 10.1083/jcb.139.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi T., Forgac M. The vacuolar (H+)-ATPases–nature's most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- Nuckels R. J., Ng A., Darland T., Gross J. M. The vacuolar-ATPase complex regulates retinoblast proliferation and survival, photoreceptor morphogenesis, and pigmentation in the zebrafish eye. Invest. Ophthalmol. Vis. Sci. 2009;50:893–905. doi: 10.1167/iovs.08-2743. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Madsen O., Perrelet A., Vassalli J. D., Anderson R. G. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J. Cell Biol. 1986;103:2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet L., Bergeron F., Boudreault A., Seidah N. G., Chretien M., Mbikay M., Lazure C. The neuroendocrine precursor 7B2 is a sulfated protein proteolytically processed by a ubiquitous furin-like convertase. J. Biol. Chem. 1994;269:19279–19285. [PubMed] [Google Scholar]
- Paroutis P., Touret N., Grinstein S. The pH of the secretory pathway: measurement, determinants, and regulation. Physiology (Bethesda) 2004;19:207–215. doi: 10.1152/physiol.00005.2004. [DOI] [PubMed] [Google Scholar]
- Schmidt W. K., Moore H. P. Ionic milieu controls the compartment-specific activation of pro-opiomelanocortin processing in AtT-20 cells. Mol. Biol. Cell. 1995;6:1271–1285. doi: 10.1091/mbc.6.10.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonderwoert V. T., Holthuis J. C., Tanaka S., Tooze S. A., Martens G. J. Inhibition of the vacuolar H+-ATPase perturbs the transport, sorting, processing and release of regulated secretory proteins. Eur. J. Biochem. 2000;267:5646–5654. doi: 10.1046/j.1432-1327.2000.01648.x. [DOI] [PubMed] [Google Scholar]
- Schoonderwoert V. T., Jansen E. J., Martens G. J. The fate of newly synthesized V-ATPase accessory subunit Ac45 in the secretory pathway. Eur. J. Biochem. 2002;269:1844–1853. doi: 10.1046/j.1432-1033.2002.02831.x. [DOI] [PubMed] [Google Scholar]
- Schoonderwoert V. T., Martens G. J. Proton pumping in the secretory pathway. J. Membr. Biol. 2001;182:159–169. doi: 10.1007/s00232-001-0040-2. [DOI] [PubMed] [Google Scholar]
- Schoonderwoert V. T., Martens G. J. Targeted disruption of the mouse gene encoding the V-ATPase accessory subunit Ac45. Mol. Membr. Biol. 2002;19:67–71. doi: 10.1080/09687680110112910. [DOI] [PubMed] [Google Scholar]
- Strating J. R., Bouw G., Hafmans T. G., Martens G. J. Disparate effects of p24alpha and p24delta on secretory protein transport and processing. PLoS ONE. 2007;2:e704. doi: 10.1371/journal.pone.0000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strating J. R., Martens G. J. Incomplete posttranslational prohormone modifications in hyperactive neuroendocrine cells. BMC Cell Biol. 2009;10:35. doi: 10.1186/1471-2121-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supek F., Supekova L., Mandiyan S., Pan Y. C., Nelson H., Nelson N. A novel accessory subunit for vacuolar H(+)-ATPase from chromaffin granules. J. Biol. Chem. 1994;269:24102–24106. [PubMed] [Google Scholar]
- Tanaka S., Yora T., Nakayama K., Inoue K., Kurosumi K. Proteolytic processing of pro-opiomelanocortin occurs in acidifying secretory granules of AtT-20 cells. J. Histochem. Cytochem. 1997;45:425–436. doi: 10.1177/002215549704500310. [DOI] [PubMed] [Google Scholar]
- Taupenot L., Harper K. L., O'Connor D. T. Role of H+-ATPase-mediated acidification in sorting and release of the regulated secretory protein chromogranin A: evidence for a vesiculogenic function. J. Biol. Chem. 2005;280:3885–3897. doi: 10.1074/jbc.M408197200. [DOI] [PubMed] [Google Scholar]
- van Rosmalen J. W., Martens G. J. Transgene expression of prion protein induces crinophagy in intermediate pituitary cells. Dev. Neurobiol. 2007;67:81–96. doi: 10.1002/dneu.20330. [DOI] [PubMed] [Google Scholar]
- van Zoest I. D., Heijmen P. S., Cruijsen P. M., Jenks B. G. Dynamics of background adaptation in Xenopus laevis: role of catecholamines and melanophore-stimulating hormone. Gen. Comp. Endocrinol. 1989;76:19–28. doi: 10.1016/0016-6480(89)90028-2. [DOI] [PubMed] [Google Scholar]
- Vindrola O., Lindberg I. Biosynthesis of the prohormone convertase mPC1 in AtT-20 cells. Mol. Endocrinol. 1992;6:1088–1094. doi: 10.1210/mend.6.7.1508222. [DOI] [PubMed] [Google Scholar]
- Wagner C. A., Finberg K. E., Breton S., Marshansky V., Brown D., Geibel J. P. Renal vacuolar H+-ATPase. Physiol. Rev. 2004;84:1263–1314. doi: 10.1152/physrev.00045.2003. [DOI] [PubMed] [Google Scholar]
- Xu J., Cheng T., Feng H. T., Pavlos N. J., Zheng M. H. Structure and function of V-ATPases in osteoclasts: potential therapeutic targets for the treatment of osteolysis. Histol. Histopathol. 2007;22:443–454. doi: 10.14670/HH-22.443. [DOI] [PubMed] [Google Scholar]
- Zhou A., Mains R. E. Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2. J. Biol. Chem. 1994;269:17440–17447. [PubMed] [Google Scholar]