Abstract
Strategies were developed by which mesoporous microparticles were modified on their external surfaces with tetraethylene glycol (TEG), a protein, or both, leaving the pore surfaces available for modification with a separate moiety, such as a dye. Only particles bifunctionally modified with both TEG and a cell-specific antibody were taken up specifically by a targeted cancer cell line. In contrast to similarly functionalized nanoparticles, endocytosed microparticles were not contained within a lysosome.
Keywords: biomedical applications, drug delivery, silica, surface modification, porous materials
Introduction
Modification with ethylene glycol oligomers is a commonly used method to improve the biocompatibility and retention of biomaterials in living systems.1–3 In addition, tissue-specific delivery agents offer precise delivery of therapeutic payloads, guided by targeting groups such as folate,4,5 antibodies,6–8 peptides,9 or carbohydrates.10,11 Many researchers have sought means of combining both types of functional groups to achieve combined biocompatibility and targeting. In our group, we use mesoporous silica particles, which carry the additional challenge of modifying the internal (pore) and external surfaces of porous silica particles with different functional groups. For example, pore-blocking with structure-directing agents12 or bulky groups13,14 can be used to preferentially modify only the exteriors of particles, and Bein has elegantly shown that the radial distribution of multiple functional groups can be controlled in porous silica over a range of particle sizes.15–17 Unfortunately, current methods for adding multiple functional groups to the outside of mesoporous silica particles involve simultaneously grafting two types of organosilanes to the silica, which cannot provide a controlled spatial distribution of functional groups. Controlling the location and type of multiple functionalities is important in the development of mesoporous silica particles as drug delivery devices.
Nanoparticle-based drug-delivery agents have made strides in the past decade,18,19 but questions remain about the acute and/or chronic toxicities of nanoparticles.20–23 As an alternative to nanoparticles, particles with diameters in the micrometer range (microparticles) could avoid many of the toxicity issues of nanoparticles while retaining the ability to be functionalized with the moieties for cell uptake and targeting that are important in drug delivery. Although a variety of mesoporous particles are available for these experiments, we used a type of mesoporous silica called APMS (acid-prepared mesoporous spheres).13,24–27 This material has a spherical particle morphology that is easily observed by microscopy, and a particle diameter that can be varied from 1 to 10 μm by simple manipulation of synthesis conditions. In addition, the pore structure is disordered and highly interconnected, allowing molecules to diffuse easily throughout the particles’ interiors, and the pore diameter can also be varied between 2 and 10 nm. Other microparticles for drug delivery have previously been prepared from biodegradable polymers,28,29 but mesoporous silica microparticles are an especially attractive delivery device because the large internal pore volume and surface area of these materials allow large amounts of molecular material to be adsorbed and released. In contrast to crystalline silicas,30,31 numerous studies have shown no adverse long-term health effects or developmental effects due to exposure to amorphous silicas by several routes of administration.32–36 Moreover, silica can be easily modified for tissue-specific targeting using a wide array of functionalization strategies.37,38
In a recent report, we showed that a surface modification with a short poly(ethylene glycol) chain, tetraethylene glycol (TEG), allowed APMS to be readily taken up by malignant mesothelioma (MM) cells in vitro and in vivo without any adverse effects.39 TEG enhanced the fusion of the particles with plasma membranes and facilitated uptake by cells. In related work, we found that TEG-modified APMS loaded with the chemotherapeutic doxorubicin were 30 to 50 times more effective in killing MM cells in vitro; fluorescence HPLC studies indicated that this was due to the fact that approximately twice as much doxorubicin was present within the cells when APMS was used as a delivery device.40 In these past experiments, only uptake (not targeting) was studied; thus, bifunctional particles modified with both TEG and a targeting antibody were not required. Herein, we describe the synthesis of porous silica microparticles with exterior surfaces that were bifunctionally modified with a biocompatible polymer and a targeting antibody, and we show that the bifunctional modification was necessary for targeted uptake to specific cancer cells.
Experimental Section
Materials and Methods
Unless otherwise noted, reagents were purchased from commercial sources and used as received. Triethylamine (Et3N), hexane, toluene, and methylene chloride (CH2Cl2) were distilled prior to use, acetonitrile (MeCN) was stored over molecular sieves, the monoclonal anti-mesothelin antibody ME141 was obtained from Affinity BioReagentsTM, and Fmoc-APTES was synthesized as reported previously.13 For scanning electron microscopy, specimens were mounted on aluminum specimen stubs using conductive graphite paint, were sputter-coated for 4 min with Au/Pd in a Polaron sputter coater (Model 5100), and were examined with a JSM 6060 SEM (JEOL USA, Inc., Peabody, MA). Transmission electron microscopy samples were fixed with Karnovsky’s fixative, stained with OsO4, and embedded in Spurr’s resin. Samples were microtomed, counter-stained with uranyl acetate, and imaged with a JEM 1210 TEM (JEOL USA, Inc.) operating at an accelerating voltage of 60kV. For confocal laser scanning microscopy (CLSM), cells were grown on cover slips, treated with the desired agents, and fixed and stained at various time points using paraformaldehyde and 4′,6-diamidino-2-phenylindole (DAPI). Microscopy was performed on a Zeiss LSM 510 META using excitation lasers at 405, 488, and/or 568 nm and oil immersion objectives of 63 or 100 X magnification. Flow cytometry was performed on single cell suspensions using a BD LSRII flow cytometer (BD Biosciences, San Jose, CA) equipped with a Sapphire 488 (Coherent, Santa Clara, CA) laser and a solid state Xcite (Lightware) laser emitting at 355 nm. See the supporting information for complete instrumental details.
Preparation of Bifunctional APMS
APMS particles were synthesized as previously reported39. Silica nanoparticles were prepared according to the method of Stöber42 and were subsequently modified with thiols by reaction with 3-mercaptopropyltrimethoxy silane in MeCN (50 °C for 18 hr) and recovery by centrifugation (Biofuge Pico, Thermo Scientific). The remainder of the sequence proceeded as described in the body text, using standard bioconjugate chemistry techniques. Complete details of the individual steps are available in the supporting information.
Cell culture
Cells were propagated in DMEM/F-12 50/50 medium supplemented with 10% fetal bovine serum, 0.1 μg/mL hydrocortisone, 2.5/mL insulin, 2.5 μg/mL transferrin, 2.5 ng/mL sodium selenite, and penicillin-streptomycin (50 U/mL penicillin G, 50 ug/mL streptomycin sulfate). Cells were grown to near confluence, the medium was removed, and medium containing 0.5% FBS was added 24 h before exposure to agents. Control dishes received medium without agents. For treatment to cells, APMS particles were resuspended in medium at a concentration of approximately 6 × 107 particles per 100 μL, mixed well, and sonicated for 5 s to disperse clumps. 50 μL of this solution were then added to cells in 12-well plates, in 2 mL media, to give a final concentration of APMS particles of 1.5 × 107 particles/mL (or 3 × 107 particles per well). For the co-culture experiments, MM and A549 cells were plated onto coverslips in separate 12-well plates. Cell monolayers on glass coverslips were allowed to grow to near-confluence. The cell coverslips were then placed in the same dish and switched to DMEM/F-12 50/50 medium containing 0.5 % FBS for 24 h before APMS-TEG(BSA) or APMS-TEG(ME1) (0.1 mg particles per mL of medium, in the same medium as the cells) was added, to give a final particle concentration of 1.5×107 particles/mL. At specific time points, the cells were harvested for analysis by flow cytometry.
Results and Discussion
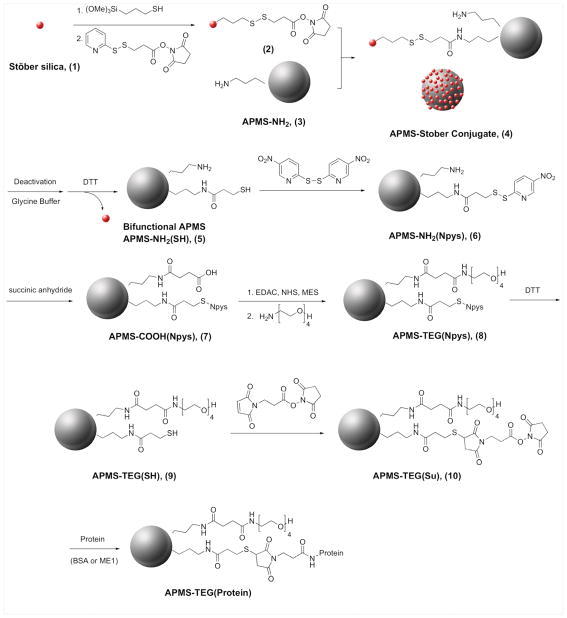
To prepare particles with bifunctionally-modified surfaces in a controlled manner, we developed a scheme that used a well-known, dense nanoparticulate type of silica first described by Stöber et al. to selectively modify the external surface of APMS (Scheme 1). Stöber silica42 and APMS39 were synthesized by previously described methods. In brief, Stöber silica (1), ~100 nm in diameter, was reacted with mercaptopropyltrimethoxysilane and then with a heterobifunctional linker (N-succinimidyl-3-(2-pyridyldithio) propionate, SPDP), producing a succinimidyl ester connected to the nanoparticle through a disulfide bridge (2). Separately, APMS was reacted with aminopropyltriethoxysilane, resulting in surface modification both inside and outside the particle (3). As shown by scanning electron microscopy (Figure 1), combining the amine-modified APMS (3) with the succinimidyl ester-modified Stöber silica (2) resulted in the attachment of the smaller Stöber particles to the APMS surface (4). The extent of APMS surface coverage could be easily controlled by adjusting the size of the Stöber silica and the ratio ofp Stöber silica to APMS. Under some conditions, a monolayer of Stöber silica was formed on the APMS surface; however, geometric considerations made it impossible to completely react with all of the amines. Thus, after cleavage of the disulfide bridge with dithiothreitol (DTT) to release the particles, the APMS particles were left with a bifunctional external surface consisting of amines and thiols (5). Most Stöber silica could be removed from the APMS surface by cycles of centrifugation and ultrasonication and finally, filtration. At this point, the thiol group was protected by reacting it with 2,2′-dithpiobis(5-nitro)pyridine (dTNP) (6), and the amine was reacted with succinic anhydride to form a carboxylic acid terminus (7). We then used a carbodiimide-mediated coupling reaction with EDAC/NHS (1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride/N-hydroxysuccinimide) to attach amino-TEG to the acid groups (8). Deprotection of the thiol protecting group with DTT produced a bifunctional surface terminated with TEG and thiol (9), and the thiol was reacted with the heterobifunctional linker BMPS (3-(maleimido) propionic acid N-succinimidyl ester) to yield TEG-modified, amine-reactive particles (10).
Scheme 1.
Reactions used to produce a bifunctionally modified APMS particle. Abbreviations: DTT, 1,4-dithiothreitol; EDAC, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride; NHS, N-hydroxysuccinimide; MES, 2-(N-morpholino) ethanesulfonic acid.
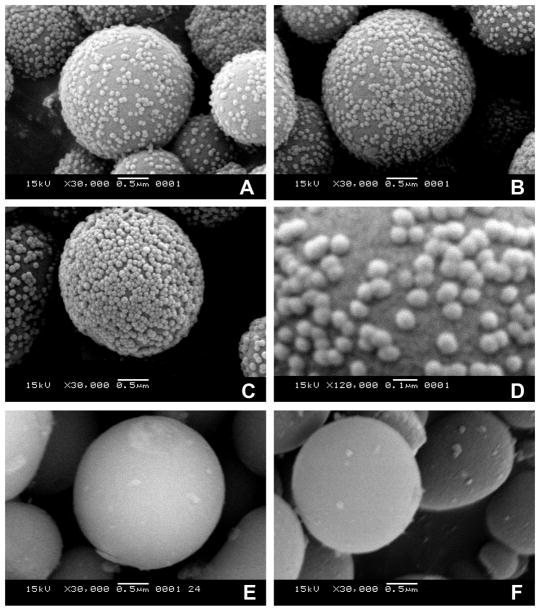
Figure 1.
Scanning electron microscopic (SEM) images of APMS-Stöber conjugates (4). Amine-modified APMS particles (3) were suspended in solutions with increasing concentrations of succinimide-modified Stöber silica nanoparticles (2): (A) 20, (B, D) 30, or (C) 40 mg/mL. Unreacted (3) is shown in (E), and an example of a conjugate after reaction with DTT is shown in (F).
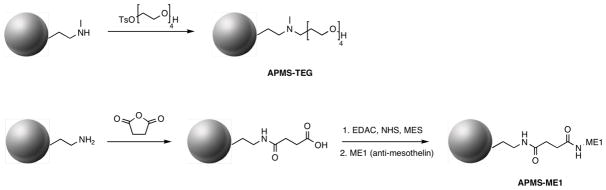
From bifunctional APMS (10), we could attach proteins, including antibodies, to the particles through the reaction of the succinimidyl esters on the surface of the particles and lysine residues on the protein. We chose mesothelin, a 40-kDa cell surface glycoprotein present on normal mesothelial cells and overexpressed in several human tumors including mesotheliomas and ovarian and pancreatic adenocarcinomas, as a tissue-specific target.43 A commercially available human monoclonal antibody for mesothelin, ME1, was thus attached to the particle surface by reacting the antibody with the final bifunctional particles 10 to yield APMS-TEG(ME1). As a non-specific negative control, bovine serum albumin (BSA) was separately reacted with 10 to make APMS-TEG(BSA). As further controls, we also prepared monofunctional particles containing only ME1 or TEG on their external surfaces (Scheme 2). To prepare APMS-TEG, APMS was first reacted with 3-(N-methylamino)propyl trimethoxysilane, then with monotosylated TEG to attach the TEG to the surface through a tertiary amine linkage. APMS-ME1 was prepared by reacting primary-amine-modified APMS with succinic anhydride to yield a carboxylate-terminated surface that was then submitted to a carbodiimide-mediated coupling (similar to the formation of 8, above) with the amine-containing side chains of lysines in ME1 to complete the covalent attachment.
Scheme 2.
Reactions used to produce monofunctionally modified APMS particles for control studies. (Abbreviations are defined in Scheme 1).
Scanning and transmission electron microscopy (Figure 2) showed the importance of using bifunctionally modified particles rather than particles modified with only ME1. APMS particles modified only with ME1 were closely associated with the cell membranes of MM cells 4 hr after the addition of the particles to the medium; however, very few particles were observed within the cells. In contrast, particles exclusively modified with TEG were readily taken up by MM cells at this time point. Thus, TEG was necessary for effective fusion with MM cellular membranes and cellular uptake, consistent with our previously published results.39 These results were also consistent with studies showing that modified nanoparticles were effective in intracellular drug delivery.5,44–46 A significant difference is that in most studies involving nanoparticles, the primary function of poly(ethylene glycol) was not to promote fusion with cell membranes but to avoid clearance by the reticuloendothelial system and hence increase in vivo lifetime of the particles.6,11,47,48
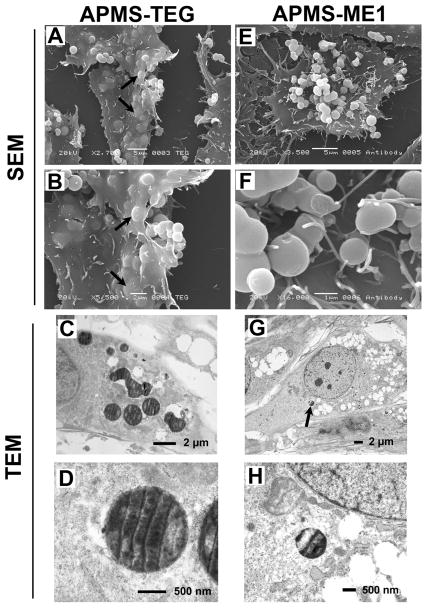
Figure 2.
Images of particles labeled with either TEG (APMS-TEG, A-D) or anti-mesothelin (APMS-ME1, E-H) interacting with cells 4 h after their addition to MM cells. SEM images showed that only particles carrying the TEG functional group were internalized by cells (arrows in A and B). APMS-TEG particles directly exposed to cytoplasm were observed in TEM (D). The arrow in image G indicates the lone APMS-ME1 particle found within an MM cell; it is enlarged in image H.
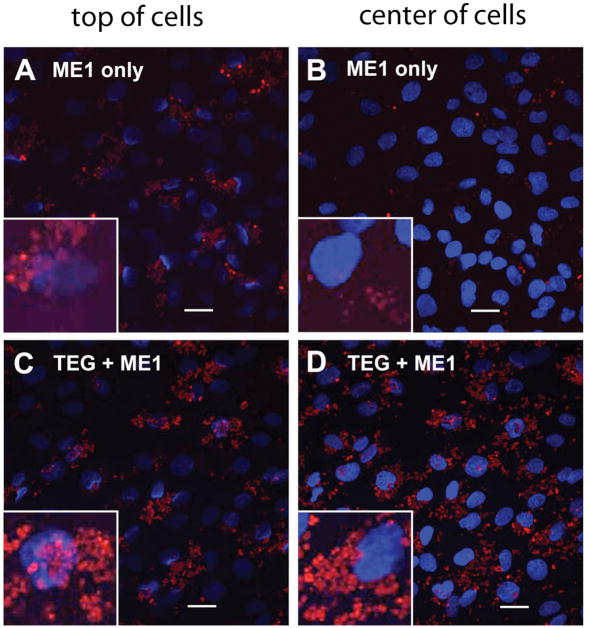
In our next set of experiments, we compared the uptake of APMS-ME1 and APMS-TEG(ME1) to study whether bifunctionally modified particles were taken up as readily as particles modified only with TEG. In these experiments, particles were labeled with a fluorescent molecule exclusively in the pores by using a diffusion-controlled deprotection strategy previously described by our group,13 and confocal laser scanning microscopy was used to provide a three-dimensional view of particle uptake by MM cells. To fluorescently tag the particles, an Fmoc-protected aminopropylsilane was reacted with APMS and a rapid deprotection of the external amines (20 min) was performed with 5% piperidine in N,N-dimethylformamide. The amines within the pores remained Fmoc-protected through the modifications in Schemes 1 or 2, after which they were exhaustively deprotected for 24 h, freeing them for further reaction with a dye. APMS-ME1 and APMS-TEG(ME1) were modified with AlexaFluor-488 succinimidyl ester in this manner. Fluorescently labeled APMS-ME1 and APMS-TEG(ME1) were then added to separate MM cell cultures and confocal microscopy was performed after 4 h, as described in the experimental section and the Supporting Information (Figure 3). While both types of particles were present at the cell surfaces, only particles modified with both ME1 and TEG were present in large numbers within the cells, confirming that the antibody alone was insufficient to promote uptake of microparticles by MM cells. This also confirmed that APMS-TEG(ME1) was able to enter MM cells as readily as particles modified with only TEG.
Figure 3.
Confocal scanning laser microscopic (CSLM) images showing interaction and uptake of modified APMS particles by MM cells. The left column shows the top layer from the orthogonal presentation of a stack of CSLM images, representing the apical surface of the plasma membrane. The right column shows an optical section from the center of a stack of CSLM images, corresponding to the location of the cell nuclei (blue). Cells were exposed to particles modified only with an antibody to mesothelin (APMS-ME1, A and B), or particles modified with both TEG and the antibody (APMS-TEG(ME1), C and D) for 4 h. While particles were found on the outer membranes of all cells, only those particles with TEG were found within the cells; background fluorescence observed in panel B is from particles located outside the focal plane. (Bar = 20 μm in each image).
The specificity of the uptake of bifunctionally modified particles by MM cells was confirmed and quantified by performing co-culture experiments5 with MM and lung cancer (A549) cells (Figure 4). A549 cells were selected because they have a similar growth rate to the type of MM cells used for this experiment and because they do not express mesothelin. Each cell type was cultured separately on glass coverslips to near confluence, and then the coverslips were transferred to the same 60 mm dish. This procedure allowed the cells to be cultured together without physical cross-contamination but to share the same culture medium during APMS uptake experiments. After adding the particles, the co-culture was placed on an oscillating platform to ensure that uptake was not influenced simply by the location at which particles were administered. We treated both cell types with TEG particles bifunctionally modified with either the anti-mesothelin antibody, APMS-TEG(ME1), or a non-specific protein control, bovine serum albumin, APMS-TEG(BSA). Both particle types were modified with AlexaFluor-488 in the pores, described above, for the detection of cell uptake by fluorescence-assisted cell sorting. When the co-cultured cells were exposed to APMS-TEG(ME1) for 8 h, approximately 52% of particles were associated with MM cells whereas less than 23% of APMS-TEG(BSA) were observed within MM cells (Figure 4C). In contrast, the relative uptake of APMS-TEG(ME1) and APMS-TEG(BSA) by A549 lung cancer cells was not significantly different (9% and 13%, respectively) from each other or from MM cells exposed to the non-targeted APMS-TEG-BSA.
Figure 4.
Fluorescence-assisted cell sorting (FACS) data from cell co-culture experiments. Cell nuclei were stained with Hoechst 33342, and bifunctionally modified APMS particles with either ME1 or a non-specific protein (bovine serum albumin, BSA) were labeled with AlexaFluor-488. The x and y axes indicate increasing intensity of Hoechst 33342 and AlexaFluor-488, respectively; thus, cells associated with particles are identified in the top right quadrant of each plot. (A) Experiments comparing MM cells or A549 cells alone showed slightly different fluorescence backgrounds; therefore “gating” or fluorescence limits used for cell sorting were established separately for each cell type. (B) Since different batches of modified APMS were used, the fluorescence response for each batch was different. However, no overlap with the quadrants used to identify cells was found. (C) Cells were co-cultured and treated with either APMS-ME1(TEG) or APMS-TEG(BSA); the numbers in each quadrant correspond to the percentage of cells in each quadrant as determined by the individual gating for each cell type. Axis labels in (B) and (C) are identical to those in (A).
Conclusions
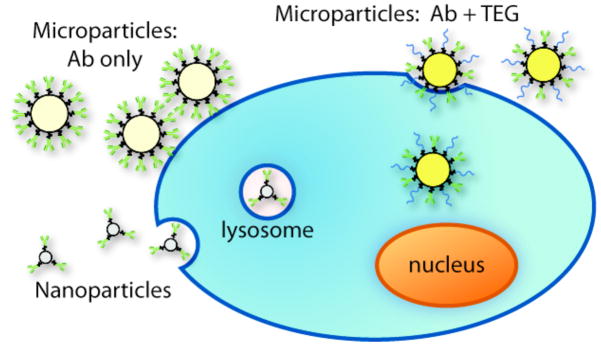
We have presented a new method for preparing porous silica microparticles with a bifunctional surface of both biocompatible polymers and proteins (either an antibody specific for MM cells or a non-specific protein). Our findings are summarized in Scheme 3. Modification with only the targeting protein resulted in the association of microparticles with the target cells, but they were not internalized by those cells. Thus, modification with both TEG and anti-mesothelin were necessary to successfully achieve targeting and uptake of the particles by MM cells, as shown by microscopy and fluorescence-assisted cell sorting in the presence of non-targeted cells. Also, unlike typical nanoparticles, the microparticles were not bound in lysosomes after uptake and therapeutic payloads could be delivered directly to the cytosol without degradation. The diameter of the particles used in this study are ideal for in vivo applications in which entrance into the bloodstream and systemic distribution within the body is not desired. The diffuse, intracavitary location of MM tumors make them an ideal malignancy for therapy using modified microparticles of the type described here. We are currently studying targeted delivery of chemotherapeutics in vivo using a human xenograft model in mice, and we envision that APMS particles and other types of microparticles can be adapted for treatment of a number of malignant and non-malignant diseases.
Scheme 3.
Graphic illustration of differences among interactions of modified particles with cells.
Supplementary Material
Acknowledgments
This project was funded by the National Institutes of Health under grant numbers T32ES0071 and 1R41-CA126155-01A1 and by the Mesothelioma Applied Research Foundation. We thank Michelle von Turkovich, Colette Charland, and the UVM Microscopy Imaging Center for assistance with microscopy and flow cytometry.
Footnotes
Supporting Information Available
Please see the supporting information for full instrumental and synthetic details, as well as characterization data of the particles. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Science. 1994;263:1600. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 2.Harris JM, Chess RB. Nat Rev Drug Discov. 2003;2:214. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 3.Gabizon A, Shmeeda H, Barenholz Y. Clin Pharmacokinet. 2003;42:419. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 4.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. ACS Nano. 2008;2:889. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saul JM, Annapragada A, Natarajan JV, Bellamkonda RV. J Control Release. 2003;92:49. doi: 10.1016/s0168-3659(03)00295-5. [DOI] [PubMed] [Google Scholar]
- 6.Maruyama K, Takizawa T, Yuda T, Kennel SJ, Huang L, Iwatsuru M. Biochim Biophys Acta-Biomembr. 1995;1234:74. doi: 10.1016/0005-2736(94)00263-o. [DOI] [PubMed] [Google Scholar]
- 7.Nobs L, Buchegger F, Gurny R, Allemann E. J Pharm Sci. 2004;93:1980. doi: 10.1002/jps.20098. [DOI] [PubMed] [Google Scholar]
- 8.Gu FX, Karnik R, Wang AZ, Alexis F, Levy-Nissenbaum E, Hong S, Langer RS, Farokhzad OC. Nano Today. 2007;2:14. [Google Scholar]
- 9.Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, Wrasidlo W, Cheresh DA. Proc Natl Acad Sci U S A. 2008;105:9343. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikkeri R, Lepenies B, Adibekian A, Laurino P, Seeberger PH. J Am Chem Soc. 2009;131:2110. doi: 10.1021/ja807711w. [DOI] [PubMed] [Google Scholar]
- 11.Akerman ME, Chan WCW, Laakkonen P, Bhatia SN, Ruoslahti E. Proc Natl Acad Sci U S A. 2002;99:12617. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Juan F, Ruiz-Hitzky E. Adv Mater. 2000;12:430. [Google Scholar]
- 13.Cheng K, Landry CC. J Am Chem Soc. 2007;129:9674. doi: 10.1021/ja070598b. [DOI] [PubMed] [Google Scholar]
- 14.Shephard DS, Zhou WZ, Maschmeyer T, Matters JM, Roper CL, Parsons S, Johnson BFG, Duer MJ. Angew Chem-Int Edit. 1998;37:2719. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2719::AID-ANIE2719>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Cauda V, Schlossbauer A, Kecht J, Zurner A, Bein T. J Am Chem Soc. 2009;131:11361. doi: 10.1021/ja809346n. [DOI] [PubMed] [Google Scholar]
- 16.Gartmann N, Bruhwiler D. Angew Chem-Int Edit. 2009;48:6354. doi: 10.1002/anie.200902436. [DOI] [PubMed] [Google Scholar]
- 17.Kecht J, Schlossbauer A, Bein T. Chem Mat. 2008;20:7207. [Google Scholar]
- 18.De M, Ghosh PS, Rotello VM. Adv Mater. 2008;20:4225. [Google Scholar]
- 19.Rosi NL, Mirkin CA. Chem Rev. 2005;105:1547. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 20.Lewinski N, Colvin V, Drezek R. Small. 2008;4:26. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick JAJ, Andreko SK, Ernst LA, Waggoner AS, Ballou B, Bruchez MP. Nano Lett. 2009 doi: 10.1021/nl901534q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberdorster G, Stone V, Donaldson K. Nanotoxicology. 2007;1:2. [Google Scholar]
- 23.Handy RD, Owen R, Valsami-Jones E. Ecotoxicology. 2008;17:315. doi: 10.1007/s10646-008-0206-0. [DOI] [PubMed] [Google Scholar]
- 24.Gallis KW, Araujo JT, Duff KJ, Moore JG, Landry CC. Adv Mater. 1999;11:1452. [Google Scholar]
- 25.Gallis KW, Landry CC. 6, 334, 988. United States Patent. 2002
- 26.Nassivera T, Eklund AG, Landry CC. J Chromatogr A. 2002;973:97. doi: 10.1016/s0021-9673(02)01200-1. [DOI] [PubMed] [Google Scholar]
- 27.Nassivera TW, Landry CC. 2006118490 United States Patent Application.
- 28.Pekarek KJ, Jacob JS, Mathiowitz E. Nature. 1994;367:258. doi: 10.1038/367258a0. [DOI] [PubMed] [Google Scholar]
- 29.Anderson JM, Shive MS. Adv Drug Deliv Rev. 1997;28:5. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 30.Kogan FM, Nikitina OV. Environ Health Perspect. 1994;102:205. doi: 10.1289/ehp.94102s5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams AO, Knapton AD. Hepatology. 1996;23:1268. doi: 10.1002/hep.510230548. [DOI] [PubMed] [Google Scholar]
- 32.Mossman BT, Churg A. Am J Respir Crit Care Med. 1998;157:1666. doi: 10.1164/ajrccm.157.5.9707141. [DOI] [PubMed] [Google Scholar]
- 33.Johnston CJ, Driscoll KE, Finkelstein JN, Baggs R, O’Reilly MA, Carter J, Gelein R, Oberdorster G. Toxicol Sci. 2000;56:405. doi: 10.1093/toxsci/56.2.405. [DOI] [PubMed] [Google Scholar]
- 34.Warheit DB. J Environ Pathol Toxicol Oncol. 2001;(Suppl 1):133. [PubMed] [Google Scholar]
- 35.Kim JS, Yoon TJ, Kim BG, Park SJ, Kim HW, Lee KH, Park SB, Lee JK, Cho MH. Toxicol Sci. 2006;89:338. doi: 10.1093/toxsci/kfj027. [DOI] [PubMed] [Google Scholar]
- 36.Tan K, Cheang P, Ho IAW, Lam PYP, Hui KM. Gene Ther. 2007;14:828. doi: 10.1038/sj.gt.3302937. [DOI] [PubMed] [Google Scholar]
- 37.Vallet-Regi M, Balas F, Arcos D. Angew Chem-Int Edit. 2007;46:7548. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- 38.Slowing II, Vivero-Escoto JL, Wu CW, Lin VSY. Adv Drug Deliv Rev. 2008;60:1278. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Blumen SR, Cheng K, Ramos-Nino ME, Taatjes DJ, Weiss DJ, Landry CC, Mossman BT. Am J Respir Cell Mol Biol. 2007;36:333. doi: 10.1165/rcmb.2006-0319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hillegass JM, Blumen SR, Cheng K, MacPherson MB, Aleexeva V, Lathrop SA, Beuschel SL, Steinbacher JL, Butnor KJ, Ramos-Nino ME, Shukla A, James TA, Weiss DJ, Taatjes DJ, Landry CC, Mossman BT. 2009 submitted. [Google Scholar]
- 41.Ohara CJ, Corson JM, Pinkus GS, Stahel RA. Am J Pathol. 1990;136:421. [PMC free article] [PubMed] [Google Scholar]
- 42.Stöber W, Fink A, Bohn E. J Coll Interf Sci. 1968;26:62. [Google Scholar]
- 43.Hassan R, Bera T, Pastan I. Clin Cancer Res. 2004;10:3937. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 44.Saad M, Garbuzenko OB, Ber E, Chandna P, Khandare JJ, Pozharov VP, Minko T. J Control Release. 2008;130:107. doi: 10.1016/j.jconrel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao ZH, Tong R, Mishra A, Xu WC, Wong GCL, Cheng JJ, Lu Y. Angew Chem-Int Edit. 2009;48:6494. doi: 10.1002/anie.200901452. [DOI] [PubMed] [Google Scholar]
- 46.Rosenholm JM, Meinander A, Peulhu E, Niemi R, Eriksson JE, Sahlgren C, Linden M. ACS Nano. 2009;3:197. doi: 10.1021/nn800781r. [DOI] [PubMed] [Google Scholar]
- 47.Mok H, Bae KH, Ahn CH, Park TG. Langmuir. 2009;25:1645. doi: 10.1021/la803542v. [DOI] [PubMed] [Google Scholar]
- 48.Tong GJ, Hsiao SC, Carrico ZM, Francis MB. J Am Chem Soc. 2009;131:11174. doi: 10.1021/ja903857f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.