Abstract
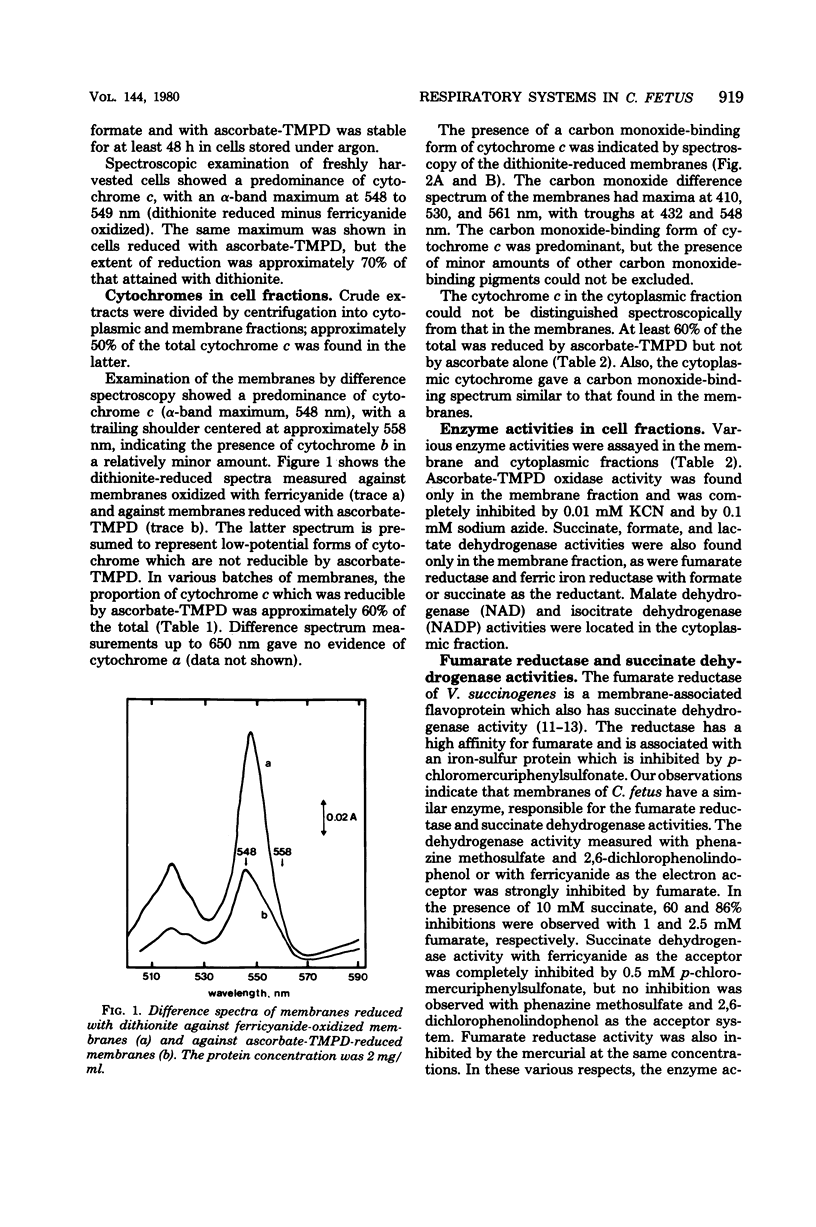
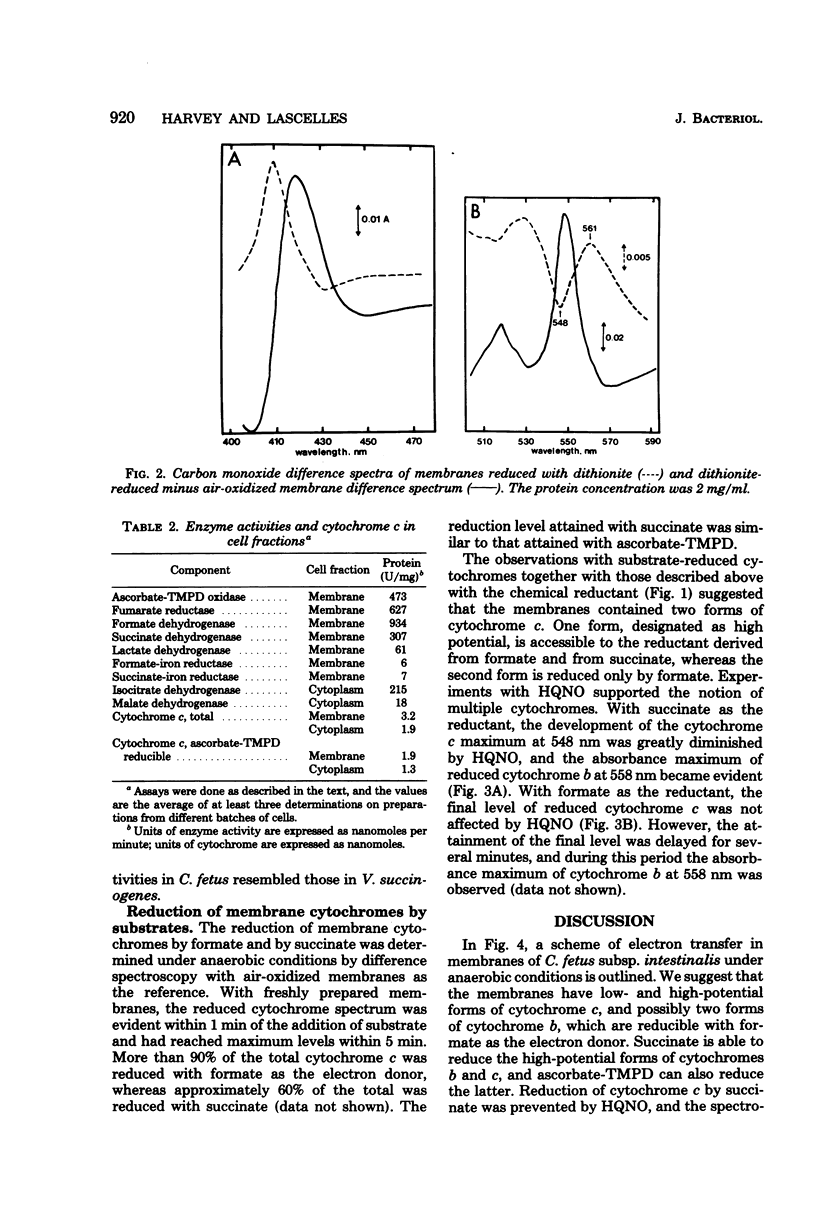
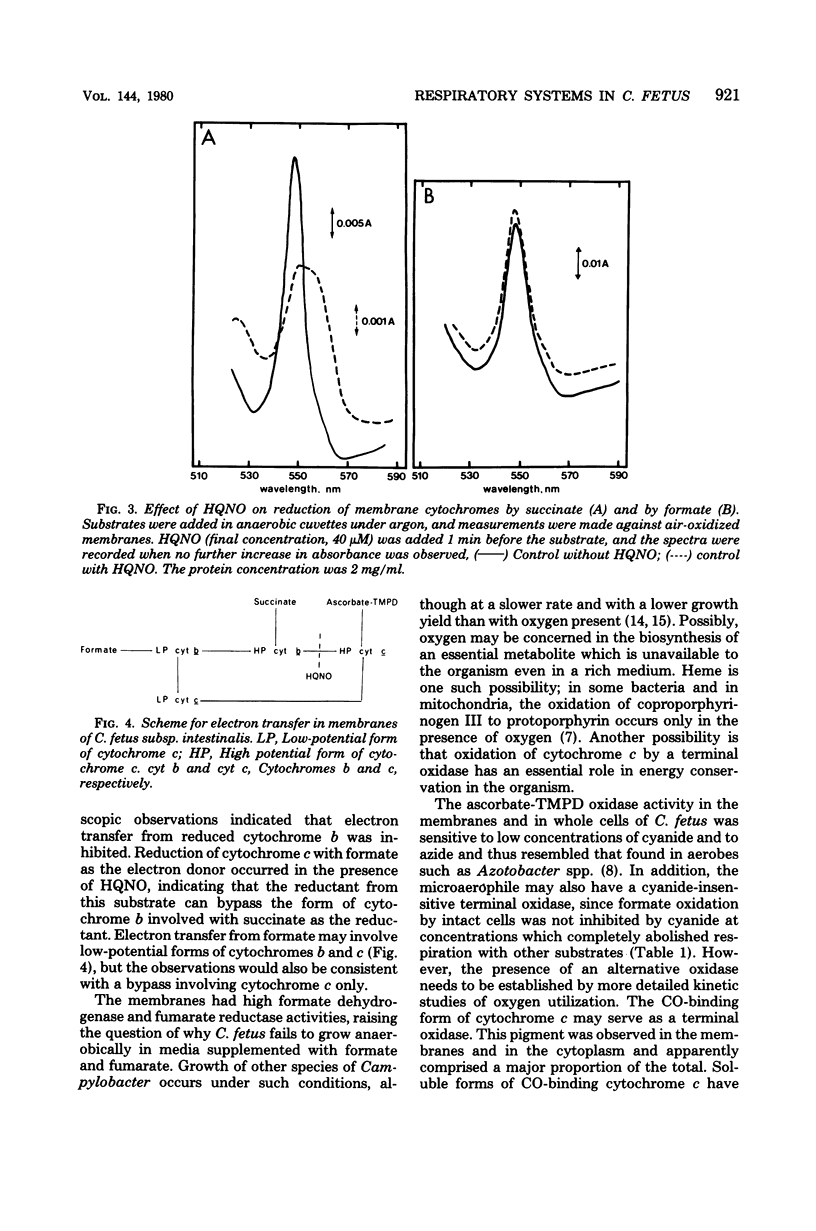
Cell suspensions of Campylobacter fetus subsp. intestinalis grown microaerophilically in complex media consumed oxygen in the presence of formate, succinate, and DL-lactate, and membranes had the corresponding dehydrogenase activities. The cells and membranes also had ascorbate-N,N,N',N'-tetramethyl-p-phenylenediamine oxidase activity which was cyanide sensitive. The fumarate reductase activity in the membranes was inhibited by p-chloromercuriphenylsulfonate, and this enzyme was probably responsible for the succinate dehydrogenase activity. Cytochrome c was predominant in the membranes, and a major proportion of this pigment exhibited a carbon monoxide-binding spectrum. Approximately 60% of the total membrane cytochrome c, measured with dithionite as the reductant, was also reduced by ascorbate-N,N,N',N'-tetramethyl-p-phenylenediamine. A similar proportion of the membrane cytochrome c was reduced by succinate under anaerobic conditions, whereas formate reduced more than 90% of the total cytochrome under these conditions. 2-Heptyl-4-hydroxyquinoline-N-oxide inhibited reduction of cytochrome c with succinate, and the reduced spectrum of cytochrome b became evident. The inhibitor delayed reduction of cytochrome c with formate, but the final level of reduction was unaffected. We conclude that the respiratory chain includes low- and high-potential forms of cytochromes c and b; the carbon monoxide-binding form of cytochrome c might function as a terminal oxidase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C. The microbial metabolism of C1 compounds. The cytochromes of Pseudomaonas AM1. Biochem J. 1975 Feb;146(2):289–298. doi: 10.1042/bj1460289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdre J. H., Krieg N. R., Hoffman P. S., Smibert R. M. Stimulatory effect of dihydroxyphenyl compounds on the aerotolerance of Spirillum volutans and Campylobacter fetus subspecies jejuni. Appl Environ Microbiol. 1976 Jan;31(1):127–133. doi: 10.1128/aem.31.1.127-133.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A., Jr, Lascelles J. Reduction of iron and synthesis of protoheme by Spirillum itersonii and other organisms. J Bacteriol. 1977 Feb;129(2):815–820. doi: 10.1128/jb.129.2.815-820.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Harder W., Attwood M. M. Biology, physiology and biochemistry of hyphomicrobia. Adv Microb Physiol. 1978;17:303–359. doi: 10.1016/s0065-2911(08)60060-0. [DOI] [PubMed] [Google Scholar]
- Hoffman P. S., Krieg N. R., Smibert R. M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. I. Physiological aspects of enhanced aerotolerance. Can J Microbiol. 1979 Jan;25(1):1–7. doi: 10.1139/m79-001. [DOI] [PubMed] [Google Scholar]
- KING T. E. RECONSTITUTION OF RESPIRATORY CHAIN ENZYME SYSTEMS. XI. USE OF ARTIFICIAL ELECTRON ACCEPTORS IN THE ASSAY OF SUCCINATE-DEHYDROGENATING ENZYMES. J Biol Chem. 1963 Dec;238:4032–4036. [PubMed] [Google Scholar]
- Knowles C. J., Calcott P. H., MacLeod R. A. Periplasmic CO-binding c-type cytochrome in a marine bacterium. FEBS Lett. 1974 Dec 1;49(1):78–83. doi: 10.1016/0014-5793(74)80636-8. [DOI] [PubMed] [Google Scholar]
- Niekus H. G., de Vries W., Stouthamer A. H. The effect of different dissolved oxygen tensions on growth and enzyme activities of Campylobacter sputorum subspecies bubulus. J Gen Microbiol. 1977 Dec;103(2):215–222. doi: 10.1099/00221287-103-2-215. [DOI] [PubMed] [Google Scholar]
- Smibert R. M. The genus Campylobacter. Annu Rev Microbiol. 1978;32:673–709. doi: 10.1146/annurev.mi.32.100178.003325. [DOI] [PubMed] [Google Scholar]
- Smith L. Bacterial cytochromes and their spectral characterization. Methods Enzymol. 1978;53:202–212. doi: 10.1016/s0076-6879(78)53025-5. [DOI] [PubMed] [Google Scholar]
- Weston J. A., Knowles C. J. A soluble CO-binding c-type cytochrome from the marine bacterium Beneckea natriegens. Biochim Biophys Acta. 1973 Apr 27;305(1):11–18. doi: 10.1016/0005-2728(73)90226-0. [DOI] [PubMed] [Google Scholar]
- Widdowson D., Anthony C. The microbial metabolism of C1 compounds. The electron-transport chain of Pseudomonas am1. Biochem J. 1975 Nov;152(2):349–356. doi: 10.1042/bj1520349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Carithers R. P. Bacterial iron-sulfur proteins. Microbiol Rev. 1979 Sep;43(3):384–421. doi: 10.1128/mr.43.3.384-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



