Abstract
Leishmania infantum is a causative agent of endemic zoonotic visceral leishmaniasis (VL) in regions of South America and the Mediterranean. Dogs are the major reservoirs for Leishmania infantum in these regions, and control of disease in dogs could have a significant impact on human disease. Although dogs share many symptoms of VL with humans as a result of L. infantum infection, they also show some unique clinical manifestations, which are often a combination of visceral and cutaneous leishmaniasis, suggesting different mechanisms of disease development in dogs and humans. Here, we compare antibody responses of dogs and humans with VL to various defined leishmanial antigens. Parasite lysate and K39, the two most commonly used antigens for serodiagnosis of VL, detected the highest levels of antibodies in both humans and dogs with VL, whereas the recognition patterns of these antigens were distinct between the hosts. Among other defined antigens tested, LmSTI1 and CPB detected higher levels of antibodies in dogs and humans, respectively. These results indicate there is a difference between humans and dogs in antigen recognition patterns during VL; we infer that different strategies may need to be used in development of vaccines and diagnostics for humans and for dogs. In addition, we show a correlation between antibody titers to several antigens and severity of clinical symptoms during canine VL.
Keywords: Zoonosis, Leishmania infantum, Antigen, Dog
Introduction
Zoonoses are of considerable concern as a source of recurring human infection as well as future epidemics (Seimenis et al., 2006). Control of these animal diseases is a well-recognized means to prevent their spread to humans. Some diagnostic tests may not be useful for detecting infection in both humans and animal reservoirs, and some vaccines may not be effective to prevent / treat diseases in both humans and other animals. Understanding immune responses in multiple hosts may be a key to the detection and control of zoonotic diseases.
Zoonotic visceral leishmaniasis (VL), caused by the protozoan parasite Leishmania infantum (chagasi), is a vector-borne disease found in South America and areas around the Mediterranean (Chappuis et al., 2007; Lukes et al., 2007). In humans, active VL is characterized by hepato-splenomegaly, lethargy, fever, anemia, and weight loss and is generally fatal unless properly treated (Herwaldt, 1999). Dogs are the major reservoirs for L. infantum in these regions (Dantas-Torres, 2007; Moreno and Alvar, 2002), and control of the disease in dogs could have a significant impact on human disease (Dantas-Torres, 2006; Gavgani et al., 2002; Gramiccia and Gradoni, 2005; Reithinger and Davies, 2002). Although dogs and humans with VL share some clinical symptoms, dogs may also manifest symptoms not generally observed in human VL such as conjunctivitis, ocular signs, skin lesions, alopecia and nail abnormality, including onychogryphosis (Gomes et al., 2008). Although L. infantum sometimes causes cutaneous leishmaniasis (CL) in humans as well, individual patients rarely show the mixed symptoms characteristic of canine VL, and these two types of disease are often caused by distinct zymodemes of L. infantum (Pratlong et al., 2004). There is also a difference between these two hosts in terms of response to chemotherapy. In humans, chemotherapy using pentavalent antimony or amphotericin B generally works well to resolve the disease, and cured patients usually develop protective responses to future infections. In contrast, canine VL is not uniformly cured by chemotherapy well, and even responsive canine cases often relapse (Baneth and Shaw, 2002). In spite of the differences in disease responses to treatment and clinical outcome, there have been few studies comparing immune responses of these two mammalian hosts.
Immunological aspects commonly seen in humans and dogs during VL are the presence of strong antigen-specific humoral responses and the lack of cell-mediated responses (Barbieri, 2006; Nylen and Sacks, 2007). In this study, we sought to examine immunological differences between the two hosts during VL by evaluating their antibody responses to various leishmanial antigens, which are currently in use or being examined for vaccine or diagnostic purposes for VL.
Materials and Methods
Humans and dogs with VL in Brazil
Brazilian VL patients were first clinically diagnosed at Hospital Univ. Prof. Edgard Santos, Universidade Federal da Bahia, Salvador, Bahia, Brazil based on presence of fever, anemia, splenomegaly or lymphadenopathy and by microscopic examination of Giemsa-stained smears for parasites in aspirates from bone marrow or spleen. Plasma was prepared from peripheral blood collected from 21 parasitologically confirmed VL patients with their informed consent to use the samples for research purposes. Collection and usage of these human samples were approved by the Institutional Review Board ethical committee at Universidade Federal da Bahia.
Twenty-six dogs with VL were included in this study. These dogs were from Monte Gordo, Bahia, Brazil, an area endemic for L. infantum. The dogs presented with clinical symptoms of VL including cachexia, alopecia, splenomegaly, lymphadenopathy and skin lesion. VL was confirmed by the presence of parasites in bone marrow, lymph node, or spleen upon examination of Giemsa-stained smears. Peripheral blood was collected from these dogs for plasma samples with their owners’ consents. Neither humans nor dogs in this study previously had VL or were they under current drug treatment at the time of sample collection.
As a control, plasma samples from healthy human (eight) and dog donors (eight) from non-endemic areas in Brazil were used. None of these healthy donors had a previous history of leishmaniasis at the time of sample collection.
Clinical scoring
Severity of canine VL was enumerated based on clinical symptoms including alopecia, onychogryphosis, splenomegaly, lymphadenopathy, conjunctivitis, and presence of oral mucosal lesions using a scoring system as shown in Table I. Overall scores were calculated as sums of scores from individual criteria.
Table I.
Scoring of disease severity in canine viscero-cutaneous leishmaniasis
| Category | Clinical Score |
||
|---|---|---|---|
| 0 | 1 | 2 | |
| Hair | Good or excellent | Not good | Bad (alopecia, desquamation, etc) |
| Weight | Normal or obese | Skinny | Cachexia |
| Nails | Normal | - | Excessive growth |
| Spleen | Normal | - | Enlarged (palpable) |
| Lymph nodes | Not palpable/Small | Enlarged | Very Enlarged |
| Oral mucosa | Normal | Hyperemia or Pale | - |
| Oral lesions | Absent | Present | - |
| Conjunctivitis | Absent | Mild (unilateral, serous or mucous) | Severe (bilateral, purulent) |
ELISA
Previously characterized leishmanial antigens K39 (Burns et al., 1993), K26 (Bhatia et al., 1999), SMT (Goto et al., 2007), TSA (Webb et al., 1998), LmSTI1 (Webb et al., 1996), CPB (Rafati et al., 2003), A2 (Ghedin et al., 1997), KMP11 (Berberich et al., 1997), as well as L. infantum parasite lysate (SLA), were used for antibody detection by ELISA. All the proteins other than SLA were produced as recombinant proteins in E. coli. To prepare SLA, L. infantum promastigotes were cultured in MEM as previously described (Goto et al., 2007), and the parasites in a late log or stationary phase were harvested and washed three times with phosphate-buffered saline (PBS) by centrifuging at 1,600 g for 10 min at 4 °C. Cell pellet was resuspended in PBS and then sonicated on ice. The sample was centrifuged at 10,000 g for 30 min at 4 °C. The supernatant was collected and passed through a 0.22 μm pore size membrane. Protein concentration of SLA was determined by BCA protein assay (Pierce Biotechnology Inc., Rockford, IL) and adjusted to 1 mg/ml with PBS.
Antigens were diluted in ELISA coating buffer (sodium carbonate buffer, pH 9.6) and Nunc 96-well polysorp plates (Thermo Fisher Scientific Inc., Waltham, MA) were coated for 4 h at room temperature (RT) with SLA (1 μg/well), K39 (50 ng/well) or other antigens (200 ng/well) followed by overnight blocking at 4 °C with PBS containing 0.05% Tween-20 and 1% bovine serum albumin (200 μl/well). The plates were washed five times with PBS containing 0.1% Tween-20 and once with PBS. Prior to the plate washing, human and dog plasma samples were diluted at 1/100 with PBS containing 0.05% Tween-20 and 0.1% bovine serum albumin, then further diluted by five-fold serial dilution up to 1/312,500. Those diluted plasma samples (100 μl/well) were added to the plates and incubated for 2 h at RT. The plates were washed as described above prior to incubation with 100 μl/well of HRP-conjugated protein G (1/5,000 dilution: Invitrogen corporation, Carlsbad, CA) for 1 h at RT to detect bound primary antibodies. The plates were washed as described above and developed with 100 μl/well of tetramethylbenzidine peroxidase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD), and the enzyme-substrate reaction was stopped after 4 min by adding 50 μl/well of 1N H2SO4. The plates were read by a microplate reader at 450 nm (570 nm reference). Reciprocal endpoint titers to individual antigens were calculated with GraphPad Prism 4 software (GraphPad Software, Inc., La Jolla, CA) using a cutoff value of the higher of either 0.1 or the mean + 3SD at 1:100 dilution of healthy controls. Endpoint titers of samples were recorded as <100 if OD values of the samples were lower than the cutoff value at 1:100 or >312,500 if higher than that at 1:312,500 dilution. In these two cases, titers of 100 or 312,500 were used for graphing.
Statistical analyses
Either an unpaired t-test or Mann-Whitney test was used for comparison in antibody titers to individual antigens between humans and dogs depending on the nature of the data (e.g., whether data fit as Gaussian distribution). The correlation between two antibody titers for two antigens or between antibody titer and clinical score was analyzed by the Spearman correlation test. All the statistical analyses were performed with the GraphPad Prism 4 software.
Results
Distinct serological responses of humans and dogs to defined antigens
Cutoff values were calculated for individual antigens based on the responses of healthy donor. Using such cutoff values, reciprocal antibody titers of all the healthy donors (8 humans / 8 dogs) were below 100 to any antigens tested in this study (data not shown). First, we focused on SLA and K39 to seek different patterns between humans and dogs in antibody responses during VL. Both humans and dogs with VL had antibody responses to these two antigens (Fig. 1A and B). Among antigens used in this study, these two are the most strongly recognized antigens by the two mammalian hosts. When responses of the two hosts to each antigen were compared, there were no statistically significant differences (Fig. 1B). In contrast, when ratios of antibody titers to SLA and to K39 were analyzed in individual humans and dogs, there was a clear difference in antigenic dominance; relative to the responses to SLA, humans showed stronger responses to K39, whereas the dominance of K39 was much less in dogs (Fig. 1C).
Figure 1.
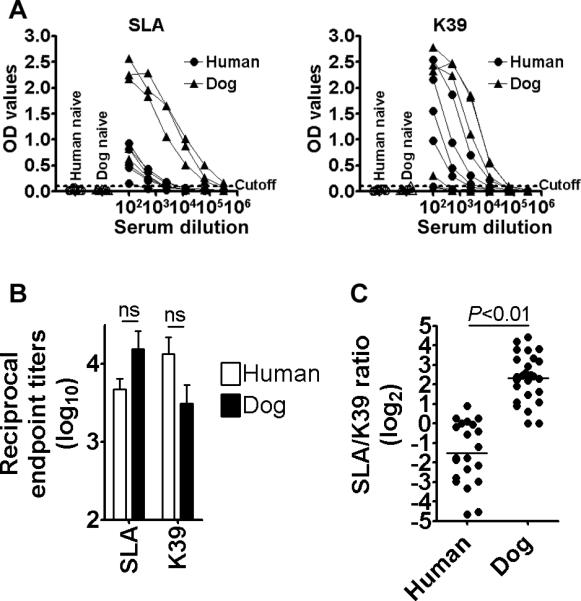
Seroreactivity of humans and dogs with VL to parasite lysate (SLA) and K39. (A) Antibody endpoint titers to these antigens were determined by ELISA. The cutoff values for individual antigens used for calculating endpoint titers were the higher of either 0.1 or the mean + 3SD of OD values of human and dog controls (n=8 each). Cutoff values for SLA and K39 are shown as dotted lines. Five each of humans (•) and dogs (▲) with VL are shown as an example for reactivity of serially diluted plasma samples to these antigens. (B) Mean and SEM of reciprocal endpoint titers in individual groups are shown. (C) Ratios of anti-SLA and anti-K39 titers in individuals were calculated and are shown as dots. Bars represent mean values of individual groups.
Next, antibody responses to seven other defined antigens were examined (Fig. 2). No significant differences in antibody titers were found between the two mammalian hosts to five of the antigens, K26, KMP11, SMT, TSA or A2. Differences between humans and dogs were found in responses to two antigens, LmSTI1 and CPB, detecting higher levels of antibodies in dogs and humans, respectively.
Figure 2.
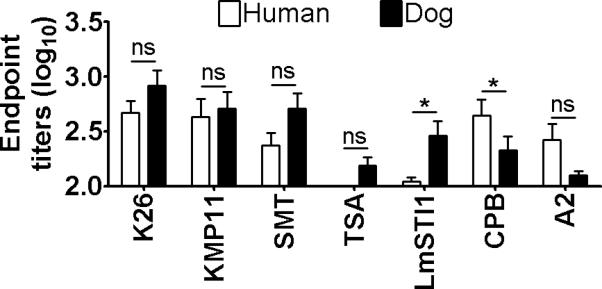
Seroreactivity of humans and dogs with VL to Leishmania defined antigens. Antibody endpoint titers to Leishmania antigens were determined by ELISA using sera from humans and dogs with VL. Mean and SEM of reciprocal endpoint titers in individual groups are shown. Asterisks represent a statistically significant difference (P<0.05) between antibody titers of humans and of dogs for the particular antigen.
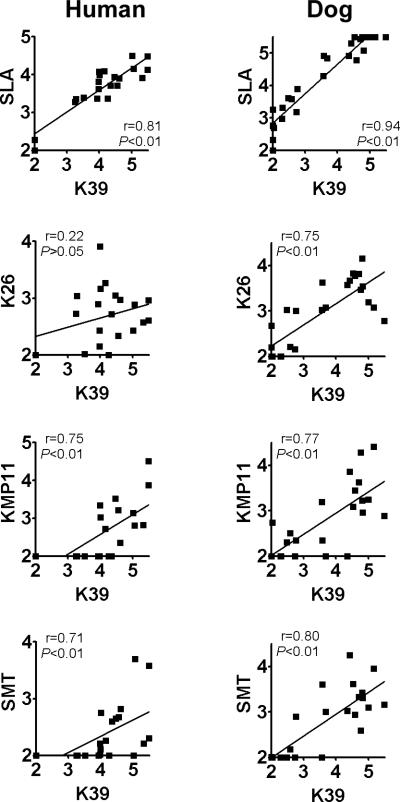
The correlations between human and dog antibody titers to the five most strongly recognized antigens (SLA, K39, K26, KMP11 and SMT) were also analyzed (Fig 3). Overall, better correlations in antibody titers were found in dogs compared with humans. In dogs, anti-K39 titer correlated well with anti-SLA (r=0.94, P<0.01), anti-K26 (r=0.75, P<0.01), anti-KMP11 (r=0.77, P<0.01) and anti-SMT titer (r=0.80, P<0.01). On every antigen, Spearman r value in the correlation analysis was lower in humans than dogs. In humans, anti-K39 titer correlated with anti-SLA (r=0.81, P<0.01), antige-KMP11 (r=0.75, P<0.01), and anti-SMT titer (r=0.71, P<0.01), but did not correlate with anti-K26 titer (r=0.22, P>0.05). Overall, these correlations were stronger in dogs than in humans.
Figure 3.
Correlation between plasma antibody titers to SLA and defined antigens in humans and dogs with VL. Reciprocal endpoint titers (in log10) of antibodies to SLA, K39, K26, KMP11 and SMT were plotted. Spearman r values and P values of the correlation analyses are shown.
Correlation between disease severity and antibody titers
We analyzed canine antibody titers to various antigens to examine the correlation of titers with a dog's clinical score. Figure 4 shows that there is a positive correlation between clinical score and anti-SLA titer (r=0.57, P<0.01). The three defined antigens K39, K26 and SMT also showed positive correlations between antigen-specific antibody titer and clinical score (K39: r=0.57, P<0.01, K26: r=0.52, P<0.01, SMT: r=0.67, P<0.01). Anti-SMT titer showed the best correlation with the clinical score.
Figure 4.
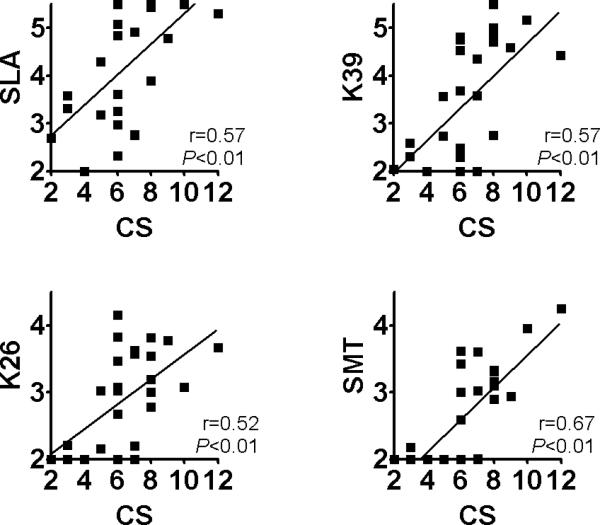
Correlation between antibody titers to Leishmania antigens and clinical scores during canine VL. Individual dogs with VL were plotted based on reciprocal endpoint titers (in log10) of antibodies to Leishmania antigens and the clinical scores. Spearman r values and P values of the correlation analyses are shown.
Discussion
Little effort has been expended to understand zoonotic VL by comparing immune responses of humans and dogs, despite the importance of dogs as a reservoir for Leishmania infantum parasites. We believe this study is the first comparing the two mammalian hosts based on immune responses to multiple defined Leishmania antigens. Results in this study have shown the distinct antigen recognition patterns during VL between humans and dogs. These results have important implications for understanding the disease and designing vaccines and diagnostics for the disease.
As reinforced in this study, K39 has been known as an exceptionally strong antigen as recognized by sera of active human VL patients. This antigen detects antibodies in active VL patients more efficiently than does SLA (Badaro et al., 1996; Burns et al., 1993). That K39 is the only defined antigen used globally for commercially available serodiagnostic tests is a reflection of this fact. In contrast to the human results, dominance of anti-K39 response in canine VL seemed to be lower. This observation agrees with previous studies showing a dipstick test with parasite crude lysate had better sensitivity than K39 in dogs (Reithinger et al., 2002; Schallig et al., 2004). Using lysate antigen, however, is often problematic for reproducible specificity and manufacturability (Gomes et al., 2008). Identification of additional defined antigens to complement K39 may promote development of a canine VL diagnostic test with higher sensitivity and specificity.
While dogs showed equivalent or stronger antibody responses to parasite lysate as well as many of defined antigens than did humans, the two antigens CPB and A2 were recognized more weakly in dogs (whereas the difference was not significant in A2). There have been reports that asymptomatic dogs have higher antibody titers to A2 and CPB than symptomatic dogs (Nakhaee et al., 2004; Porrozzi et al., 2007), suggesting that anergic responses to these antigens is associated with disease promotion in dogs. A2 and CPB are thought to be virulence factors involved in parasite propagation in vivo (Alexander et al., 1998; Zhang and Matlashewski, 1997). Differences in immune responses to Leishmania antigens between humans and dogs may be derived from different survival mechanisms of the parasites in these hosts; it is intriguing to further characterize such differentially recognized antigens in terms of expression levels and functions in such hosts.
Biomarkers may be useful for objective diagnosis of diseases. For their diagnosis some diseases rely solely on clinical symptoms, which make diagnosis subjective. VL is usually suspected first by clinical observations of fever, anemia and hepato-splenomegaly, symptoms that are shared with other infectious diseases. Confirmative diagnosis of canine VL requires microscopic examination of aspirates from the bone marrow, spleen, lymph node, or a skin lesion. Tissue biopsy for this confirmation is invasive and requires trained personnel for collection and microscopic evaluation. Therefore, serology is a valuable alternative to direct parasitological examination. It has been reported that higher antibody levels to SLA, K39 and K26 are found in symptomatic VL than in asymptomatic parasite infection (Badaro et al., 1996; Porrozzi et al., 2007). However, few studies have focused on the relationship between disease severity and immune responses. Our present study demonstrated that anti-SMT, anti-K39, and anti-K26 titers, as well as anti-SLA titer, correlate with canine disease severity in our study pool of dogs. Quinnell et al. have reported that canine VL severity correlates positively with IL-4 levels and anti-Leishmania IgG responses, and negatively with cellular proliferative response (Quinnell et al., 2001). Taken together, antigen-specific humoral responses are related to disease severity in canine VL. With the exception of K39, all the defined antigens used in this study are also considered to be candidate antigens for a vaccine (Basu et al., 2005; Campos-Neto et al., 2001; Ghosh et al., 2001; Rafati et al., 2006; Stager et al., 2000; Webb et al., 1998). Successful vaccinations with these antigens may augment antigen-specific Th1 responses, unless otherwise Leishmania infection results in the induction of humoral responses to these antigens and lack of cell-mediated responses.
Acknowledgements
We thank Dr. Melissa Abbehussen at the Clinvet, Monte Gordo, Bahia, Brazil for sample collections at the veterinary hospital. This work was supported by the National Institutes of Health grant AI25038 and a grant from the Bill and Melinda Gates Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Alexander J, Coombs GH, Mottram JC. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J Immunol. 1998;161:6794–6801. [PubMed] [Google Scholar]
- Badaro R, Benson D, Eulalio MC, Freire M, Cunha S, Netto EM, Pedral-Sampaio D, Madureira C, Burns JM, Houghton RL, David JR, Reed SG. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J Infect Dis. 1996;173:758–761. doi: 10.1093/infdis/173.3.758. [DOI] [PubMed] [Google Scholar]
- Baneth G, Shaw SE. Chemotherapy of canine leishmaniosis. Vet Parasitol. 2002;106:315–324. doi: 10.1016/s0304-4017(02)00115-2. [DOI] [PubMed] [Google Scholar]
- Barbieri CL. Immunology of canine leishmaniasis. Parasite Immunol. 2006;28:329–337. doi: 10.1111/j.1365-3024.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- Basu R, Bhaumik S, Basu JM, Naskar K, De T, Roy S. Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1- and Th2-like responses in visceral leishmaniasis. J Immunol. 2005;174:7160–7171. doi: 10.4049/jimmunol.174.11.7160. [DOI] [PubMed] [Google Scholar]
- Berberich C, Requena JM, Alonso C. Cloning of genes and expression and antigenicity analysis of the Leishmania infantum KMP-11 protein. Exp Parasitol. 1997;85:105–108. doi: 10.1006/expr.1996.4120. [DOI] [PubMed] [Google Scholar]
- Bhatia A, Daifalla NS, Jen S, Badaro R, Reed SG, Skeiky YA. Cloning, characterization and serological evaluation of K9 and K26: two related hydrophilic antigens of Leishmania chagasi. Mol Biochem Parasitol. 1999;102:249–261. doi: 10.1016/s0166-6851(99)00098-5. [DOI] [PubMed] [Google Scholar]
- Burns JM, Jr., Shreffler WG, Benson DR, Ghalib HW, Badaro R, Reed SG. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc Natl Acad Sci U S A. 1993;90:775–779. doi: 10.1073/pnas.90.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Neto A, Porrozzi R, Greeson K, Coler RN, Webb JR, Seiky YA, Reed SG, Grimaldi G., Jr. Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infect Immun. 2001;69:4103–4108. doi: 10.1128/IAI.69.6.4103-4108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F. Leishmune vaccine: the newest tool for prevention and control of canine visceral leishmaniosis and its potential as a transmission-blocking vaccine. Vet Parasitol. 2006;141:1–8. doi: 10.1016/j.vetpar.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet Parasitol. 2007;149:139–146. doi: 10.1016/j.vetpar.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Gavgani AS, Hodjati MH, Mohite H, Davies CR. Effect of insecticide-impregnated dog collars on incidence of zoonotic visceral leishmaniasis in Iranian children: a matched-cluster randomised trial. Lancet. 2002;360:374–379. doi: 10.1016/s0140-6736(02)09609-5. [DOI] [PubMed] [Google Scholar]
- Ghedin E, Zhang WW, Charest H, Sundar S, Kenney RT, Matlashewski G. Antibody response against a Leishmania donovani amastigote-stage-specific protein in patients with visceral leishmaniasis. Clin Diagn Lab Immunol. 1997;4:530–535. doi: 10.1128/cdli.4.5.530-535.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Zhang WW, Matlashewski G. Immunization with A2 protein results in a mixed Th1/Th2 and a humoral response which protects mice against Leishmania donovani infections. Vaccine. 2001;20:59–66. doi: 10.1016/s0264-410x(01)00322-x. [DOI] [PubMed] [Google Scholar]
- Gomes YM, Paiva Cavalcanti M, Lira RA, Abath FG, Alves LC. Diagnosis of canine visceral leishmaniasis: biotechnological advances. Vet J. 2008;175:45–52. doi: 10.1016/j.tvjl.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Goto Y, Bogatzki LY, Bertholet S, Coler RN, Reed SG. Protective immunization against visceral leishmaniasis using Leishmania sterol 24-c-methyltransferase formulated in adjuvant. Vaccine. 2007;25:7450–7458. doi: 10.1016/j.vaccine.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramiccia M, Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. Int J Parasitol. 2005;35:1169–1180. doi: 10.1016/j.ijpara.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- Lukes J, Mauricio IL, Schonian G, Dujardin JC, Soteriadou K, Dedet JP, Kuhls K, Tintaya KW, Jirku M, Chocholova E, Haralambous C, Pratlong F, Obornik M, Horak A, Ayala FJ, Miles MA. Evolutionary and geographical history of the Leishmania donovani complex with a revision of current taxonomy. Proc Natl Acad Sci U S A. 2007;104:9375–9380. doi: 10.1073/pnas.0703678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J, Alvar J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol. 2002;18:399–405. doi: 10.1016/s1471-4922(02)02347-4. [DOI] [PubMed] [Google Scholar]
- Nakhaee A, Taheri T, Taghikhani M, Mohebali M, Salmanian AH, Fasel N, Rafati S. Humoral and cellular immune responses against Type I cysteine proteinase of Leishmania infantum are higher in asymptomatic than symptomatic dogs selected from a naturally infected population. Vet Parasitol. 2004;119:107–123. doi: 10.1016/j.vetpar.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007;28:378–384. doi: 10.1016/j.it.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Porrozzi R, Santos da Costa MV, Teva A, Falqueto A, Ferreira AL, dos Santos CD, Fernandes AP, Gazzinelli RT, Campos-Neto A, Grimaldi G., Jr. Comparative evaluation of enzyme-linked immunosorbent assays based on crude and recombinant leishmanial antigens for serodiagnosis of symptomatic and asymptomatic Leishmania infantum visceral infections in dogs. Clin Vaccine Immunol. 2007;14:544–548. doi: 10.1128/CVI.00420-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratlong F, Rioux JA, Marty P, Faraut-Gambarelli F, Dereure J, Lanotte G, Dedet JP. Isoenzymatic analysis of 712 strains of Leishmania infantum in the south of France and relationship of enzymatic polymorphism to clinical and epidemiological features. J Clin Microbiol. 2004;42:4077–4082. doi: 10.1128/JCM.42.9.4077-4082.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnell RJ, Courtenay O, Shaw MA, Day MJ, Garcez LM, Dye C, Kaye PM. Tissue cytokine responses in canine visceral leishmaniasis. J Infect Dis. 2001;183:1421–1424. doi: 10.1086/319869. [DOI] [PubMed] [Google Scholar]
- Rafati S, Nakhaee A, Taheri T, Ghashghaii A, Salmanian AH, Jimenez M, Mohebali M, Masina S, Fasel N. Expression of cysteine proteinase type I and II of Leishmania infantum and their recognition by sera during canine and human visceral leishmaniasis. Exp Parasitol. 2003;103:143–151. doi: 10.1016/s0014-4894(03)00097-3. [DOI] [PubMed] [Google Scholar]
- Rafati S, Zahedifard F, Nazgouee F. Prime-boost vaccination using cysteine proteinases type I and II of Leishmania infantum confers protective immunity in murine visceral leishmaniasis. Vaccine. 2006;24:2169–2175. doi: 10.1016/j.vaccine.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Reithinger R, Davies CR. Canine leishmaniasis: novel strategies for control. Trends Parasitol. 2002;18:289–290. doi: 10.1016/s1471-4922(02)02296-1. [DOI] [PubMed] [Google Scholar]
- Reithinger R, Quinnell RJ, Alexander B, Davies CR. Rapid detection of Leishmania infantum infection in dogs: comparative study using an immunochromatographic dipstick test, enzyme-linked immunosorbent assay, and PCR. J Clin Microbiol. 2002;40:2352–2356. doi: 10.1128/JCM.40.7.2352-2356.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallig HD, Cardoso L, Hommers M, Kroon N, Belling G, Rodrigues M, Semiao-Santos SJ, Vetter H. Development of a dipstick assay for detection of Leishmania-specific canine antibodies. J Clin Microbiol. 2004;42:193–197. doi: 10.1128/JCM.42.1.193-197.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seimenis A, Morelli D, Mantovani A. Zoonoses in the Mediterranean region. Ann Ist Super Sanita. 2006;42:437–445. [PubMed] [Google Scholar]
- Stager S, Smith DF, Kaye PM. Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J Immunol. 2000;165:7064–7071. doi: 10.4049/jimmunol.165.12.7064. [DOI] [PubMed] [Google Scholar]
- Webb JR, Campos-Neto A, Ovendale PJ, Martin TI, Stromberg EJ, Badaro R, Reed SG. Human and murine immune responses to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infect Immun. 1998;66:3279–3289. doi: 10.1128/iai.66.7.3279-3289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JR, Kaufmann D, Campos-Neto A, Reed SG. Molecular cloning of a novel protein antigen of Leishmania major that elicits a potent immune response in experimental murine leishmaniasis. J Immunol. 1996;157:5034–5041. [PubMed] [Google Scholar]
- Zhang WW, Matlashewski G. Loss of virulence in Leishmania donovani deficient in an amastigote-specific protein, A2. Proc Natl Acad Sci U S A. 1997;94:8807–8811. doi: 10.1073/pnas.94.16.8807. [DOI] [PMC free article] [PubMed] [Google Scholar]