PLCγ03B3 binds Spry1 and Spry2. Overexpression of Spry decreased PLCγ03B3 activity and IP3 and DAG production, whereas Spry-deficient cells yielded more IP3. Spry overexpression inhibited T-cell receptor signaling and Spry1 null T-cells hyperproliferated with TCR ligation. Through action of PLCγ03B3, Spry may influence signaling through multiple receptors.
Abstract
Sprouty (Spry) proteins are negative regulators of receptor tyrosine kinase signaling; however, their exact mechanism of action remains incompletely understood. We identified phosphatidylinositol-specific phospholipase C (PLC)-γ as a partner of the Spry1 and Spry2 proteins. Spry–PLCγ interaction was dependent on the Src homology 2 domain of PLCγ and a conserved N-terminal tyrosine residue in Spry1 and Spry2. Overexpression of Spry1 and Spry2 was associated with decreased PLCγ phosphorylation and decreased PLCγ activity as measured by production of inositol (1,4,5)-triphosphate (IP3) and diacylglycerol, whereas cells deficient for Spry1 or Spry1, -2, and -4 showed increased production of IP3 at baseline and further increased in response to growth factor signals. Overexpression of Spry 1 or Spry2 or small-interfering RNA-mediated knockdown of PLCγ1 or PLCγ2 abrogated the activity of a calcium-dependent reporter gene, suggesting that Spry inhibited calcium-mediated signaling downstream of PLCγ. Furthermore, Spry overexpression in T-cells, which are highly dependent on PLCγ activity and calcium signaling, blocked T-cell receptor-mediated calcium release. Accordingly, cultured T-cells from Spry1 gene knockout mice showed increased proliferation in response to T-cell receptor stimulation. These data highlight an important action of Spry, which may allow these proteins to influence signaling through multiple receptors.
INTRODUCTION
Cell proliferation and fate are in large part controlled through the actions of receptor tyrosine kinases (RTKs). Ligation of such receptors by their cognate ligands activates several intracellular signaling pathways, including the Ras/mitogen-activated protein (MAP) kinase (MAPK)-, the phosphatidyl inositol/AKT-, and phosphatidylinositol-specific phospholipase C (PLC)-mediated pathways (Fantl et al., 1993; Schlessinger, 2000). The extent of RTK activation is dependent on factors such as the concentration and availability of ligand and the number of receptors on the cell surface. In addition, many negative regulatory mechanisms have evolved to limit signaling by RTKs. These mechanisms include the degradation of receptors through the action of c-Cbl and related ubiquitin ligases, the induction of inhibitory membrane-associated molecules such as SEF (Similar expression to FGF gene), and the secretion of proteins that sequester ligand as in the Drosophila protein Argos (Ledda and Paratcha, 2007). The sprouty gene was first identified as an antagonist of tracheal branching in the fly (Hacohen et al., 1998) and subsequently was shown to be a general inhibitor of RTKs (Casci et al., 1999; Kramer et al., 1999; Reich et al., 1999). There are four Spry genes in higher vertebrates with only partial overlap in expression pattern (Minowada et al., 1999) and some distinct biochemical properties (Mason et al., 2006; Edwin et al., 2009). Sprouty genes are induced in response to RTK signaling through both MAP kinase (Ozaki et al., 2001) and calcium signaling pathways (Abe and Naski, 2004), and vertebrate Spry proteins were shown to consistently antagonize signaling through the fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), glial cell-derived neurotrophic factor (GDNF), and vascular endothelial growth factor (VEGF) receptors (Mason et al., 2006; Cabrita and Christofori, 2008). Targeted deletion of Spry genes in mice yields defects in the development of many organs, including kidney, cochlea, and tooth due to excessive signaling through specific RTKs, which can be rescued by genetic reduction of RTK signaling (Basson et al., 2005, 2006; Shim et al., 2005; Klein et al., 2006, 2008). The molecular mechanism by which Spry proteins inhibit RTK signaling remains uncertain. Spry proteins can interact with multiple components of the Ras/MAPK pathway, including Grb2 (Hanafusa et al., 2002; Tefft et al., 2002), FRS2 (Tefft et al., 2002), Shp2 (Tefft et al., 2002), c-Cbl (Wong et al., 2001; Fong et al., 2003; Hall et al., 2003; Rubin et al., 2003; Mason et al., 2004), Raf1 (Tefft et al., 2002; Sasaki et al., 2003), and GAP1 (Tefft et al., 2002). These interactions only partially explain the ability of Spry proteins to inhibit Ras and MAP kinase activation.
The two isoforms of PLCγ, PLCγ1 and PLCγ2, are both activated by phosphorylation through growth factor receptor tyrosine kinases as well as nonreceptor tyrosine kinases (Wells et al., 2003). PLCγ2 is primarily expressed in cells of hematopoietic lineage, whereas PLCγ1 is ubiquitously expressed (Wilde and Watson, 2001). Although PLCγ1 is critical for many cells, it is the predominant isoform in T-cells, whereas PLCγ2 is important in B-cells, mast cells, and platelets (Kurosaki et al., 2000). PLCγ1 has an essential role in mammalian development because mice deficient for PLCγ1 die by embryonic day 9 (Ji et al., 1998). The PLCγ2 gene knockout showed restricted growth and abnormalities in B-, mast, and natural killer cells and a block in crystallizable fragment receptor (FcεR)-mediated responses (Wang et al., 2000). PLCγ1 is among the signaling molecules that bind to the intracellular domain of activated growth factor receptors. Subsequent tyrosine phosphorylation of PLCγ1 leads to increased enzymatic activity (Peters et al., 1992; Ronnstrand et al., 1992; Rotin et al., 1992; Middlemas et al., 1994). Activated PLCγ1 hydrolyzes phosphatidylinositol-4,5-bisphosphate (PIP2) into inositol (1,4,5)-triphosphate (IP3) and diacylglycerol (DAG), which are important second messengers (Rhee, 2001). IP3 binds to a receptor on the endoplasmic reticulum, causing the release of Ca2+ into the cytosol, whereas DAG recruits protein kinase C (PKC) isoforms to plasma membranes and facilitates its activation and engagement in signaling. Both the increase of Ca2+ levels and activation of PKC are important for cell differentiation, proliferation, and growth (Hardingham and Bading, 1999; Oliva et al., 2005). PLCγ-mediated increase of DAG can lead to activation of specific Ras-guanine nucleotide exchange factors and stimulation of the MAP kinase pathway (Bivona et al., 2003). Furthermore, calcium-stimulated activation of PKC can lead to the phosphorylation and activation of c-Raf in a Ras-independent manner (Marais et al., 1998).
To clarify mechanisms by which Spry regulates signaling networks, we searched for proteins that interact with Spry that may mediate its ability to inhibit signal transduction. We identified PLCγ as a protein that interacts with Spry1 and -2, particularly after growth factor stimulation of cells. Here, we showed that overexpression of Spry1 and Spry2 was associated with decreased PLCγ activity as measured by the production of IP3 or DAG after receptor stimulation. Deletion of Spry genes in murine embryonic fibroblasts or mast cells was associated with increased PLCγ activity. These findings highlight another important function of Spry proteins and indicate their ability to regulate several branches of signaling downstream of multiple cell surface receptors.
MATERIALS AND METHODS
Cell Lines
NIH 3T3 cells inducible for expression of murine Spry1 (NIH 3T3-Spry1) or Spry2 (NIH 3T3-Spry2) were created by stable transfection of NIH-3T3 cells with Spry1 inserted into pBIG2i (Strathdee et al., 1999). Spry1flox/flox (Basson et al., 2005), Spry2flox/flox (Shim et al., 2005), and Spry4flox/flox (Klein et al., 2006) mice were interbred to create mice homozygous for all three alleles. Fibroblasts created from these mouse embryos were immortalized by passage under a 3T3 protocol and infected with recombinant adenoviruses AdCMV5-eGFP or AdCMVCre-eGFP (Gene Transfer Vector Core, University of Iowa, Iowa City, IA) to delete the Sprouty alleles. Mast cells were created from Spry2 gene knockout mice (Basson et al., 2005; Shim et al., 2005) by culturing bone marrow cells for 4 wk in Iscove's modified Dulbecco media + glutamine containing 10 ng/ml interleukin (IL)-3 and 20 ng/ml murine stem cell factor (SCF). Spry1,2,4flox/flox fibroblasts treated with adenovirus vector or adenovirus-expressed Cre vector and Spry2 wt and Spry2 null mast cells were genotyped to confirm the deletion of respective Spry genes. Genotyping polymerase chain reaction primers and conditions are indicated in Supplemental Data.
Immunoblot and Immunoprecipitation Analysis
The following antibodies were used for the study: phospho-42/44 MAP kinase, phospho-AKT, phospho-Raf (Cell Signaling Technology); FLAG (Sigma-Aldrich, St. Louis, MO); hemagglutinin (HA; Roche Diagnostics, Indianapolis, IN); PLCγ1, PLCγ2, PLCβ1, PLCδ1 (Santa Cruz Biotechnology, Santa Cruz, CA); and phospho-PLCγ1 and phospho-PLCγ2 (Cell Signaling Technology). Rabbit polyclonal sera against Spry1 and Spry2 were described previously (Gross et al., 2001). Immunoprecipitations and immunoblots were performed after transient transfection of 293T cells as described previously (Mason et al., 2004). The immunoblots were visualized by the enhanced chemiluminescence detection system (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom).
Luciferase Assay
NIH 3T3 cells engineered to express Spry1 under doxycycline control were plated 24 h before transfection in DMEM with 10% serum. The cells were transiently cotransfected in duplicate with nuclear factor of activated T-cells (NFAT) luciferase reporter (5 μg) and Renilla (50 ng) plasmids by using FuGENE Transfection Reagent (GE Healthcare). Serum-starved (0.2% serum-containing media ± doxycycline) cells were left unstimulated or stimulated with either basic (b)FGF (20 ng/ml) or PDGF BB (20 ng/ml) for 4 h. The cells were then assayed using the Dual-Luciferase Assay kit (Promega, Madison, WI), normalizing firefly luciferase activity to Renilla luciferase activity. For assessing luciferase activity with Spry2, the Spry1-inducible NIH 3T3 cells were transiently cotransfected in duplicate with an NFAT luciferase reporter (5 μg), Renilla (50 ng), and Spry2 (1 μg) expression plasmids by using FuGENE Transfection Reagent (GE Healthcare). The medium was replaced with starvation medium (0.2% serum-containing media, without doxycycline) for 24 h after transfection. Cells were either left unstimulated or stimulated with bFGF (20 ng/ml) or PDGF BB (20 ng/ml) for 4 h. Equal quantities of cell lysate were then assayed using the Dual-Luciferase Assay kit (Promega), normalizing luciferase activity to Renilla luciferase activity.
Small-interfering RNA (siRNA)-mediated PLCγ1 and PLCγ2 Knockdown
siGENOME SMARTpool duplex RNA oligonucleotides targeting mouse PLCγ1 and PLCγ2 or scrambled control siRNA were purchased from Dharmacon RNA Technologies (Lafayette, CO). NIH 3T3 cells were treated with indicated siRNA by using HiPerFect (QIAGEN, Valencia, CA) for 24 h followed by transfections with the indicated plasmids. Cells were analyzed for the luciferase activity or PLCγ1 and PLCγ2 protein levels by immunoblot as described above.
Calcium Mobilization Assay
Jurkat T-cells (2 × 106) were transfected with 1 μg of enhanced green fluorescent protein (EGFP) and 3 μg of Spry1 or empty vector with DMRIE-C transfection reagent (Invitrogen, Carlsbad, CA). After 24 h, the cells were incubated with 5 μM indo-1-acetoxymethyl ester (indo-1; Invitrogen) in RPMI 1640 medium (Invitrogen) at 37°C for 30 min. The cells were then washed and resuspended in minimal essential medium without phenol red. Cells were incubated at 37°C for 5 min before flow cytometry measurements were taken. Baselines were acquired, and anti-CD3 antibody was added (3 μg/ml) after 1 min. Data were collected for another 4 min. Calcium levels were plotted as a ratio between calcium-bound indo-1 and unbound indo-1 versus time. The data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Inositol Phosphate Production Assay
Fibroblasts were seeded in a 10-cm dish with DMEM containing 10% fetal bovine serum (FBS). After 20 h, cells were serum starved (0.2% FBS) with fresh medium containing 2 μCi/ml myo-[3H]inositol (PerkinElmer Life and Analytical Sciences, Boston, MA) and incubated overnight. Cells were washed twice and preincubated for 1 h in media containing 20 mM LiCl and 1 mg/ml bovine serum albumin (BSA), followed by stimulation with PDGF BB (20 ng/ml) for the indicated time. Mast cells were seeded in Iscove's modified Dulbecco media + glutamine containing 10 ng/ml IL-3 and 20 ng/ml murine SCF for overnight incubation. Cells were serum starved (0.2% FBS) with fresh medium containing 2 μCi/ml myo-[3H]inositol (PerkinElmer Life and Analytical Sciences) plus immunoglobulin (Ig)E anti-2,4-dinitrophenyl (DNP) and incubated overnight. The cells were washed twice and preincubated for 1 h in media containing 20 mM LiCl and 1 mg/ml BSA, followed by stimulation with DNP-BSA. After treatment with PDGF or anti-DNP, stimulation media were removed from the cells, and 2 ml of ice-cold 0.4 M HClO4 was added to the cells for 5 min. The samples were then neutralized with 2 ml of 0.72 M KOH + 0.6 M KHCO3, and the precipitate was removed by centrifugation. The resulting supernatant was applied to an ion exchange column (AGI-X8, catalog no. 143-2445; Bio-Rad Laboratories, Hercules, CA) and sequentially eluted with 0.2, 0.6, and 1.2 M ammonium formate/0.1 M formic acid to isolate inositol monophosphate (IP1), inositol diphosphate (IP2), and IP3, respectively. Radioactivity in the fractions was measured by scintillation counting (LS-6500 counter; Beckman Coulter, Fullerton, CA; Paris and Pouyssegur, 1987) and normalized to total radioactivity incorporation in each experiment.
DAG Signaling and Confocal Microscopy
RBL-2H3, a rat mast cell line, was cultured in DMEM containing 10% FBS and transfected with plasmids encoding green fluorescent protein (GFP)-tagged Cys-domains of PKC (Oancea et al., 1998) and DsRed Spry2 by using Superfect (QIAGEN). Cells were plated on glass-bottomed dishes (MatTek, Ashland, MA), and the next day the media were changed to extracellular buffer (5 mM KCl, 125 mM NaCl, 20 mM HEPES, pH 7.4, 1.5 mM CaCl2, 1.5 mM MgCl2, and 10 mM glucose). Cell stimulation via the FcεR1 was carried out by incubation with 1 μg/ml IgE anti-DNP (Sigma-Aldrich) for 8 h, followed by antigenic cross-linking of bound IgE by using 20 μg/ml DNP-BSA (Invitrogen). Fluorescence confocal microscopy (model LSM 510 Meta; Carl Zeiss, Jena, Germany) was used to monitor the translocation of the GFP-fusion construct in response to stimulus. Timed series of images were recorded before and after stimulation of cells, and midsections of the cells are shown in all the images.
T-Cell Isolation
T-cells were isolated from Spry1−/−, Spry1+/−, and Spry1+/+ mice by disaggregation of lymph nodes into air-buffered media containing 1% FBS, washed, counted, and resuspended in complete RPMI 1640 medium (10% FBS, glutamine, and Pen/Strep). Total lymph node cells (5 × 105/well) were cultured in round-bottomed 96-well plates and stimulated in triplicate wells with soluble anti-CD3 (145-2C11 at the indicated concentrations) in combination with anti-CD28 (1 μg/ml) antibodies for 72 h. Cultures were pulsed with 0.5 μCi of [3H]thymidine in the last 18 h of culture, harvested, and counted in a beta counter with scintillation.
Flow Cytometry
Jurkat T-cells (2 × 106) were transfected with 1 μg of EGFP and 3 μg of Spry1 or Spry2 or empty vector with DMRIE-C transfection reagent (Invitrogen). After 48 h, cells were stimulated with 3 μg/ml anti-CD3 antibody (R&D Systems, Minneapolis, MN) for 16 h. Cells were then washed and suspended in 300 μl of media with 25 μl of phycoerythrin-conjugated anti-human CD69 antibody (BD Biosciences Pharmingen, San Diego, CA) for 30 min in the dark. CD69 expression was analyzed by flow cytometry (FACscan; BD Biosciences, San Jose, CA) after gating on GFP-positive cells.
RESULTS
PLCγ1 and PLCγ2 Associate with Spry1 and Spry2
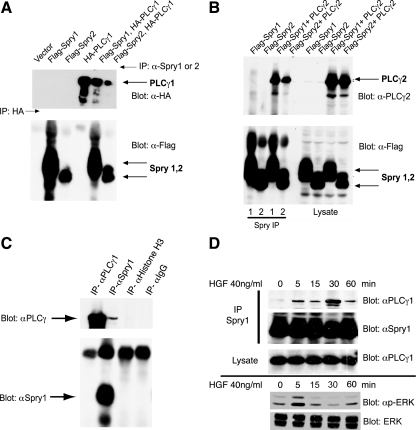
A search of the Alliance for Cell Signaling database (Saunders et al., 2008) of a large-scale yeast two-hybrid screen indicated interaction between Sprouty2 and PLCγ2. To confirm this interaction, human embryonic kidney (HEK)293 cells were transiently cotransfected with plasmids encoding epitope-tagged Spry1, Spry2, PLCγ1, and PLCγ2. PLCγ1 (Figure 1A) and PLCγ2 (Figure 1B) were readily coprecipitated with Spry1 or Spry2. Interaction between endogenous Spry1 and PLCγ1 was detected in murine IMCD3 cells that express high levels of Spry1 (Basson et al., 2006; Figure 1C, lane 2). In IMCD3 cells starved for growth factor, the Spry1–PLCγ1 interaction was minimal (Figure 1D, lane 1), but after stimulation with hepatocyte growth factor (HGF), which stimulates extracellular signal-regulated kinase (ERK) activity in these cells (Figure 1D, bottom), the interaction was readily detected, reaching a maximum after 30 min (Figure 1D). The inducible nature of the Spry1–PLCγ1 interaction also was reconstructed in an FGF-dependent system in HEK293 cells, by using epitope-tagged Spry1 and PLCγ (data not shown).
Figure 1.
PLCγ1 and PLCγ2 coimmunoprecipitate with Spry1 and Spry2. (A) HEK293 cells were transfected with plasmids expressing epitope-tagged Spry and PLCγ as indicated. Spry1 and Spry2 were immunoprecipitated with anti-Spry1 or anti-Spry2 antibody. HA-PLCγ1 was immunoprecipitated (lane 4) with anti-HA antibody. The immunoprecipitates were probed with anti-HA antibody to detect coimmunoprecipitated PLCγ1 (top) and with anti-FLAG antibody to detect Spry1 and Spry2 (bottom). (B) HEK293 cells were cotransfected with cDNAs encoding FLAG-tagged Spry1 or FLAG-tagged Spry2 and PLCγ2 as indicated. After 48 h, lysates were prepared, and immunoprecipitations were performed using specific anti-Spry1 or anti-Spry2 antibody (left). Total cell lysate (50 μg) was resolved in 10% SDS-polyacrylamide gel electrophoresis gel and probed with anti-PLCγ2 and anti-FLAG antibodies to show the expression level of the transfectants (right). (C) mIMCD3 cells growing in media containing 10% FBS were lysed and subjected to immunoprecipitation with anti-Spry1 and anti-PLCγ1 antibodies or IgG or anti histone H3 controls, followed by immunoblotting with anti-Spry1 or PLCγ1. (D) Murine IMCD3 cells were serum starved for 18 h followed by HGF (40 ng/ml) stimulation for the indicated time. Endogenous Spry1 and PLCγ1 interaction was demonstrated by immunoprecipitation with anti-Spry1 antibody and immunoblotting for PLCγ1. Total cell lysate was blotted with PLCγ1 to show the endogenous level of PLCγ1 in the cell lysates after stimulation with HGF. Bottom, lysate from a second experiment was immunoblotted for activated phosphorylated (p)-ERK and total ERK to show the time course of activation of signaling in response to HGF.
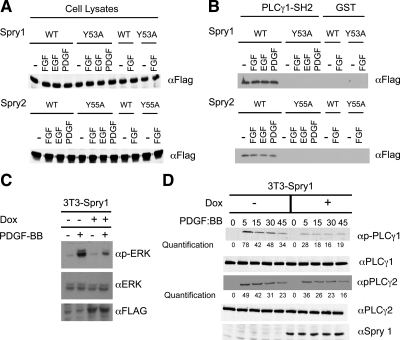
Given the presence of Src homology 2 (SH2) domains in PLCγ and that Spry proteins can bind to c-Cbl and other SH2 domain-containing proteins, a glutathione transferase (GST) fusion protein consisting of the C-terminal SH2 domain of PLCγ (Reddi et al., 2007) was produced in Escherichia coli (Supplemental Figure 1) and incubated with cell lysate isolated from 293T cells transfected with either wild-type Spry1, Spry2, or versions of the Spry proteins in which conserved N-terminal tyrosine residues located within an SH2 binding domain were mutated (Y53A and Y55A, respectively; Mason et al., 2004). Immunoblot analysis showed almost equal expression of wild-type and mutant forms of Spry1 and Spry2 (Figure 2A). GST-PLCγ1-SH2-C was bound to wild-type but not with the tyrosine mutant forms of Spry (Figure 2B). Curiously, Spry1 or Spry2 derived from extracts of serum-starved cells could still bind to the GST-PLCγ1 protein. This could denote the presence of residual tyrosine phosphorylated Spry in the cell extracts even after serum starvation. Accordingly, a small amount of Spry–PLCγ1 interaction was detected by coprecipitation of extracts of serum-starved cells (Figure 1D, t = 0). After growth factor stimulation, PLCγ1 and PLCγ2 bind to growth factor receptors through an SH2 domain and become phosphorylated (Carpenter and Ji, 1999). To determine the consequences of the Spry1–PLCγ1 interaction, we measured the activation state of PLCγ as marked by Y783 phosphorylation. As we reported previously, a doxycycline-responsive system for the conditional expression of Spry1 strongly inhibited MAP kinase activation after growth factor stimulation (Figure 2; Gross et al., 2001; Mason et al., 2004). In the absence of doxycycline, PDGF rapidly stimulated PLCγ1 and PLCγ2 tyrosine phosphorylation (Figure 2D, left). However, in the presence of Spry1, PLCγ1 and PLCγ2 phosphorylation was inhibited 33–60%, depending on the time after stimulation (Figure 2D, right). As reported previously, expression of PLCβ1 and PLCδ1 was almost undetectable in these cells (Supplemental Figure 2; Smith et al., 1990); hence, it is unlikely that Spry1 or Spry2 affects signaling through these other members of the PLC family in fibroblasts. Collectively, these data demonstrate that Spry interacts with the PLCγ protein, and this is associated with decreased phosphorylation and presumably activation of this enzyme.
Figure 2.
Sprouty–PLCγ1 interaction requires an N-terminal conserved tyrosine residue of Sprouty. (A) 293T cells were transfected with either wild type or mutant Spry1 or Spry 2 as indicated. Thirty-six hours after transfection, cells were starved with 0.2% serum-containing medium for 12 h, and cells were stimulated with indicated growth factors for 10 min. Aliquots (50 μg) of whole cell lysate from unstimulated or stimulated cells were subjected to immunoblotting with anti-FLAG antibody to visualize Spry1 (top) and Spry2 (bottom) expression. (B) 293T cells were transfected with FLAG-tagged wild-type (WT) Spry1 or Spry2 or N-terminal tyrosine mutants as indicated. The cells were starved with 0.2% FBS-containing medium for 12 h and were stimulated with indicated growth factors for 10 min. The cell lysates from stimulated or unstimulated cells were incubated with 20 μg of GST or GST-PLCγ1-SH2-C fusion proteins. The GST-bound proteins were eluted in SDS loading buffer, and the bound Spry was detected by immunoblotting with anti-FLAG antibody. (C) Spry1 expression in a stable 3T3 cell line was induced by addition of doxycycline to starvation media containing 0.2% FBS and then stimulated with PDGF BB (20 ng/ml) for 15 min. Cell lysates were probed for total and phospho-ERK. (D) 3T3 cells were stimulated for increasing periods of time with PDGF BB (20 ng/ml) in the presence or absence of doxycycline (Dox) to induce Spry1 expression. Cell lysates were immunoblotted for total and phosphorylated PLCγ1 and PLCγ2 as indicated.
Sprouty Inhibits IP3 Production, Calcium Release, and Calcium-dependent Transcription
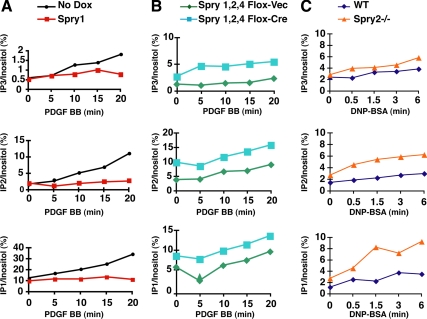
Having shown that Spry1 and Spry2 interact with PLCγ1 and PLCγ2, we asked whether signals downstream of PLCγ were affected by Spry. PLCγ catalyzes the breakdown of PIP2 into DAG and IP3. We measured directly the effect of Spry expression on inositol phosphate production in several systems. In NIH 3T3 cells treated with PDGF, there was a time-dependent increase in the concentration of IP3 as well as the IP3 metabolites IP2 and IP1 that result from the action of phosphatases on IP3. When Spry1 expression was induced (Supplemental Figure 3A) in these cells, abrogation of IP3 and IP3 metabolite production in response to PDGF was observed (Figure 3A and Supplemental Table 1A). As a complimentary experiment, we measured inositol phosphate production in a murine embryo fibroblasts (MEFs) loss-of-function system in which LoxP site-flanked Spry1, -2, and -4 alleles were all excised by infection with adenovirus-Cre. Spry1,2,4 null fibroblasts (Supplemental Figure 3, B and D) had elevated basal levels of IP1, IP2, and IP3 after serum starvation compared with MEFs infected with adenovirus-GFP (Figure 3B and Supplemental Table 1B). Stimulation of these cells with PDGF led to a further increase in inositol phosphate production. To determine whether elimination of even a single Sprouty gene could lead to an effect on IP3 production, we studied mast cells derived from Spry2 null mice (Supplemental Figure 3C). Fc receptor stimulation activates PLCγ1 and PLCγ2 in mast cells (Barker et al., 1998). In response to Fc receptor stimulation, Spry2−/− mast cells showed increased production of IP3 and metabolites compared with cultured cells from Spry2+/+ replete littermate controls (Figure 3C and Supplemental Table 1C). Similar levels of Fc receptor in cell surface were measured in Spry2+/+ and Spry2−/− mast cells (data not shown). Collectively, these data indicate that Spry proteins regulate IP3 production in the cell, consistent with the inhibition of PLCγ activity.
Figure 3.
Spry inhibits inositol phosphate (IP) production after receptor stimulation. (A) NIH 3T3 cells cultured in the presence of [3H]inositol were serum starved and stimulated with PDGF BB (20 ng/ml) for increasing periods in the presence or absence of doxycycline (Dox) to induce Spry1 expression. Lipids were collected from the cell lysates, and radiolabeled IPs were fractionated and counted by liquid scintillation. Inositol phosphate levels are expressed as a percentage of total radioactive inositol incorporation. (B) Spry1,2,4 null or control adenovirus-GFP fibroblasts were serum starved and treated with PDGF BB (20 ng/ml) for the indicated times and assayed for IPs. (C) A bone marrow mast cell line derived from a wild-type or Spry2−/− mouse was treated with IgE followed by dinitrophenol-human serum albumin to cross-link Fc receptors. Cell lysates were prepared at the indicated times and assayed for IPs. All experiments were performed three times (see Supplemental Table 1, A–C) with similar results obtained.
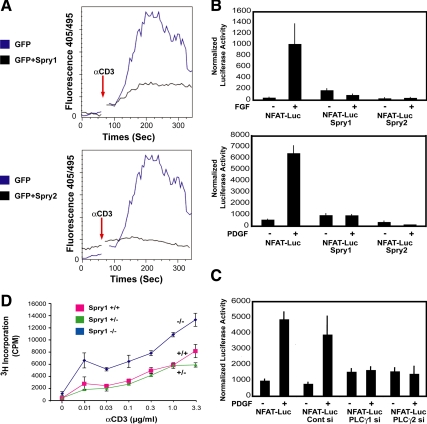
IP3 normally stimulates release of Ca2+ from intracellular stores by binding to specific channel/receptor on the endoplasmic reticulum (Foskett et al., 2007). To directly assess the ability of Spry to affect calcium release, we used Jurkat T-cells, which express PLCγ1. T-cell receptor (TCR)-mediated signaling and activation of MAP kinase is highly dependent on PLCγ in these cells (Bivona et al., 2003; Perez de Castro et al., 2004). Jurkat cells transfected with Spry1 or Spry2 along with GFP or with GFP alone were loaded with the calcium-sensitive dye indo-1 and stimulated with anti-CD3 antibody. Changes in intracellular calcium levels over time were monitored by flow cytometry, gating GFP-positive cells. TCR engagement with anti-CD3 antibody induced a rapid increase in calcium concentration in the control Jurkat cells, peaking at 200 s after stimulation. In cells transfected with Spry1 or Spry2, calcium mobilization was strongly suppressed (Figure 4A). These data suggest that Spry1 and Spry2 prevent the coupling of TCR signaling to PLCγ1 activation and Ca2+ mobilization in Jurkat T-cells.
Figure 4.
Sprouty inhibits intracellular calcium mobilization and signaling in T-cells and fibroblasts. (A) Jurkat T-cells were cotransfected with a GFP expression vector (1 μg) along with either empty pcDNA (3 μg) or Spry1 or Spry2 expression vector (3 μg) as indicated. The cells were then loaded with indo-1 and after baseline measurements of 60 s and stimulated with anti-CD3 (3 mg/ml) antibody. Measurements were taken for up to 300 s. Intracellular calcium mobilization was analyzed by fluorescence-activated cell sorting analysis by monitoring the fluorescence emission ratio of the indo-1 bound to Ca2+ versus the free form at 405 and 495 nM, respectively. The experiment was performed three times with similar results obtained. (B) Spry1-inducible NIH 3T3 cells were transiently cotransfected with a reporter containing an NFAT-responsive element (5 μg) and Renilla (50 ng), starved for 24 h in media containing 0.2% FBS, and stimulated either with bFGF (20 ng/ml; top) or PDGF BB (20 ng/ml; bottom) for 4h. Spry1 was induced by doxycycline addition to the starvation media; Spry2 was expressed by transient transfection of a Spry2 expression plasmid in cells cultured in the absence of doxycycline. After growth factor addition, equal quantities of cell extracts (50 μg) were assayed using the Dual-Luciferase Assay kit (Promega), normalizing luciferase activity to Renilla luciferase activity. The experiments were repeated three times, and similar results were obtained. Luc, luciferase. (C) NIH 3T3 cells were treated with siGENOME SMARTpool directed against PLCγ1 or PLCγ2 for 24 h followed by transient transfection with NFAT luciferase reporter (5 μg) and Renilla (50 ng) plasmids by using FuGENE Transfection Reagent (GE Healthcare) in duplicate. Serum-starved (0.2% FBS-containing serum media) cells were either left unstimulated or stimulated with PDGF BB (20 ng/ml) for 4 h. Luciferase activity was assayed as described above. (D) T-cells from Spry1−/− mice were cultured in triplicate with the indicated amount of anti-CD3 antibody in combination with anti-CD28 (1 μg/ml) antibody for 72 h, and [3H]thymidine incorporation was quantified by liquid scintillation counting.
To determine whether Spry led to changes in calcium-mediated signaling, we measured the transcriptional activity of the NFAT transcription factor, which is regulated by the calcium-sensitive phosphatase calcineurin (Feske et al., 2001). NIH 3T3 fibroblasts engineered to conditionally express Spry1 were transfected with a reporter gene harboring a NFAT binding site along with Renilla luciferase internal control. This reporter was robustly induced by the addition of PDGF or FGF. When Spry1 was induced by doxycycline addition, the reporter gene activity was severely reduced, and no induction in response to FGF or PDGF was noted. Similarly when these cells were transiently transfected with a Spry2 expression vector, the activity of the NFAT luciferase reporter was inhibited (Figure 4B), suggesting that Spry inhibited the PLCγ-generated calcium signals downstream of these growth factors. Accordingly, siRNA-mediated depletion of endogenous PLCγ1 or PLCγ2 (Supplemental Figure 4, A and B) blocked PDGF-mediated activation of the reporter gene to a similar extent as overexpression of Spry1 or Spry2 (Figure 4C). The combination of knockdown of PLCγ and overexpression of Spry1 or Spry2 also showed complete inhibition of NFAT reporter activity (Supplemental Figure 4C, top and bottom). These data suggest that PLCγ1 and PLCγ2 act together to stimulate calcium release in the cell, and overexpression of Spry phenocopies the loss of function of one of these proteins, consistent with the notion that Spry1 and Spry2 interfere with PLCγ function.
To determine the biological significance of the effect of Spry on T-cell receptor signaling that largely depends on calcium release, T-cells were isolated from Spry1 null animals and treated with graded doses of anti-CD3 antibody. Proliferation was measured by incorporation of [3H]thymidine. As seen in Figure 4D, T-cells from Spry1−/− mice showed increased proliferation at every dose of agonistic anti-CD3 antibody. Similarly, T-cells from mice homozygous for a LoxP-flanked Spry1 allele (Basson et al., 2005, 2006) and deleted for Spry1 by crossing with CD4-Cre mice showed increased proliferation in response to CD3 ligation (data not shown).
Spry Modulates the Production of Diacylglycerol
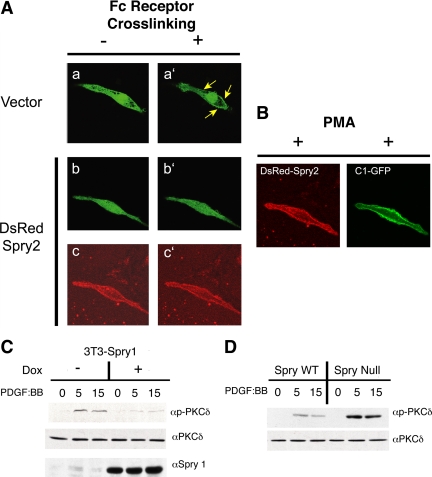
To further demonstrate the ability of Spry to inhibit PLCγ activity, we determined the ability of Spry to affect production of DAG, the other product of PLCγ-mediated hydrolysis of PIP2, by using an assay that reflects the cell biological effects of DAG. When DAG is generated at the plasma membrane regions, it recruits signaling proteins such as PKC isoforms. A fusion protein consisting of the C1 domain of PKCγ protein fused to GFP binds DAG and translocates from cytosol to the plasma membrane after IgE (FcεRI) receptor stimulation of RBL-2H3 mast cells (Oancea et al., 1998). The PKCγ-C1-GFP construct was transfected into this mast cell line with or without DsRed Spry2, and in more than a dozen individual cells both conditions were analyzed by live-cell confocal microscopy, with images taken at 10-s intervals after IgE receptor stimulation. Examination of a representative cell transfected with the GFP reporter protein alone showed that before Fc cross-linking, PKCγ-C1-GFP was homogenously distributed across the cytosol and the nucleus (Figure 5Aa). After addition of DNP-bovine serum albumin to cross-link DNP-specific IgE-primed Fc receptors, PKCγ-C1-GFP rapidly relocalized to the plasma membrane (Figure 5A, compare a and a′, arrows). By contrast, when mast cells were transfected with Spry2, the redistribution of PKCγ-C1-GFP to the plasma membrane was prevented (Figure 5A, b and b′). DsRed-tagged Spry2 itself was localized at the plasma membrane in unstimulated as well as cross-linked cells (Figure 5A, c and c′). Analysis of multiple individual cells by time course microscopy showed that expression of Spry2 almost always (11/13 cells tested) inhibited the translocation of the PKC probe (Table 1). By contrast, most cells (12/16) stimulated by Fc cross-linking but not transfected with Spry2 showed migration of the probe to the plasma membrane. When phorbol 12-myristate 13-acetate (PMA), a mimic of DAG, was applied to mast cells expressing DsRed Spry2, PKCγ-C1-GFP readily relocated to the plasma membrane (Figure 5B). This showed that the ability of Spry2 to localize at the plasma membrane by itself did not block the ability of PKCγ-C1-GFP to accumulate at the plasma membrane. Collectively, these data suggest that Spry expression inhibits the generation of DAG in the cell and not the DAG-dependent translocation of PKCγ to the membrane, a finding consistent with the ability of Spry to inhibit PLCγ activity.
Figure 5.
Spry2 prevents relocalization of a DAG reporter protein to the plasma membrane and inhibits signaling downstream of DAG. (A) Fluorescent images of RBL-2H3 mast cells transfected with a DAG binding reporter protein construct (PKCγ-C1-GFP) without (a and a′) or with (b and b′) transfection of Spry2 before (a and b) and after (a′ and b′) cross-linking of the IgE receptors with DNP-BSA. c and c′ show DsRed Spry2 expression in the mast cell presented in b and b′. (B) The mast cell from b and b′ was treated with PMA showing membrane localization of DsRed Spry2 and PKCγ-C1-GFP after stimulation. Sixteen vector-transfected cells and 13 Spry2-transfected cells were analyzed as described above with results presented in Table 1. (C) NIH 3T3 cells engineered to express Spry1 under doxycycline control treated overnight with doxycycline in starvation media, treated with PDGF BB (20 ng/ml) for the indicated times, and then cell lysates were immunoblotted with antibodies directed against PKCδ and phospho-threonine 505-modified PKCδ. (D) Sprouty1,2,4 null or control fibroblasts were serum starved and treated with PDGF BB (20 ng/ml) for the indicated times. Cell lysates were immunoblotted for PKCδ and phospho-threonine 505-PKCδ. WT, wild type.
Table 1.
Localization of PKC-GFP probe after mast cell stimulation
| Probe at membrane | Probe in cytoplasm | |
|---|---|---|
| Vector | 12 | 4 |
| Spry2* | 2 | 11 |
* p < 0.005 by chi-square.
Increased PLCγ activity leads to the recruitment of protein kinase C molecules, including PKCδ, to the plasma membrane (Griner and Kazanietz, 2007), and DAG-mediated allosteric changes in PKCδ cooperate with the action of the serine-threonine kinase PDK1 to phosphorylate and activate the kinase (Le Good et al., 1998). Accordingly, overexpression of Spry1 in NIH 3T3 cells was associated with decreased phosphorylation of PKCδ on threonine 505, a modification associated with activation of the enzyme (Figure 5C). By contrast in Spry1,2,4 knockout MEFs, PKCδ phosphorylation was increased after growth factor receptor activation compared with control MEFs (Figure 5D). Collectively, these data indicate that Spry modulates the activity of PLCγ and downstream pathways, including the activity of PKC.
Spry Suppresses CD69, a T-Cell Marker for Activation of the Ras/MAPK Pathway
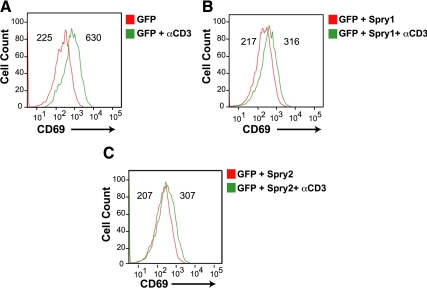
Activation of the Ras/MAP kinase pathway by TCR stimulation leads to the up-regulation of cell surface activation markers such as CD69 (Testi et al., 1994). Previous work indicated that TCR-mediated activation of Ras/MAP kinase was highly dependent on PLCγ1 activity and activation of a DAG-dependent Ras-GRP (Bivona et al., 2003). Jurkat T-cells were transfected with a GFP expression vector alone or with a Spry1 or Spry2 expression vector and were left untreated or treated with anti-CD3. In response to CD3 stimulation, GFP-transfected cells showed a threefold increase in CD69 expression (Figure 6A). In accordance with its ability to inhibit PLCγ activity, overexpression of Spry1 (Figure 6B) or Spry2 (Figure 6C) in Jurkat T-cells robustly blunted the induction of CD69 after CD3 stimulation. These results show that Spry1 and Spry 2 inhibit TCR-mediated activation of the Ras/MAPK pathway.
Figure 6.
Spry suppresses induction of CD69, an MAP-kinase dependent marker in T-cells. Jurkat cells were transfected with GFP alone (A), with GFP and Spry1 (B), or with GFP and Spry2 (C) and left unstimulated or stimulated for 16 h with anti-CD3; stained with phycoerythrin-conjugated anti-CD69 expression, and analyzed by flow cytometry. Histograms of cell number versus fluorescence intensity are plotted, and mean fluorescence intensity of each curve is presented. The experiments were repeated three times, and similar results were obtained.
DISCUSSION
The Spry proteins seem to limit signaling through interactions with several partner proteins. We identified an interaction between Spry proteins and PLCγ that further characterizes the inhibitory action of the Spry proteins on signaling downstream of many different receptor tyrosine kinases, including the FGF receptor, PDGF receptor, T-cell receptor, and Fc receptor. Calcium signaling pathways as well as the Ras/MAP kinase pathway stimulate Spry expression, presumably as part of a negative feedback loop. Specifically, Spry1 and Spry2 expression can be induced by FGF through calcium-dependent pathways. Mutant forms of the FGF receptor 3 receptor were unable to bind and activate PLCγ1. They only weakly induced Spry1 and Spry2 in contrast to the robust induction by wild-type receptor (Abe and Naski, 2004). Signaling through phosphotidyinositol hydrolysis also affects the localization of Spry. Spry2 in particular was shown to bind to PIP2, which may attract Spry to the plasma membrane ruffles after active signaling, allowing it to further limit signaling (Lim et al., 2002).
In this work, we showed that Spry1 and Spry2 directly interacts with PLCγ and affects the PLCγ-mediated signaling. Spry overexpression was associated with decreased PLCγ phosphorylation and decreased PLCγ activity as shown by decreased IP3 generation, calcium release, and DAG production. Cells deleted for one or multiple Spry genes showed increased IP3 production in response to growth factor receptor activation. There have been previous indications that Spry may affect signaling through the PLCγ pathway. Nutt et al. (2001) showed that in Xenopus oocytes, overexpression of Xenopus Spry2 (Xspry2) inhibited Ca2+ signaling but failed to affect MAP kinase signaling. In contrast, in the same system Drosophila sprouty inhibited MAP kinase but not Ca2+ signaling. Another report using Xspry2 showed inhibition of Ca2+ signaling and PKCδ activity, whereas Xspred proteins inhibited MAP kinase signaling (Sivak et al., 2005). These studies suggested that Spry proteins inhibited either Ca2+ signaling via the PLCγ pathway or MAPK signaling through Ras but not both the signaling pathways in the same system. Our data indicate that murine Spry1 and Spry2 can affect the activity of PLCγ as well as MAP kinase. This may explain the ability of Spry to affect signaling downstream of a diverse set of receptors. Furthermore, the ability of Spry to inhibit PLCγ-mediated signaling may contribute to its ability to affect ERK activation. Inhibition of PLCγ activity can alter downstream signaling by reducing DAG levels. Reduction in the DAG level limits the activity of Ras-Grp1, a known guanine nucleotide exchange factor involved in the conversion of inactive guanosine diphosphate (GDP)-bound Ras to active guanosine triphosphate (GTP)-bound Ras (Roose et al., 2005). Decreased Ras-Grp1 activity can lead to lower levels of active GTP-bound Ras. In addition, DAG normally stimulates PKC isoforms that phosphorylate and stimulate Raf (Schonwasser et al., 1998); hence, decreased PLCγ activity in response to Spry can in this way also leads to decreased ERK activity.
The inhibitory effect of the Spry1–PLCγ interaction also was reflected by the reduced transcriptional activity of NFAT. NFAT activity is modulated by cytoplasmic Ca2+ concentration, which is increased by IP3-mediated release of calcium from intracellular stores (Masuda et al., 1998; Crabtree and Olson, 2002). Increases in cytoplasmic Ca2+ concentration induce NFAT dephosphorylation and NFAT translocation to the nucleus where it binds to cis-regulatory elements of target genes. In accordance with our results, Choi et al. (2006) also found that Spry1 overexpression inhibited NFAT activation, suggesting that Spry1 limited calcium release after signaling. Lee et al. (2009) showed an association of Spry1 overexpressed in T-cells with Linker for activation of T cells (LAT), a tyrosine kinase linking the T-cell receptor to downstream signaling, as well as PLCγ1. In contrast to our results in fibroblasts, this group found that Spry1 overexpression decreased LAT but not PLCγ1 tyrosine phosphorylation. By contrast our gain-of-function and loss-of-function studies link Spry1 and Spry2 to PLCγ activity and downstream signaling. Spry may affect phosphotodylinositol-mediated signaling by more than one mechanism. One group found that that Spry4 inhibited VEGF-stimulated PIP2 hydrolysis by PLCγ but not ERK activation. This activity seemed to be related to the ability of Spry4 to bind PIP2, perhaps sequestering this substrate from PLCγ. Unlike our results with Spry1 and Spry2, Spry4 expression was not associated with decreased PLCγ phosphorylation, and the interaction between Spry4 and PLCγ was not explored (Ayada et al., 2009).
We showed previously by targeted deletion of the Spry1 gene that Spry1 specifically inhibited the action of GDNF via the Ret receptor during kidney development (Basson et al., 2005, 2006). A recent study showed that loss of Spry1 in mice rescued renal agenesis resulting from a mutant allele of Ret that could no longer directly activate Ras via Grb2 and SOS but that could only signal through the PLCγ pathway (Rozen et al., 2009). This observation is consistent with our data and suggests that Spry1 inhibits PLCγ-mediated signaling in vivo.
In IMCD3 cells, endogenous Spry1 interacted with PLCγ modestly under serum starvation conditions and more robustly after growth factor stimulation, which leads to Spry tyrosine phosphorylation (Gross et al., 2001). Interaction between the SH2 domain of PLCγ1 and Spry1 in vitro was dependent, at least in part, on the conserved N-terminal Y53 residue of the protein (Y55 in Spry2). This N-terminal tyrosine residue is also critical for interaction of Spry with proteins such as c-Cbl (Fong et al., 2003), Shp2 (Hanafusa et al., 2004), and PP2A (Lao et al., 2007). The interplay between Spry and c-Cbl can modulate epidermal growth factor receptor levels (Haglund et al., 2005), but c-Cbl is not required for the inhibitory action of Spry on signaling (Mason et al., 2004). Shp2 may dephosphorylate the N-terminal tyrosine and inhibit Spry function (Hanafusa et al., 2004). PP2A binding through the N terminus is associated with serine–threonine dephosphorylation of Spry2, which facilitates its ability to bind Grb2, potentially interfering with the coupling of receptor tyrosine kinases to Ras activation (Lao et al., 2007). The interaction of Spry with PLCγ through its N-terminal domain represents another way for Spry proteins to modulate signal transduction.
We found that Spry1- and Spry2-expressing cells inefficiently coupled TCR signaling to calcium mobilization. Furthermore, CD69 expression, an indication of Ras/MAP kinase activation in T-cells (Dumont et al., 1998) was inhibited by enforced expression of Spry1 or Spry2. Conversely, loss of Spry1 expression led to increased proliferation of T-cells after stimulation of the T-cell receptor. Given that T-cells are highly dependent on DAG-stimulated Ras-GRP to activate Ras, this also suggests that Spry1 and Spry2 inhibited PLCγ activity. Spry1 expression is induced in response to TCR signaling (Choi et al., 2006), suggesting that, as in other receptor-mediating signaling pathways, Spry proteins act as part of a negative feedback loop to limit receptor mediated signaling and to modulate immune responses. In support of these results, Spry1 is up-regulated in anergic T-cells that fail to proliferate in response to antigen (Powell, unpublished data). T-cell anergy is characterized by diminished intercellular Ca2+ mobilization, in response to improper signaling from the TCR (Macian et al., 2004). Spry, being a negative regulator of calcium signaling, could help to enforce anergy.
Having no evident function as an enzyme, the role of Spry proteins as regulators of signaling pathways remains to be fully elucidated. Spry proteins interact with multiple signaling molecules, including Grb2, Shp2, SIAH, PTP1B, caveolin, TESK1, and Raf (Mason et al., 2006; Edwin et al., 2009). The identification of partner proteins has yielded important insight into Spry function. Interplay between Spry and the PLCγ represents one important mode of action of these relatively small but complicated molecules in diverse cell systems.
Supplementary Material
ACKNOWLEDGMENTS
We thank Gail Martin (Department of Anatomy and program in Development Biology, University of California, San Francisco) for Sprouty2 and Sprouty4 mutant mice and Pallavi Chaturvedi for helpful suggestions. This study was supported by National Institutes of Health grant CA-59998, the Illinois Department of Public Health, the Lynn Sage Cancer Research Foundation, and Northwestern Memorial Foundation (to J.D.L.); National Institutes of Health grant P01AI-072677 (to J.P.); and the Medical Research Council (M.A.B. and M.L.).
Abbreviations used:
- DAG
diacylglycerol
- FBS
fetal bovine serum
- IP1
inositol monophosphate
- IP2
inositol diphosphate
- IP3
inositol (1,4,5)-triphosphate
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PKC
protein kinase C
- PLC
phospholipase C
- PMA
phorbol 12-myristate 13-acetate.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-02-0123) on August 18, 2010.
REFERENCES
- Abe M., Naski M. C. Regulation of sprouty expression by PLCγ and calcium-dependent signals. Biochem. Biophys. Res. Commun. 2004;323:1040–1047. doi: 10.1016/j.bbrc.2004.08.198. [DOI] [PubMed] [Google Scholar]
- Ayada T., Taniguchi K., Okamoto F., Kato R., Komune S., Takaesu G., Yoshimura A. Sprouty4 negatively regulates protein kinase C activation by inhibiting phosphatidylinositol 4,5-biphosphate hydrolysis. Oncogene. 2009;28:1076–1088. doi: 10.1038/onc.2008.464. [DOI] [PubMed] [Google Scholar]
- Barker S. A., Caldwell K. K., Pfeiffer J. R., Wilson B. S. Wortmannin-sensitive phosphorylation, translocation, and activation of PLCgamma1, but not PLCgamma2, in antigen-stimulated RBL-2H3 mast cells. Mol. Biol. Cell. 1998;9:483–496. doi: 10.1091/mbc.9.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson M. A., et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev. Cell. 2005;8:229–239. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Basson M. A., Watson-Johnson J., Shakya R., Akbulut S., Hyink D., Costantini F. D., Wilson P. D., Mason I. J., Licht J. D. Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev. Biol. 2006;299:466–477. doi: 10.1016/j.ydbio.2006.08.051. [DOI] [PubMed] [Google Scholar]
- Bivona T. G., Perez De Castro I., Ahearn I. M., Grana T. M., Chiu V. K., Lockyer P. J., Cullen P. J., Pellicer A., Cox A. D., Philips M. R. Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature. 2003;424:694–698. doi: 10.1038/nature01806. [DOI] [PubMed] [Google Scholar]
- Cabrita M. A., Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Ji Q. Phospholipase C-gamma as a signal-transducing element. Exp. Cell Res. 1999;253:15–24. doi: 10.1006/excr.1999.4671. [DOI] [PubMed] [Google Scholar]
- Casci T., Vinos J., Freeman M. Sprouty, an intracellular inhibitor of Ras signaling. Cell. 1999;96:655–665. doi: 10.1016/s0092-8674(00)80576-0. [DOI] [PubMed] [Google Scholar]
- Choi H., Cho S. Y., Schwartz R. H., Choi K. Dual effects of Sprouty1 on TCR signaling depending on the differentiation state of the T cell. J. Immunol. 2006;176:6034–6045. doi: 10.4049/jimmunol.176.10.6034. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R., Olson E. N. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(suppl):S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Dumont F. J., Staruch M. J., Fischer P., DaSilva C., Camacho R. Inhibition of T cell activation by pharmacologic disruption of the MEK1/ERK MAP kinase or calcineurin signaling pathways results in differential modulation of cytokine production. J. Immunol. 1998;160:2579–2589. [PubMed] [Google Scholar]
- Edwin F., Anderson K., Ying C., Patel T. B. Intermolecular interactions of Sprouty proteins and their implications in development and disease. Mol. Pharmacol. 2009;76:679–691. doi: 10.1124/mol.109.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl W. J., Johnson D. E., Williams L. T. Signalling by receptor tyrosine kinases. Annu. Rev. Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- Feske S., Giltnane J., Dolmetsch R., Staudt L. M., Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- Fong C. W., Leong H. F., Wong E. S., Lim J., Yusoff P., Guy G. R. Tyrosine phosphorylation of Sprouty2 enhances its interaction with c-Cbl and is crucial for its function. J. Biol. Chem. 2003;278:33456–33464. doi: 10.1074/jbc.M301317200. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., White C., Cheung K. H., Mak D. O. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griner E. M., Kazanietz M. G. Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- Gross I., Bassit B., Benezra M., Licht J. D. Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. J. Biol. Chem. 2001;276:46460–46468. doi: 10.1074/jbc.M108234200. [DOI] [PubMed] [Google Scholar]
- Hacohen N., Kramer S., Sutherland D., Hiromi Y., Krasnow M. A. Sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92:253–263. doi: 10.1016/s0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- Haglund K., Schmidt M. H., Wong E. S., Guy G. R., Dikic I. Sprouty2 acts at the Cbl/CIN85 interface to inhibit epidermal growth factor receptor downregulation. EMBO Rep. 2005;6:635–641. doi: 10.1038/sj.embor.7400453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. B., Jura N., DaSilva J., Jang Y. J., Gong D., Bar-Sagi D. hSpry2 is targeted to the ubiquitin-dependent proteasome pathway by c-Cbl. Curr. Biol. 2003;13:308–314. doi: 10.1016/s0960-9822(03)00086-1. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Torii S., Yasunaga T., Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat. Cell Biol. 2002;4:850–858. doi: 10.1038/ncb867. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Torii S., Yasunaga T., Matsumoto K., Nishida E. Shp2, an SH2-containing protein-tyrosine phosphatase, positively regulates receptor tyrosine kinase signaling by dephosphorylating and inactivating the inhibitor Sprouty. J. Biol. Chem. 2004;279:22992–22995. doi: 10.1074/jbc.M312498200. [DOI] [PubMed] [Google Scholar]
- Hardingham G. E., Bading H. Calcium as a versatile second messenger in the control of gene expression. Microsc. Res. Tech. 1999;46:348–355. doi: 10.1002/(SICI)1097-0029(19990915)46:6<348::AID-JEMT3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Ji Q. S., Ermini S., Baulida J., Sun F. L., Carpenter G. Epidermal growth factor signaling and mitogenesis in Plcg1 null mouse embryonic fibroblasts. Mol. Biol. Cell. 1998;9:749–757. doi: 10.1091/mbc.9.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein O. D., Lyons D. B., Balooch G., Marshall G. W., Basson M. A., Peterka M., Boran T., Peterkova R., Martin G. R. An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development. 2008;135:377–385. doi: 10.1242/dev.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein O. D., Minowada G., Peterkova R., Kangas A., Yu B. D., Lesot H., Peterka M., Jernvall J., Martin G. R. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev. Cell. 2006;11:181–190. doi: 10.1016/j.devcel.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S., Okabe M., Hacohen N., Krasnow M. A., Hiromi Y. Sprouty: a common antagonist of FGF and EGF signaling pathways in Drosophila. Development. 1999;126:2515–2525. doi: 10.1242/dev.126.11.2515. [DOI] [PubMed] [Google Scholar]
- Kurosaki T., Maeda A., Ishiai M., Hashimoto A., Inabe K., Takata M. Regulation of the phospholipase C-gamma2 pathway in B cells. Immunol. Rev. 2000;176:19–29. doi: 10.1034/j.1600-065x.2000.00605.x. [DOI] [PubMed] [Google Scholar]
- Lao D. H., Yusoff P., Chandramouli S., Philp R. J., Fong C. W., Jackson R. A., Saw T. Y., Yu C. Y., Guy G. R. Direct binding of PP2A to Sprouty2 and phosphorylation changes are a prerequisite for ERK inhibition downstream of fibroblast growth factor receptor stimulation. J. Biol. Chem. 2007;282:9117–9126. doi: 10.1074/jbc.M607563200. [DOI] [PubMed] [Google Scholar]
- Le Good J. A., Ziegler W. H., Parekh D. B., Alessi D. R., Cohen P., Parker P. J. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- Ledda F., Paratcha G. Negative regulation of receptor tyrosine kinase (RTK) signaling: a developing field. Biomark Insights. 2007;2:45–58. [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Lee J. E., Oh Y. M., Park J. B., Choi H., Choi C. Y., Kim I. H., Lee S. H., Choi K. Recruitment of Sprouty1 to immune synapse regulates T cell receptor signaling. J. Immunol. 2009;183:7178–7186. doi: 10.4049/jimmunol.0803799. [DOI] [PubMed] [Google Scholar]
- Lim J., Yusoff P., Wong E. S., Chandramouli S., Lao D. H., Fong C. W., Guy G. R. The cysteine-rich sprouty translocation domain targets mitogen-activated protein kinase inhibitory proteins to phosphatidylinositol 4,5-bisphosphate in plasma membranes. Mol. Cell. Biol. 2002;22:7953–7966. doi: 10.1128/MCB.22.22.7953-7966.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F., Im S. H., Garcia-Cozar F. J., Rao A. T-cell anergy. Curr. Opin. Immunol. 2004;16:209–216. doi: 10.1016/j.coi.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Marais R., Light Y., Mason C., Paterson H., Olson M. F., Marshall C. J. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science. 1998;280:109–112. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- Mason J. M., Morrison D. J., Bassit B., Dimri M., Band H., Licht J. D., Gross I. Tyrosine phosphorylation of Sprouty proteins regulates their ability to inhibit growth factor signaling: a dual feedback loop. Mol. Biol. Cell. 2004;15:2176–2188. doi: 10.1091/mbc.E03-07-0503. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mason J. M., Morrison D. J., Basson M. A., Licht J. D. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Masuda E. S., Imamura R., Amasaki Y., Arai K., Arai N. Signalling into the T-cell nucleus: NFAT regulation. Cell Signal. 1998;10:599–611. doi: 10.1016/s0898-6568(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Middlemas D. S., Meisenhelder J., Hunter T. Identification of TrkB autophosphorylation sites and evidence that phospholipase C-gamma 1 is a substrate of the TrkB receptor. J. Biol. Chem. 1994;269:5458–5466. [PubMed] [Google Scholar]
- Minowada G., Jarvis L. A., Chi C. L., Neubuser A., Sun X., Hacohen N., Krasnow M. A., Martin G. R. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- Nutt S. L., Dingwell K. S., Holt C. E., Amaya E. Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning. Genes Dev. 2001;15:1152–1166. doi: 10.1101/gad.191301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oancea E., Teruel M. N., Quest A. F., Meyer T. Green fluorescent protein (GFP)-tagged cysteine-rich domains from protein kinase C as fluorescent indicators for diacylglycerol signaling in living cells. J. Cell Biol. 1998;140:485–498. doi: 10.1083/jcb.140.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva J. L., Griner E. M., Kazanietz M. G. PKC isozymes and diacylglycerol-regulated proteins as effectors of growth factor receptors. Growth Factors. 2005;23:245–252. doi: 10.1080/08977190500366043. [DOI] [PubMed] [Google Scholar]
- Ozaki K., Kadomoto R., Asato K., Tanimura S., Itoh N., Kohno M. ERK pathway positively regulates the expression of Sprouty genes. Biochem. Biophys. Res. Commun. 2001;285:1084–1088. doi: 10.1006/bbrc.2001.5295. [DOI] [PubMed] [Google Scholar]
- Paris S., Pouyssegur J. Further evidence for a phospholipase C-coupled G protein in hamster fibroblasts. Induction of inositol phosphate formation by fluoroaluminate and vanadate and inhibition by pertussis toxin. J. Biol. Chem. 1987;262:1970–1976. [PubMed] [Google Scholar]
- Perez de Castro I., Bivona T. G., Philips M. R., Pellicer A. Ras activation in Jurkat T cells following low-grade stimulation of the T-cell receptor is specific to N-Ras and occurs only on the Golgi apparatus. Mol. Cell. Biol. 2004;24:3485–3496. doi: 10.1128/MCB.24.8.3485-3496.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K. G., Marie J., Wilson E., Ives H. E., Escobedo J., Del Rosario M., Mirda D., Williams L. T. Point mutation of an FGF receptor abolishes phosphatidylinositol turnover and Ca2+ flux but not mitogenesis. Nature. 1992;358:678–681. doi: 10.1038/358678a0. [DOI] [PubMed] [Google Scholar]
- Reddi A. L., Ying G., Duan L., Chen G., Dimri M., Douillard P., Druker B. J., Naramura M., Band V., Band H. Binding of Cbl to a phospholipase Cgamma1-docking site on platelet-derived growth factor receptor beta provides a dual mechanism of negative regulation. J. Biol. Chem. 2007;282:29336–29347. doi: 10.1074/jbc.M701797200. [DOI] [PubMed] [Google Scholar]
- Reich A., Sapir A., Shilo B. Sprouty is a general inhibitor of receptor tyrosine kinase signaling. Development. 1999;126:4139–4147. doi: 10.1242/dev.126.18.4139. [DOI] [PubMed] [Google Scholar]
- Rhee S. G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnstrand L., Mori S., Arridsson A. K., Eriksson A., Wernstedt C., Hellman U., Claesson-Welsh L., Heldin C. H. Identification of two C-terminal autophosphorylation sites in the PDGF beta-receptor: involvement in the interaction with phospholipase C-gamma. EMBO J. 1992;11:3911–3919. doi: 10.1002/j.1460-2075.1992.tb05484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose J. P., Mollenauer M., Gupta V. A., Stone J., Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol. Cell. Biol. 2005;25:4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D., Margolis B., Mohammadi M., Daly R. J., Daum G., Li N., Fischer E. H., Burgess W. H., Ullrich A., Schlessinger J. SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C gamma. EMBO J. 1992;11:559–567. doi: 10.1002/j.1460-2075.1992.tb05087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen E. J., Schmidt H., Dolcet X., Basson M. A., Jain S., Encinas M. Loss of Sprouty1 rescues renal agenesis caused by Ret mutation. J. Am. Soc. Nephrol. 2009;20:255–259. doi: 10.1681/ASN.2008030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C., Litvak V., Medvedovsky H., Zwang Y., Lev S., Yarden Y. Sprouty fine-tunes EGF signaling through interlinked positive and negative feedback loops. Curr. Biol. 2003;13:297–307. doi: 10.1016/s0960-9822(03)00053-8. [DOI] [PubMed] [Google Scholar]
- Sasaki A., Taketomi T., Kato R., Saeki K., Nonami A., Sasaki M., Kuriyama M., Saito N., Shibuya M., Yoshimura A. Mammalian Sprouty4 suppresses Ras-independent ERK activation by binding to Raf1. Nat. Cell Biol. 2003;5:427–432. doi: 10.1038/ncb978. [DOI] [PubMed] [Google Scholar]
- Saunders B., Lyon S., Day M., Riley B., Chenette E., Subramaniam S., Vadivelu I. The Molecule Pages database. Nucleic Acids Res. 2008;36:D700–706. doi: 10.1093/nar/gkm907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Schonwasser D. C., Marais R. M., Marshall C. J., Parker P. J. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol. Cell. Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim K., Minowada G., Coling D. E., Martin G. R. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev. Cell. 2005;8:553–564. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Sivak J. M., Petersen L. F., Amaya E. FGF signal interpretation is directed by Sprouty and Spred proteins during mesoderm formation. Dev. Cell. 2005;8:689–701. doi: 10.1016/j.devcel.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Liu Y. L., Kim H., Rhee S. G., Kung H. F. Inhibition of serum- and ras-stimulated DNA synthesis by antibodies to phospholipase C. Science. 1990;247:1074–1077. doi: 10.1126/science.2408147. [DOI] [PubMed] [Google Scholar]
- Strathdee C. A., McLeod M. R., Hall J. R. Efficient control of tetracycline-responsive gene expression from an autoregulated bi-directional expression vector. Gene. 1999;229:21–29. doi: 10.1016/s0378-1119(99)00045-1. [DOI] [PubMed] [Google Scholar]
- Tefft D., Lee M., Smith S., Crowe D. L., Bellusci S., Warburton D. mSprouty2 inhibits FGF10-activated MAP kinase by differentially binding to upstream target proteins. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;283:L700–L706. doi: 10.1152/ajplung.00372.2001. [DOI] [PubMed] [Google Scholar]
- Testi R., D'Ambrosio D., De Maria R., Santoni A. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol. Today. 1994;15:479–483. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- Wang D., et al. Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity. 2000;13:25–35. doi: 10.1016/s1074-7613(00)00005-4. [DOI] [PubMed] [Google Scholar]
- Wells A. D., Liu Q. H., Hondowicz B., Zhang J., Turka L. A., Freedman B. D. Regulation of T cell activation and tolerance by phospholipase C gamma-1-dependent integrin avidity modulation. J. Immunol. 2003;170:4127–4133. doi: 10.4049/jimmunol.170.8.4127. [DOI] [PubMed] [Google Scholar]
- Wilde J. I., Watson S. P. Regulation of phospholipase C gamma isoforms in haematopoietic cells: why one, not the other? Cell Signal. 2001;13:691–701. doi: 10.1016/s0898-6568(01)00191-7. [DOI] [PubMed] [Google Scholar]
- Wong E. S., Lim J., Low B. C., Chen Q., Guy G. R. Evidence for direct interaction between Sprouty and Cbl. J. Biol. Chem. 2001;276:5866–5875. doi: 10.1074/jbc.M006945200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.