Abstract
Background
White matter hyperintensity volume (WMHV), cerebral infarcts, and total brain volume (TBV) are associated with cognitive function, but few studies have examined these associations in the general population or whether they differ by race.
Objective
To examine the association of WMHV, cerebral infarcts, and TBV with global cognition and cognition in 5 separate domains in a biracial population sample.
Setting
A biracial community population of Chicago, Illinois.
Design
Cross-sectional population study.
Participants
The study population comprised 575 participants from the Chicago Health and Aging Project (CHAP).
Main Outcome Measures
Volumetric magnetic resonance imaging (MRI) measures of WMHV, TBV, and cerebral infarcts and detailed neuropsychological testing assessments of global cognition and 5 cognitive domains.
Results
Overall and among those without dementia, cognition was inversely associated with WMHV and number of infarcts but was positively associated with TBV. When all 3 measures were simultaneously added to the model, the association of global cognition with WMHV and TBV remained significant and unchanged but was no longer significant with infarcts. Among subjects without dementia, all 3 MRI measures were associated with performance in multiple cognitive domains, specifically perceptual speed. However, among subjects with dementia, only TBV was associated with cognition and performance in multiple cognitive systems. Race did not significantly modify any of these associations.
Conclusions
In this biracial general population sample, the associations of MRI measures with cognition differed according to clinical status of subjects (stronger among subjects without dementia) and were not modified by race. These associations did not affect all cognitive domains equally but were more consistent with impairments in perceptual speed.
WHITE MATTER HYPERintensities (WMHs), cerebral infarcts, and total brain volume (TBV) have been associated with cognitive function,1–7 cognitive decline,8–15 and dementia.2,16,17 Each reportedly affects certain cognitive domains more than others.18–25 Few studies have systematically examined these associations among subjects from the general population with adequate minority representation.4,5,20 We used data from 575 older individuals from a biracial (white and African American) population in the Chicago Health and Aging Project (CHAP), an ongoing epidemiological study of chronic diseases in elderly individuals, to examine the association of each magnetic resonance imaging (MRI) measure with cognition globally and in 5 cognitive domains and whether these associations were modified by race or varied with clinical diagnosis.
METHODS
STUDY POPULATION
CHAP is a longitudinal population study of common chronic health problems among African American and white older adults. Its design and population characteristics have been previously reported.26,27 Each CHAP data collection cycle has (1) an in-home population interview, with brief tests of physical and cognitive function, and (2) clinical evaluation of a stratified random sample (approximately one-sixth) of subjects that includes detailed neuropsychological testing, a neurological examination, medical history, laboratory testing, and expert clinical assessment for dementia. Clinical evaluations usually take place in subjects' homes, conducted by a team of examiners led by a senior neurologist (N.T.A.). Structured neurological examinations and medical histories were performed by specially trained nurse clinicians. A senior neuropsychologist (R.S.W.), blinded to age, sex, race, and clinical data other than the subjects' educational level, occupation, and information about sensory or motor deficits, reviewed the results of 17 cognitive performance tests and summarized impairment in each of 5 areas (orientation, attention, memory, language, and perception). Diagnosis of dementia required the study neurologist's or geriatrician's assessment of loss of cognitive function and impairment in 2 or more areas on cognitive performance testing. The diagnosis of Alzheimer disease (AD) used the criteria of the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association,28 except that subjects who met these criteria and had another condition impairing cognition were retained. Vascular dementia diagnosis followed the National Institute of Neurological Association–Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria.29
Of 1260 persons who completed the clinical evaluation, 663 participated MRI evaluation. Those who did not (n=597) were older (mean [SD] age, 81.5 [6.5] vs 80.1 [5.9] years; t1211=4.1; P>.001); less educated (mean [SD] years of education, 12.4 [3.5] vs 12.9 [3.7]; t1258=−2.3; P=.02); and more likely to be women (396 of 775 women vs 201 of 485 men; ; P=.008). No differences regarding participation were noted for race (P=.74).
The study sample comprised 575 persons for whom complete neuropsychological and MRI data were available for analyses. The 2 groups of persons with and without complete cognitive and/or neuroimaging data did not differ in clinical characteristics (age, education, sex, or race). Those included in these analyses had a mean (SD) age of 79.8 (5.9) years; completed a mean (SD) of 13.0 (3.7) years of education; and 57.0% were women (328 of 575) and 58.3%, African American (335 of 575).
There were 81 dementia cases: 6 non-AD dementia cases (7.4%) (2 vascular dementia, 1 Parkinson disease, and 3 of unknown subtype) and 75 AD dementia cases (92.6%) (61 AD and 14 AD and another condition [9 with a diagnosis of clinical stroke, 4 with depression, and 1 with Parkinson disease]). Because results from analyses performed in the AD dementia group and the full dementia group were comparable (there were too few cases of non-AD dementia to perform analyses), all analyses in this article were performed with the full dementia group. Signed informed consent was obtained from each subject, and the institutional review board of Rush University Medical Center, Chicago, Illinois. approved the study.
COGNITIVE ASSESSMENT
The 17 cognitive function tests used in analyses (Table 1) assessed cognitive abilities that may be differentially affected by aging and AD, including 7 episodic memory measures: Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Word List Memory, recall and recognition,30 and immediate and delayed recall of Story A from the Logical Memory subtest of the Wechsler Memory Scale–Revised31 and the East Boston Story32; 3 semantic memory measures (a 15-item version of the Boston Naming Test,33 verbal fluency, and a short form of the National Adult Reading Tests)34; 3 working memory measures (digit span forward, digit span backward, and digit ordering)35; 2 perceptual speed measures (an oral version of the Symbol Digit Modalities Test36 and Number Comparison Test37); and 2 visuo-spatial ability measures (a short form of the Judgment of Line Orientation Test38 and Standard Progressive Matrices39).
Table 1.
Psychometric Characteristics of Cognitive Tests in a Stratified Random Sample of the Populationa
| Cognitive Domain, Test | Mean (SD) (Range) |
|---|---|
| Episodic memory | |
| Word list memory | 12.45 (6.01) (0–27) |
| Word list recall | 3.11 (2.79) (0–10) |
| Word list recognition | 7.71 (3.10) (0–10) |
| East Boston immediate | 7.07 (3.53) (0–12) |
| East Boston delayed | 6.02 (3.40) (0–12) |
| Logical memory: Ia | 6.65 (4.94) (0–21) |
| Logical memory: IIb | 4.99 (4.68) (0–20) |
| Semantic memory | |
| Boston naming | 11.03 (3.57) (0–15) |
| Category fluency | 22.90 (11.42) (0–58) |
| Reading test | 7.23 (4.95) (0–15) |
| Working memory | |
| Digits span forward | 6.44 (2.51) (0–12) |
| Digits span backward | 4.01 (2.45) (0–12) |
| Digit ordering | 5.03 (2.68) (0–12) |
| Perceptual speed | |
| Symbol digit | 21.52 (15.69) (0–59) |
| Number comparison | 15.54 (9.39) (0–42) |
| Visuospatial ability | |
| Judgment of line orientation | 6.64 (4.62) (0–15) |
| Standard progressive matrices | 7.63 (3.10) (0–12) |
Used in computing zscores for cognitive measures.
In a previous study of 1399 persons without dementia in this cohort,40 we performed a factor analysis of the 17 tests with varimax rotation. The hypothesized factor analytic grouping of these tests showed good agreement with the empirical results of the factor analysis: the Rand statistic,41 a measure of goodness of fit ranging from −1 to 1, was 0.62 (P>.001). To minimize floor and ceiling effects, we constructed summary measures of global cognition and each of 5 cognitive domains into which the tests could hypothetically be grouped, rather than using individual tests scores in analyses. Each summary measure was constructed by converting raw scores from individual tests to z scores using the mean and standard deviation from the baseline clinical evaluation of all participants in the CHAP study and averaging the z scores. Valid summary measures required valid scores on at least half of the component tests. The global cognitive summary averaged the z scores of all 17 tests. Several studies characterizing cognitive function using this approach in this and other cohorts have been previously reported.42–45
MRI EVALUATION
Subjects were imaged on a General Electric 1.5-T scanner (Excite platform, version 11; General Electric Healthcare, Milwaukee, Wisconsin), and the following imaging sequences were obtained: (1) fluid-attenuated inversion recovery (FLAIR): repetition time (TR)=11 000 milliseconds (ms), echo time (TE)=144 ms, inversion time (TI)=2250 ms, 22-cm field of view (FOV), 3-mm slice thickness, 192×256 acquisition matrix; (2) SPGR: TE minimum, 20° flip angle, 24-cm FOV, and 1.5-mm slice thickness, with 256×256 acquisition matrix; and (3) double-spin echo: TR=2100 ms, TE=30/92 ms, 22-cm FOV, and 4-mm slice thickness, with 256/192 acquisition matrix. Images were oriented parallel to a hypothetical line connecting the anterior commissure and posterior commissure. After acquiring the MRI scans, the digital information was transferred to a central laboratory directed by one of us (C.D.) for processing and analysis. Imaging analyses were performed blind to personal identifying information and used QUANTA 6.2, operating on a Sun Microsystems (Santa Clara, California) Ultra 5 workstation.
White matter hyperintensity segmentation was performed using a 2-step process as reported previously.46–48 Briefly, nonbrain elements were manually removed from the image, and the resulting measure of the cranial vault was defined as the total cranial volume to correct for head size differences among subjects. The first step in image segmentation required identifying brain matter. Image intensity nonuniformities were then removed, and the corrected image was modeled as a mixture of 2 gaussian probability functions, with the segmentation threshold determined at the minimum probability between the two.46,49 Once brain matter segmentation was achieved, a single gaussian distribution was fitted to image data, and a segmentation threshold for WMH volume (WMHV) was determined a priori as 3.5 SDs in pixel intensity above the mean of the fitted distribution of brain parenchyma.50 Morphometric erosion of 2 exterior image pixels was also applied to the brain matter image before modeling to remove the effect of partial volume cerebrospinal fluid pixels on WMH determination. White matter hyperintensity volume was calculated as a proportion of total cranial volume (to account for head size variation among participants) and log transformed (natural log) to achieve a normal distribution (skew, −0.21). Total brain volume was computed as the ratio of total brain parenchymal volume to total cranial volume and had approximately a normal distribution (skew, −0.10). The presence or absence of cerebral infarcts was determined manually by the operator based on the lesion's size and imaging characteristics.51 The image analysis system allowed for superimposition of the FLAIR image, proton-density image, and T2-weighted image at 3-times magnified view to assist in interpreting lesion characteristics. Signal void, seen on T2-weighted images, was interpreted to indicate a vessel. Lesions 3 mm or larger were considered brain infarcts. Other necessary imaging characteristics included (1) cerebrospinal fluid density on the subtraction image and (2) whether the infarct was in the basal ganglia, distinct separation from the circle of Willis vessels and perivascular spaces. Interrater reliability for the MRI measures have been previously published,52–54 and intrarater and interrater reliabilities for this study were consistently above 0.90. Because the number of cerebral infarcts had a skewed distribution, for analyses we used as a reference group those without infarcts. We then compared that group with the group with 1 infarct and the group with more than one,55 as well as with those with only small infarcts (<1 cm), only large infarcts (≥1 cm), and both small and large infarcts.
STATISTICAL ANALYSIS
Using linear regression analyses, we first assessed the association of MHV and our measure of TBV with Pearson correlation coefficients and the association of WMHV and TBV with having cerebral infarcts (1 and >1) To test for differences in demographics and neuroimaging measures among African Americans and whites and those with and without dementia, we used t tests or analysis of variance for continuous variables and χ2 tests for categorical data. P<.05 was considered statistically significant unless otherwise specified.
We next assessed the associations of WMHV, TBV, and cerebral infarcts with cognition globally and within each of the 5 cognitive systems, using linear regression analyses, considering each MRI measure individually and the 3 jointly. All core models controlled for age, sex, race, education, and time elapsed between the clinical evaluation and brain MRI. Because there were 6 outcomes, we used a P value of .008 (.05/6) for a 2-sided test as a cutoff score of significance for these analyses. In additional analyses, we evaluated whether these associations varied according to the presence of dementia or by race/ethnicity, using interaction terms. Model assumptions about linearity, normality, independence, and homoscedasticity of errors were assessed graphically and analytically and were adequately met. Analyses were performed using SAS/STAT software version 8.56
RESULTS
Increased WMHV was associated with decreased TBV (r573=−0.22; P<.001) and having single (coefficient estimate [SE], 59.3 [10.4]; P<.001) or multiple (coefficient estimate [SE], 101.5 [14.7]; P<.001) infarcts. Decreased TBV was associated with having multiple infarcts (coefficient estimate [SE], −1.84 [0.58]; P=.002) and showed a trend toward association with having a single infarct (coefficient estimate [SE], −0.72 [0.41]; P=.08). Increased age was associated with higher WMHV (r573=0.31; P<.001), lower TBV (r573=−0.41; P<.001), and having 1 infarct (F2,577=3.43; P=.03). Lower levels of education were associated with lower TBV (r573=0.09; P=.04) and having 1 infarct (F2,577=4.4; P=.01). Male sex was associated with lower TBV (t573=5.46; P<.001).
Demographic and neuroimaging characteristics of the sample are given in Table 2, stratified by race. African Americans had fewer years of education and lower cognitive test scores. There was a trend toward a greater proportion of whites with multiple infarcts, but no significant differences were noted in any neuroimaging measures across the racial groups.
Table 2.
Demographic, Neuropsychological, and Magnetic Resonance Imaging Characteristics of 575 Participants Stratified by Race/Ethnicity
| Variable | All Subjects (N = 575) | African American (n = 335) | White (n = 240) | P Value for Differencea |
|---|---|---|---|---|
| Age, mean (SD), y | 79.8 (5.9) | 79.5 (6.0) | 80.1 (5.8) | .22 |
| Education, mean (SD), y | 13.0 (3.7) | 11.6 (3.3) | 14.9 (3.4) | <.001 |
| MMSE score (maximum = 30), mean (SD) | 26.2 (3.8) | 25.4 (4.0) | 27.3 (3.4) | <.001 |
| Global cognitive score, mean (SD) | 0.46 (0.6) | 0.74 (0.5) | 0.74 (0.5) | <.001 |
| WMHV, mean (SD) | −5.13 (1.09) | −5.16 (1.10) | −5.09 (1.07) | .49 |
| TBV, mean (SD) | 74.29 (5.85) | 74.94 (4.72) | 74.00 (4.40) | .20 |
| Male sex, No. (%) | 247 (43.0) | 146 (43.6) | 101 (42.1) | .62 |
| 1 Infarct, No. (%) | 108 (18.8) | 62 (18.5) | 45 (18.8) | NA |
| >1 Infarct, No. (%) | 47 (8.2) | 21 (6.3) | 27 (11.3) | .09b |
Abbreviations: MMSE, Mini-Mental State Examination; NA, not applicable; TBV, total brain volume (TBV = total brain parenchymal volume/total cranial volume); WMHV, white matter hyperintensity volume (WMHV = natural log [white matter hyperintensity volume/total cranial volume]).
Pvalue based on t test for continuous variables and χ2 test for categorical variables.
Based on group by number of infarcts (0, 1, or >1); χ2 association test.
MRI MEASURES AND GLOBAL COGNITIVE FUNCTION
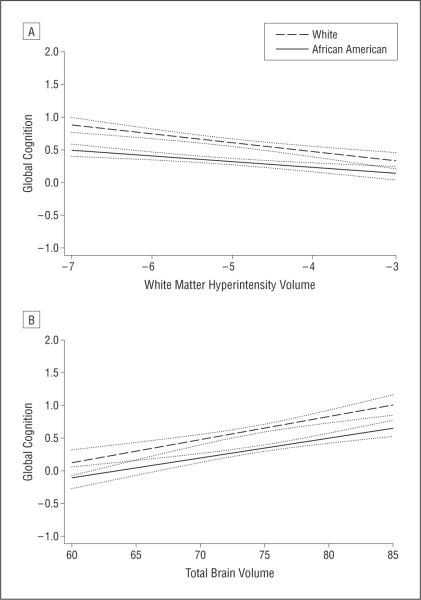
Among all subjects, higher WMHV and having more than 1 infarct were associated with lower global cognitive function, and higher TBV was associated with better cognitive function (Table 3). When all 3 MRI measures were considered simultaneously in a model, the associations of WMHV (adjusted estimate [SE], −0.109 [0.018]; P<.001) and TBV (adjusted estimate [SE], 0.031 [0.005]; P<.001) with global cognition were not substantially changed, but infarcts were no longer associated with global cognition (adjusted estimate [SE], −0.050 [0.066]; P=.45). For all 3 MRI measures, the association with global cognitive function was not modified by race/ethnicity (Table 4). The Figure shows predicted cognition vs each MRI measure for African Americans and whites. Although the intercepts of the 2 lines differed significantly, the relationship between MRI measure and cognition was similar in both groups.
Table 3.
Association of Magnetic Resonance Imaging Measures to Global Cognitive Function and 5 Different Cognitive Domainsa
| Total Sample (N = 575) |
No Dementia (n = 494) |
Dementia (n = 81) |
||||
|---|---|---|---|---|---|---|
| Cognitive Function, Model Term | Estimate (SE) | P Value | Estimate (SE) | P Value | Estimate (SE) | P Value |
| Global cognitive function | ||||||
| WMHV | −0.108 (0.018) | <.001 | −0.058 (0.015) | <.001 | −0.035 (0.049) | .48 |
| 1 Infarct | 0.043 (0.047) | .37 | 0.016 (0.039) | .68 | 0.090 (0.117) | .43 |
| >1 Infarct | −0.215 (0.067) | .002 | −0.154 (0.059) | .009 | −0.157 (0.138) | .26 |
| TBV | 0.032 (0.005) | <.001 | 0.017 (0.004) | <.001 | 0.035 (0.009) | <.001 |
| Episodic memory | ||||||
| WMHV | −0.125 (0.022) | <.001 | −0.080 (0.020) | <.001 | 0.033 (0.064) | .61 |
| 1 Infarct | 0.104 (0.059) | .08 | 0.045 (0.051) | .38 | 0.282 (0.152) | .07 |
| >1 Infarct | −0.238 (0.085) | .005 | −0.159 (0.077) | .04 | −0.249 (0.174) | .16 |
| TBV | 0.029 (0.006) | <.001 | 0.010 (0.006) | .07 | 0.034 (0.012) | .007 |
| Semantic memory | ||||||
| WMHV | −0.099 (0.023) | <.001 | −0.047 (0.021) | .03 | −0.115 (0.080) | .16 |
| 1 Infarct | 0.040 (0.059) | .054 | 0.019 (0.054) | .718 | −0.038 (0.187) | .84 |
| >1 Infarct | −0.179 (0.084) | .04 | −0.155 (0.081) | .06 | −0.126 (0.215) | .56 |
| TBV | 0.031 (0.006) | <.001 | 0.017 (0.006) | .004 | 0.047 (0.014) | .001 |
| Working memory | ||||||
| WMHV | −0.022 (0.024) | .006 | −0.022 (0.024) | .37 | −0.036 (0.075) | .63 |
| 1 Infarct | −0.019 (0.063) | .76 | −0.040 (0.063) | .53 | −0.031 (0.182) | .87 |
| >1 Infarct | −0.051 (0.216) | .03 | −0.170 (0.096) | .08 | −0.051 (0.216) | .81 |
| TBV | 0.029 (0.006) | <.001 | 0.016 (0.070) | .02 | 0.035 (0.015) | .02 |
| Perceptual speed | ||||||
| WMHV | −0.156 (0.028) | <.001 | −0.103 (0.028) | <.003 | −0.011 (0.071) | .88 |
| 1 Infarct | −0.205 (0.172) | .57 | 0.033 (0.074) | .65 | 0.205 (0.172) | .24 |
| >1 Infarct | −0.375 (0.106) | 0.1 | −0.325 (0.116) | .004 | −0.102 (0.199) | .61 |
| TBV | 0.053 (0.007) | <.001 | 0.041 (0.008) | <.001 | 0.033 (0.014) | .02 |
| Visuospatial ability | ||||||
| WMHV | −0.110 (0.024) | <.001 | −0.066 (0.024) | .006 | −0.098 (0.077) | .21 |
| 1 Infarct | −0.051 (0.063) | .43 | −0.069 (0.626) | .27 | −0.060 (0.195) | .76 |
| >1 Infarct | −0.123 (0.091) | .18 | −0.088 (0.095) | .36 | 0.058 (0.225) | .80 |
| TBV | 0.033 (0.006) | <.001 | 0.022 (0.007) | <.001 | 0.032 (0.015) | .04 |
Abbreviations: TBV, total brain volume (TBV = total brain parenchymal volume/total cranial volume); WMHV, white matter hyperintensity volume (WMHV = natural log [white matter hyperintensity volume/total cranial volume]).
Estimated from separate linear regression models that controlled for age, sex, education, race, and time elapsed between clinical evaluation and magnetic resonance imaging. The α level was set at .008 to account for the 6 outcome measures (ie, .05/6 = .008).
Table 4.
Linear Regression Models Examining the Associations of MRI Measures With Global Cognitive Function and Potential Modification by Race/Ethnicitya
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| Model Terms | Estimate (SE) | P Value | Estimate (SE) | P Value |
| Race | −0.295 (0.040) | <.001 | −0.045 (0.174) | .80 |
| WMHV | −0.108 (0.018) | <.001 | −0.137 (0.027) | <.001 |
| WMHV × race | NA | NA | 0.049 (0.033) | .14 |
| Race | −0.302 (0.040) | <.001 | 0.071 (0.589) | .90 |
| TBV | 0.032 (0.005) | <.001 | 0.035 (0.007) | <.001 |
| TBV × race | NA | NA | −0.005 (0.008) | .53 |
| Race | −0.300 (0.042) | <.001 | −0.274 (0.047) | <.001 |
| 1 Infarct | −0.215 (0.068) | .002 | −0.042 (0.095) | .66 |
| >1 Infarct | NA | NA | −0.216 (0.136) | .11 |
Abbreviations: MRI, magnetic resonance imaging; NA, not applicable; TBV, total brain volume (TBV = total brain parenchymal volume/total cranial volume); WMHV, white matter hyperintensity volume (WMHV = natural log [white matter hyperintensity volume/total cranial volume]).
For each MRI measure, model 2 adds an interaction term to assess whether race modifies the association of each measure with level of cognitive function. All models controlled for age, sex, race, education, and time elapsed between the clinical evaluation and MRI.
Figure.
Predicted relation between white matter hyperintensity volume and global cognitive function (A) and total brain volume and global cognitive function (B) for African Americans (solid line) and whites (dashed line). White matter hyperintensity volume=natural log (white matter hyperintensity volume/total cranial volume). Total brain volume=total brain parenchymal volume/total cranial volume. Dotted lines are 95% confidence intervals for the regression line.
In separate analyses of those with (n=81) and without (n=494) dementia, subjects with dementia were older, had fewer years of education, were more likely to be African American, and had lower WMHV and TBV (Table 5). Overall, the associations of MRI measures with global cognition were stronger among subjects without dementia (Table 3). Among persons without dementia, those with higher WMHV had a strong inverse association with global cognition, but among persons with dementia, the association was not significant. Having multiple infarcts also had an inverse association with global cognition among subjects without dementia but not those with dementia. Because infarct size is thought to be associated with poor cognitive function and dementia, we conducted additional analyses in our dementia and nondementia groups with a measure of infarct size. There was no association of infarct size with global cognition in both the nondementia and dementia groups (results not shown). Total brain volume was positively associated with better cognition among those both with dementia and without.
Table 5.
Sample Characteristics for Persons According to the Presence or Absence of Dementia
| Variable | No Dementia (n = 494) | Dementia (n = 81) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 79.5 (5.9) | 81.8 (5.4) | <.001 |
| Education, mean (SD), y | 13.1 (3.6) | 11.8 (4.3) | .006 |
| WMHV, mean (SD) | −5.22 (1.08) | −4.50 (0.93) | <.001 |
| TBV, mean (SD) | 74.65 (4.4) | 71.97 (5.3) | <.001 |
| Male sex, No. (%) | 213 (43.1) | 34 (42.0) | .98 |
| African American, No. (%) | 280 (56.7) | 55 (67.9) | .02 |
| 1 Infarct, No. (%) | 93 (18.8) | 14 (17.3) | NA |
| >1 Infarct, No. (%) | 37 (7.5) | 11 (13.5) | .18a |
Abbreviations: NA, not applicable; TBV, total brain volume (TBV = total brain parenchymal volume/total cranial volume); WMHV, white matter hyperintensity volume (WMHV = natural log [white matter hyperintensity volume/total cranial volume]).
Based on group by number of infarcts (0, 1, or >1); χ2 association test.
Among subjects without dementia, if all 3 MRI measures were considered together in a single model, the association of cerebral infarcts with cognitive function was no longer significant, whereas the association of WMHV and TBV with cognition remained significant and essentially unchanged (Table 6).
Table 6.
Association of WMHV, TBV, and Multiple Infarcts With Global Cognitive Functiona
| Model, Predictors | Estimate (SE) | P Value |
|---|---|---|
| Model 1 | ||
| WMHV | −0.058 (0.015) | <.001 |
| Model 2 | ||
| WMHV | −0.056 (0.015) | <.001 |
| TBV | 0.016 (0.004) | <.001 |
| Model 3 | ||
| WMHV | −0.055 (0.016) | <.001 |
| TBV | 0.017 (0.004) | <.001 |
| >1 Infarct | −0.085 (0.060) | .16 |
Abbreviations: TBV, total brain volume (TBV = total brain parenchymal volume/total cranial volume); WMHV, white matter hyperintensity volume (WMHV = natural log [white matter hyperintensity volume/total cranial volume]).
Estimated from separate linear regression models that controlled for age, sex, education, race, and time elapsed between clinical evaluation and magnetic resonance imaging.
MRI MEASURES AND AREAS OF COGNITIVE FUNCTION
In persons without dementia, having multiple cerebral infarcts was inversely associated with perceptual speed but not with performance in any other cognitive domain (Table 3). In separate analyses, we added a term for our measures of infarct size. Overall, there was no association of infarct size with any of the 5 cognitive domains in both the nondementia and dementia groups (results not shown). The association of WMHV with cognitive domain performance appeared stronger among persons without dementia. There were significant associations noted with performance in 3 of the 5 domains in this subgroup but with none of the 5 domains among persons with dementia.
COMMENT
In this cross-sectional study of more than 575 elderly individuals from a biracial population sample, we found that when considered singly, WMHV, TBV, and having multiple cerebral infarcts were associated with global cognitive function. Overall and among those without dementia, cognition was inversely associated with WMHV and number of infarcts but was positively associated with TBV. When all 3 measures were simultaneously added to the model, the association of global cognition with WMHV and TBV remained significant and unchanged but was no longer significant with infarcts. There was a non-significant trend for having multiple infarcts in a higher proportion of white subjects, but WMHV was similar in both groups. While African Americans performed more poorly on all cognitive tests, the associations of all 3 MRI measures with cognitive function were not modified by race. Finally, the inverse associations of both WMHV and multiple infarcts with global cognition were much stronger among subjects without dementia, and the strength of the associations with cognition varied across specific cognitive domains.
Few studies have examined the associations of multiple MRI measures with cognitive function in general population samples of older persons. Results of the 3 studies published in the last decade suggest that WMHV, TBV, and infarcts each are associated with cognitive function, but when considered simultaneously, such associations are mixed.2,17 One study found that the association of cognition with WMHV was independent of its association with infarcts but not with TBV17; another found that the association of global cognition with infarcts was independent of both its associations with TBV and WMHV.2 Neither of these studies used quantitative measures of WMHV or TBV; thus, the extent to which the differences in findings are attributable to methodological differences is uncertain. The results presented from this study, that the strength of the association of WMHV with cognitive function was unchanged in the presence of cerebral infarcts, are in keeping with those of others5 and suggest that WMHV may better reflect overall vascular brain injury and therefore have a stronger association with cognitive function.
The association of WMHV with global cognition and with performance in specific cognitive domains (episodic memory, perceptual speed, and visuospatial ability) was much stronger among subjects without dementia. A possible partial explanation is the disparity in these groups' sizes (81 subjects with dementia and 494 without). Another possibility, however, is that the association of WMHV with cognition may be more important at earlier stages (ie, before subjects meet criteria for clinical diagnosis of dementia). Recent data suggest that higher WMHV is associated with increased risk of progression from no cognitive impairment to mild cognitive impairment but not from mild cognitive impairment to dementia.13,57 The underlying mechanism may relate to vascular brain injury, in view of the association of WMHV with vascular disease10,23,48,51 and the reported association of vascular disease and vascular risk factors with impaired cognition.25 White matter hyperintensities are thought to reflect diffuse ischemic changes in the brain, which disrupt both the frontal subcortical circuits that subserve predominantly executive cognitive abilities58–60 and the connections between the frontal lobes and other cortical regions. White matter hyperintensity distribution over many anatomical areas of the brain could explain the correspondingly wide effects on the cognitive systems subserved by these areas, with maximal effects occurring before the pathological processes associated with dementia (reflected by brain volume loss) begin. Also possible is that many subjects with dementia had mixed pathological features61 including AD. This could explain the continued significant association between brain atrophy and cognition, but not WMHs or infarcts and cognition, in this subgroup, since brain atrophy is affected by both pathological processes.62
Some studies suggest that WMHs and TBV are associated with function in multiple cognitive domains,18,19,23,24 in contrast to cerebral infarcts, which affect predominantly measures of executive function.2,12,25 Our results generally agree: the measure of executive function performance (perceptual speed) was the domain most consistently associated with each MRI measure. We found that having multiple infarcts was associated with performance in episodic memory (typically prominently affected in AD) in the entire sample, and preliminary data suggest a similar, albeit weaker, association among subjects without dementia. These results are similar to those reported from 2 recent studies in older persons without dementia.63,64 The underlying mechanisms by which cerebral infarcts could affect cognition in elderly individuals, specifically in the domain of memory, are unclear; one possibility is that infarcts have an additive effect on subclinical preexisting AD pathology that varies with the anatomical locations of the infarcts. Although a high proportion of our subjects without dementia (130 of 494) had infarcts seen on MRI, information on the location of these infarcts will be needed to address this issue further.
Few studies have examined differences in brain MRI findings between African Americans and whites.7,62,65-67
In the present study, we examined 2 potential differences: first, differences in the amount or degree of a brain measure between these racial groups, and second, differences according to race in the association of brain MRI measures with cognition. Unlike other studies suggesting that both WMHs and cerebral infarcts66,67 may be more prevalent among African Americans, in this sample we found a trend toward a higher proportion of whites with multiple infarcts. One possible explanation is that the overall occurrence of vascular risk factors in African Americans in our sample may have been lower than in previous studies. Further studies in this sample are planned to closely examine the association of vascular disease and risk factors and their associations with brain MRI measures.
Consistent with a previous study,5 the association of each MRI measure with cognitive function was similar between African Americans and whites. Despite differences in baseline cognitive test results that persisted after controlling for educational achievement, both groups showed similar associations between cognitive performance and MRI pathological measures, suggesting that race itself does not modify the association of MRI measures with cognitive function. Longitudinal assessment of cognitive function in relation to brain measures is needed to further examine these preliminary observations.
This study has important strengths and limitations. Strengths include the inclusion of a large, well-characterized, biracial cohort of older persons; the use of previously established and validated measures of cognitive function; and the use of identical neuroimaging protocols at the same MRI facility. Limitations include the cross-sectional design, losses to MRI participation, and use of rough measures of infarct status (number and size vs volume measurements). Finally, though this study examined the association of multiple MRI measures with cognitive function in persons with dementia (the majority of which were AD), the paucity of cases of mixed dementia and vascular dementia limited our ability to perform specific analyses to examine the association between multiple MRI measures and cognitive function in these dementia subtypes. It will be important to examine these associations in future studies with larger sample sizes.
Acknowledgments
Funding/Support: This study was supported by grant AG11101 from the National Institute on Aging, Bethesda, Maryland.
Footnotes
Additional Contributions: Ann Marie Lane assisted in community development and oversight of the project coordination; Michelle Bos, Jennifer Tarpey, and Colleen Plunkett assisted in coordination of the study; Deborah Holub and Sandra Horowitz, MD, assisted in the coordination of the neuroimaging evaluation; and Todd Beck assisted in the statistical programming.
Financial Disclosure: None reported.
REFERENCES
- 1.van der Flier WM, van der Vlies AE, Weverling-Rijnsburger AW, et al. MRI measures and progression of cognitive decline in nondemented elderly attending a memory clinic. Int J Geriatr Psychiatry. 2005;20(11):1060–1066. doi: 10.1002/gps.1392. [DOI] [PubMed] [Google Scholar]
- 2.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 3.Wright CB, Festa JR, Paik MC, et al. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke. 2008;39(3):800–805. doi: 10.1161/STROKEAHA.107.484147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seshadri S, Wolf PA, Beiser A, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004;63(9):1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 5.Mosley TH, Jr, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities study. Neurology. 2005;64(12):2056–2062. doi: 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- 6.Verdelho A, Madureira S, Ferro JM, et al. LADIS Study. Differential impact of cerebral white matter changes, diabetes, hypertension and stroke on cognitive performance among non-disabled elderly: the LADIS study. J Neurol Neurosurg Psychiatry. 2007;78(12):1325–1330. doi: 10.1136/jnnp.2006.110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.López OL. Classification of mild cognitive impairment in a population study [in Spanish] Rev Neurol. 2003;37(2):140–144. [PubMed] [Google Scholar]
- 8.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Carmelli D. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol. 2001;58(4):643–647. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 9.Swan GE, DeCarli C, Miller BL, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51(4):986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- 10.DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45(11):2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 11.Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64(6):1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- 12.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128(Pt 9):2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 13.Smith EE, Egorova S, Blacker D, et al. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol. 2008;65(1):94–100. doi: 10.1001/archneurol.2007.23. [DOI] [PubMed] [Google Scholar]
- 14.Longstreth WT, Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33(10):2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 15.De Groot JC, De Leeuw FE, Oudkerk M, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol. 2002;52(3):335–341. doi: 10.1002/ana.10294. [DOI] [PubMed] [Google Scholar]
- 16.Bigler ED, Lowry CM, Kerr B, et al. Role of white matter lesions, cerebral atrophy, and APOE on cognition in older persons with and without dementia: the Cache County, Utah, study of memory and aging. Neuropsychology. 2003;17(3):339–352. doi: 10.1037/0894-4105.17.3.339. [DOI] [PubMed] [Google Scholar]
- 17.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61(10):1531–1534. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- 18.Burton EJ, Kenny RA, O'Brien J, et al. White matter hyperintensities are associated with impairment of memory, attention, and global cognitive performance in older stroke patients. Stroke. 2004;35(6):1270–1275. doi: 10.1161/01.STR.0000126041.99024.86. [DOI] [PubMed] [Google Scholar]
- 19.Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41(14):1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 20.Au R, Massaro JM, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63(2):246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 21.Wahlund LO, Basun H, Almkvist O, Andersson-Lundman G, Julin P, Sääf J. White matter hyperintensities in dementia: does it matter? Magn Reson Imaging. 1994;12(3):387–394. doi: 10.1016/0730-725x(94)92531-3. [DOI] [PubMed] [Google Scholar]
- 22.Carmelli D, Swan GE, Reed T, Wolf PA, Miller BL, DeCarli C. Midlife cardiovascular risk factors and brain morphology in identical older male twins. Neurology. 1999;52(6):1119–1124. doi: 10.1212/wnl.52.6.1119. [DOI] [PubMed] [Google Scholar]
- 23.Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Carmelli D. Biobehavioral characteristics of nondemented older adults with subclinical brain atrophy. Neurology. 2000;54(11):2108–2114. doi: 10.1212/wnl.54.11.2108. [DOI] [PubMed] [Google Scholar]
- 24.Breteler MM, van Amerongen NM, van Swieten JC, et al. Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging: the Rotterdam Study. Stroke. 1994;25(6):1109–1115. doi: 10.1161/01.str.25.6.1109. [DOI] [PubMed] [Google Scholar]
- 25.Price TR, Manolio TA, Kronmal RA, et al. CHS Collaborative Research Group. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. The Cardiovascular Health Study. Stroke. 1997;28(6):1158–1164. doi: 10.1161/01.str.28.6.1158. [DOI] [PubMed] [Google Scholar]
- 26.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP) J Alzheimers Dis. 2003;5(5):349–355. doi: 10.3233/jad-2003-5501. [DOI] [PubMed] [Google Scholar]
- 27.Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60(2):185–189. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 28.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 29.Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D. Wechsler Memory Scale—Revised Manual. Psychological Corporation; New York, NY: 1987. [Google Scholar]
- 32.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci. 1991;57(3-4):167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lea and Febige; Philadelphia, PA: 1983. [Google Scholar]
- 34.Nelsen HE. National Adult Reading Test (NART): Test Manual. NFER-Nelsen Publishing Co Ltd; Windsor, England: 1982. [Google Scholar]
- 35.Cooper JA, Sagar HJ. Incidental and intentional recall in Parkinson's disease: an account based on diminished attentional resources. J Clin Exp Neuropsychol. 1993;15(5):713–731. doi: 10.1080/01688639308402591. [DOI] [PubMed] [Google Scholar]
- 36.Smith A. Symbol Digit Modalities Test Manual—Revised. Western Psychological; Los Angeles, CA: 1984. [Google Scholar]
- 37.Ekstrom R, French J, Harman H, Dermen D. Manual for Kit of Factor Reference Cognitive Test. Education Testing Service; Princeton, NJ: 1976. [Google Scholar]
- 38.Benton AL, Varney NR, Hamsher KD. Visuospatial judgment: a clinical test. Arch Neurol. 1978;35(6):364–367. doi: 10.1001/archneur.1978.00500300038006. [DOI] [PubMed] [Google Scholar]
- 39.Raven J, Court J, Raven J. Standard Progressive Matrices. Oxford Psychologists Press; Oxford, England: 1992. [Google Scholar]
- 40.Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, Mendes de Leon C, Evans DA. Biracial population study of mortality in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2009;66(6):767–772. doi: 10.1001/archneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rand WM. Objective criteria for the evaluation of clustering methods. J Am Stat Assoc. 1971;66(336):847–850. [Google Scholar]
- 42.Wilson RS, Bienias JL, Evans DA, Bennett DA. Religious Orders Study: overview and change in cognitive and motor speed. Aging Neuropsych and Cognition. 2004;11(2&3):280–303. [Google Scholar]
- 43.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179–193. [PubMed] [Google Scholar]
- 44.Wilson R, Barnes L, Bennett D. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol. 2003;25(5):634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- 45.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 46.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr. 1992;16(2):274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 47.DeCarli C, Murphy DG, Teichberg D, Campbell G, Sobering GS. Local histogram correction of MRI spatially dependent image pixel intensity nonuniformity. J Magn Reson Imaging. 1996;6(3):519–528. doi: 10.1002/jmri.1880060316. [DOI] [PubMed] [Google Scholar]
- 48.DeCarli C, Miller BL, Swan GE, et al. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30(3):529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 49.Murphy DG, DeCarli C, Schapiro MB, Rapoport SI, Horwitz B. Age-related differences in volumes of subcortical nuclei, brain matter, and cerebrospinal fluid in healthy men as measured with magnetic resonance imaging. Arch Neurol. 1992;49(8):839–845. doi: 10.1001/archneur.1992.00530320063013. [DOI] [PubMed] [Google Scholar]
- 50.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36(1):50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeCarli C, Reed T, Miller BL, Wolf PA, Swan GE, Carmelli D. Impact of apolipo-protein E epsilon4 and vascular disease on brain morphology in men from the NHLBI twin study. Stroke. 1999;30(8):1548–1553. doi: 10.1161/01.str.30.8.1548. [DOI] [PubMed] [Google Scholar]
- 52.Murphy DG, DeCarli C, McIntosh AR, et al. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53(7):585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- 53.DeCarli C, Murphy DG, Gillette JA, et al. Lack of age-related differences in temporal lobe volume of very healthy adults. AJNR Am J Neuroradiol. 1994;15(4):689–696. [PMC free article] [PubMed] [Google Scholar]
- 54.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60(7):1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- 56.SAS Institute Inc . SAS/STAT(r) User's Guide, Version 8. SAS Institute Inc; Cary, NC: 2000. [Google Scholar]
- 57.DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63(2):220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Englund E. Neuropathology of white matter lesions in vascular cognitive impairment. Cerebrovasc Dis. 2002;13(suppl 2):11–15. doi: 10.1159/000049144. [DOI] [PubMed] [Google Scholar]
- 59.Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109(5-6):813–836. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- 60.Pantoni L, Palumbo V, Sarti C. Pathological lesions in vascular dementia. Ann N Y Acad Sci. 2002;977:279–291. doi: 10.1111/j.1749-6632.2002.tb04827.x. [DOI] [PubMed] [Google Scholar]
- 61.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 62.Jagust WJ, Zheng L, Harvey DJ, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63(1):72–80. 18157909. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer's disease pathology, and memory function in older persons. Ann Neurol. 2007;62(1):59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- 64.Reitz C, Luchsinger JA, Tang MX, Manly J, Mayeux R. Stroke and memory performance in elderly persons without dementia. Arch Neurol. 2006;63(4):571–576. doi: 10.1001/archneur.63.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao D, Cooper L, Cai J, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology. 1997;16(3):149–162. doi: 10.1159/000368814. [DOI] [PubMed] [Google Scholar]
- 66.Bryan CS. Race and health care. J S C Med Asssoc. 1999;95(3):116–118. [PubMed] [Google Scholar]
- 67.Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65(8):1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]