Abstract
Background
Islet transplantation is a promising treatment for type 1 diabetes. Due to a shortage of suitable human pancreata, high cost, and the large dose of islets presently required for long-term diabetes reversal; it is important to maximize viable islet yield. Traditional methods of pancreas preservation have been identified as suboptimal due to insufficient oxygenation. Enhanced oxygen delivery is a key area of improvement. In this paper, we explored improved oxygen delivery by persufflation (PSF), ie, vascular gas perfusion.
Methods
Human pancreata were obtained from brain-dead donors. Porcine pancreata were procured by en bloc viscerectomy from heparinized donation after cardiac death donors and were either preserved by either two-layer method (TLM) or PSF. Following procurement, organs were transported to a 1.5-T magnetic resonance (MR) system for 31P nuclear magnetic resonance spectroscopy to investigate their bioenergetic status by measuring the ratio of adenosine triphosphate to inorganic phosphate (ATP:Pi) and for assessing PSF homogeneity by MRI.
Results
Human and porcine pancreata can be effectively preserved by PSF. MRI showed that pancreatic tissue was homogeneously filled with gas. TLM can effectively raise ATP:Pi levels in rat pancreata but not in larger porcine pancreata. ATP:Pi levels were almost undetectable in porcine organs preserved with TLM. When human or porcine organs were preserved by PSF, ATP:Pi was elevated to levels similar to those observed in rat pancreata.
Conclusion
The methods developed for human and porcine pancreas PSF homogeneously deliver oxygen throughout the organ. This elevates ATP levels during preservation and may improve islet isolation outcomes while enabling the use of marginal donors, thus expanding the usable donor pool.
Islet transplantation is an emerging treatment alternative for type 1 diabetes.1,2 Currently, clinical islet allotransplantation is limited by a shortage of suitable donor organs, loss of islets throughout the islet manufacturing and transplantation process, high cost, and the large dose of islets required for long-term diabetes reversal. Islets may be predisposed to death before or during isolation owing to improper handling of the organ during procurement and/or suboptimal cold preservation (CP) during transport. Significant research effort has focused on investigating the efficacy of the 2-layer method (TLM), the present state-of-the art for pancreas preservation.3–8 In the late 1990s, many centers reported improvements in islet isolation outcome using TLM for CP. These improvements were attributed to an increase in tissue adenosine triphosphate (ATP) attributed to enhanced tissue oxygenation with TLM compared with the previously used CP in University of Wisconsin (UW) solution alone.3–8 However, recent studies have suggested that TLM is not able to oxygenate large portions of human or porcine pancreata during CP.9,10 In addition, several large retrospective analyses have found no significant improvement in isolation outcomes for pancreata stored with TLM compared with pancreata stored in UW solution alone.11–13 Therefore, it is of great importance to develop novel methods of preservation which can better oxygenate large organs. One such method investigated here is persufflation (PSF), or vascular gas perfusion. PSF has been investigated in the heart, liver, kidney, and small intestine, but until now not reported for pancreas preservation. 14–23 We used 31P nuclear magnetic resonance (NMR) spectroscopy, a well-established technique for monitoring the amount of ATP present relative to inorganic phosphate (Pi) in tissues, to assess the efficacy of preservation of large organs with TLM compared with PSF. The bioenergetic status of pancreata was monitored and these data were compared to data collected from our previous study of murine pancreata.24 31P-NMR has been used extensively to study tumor biology, bioenergetics, and the metabolism and health status of organs, such as the heart, brain, kidney, liver, and recently, the pancreas. 24 –33 The noninvasive nature of 31P-NMR and its ability to provide information in real time make it an effective and powerful tool for monitoring the bioenergetic status of pancreata and other tissues during CP.
METHODS
Procurement
Rat
Rat pancreata were investigated as detailed by Scott et al.23 All procedures using laboratory animals were approved by the University of Minnesota IACUC.
Pig
Pig pancreata were procured by en bloc viscerectomy from heparinized donation after cardiac death Landrace donors as detailed by Ferrer et al.34 Organs were then preserved at 4°C with TLM or by PSF. All procedures using laboratory animals were approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC).
Human
Human pancreata were procured from brain-dead donors. In short, after adequate exposure of the pancreas was obtained through a cruciate abdominal incision, the preliminary dissection was performed with intact donor circulation. Once the donor was fully heparinized, the distal aortic cannula was inserted, and the infusion of the chilled preservation solution was initiated after the encircled supraceliac aorta was cross-clamped. It was left up to the liver team to decide whether portal venous infusion should be performed through cannulation of the inferior mesenteric versus portal vein. The venous system was decompressed via venotomy. The organs remained in situ until the cold infusion was complete. The pancreas was either harvested en bloc with the liver and separated on the back table or removed after the harvest of clinical organs. Research consent was obtained from all donor families before procurement.
Preservation
TLM
Whole pancreata or individual porcine lobes preserved with TLM were preserved as previously described.35 In brief, pancreata or lobes were suspended halfway between 1 L of cold preservation solution (histidine-tryptophan-ketoglutarate [HTK] solution) and perfluorodecalin that was preoxygenated for 1 hour before preservation by bubbling with 99% oxygen gas. Preservation was performed in specially constructed vessels composed of magnetic resonance (MR)–compatible materials.
Persufflation
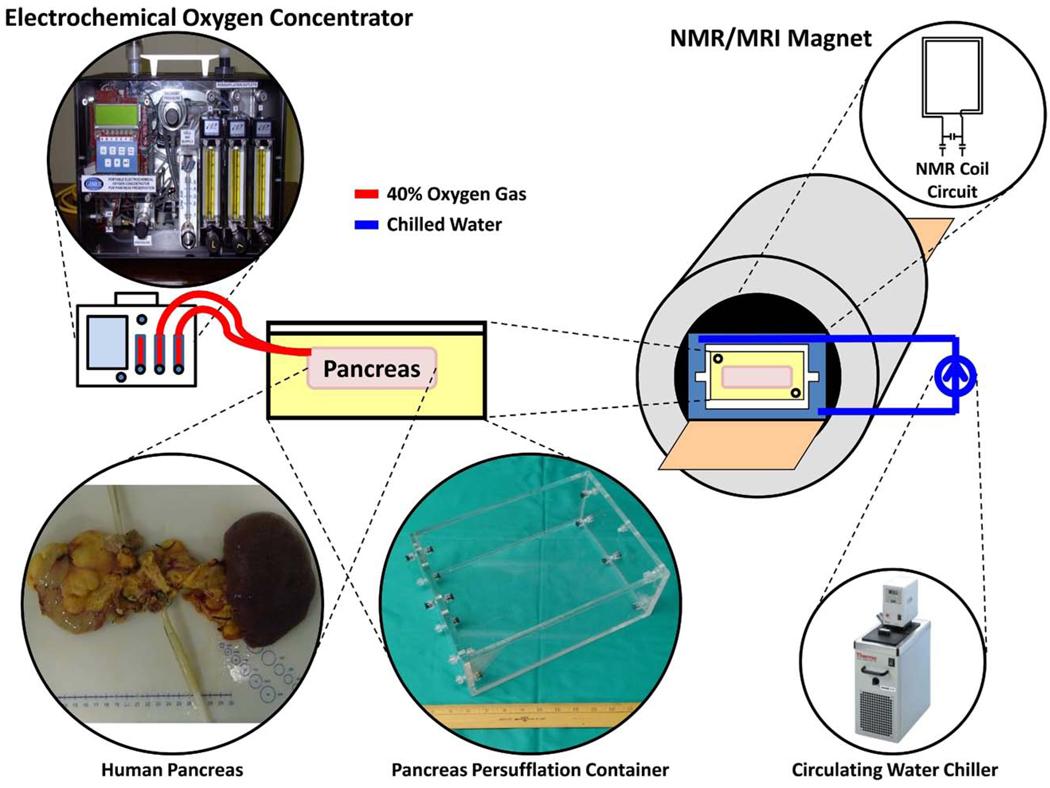
Pancreata were bathed in HTK solution and pumped with 20 mL/min 40% humidified oxygen gas to both the superior mesenteric artery and either the splenic artery (human) or celiac trunk (pig) using an electrochemical oxygen concentrator (Giner Inc., Newton, MA).
After procurement, organs were transported to a 1.5-T magnet for 31P-NMR spectroscopy to investigate their bioenergetic status by measuring the ratio of ATP to Pi (ATP:Pi). Preservation was performed in specially constructed vessels containing MR-compatible materials.
MRI
All MRI was done in a 1.5-T magnet using either a birdcage or surface coil at 63.85 MHz. A T1-weighted gradient echo sequence was used to acquire all images. Assessment of the homogeneity of PSF was done by observing the presence of gas in the vasculature by the negative contrast it provides during MRI.
31P-NMR Spectroscopy
For the pig and human organs, NMR spectroscopy was done in a 1.5-T magnet by placing a surface coil tuned to 25.85 MHz as close to the organ as possible. Rat pancreata were assessed as described by Scott et al.23 The areas of the α-, β-, and γ-ATP peaks were compared with the area of the Pi peak to monitor the bioenergetic status of the organs. A schematic diagram of how organs were placed into the magnet and maintained during preservation is shown in Fig 1.
Fig 1.
Diagram illustrating how the pancreas was placed into the magnet during data acquisition.
RESULTS
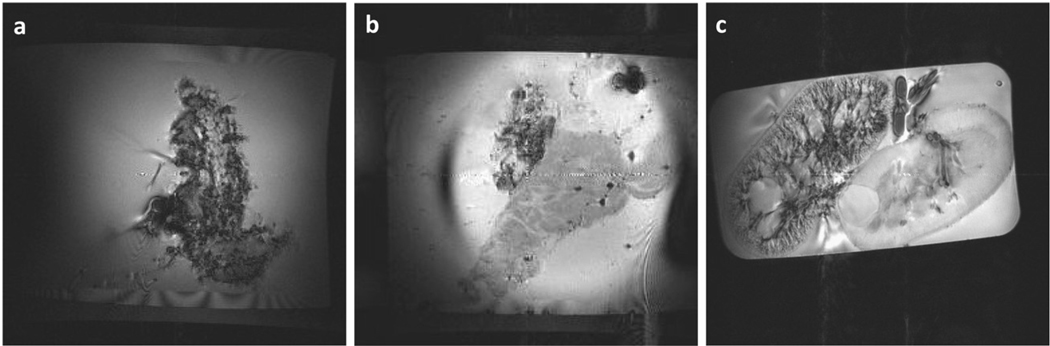
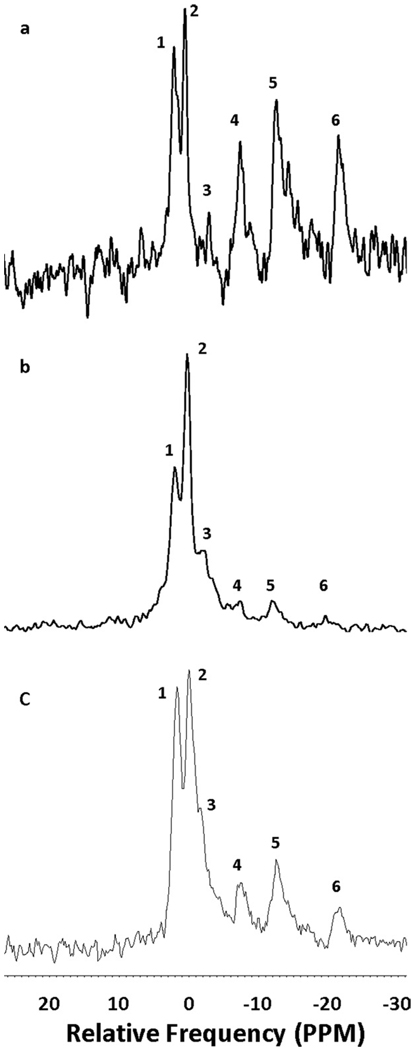
PSF organs typically exhibited the presence of negative contrast (indicating the presence of gas in the vasculature) in >90% of the tissue. Representative images from a PSF pancreas and a pancreas that exhibited significant gas leakage are shown in Fig 2. Also shown in Fig 2 is a well PSF porcine kidney showing a typical vascular branching structure and a non-PSF kidney that exhibited no negative contrast. As previously reported, rat pancreata exposed to TLM exhibited high levels of ATP:Pi, with TLM superior to other methods of static preservation.24 When we investigated porcine pancreata preserved with TLM, however, ATP levels were nearly undetectable (Fig 3). ATP levels observed from organs preserved by TLM were indistinguishable from those measured in organs preserved in HTK solution alone. The lack of ATP observed, reflects the inability of TLM to adequately supply the majority of the pancreas with oxygen in larger organs, such as from pigs or humans. However, when we investigated PSF of human pancreata we observed elevated ATP:Pi levels similar to those observed from the rat model preserved by TLM (Fig 3). When PSF was abruptly stopped and human organs were exposed to static preservation alone, ATP:Pi quickly decreased to undetectable levels similar to those observed from porcine organs preserved by TLM. This confirms that PSF was responsible for the elevated ATP:Pi observed.
Fig 2.
Gradient echo MRI of (a) a well persufflated pancreas with gas filling the vasculature indicated by dark regions, typical of what was observed in general; (b) a pancreas with poor persufflation which had a large arterial gas leak; and (c) a well persufflated kidney (left) and a nonpersufflated kidney (right), the persufflated kidney showing typical vascular branching (dark regions).
Fig 3.
31P-NMR spectra acquired from (a) a rat pancreas preserved by the two-layer method (TLM); (b) a porcine pancreas preserved by TLM; and (c) a persufflated human pancreas. Peak numbering corresponds to: 1) phosphomonoester; 2) inorganic phosphate; 3) phosphodiester; 4) γ-ATP; 5) α-ATP; and 6) β-ATP.
DISCUSSION
In this paper we used 31P-NMR spectroscopy to noninvasively assess the efficacy of different preservation protocols (TLM and PSF) in maintaining ATP levels (a direct reflection of respiration and measure of organ health and viability) in rat, porcine, and human pancreata. Previous investigation demonstrated that, in the rat pancreas model, CP with TLM results in dramatically improved ATP levels.24 The impact of TLM was investigated in porcine pancreata and compared with a novel method of pancreas preservation, PSF, in the pig and human models. ATP levels were consistently low for all organs preserved by static methods, such as TLM. However, ATP levels from human and porcine pancreata preserved by PSF were consistently higher than those observed from organs exposed to static preservation, approaching levels observed from rat pancreata preserved with TLM alone. This is due to the improved oxygenation the tissue experiences during PSF by providing humidified oxygen directly into the bulk of the solid organ through the extensive native intact vasculature. This delivery method circumvents the problem of limited oxygen diffusion into the core of larger organs (such as human or porcine pancreata) associated with the static methods of preservation. MRI indicated that PSF can actively supply most of the pancreas with gaseous oxygen during cold preservation.
ACKNOWLEDGEMENTS
The authors thank Dr T.C. Aasheim, Dr L. Guenther, and Dr T. Tanaka for help with early technique development; P.C. Williams, K. Albeck, and S. Walsch for technical assistance with the manufacturing of MR-compatible preservation containers; B. Perrault for surgical assistance; and Dr K.S. Maynard, D. Dudero, G. Wildey, M.L. Graham, L. Mutch, and H. Nelson for administrative assistance.
Supported by a grant from the National Center for Research Resources (U42 RR016598), National Institutes of Health (NIH), NIH National Institute of Diabetes and Digestive and Kidney Diseases (R43 DK070400), the Schott Foundation, and the Carol Olson Memorial Diabetes Research Fund.
REFERENCES
- 1.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:289. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Ricordi C, Hering B. Edmonton’s islet success has indeed been replicated elsewhere. Lancet. 2003;362(9391):1242. doi: 10.1016/S0140-6736(03)14526-6. [DOI] [PubMed] [Google Scholar]
- 3.Hiraoka K, Kuroda Y, Suzuki Y, et al. Outcomes in clinical pancreas transplantation with the two-layer cold storage method versus simple storage in University of Wisconsin solution. Transplant Proc. 2002;34:2688. doi: 10.1016/s0041-1345(02)03376-6. [DOI] [PubMed] [Google Scholar]
- 4.Tsujimura T, Kuroda Y, Avila JG, et al. Influence of pancreas preservation on human islet isolation outcomes: impact of the two-layer method. Transplantation. 2004;78:96. doi: 10.1097/01.tp.0000133515.37892.d5. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto S, Zhang G, Qualley S, et al. The effect of two-layer (University of Wisconsin solution/perfluorochemical) preservation method on clinical grade pancreata prior to islet isolation and transplantation. Transplant Proc. 2004;36:1037. doi: 10.1016/j.transproceed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Tsujimura T, Kuroda Y, Avila JG, et al. Resuscitation of the ischemically damaged human pancreas by the two-layer method prior to islet isolation. Transplant Proc. 2003;35:2461. doi: 10.1016/j.transproceed.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Ricordi C, Fraker C, Szust J, et al. Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation. 2003;75:1524. doi: 10.1097/01.TP.0000058813.95063.7A. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto S, Kuroda Y. Perfluorocarbon for organ preservation before transplantation. Transplantation. 2002;74:1804. doi: 10.1097/00007890-200212270-00030. [DOI] [PubMed] [Google Scholar]
- 9.Avgoustiniatos ES, Hering BJ, Papas KK. The rat pancreas is not an appropriate model for testing the preservation of the human pancreas with the two-layer method. Transplantation. 2006;81:1471. doi: 10.1097/01.tp.0000215389.64186.3f. [DOI] [PubMed] [Google Scholar]
- 10.Papas KK, Hering BJ, Guenther L, et al. Pancreas oxygenation is limited during preservation with the two-layer method. Transplant Proc. 2005;38:3501. doi: 10.1016/j.transproceed.2005.09.085. [DOI] [PubMed] [Google Scholar]
- 11.Kin T, Mirbolooki M, Salehi P, et al. Islet isolation and transplantation outcomes of pancreas preserved with University of Wisconsin solution versus two-layer method using preoxygenated perfluorocarbon. Transplantation. 2006;82:1286. doi: 10.1097/01.tp.0000244347.61060.af. [DOI] [PubMed] [Google Scholar]
- 12.Caballero-Corblan J, Eich T, Foss A, et al. No beneficial effect of the two layer method (PFCUW) compared with transportation in UW alone on the outcome of human islet isolation and transplantation: a report on 214 human pancreases. Am J Transplant. 2006;2(6 suppl 2):340. [Google Scholar]
- 13.Caballero-Corblan J, Eich T, Lundgren T, et al. No beneficial effect of two-layer storage compared with UW-storage on human islet isolation and transplantation. Transplantation. 2007;84:864. doi: 10.1097/01.tp.0000284584.60600.ab. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn-Regnier F, Fischer JH, Jeschkeit S, et al. Coronary oxygen persufflation combined with HTK cardioplegia prolongs the preservation time in heart transplantation. Eur J Cardiothorac Surg. 2000;17:71. doi: 10.1016/s1010-7940(99)00353-x. [DOI] [PubMed] [Google Scholar]
- 15.Fischer JH, Kuhn-Régnier F, Jeschkeit S, et al. Excellent recovery after prolonged heart storage by preservation with coronary oxygen persufflation: orthotopic pig heart transplantations after 14-hr storage. Transplantation. 1998;66:1450. doi: 10.1097/00007890-199812150-00007. [DOI] [PubMed] [Google Scholar]
- 16.Yotsumoto G, Jeschkeit-Schubbert S, Funcke C, et al. Total recovery of heart grafts of non-heart-beating donors after 3 hours of hypothermic coronary oxygen persufflation preservation in an orthotopic pig transplantation model. Transplantation. 2003;75:750. doi: 10.1097/01.TP.0000055217.13736.9B. [DOI] [PubMed] [Google Scholar]
- 17.Tolba RH, Schildberg FA, Schnurr C, et al. Reduced liver apoptosis after venous systemic oxygen persufflation in non-heart-beating donors. J Invest Surg. 2006;19:219. doi: 10.1080/08941930600778198. [DOI] [PubMed] [Google Scholar]
- 18.Saad S, Minor T, Kötting M, et al. Extension of ischemic tolerance of porcine livers by cold preservation including postconditioning with gaseous oxygen. Transplantation. 2001;71:498. doi: 10.1097/00007890-200102270-00003. [DOI] [PubMed] [Google Scholar]
- 19.Minor T, Akbar S, Tolba R, Dombrowski F. Cold preservation of fatty liver grafts: prevention of functional and ultrastructural impairments by venous oxygen persufflation. J Hepatol. 2000;32:105. doi: 10.1016/s0168-8278(00)80196-8. [DOI] [PubMed] [Google Scholar]
- 20.Rolles K, Foreman J, Pegg DE. A pilot clinical study of retrograde oxygen persufflation in renal preservation. Transplantation. 1989;48:339. [PubMed] [Google Scholar]
- 21.Rolles K, Foreman J, Pegg DE. Preservation of ischemically injured canine kidneys by retrograde oxygen persufflation. Transplantation. 1984;38:102. doi: 10.1097/00007890-198408000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Treckman JW, Paul A, Saad S, et al. Primary organ function of warm ischaemically damaged porcine kidneys after retrograde oxygen persufflation. Nephrol Dial Transplant. 2006;21:1803. doi: 10.1093/ndt/gfl066. [DOI] [PubMed] [Google Scholar]
- 23.Minor T, Klauke H, Isselhard W. Improved preservation of the small bowel by luminal gas oxygenation: energetic status during ischemia and functional integrity upon reperfusion. Transplant Proc. 1997;29:2994. doi: 10.1016/s0041-1345(97)00757-4. [DOI] [PubMed] [Google Scholar]
- 24.Scott WE, III, Matsumoto S, Tanaka T, et al. Real-time noninvasive assessment of pancreatic ATP levels during cold preservation. Transplant Proc. 2008;40:403. doi: 10.1016/j.transproceed.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arias-Mendoza F, Brown TR. In vivo measurement of phosphorous markers of disease. Dis Markers. 2003–2004;19:49. doi: 10.1155/2004/419095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobbins RL, Malloy CR. Measuring in-vivo metabolism using nuclear magnetic resonance. Curr Opin Clin Nutr Metab Care. 2003;6:501. doi: 10.1097/00075197-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Horn M. Cardiac magnetic resonance spectroscopy: a window for studying physiology. Methods Mol Med. 2006;124:225. [PubMed] [Google Scholar]
- 28.Barnard ML, Changani KK, Taylor-Robinson SD. The role of magnetic resonance spectroscopy in the assessment of kidney viability. Scand J Urol Nephrol. 1997;31:487. doi: 10.3109/00365599709030648. [DOI] [PubMed] [Google Scholar]
- 29.Davidson BR, Barnard ML, Changani KK, Taylor-Robinson SD. Liver transplantation: current and potential applications of magnetic resonance spectroscopy. Liver Transpl Surg. 1997;3:481. doi: 10.1002/lt.500030502. [DOI] [PubMed] [Google Scholar]
- 30.Khan SA, Cox IJ, Hamilton G, et al. In vivo and in vitro nuclear magnetic resonance spectroscopy as a tool for investigating hepatobiliary disease: a review of H and P MRS applications. Liver Int. 2005;25:273. doi: 10.1111/j.1478-3231.2005.01090.x. [DOI] [PubMed] [Google Scholar]
- 31.Gillies RJ, Morse DL. In vivo magnetic resonance spectroscopy in cancer. Annu Rev Biomed Eng. 2005;7:287. doi: 10.1146/annurev.bioeng.7.060804.100411. [DOI] [PubMed] [Google Scholar]
- 32.Yoshikawa T, Suzuki Y, Kanashiro M, et al. Objective and rapid assessment of pancreas graft viability using 31P-nuclear magnetic resonance spectroscopy combined with two-layer cold storage method. Transplantation. 2004;78:78. doi: 10.1097/01.tp.0000133516.55002.52. [DOI] [PubMed] [Google Scholar]
- 33.Siech M, Sotak CH, Letko G, Davis MA. A method for in vivo assessment of reversible rat pancreatic ischemia using 31P NMR spectroscopy at 2 Tesla. Magn Reson Imaging. 1995;13:463. doi: 10.1016/0730-725x(94)00127-o. [DOI] [PubMed] [Google Scholar]
- 34.Ferrer J, Scott WE, III, Weegman BP, et al. Pig pancreas anatomy: implications for pancreas procurement, preservation, and islet isolation. Transplantation. 2008;86:1503. doi: 10.1097/TP.0b013e31818bfda1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuroda Y, Kawamura T, Suzuki Y, et al. A new, simple method for cold storage of the pancreas using perfluorochemical. Transplantation. 1988;46:457. doi: 10.1097/00007890-198809000-00027. [DOI] [PubMed] [Google Scholar]