Abstract
Differentiation of embryonic or determined stem cell populations to adult liver fates under known conditions yields cells with some but not other adult-specific genes, aberrant regulation of one or more genes, and variation in the results from experiment to experiment. We tested the hypothesis that sets of signals produced by freshly isolated, lineage-dependent mesenchymal cell populations would yield greater efficiency and reproducibility in driving differentiation of human hepatic stem cells (hHpSCs) to adult liver fates. Subpopulations of liver-derived mesenchymal cells, purified by immunoselection technologies, included 1) angioblasts; 2) mature endothelia; 3) hepatic stellate cell precursors; 4) mature stellate cells (pericytes) and 5) myofibroblasts. Freshly immunoselected cells of each of these subpopulations were established in primary cultures under wholly defined (serum-free) conditions that we developed for short-term cultures and used them as feeders with hHpSCs. Feeders of angioblasts yielded self-replication; stellate cell precursors caused lineage restriction to hepatoblasts; mature endothelia produced differentiation to hepatocytes; and mature stellate cells and/or myofibroblasts resulted in differentiation to cholangiocytes. Paracrine signals, produced by the different feeders, were identified by biochemical, immunohistochemical, and qRT-PCR analyses and then those signals were used to replace the feeders in monolayer and 3-D cultures to elicit the desired biological responses from the hHpSCs. The defined paracrine signals proved able to yield reproducible responses from the hHpSCs and to permit differentiation to fully mature and functional parenchymal cells.
Conclusions
paracrine signals from defined mesenchymal cell populations are important for regulation of stem cell populations to specific adult fates, findings of importance for basic and clinical research as well as industrial investigations.
Keywords: epithelial-mesenchymal interactions, extracellular matrix, soluble signals, lineage restriction, expansion
Introduction
Human hepatic stem cells (hHpSCs) are uniquely positioned at the foundation of potential liver regeneration therapies because they are the only parenchymal cell subpopulation identified as capable of self-renewal combined with the capacity to generate numerous progenitors that include hepatoblasts (hHBs), committed progenitors and their descendents, mature hepatocytes and cholangiocytes (1). The hHpSCs and hepatoblasts (hHBs) have been found in livers of donors of all ages and are able to give rise to mature liver tissue in vitro and in vivo (2, 3). In addition to these determined stem cell populations, diverse stem cell populations have been identified and found able to be lineage restricted to a liver fate including embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and multiple forms of mesenchymal stem cells (MSCs) from bone marrow, adipose tissue and amniotic fluid (4-6). The efficiency of differentiation of these precursors to a liver fate, whether in vitro or in vivo, results in liver-like cells with over- or under-expression of some adult genes, aberrant regulation of genes and with the results being distinct with every preparation. These findings are discussed at length in a recent review (7).
We have used hHpSCs as a model system to define the requisite signals for lineage restricting stem cells to a liver fate. In prior studies, we established methods to isolate hHpSCs and hHBs from fetal, neonatal, pediatric, and adult human livers (2, 8). The hHpSCs are characterized by their uniform morphology, high nucleus to cytoplasmic ratio, small size (∼7-9 μm in diameter) and tightly packed colony formation. The cells weakly express albumin and cytokeratins (CK) 8 and18, and CK19, but no α-fetoprotein (AFP). The hHBs are larger (∼10-12 μm in diameter) and exhibit colonies that are cord-like, with bile canaliculi and express albumin, CK 8 and18, CK19 and AFP. The hHpSCs and hHBs have unique antigenic profiles that include epithelial cell adhesion molecule (EpCAM) for both and neural cell adhesion molecule (NCAM) for the hHpSCs versus intercellular cell adhesion molecule (ICAM) for the hHBs. More extensive characterizations of them and their descendents are given in prior reports (2). The cells can be clonogenically expanded ex vivo in a serum-free medium tailored for endodermal progenitors, Kubota's Medium (KM) (9), and have the potential to differentiate into mature functional hepatocytes and cholangiocytes in vivo.
The microenvironment of stem cell niches modulate stem cell proliferation, influence symmetric versus asymmetric division, control differentiation, protect cells from physiologic stresses, and help them contribute to tissue formation in development and in regeneration in adult life (7). The components of the stem cell microenvironment regulating these processes include distinct cell-cell interactions and paracrine signals, comprising both soluble and extracellular matrix factors, as well as the three-dimensional (3-D) architecture shaping and dictating the delivery of these cues.
The studies reported here are focused on mesenchymal companion cells providing critical paracrine signals regulating the parenchymal lineage stages. Paracrine signals were identified using purified subpopulations of the mesenchymal cells cultured under serum-free conditions. A set of these signals was then used to regulate precisely the growth and/or fates of the hHpSCs under feeder-free conditions. In a separate report, we focus on lineage-dependent soluble signals (J. Uronis and L. Reid, et al, manuscript in preparation).
Materials and Methods
Most of the detailed presentations of the methods were as described previously (2, 10) and/or given in the online supplement.
Immunoselection of subpopulations was done using magnetically activated cell sorting (MACS) according to the manufacturer instructions (Miltenyi Biotech) and with cell suspensions from human fetal livers or adult human livers. These included:
Angioblasts: CD133+ or CD117+ and co-expressing VEGFR2 (KDR) from fetal or adult livers;
Mature hepatic endothelia: CD31++ and co-expressing KDR from adult livers;
Human hepatic stellate cell (hHpSTC) precursors: CD146+ cells from fetal livers;
Mature hHpSTCs (pericytes): CD146+ cells from adult livers;
Human Hepatic stem cells (hHpSCs): [EpCAM+ NCAM+] cells from fetal and adult livers.
Results
Isolation of mesenchymal subpopulations in human livers
Human livers contain two lineages of mesenchymal cell subpopulations that are not hemopoietic cell subpopulations and are CD45 negative. Both derived from angioblasts: 1) lineage stages of endothelia and 2) hHpSTC precursors and their descendents, mature hHpSTCs (pericytes) and then myofibroblasts. Immunoselection for the different lineage stages of the two subpopulations was done by MACS with specific antigenic profiles, and the cells were used in primary co-cultures with the hHpSCs. In the Supplement Online Table 4 are provided data utilizing feeders of both cell lines and primary cultures of mesenchymal cells. Schematic images of the parenchymal and mesenchymal cell lineages are given in Figs. S5 and S6.
1). Angioblasts and endothelia (Figs. 1, S2)
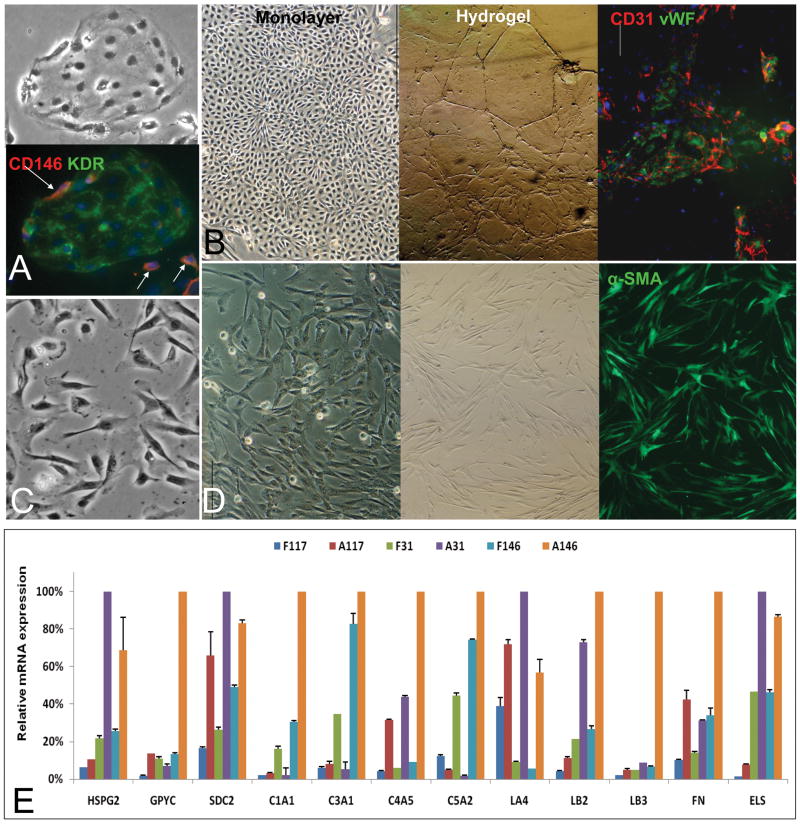
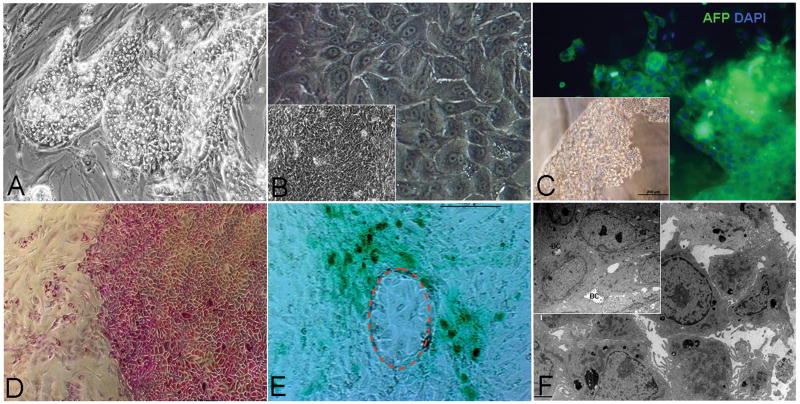
Figure 1. Mesenchymal Cell Subpopulations.
Aggregates of angioblasts (A) positive for VEGFR2 (KDR) (A) and rimmed by individual stellate cell precursors positive for CD146 (pink, arrowhead) and other markers not shown (desmin, ASMA, β-3 integrin) (2, 21). All of the mesenchymal cell populations assessed were negative for CD45 (data not shown). Mature endothelia (B) formed swirling aggregates of cells in monolayer cultures and formed tube-like structures if embedded into hyaluronans or Matrigel. The endothelia express strongly CD31 (red) and vWF (green). The nuclei are stained with DAPI (blue). The stellate cell precursors (C) that are separated from the aggregates of angioblasts are short (<10 μm), and have their nucleus on one end of a cell resembling a “comma”. Mature stellate cells are found in abundance in adult human livers cell suspensions; when freshly plated (D); they are bipolar cells that are at least 15-20 μm in length and with the nucleus centrally located. With time in culture, and especially after exposure to serum, they lengthen even more (they can be 50 μm in length and express high level of ASMA). Compare the CD146 expression in the precursors to the activated stellate cells. E: qRT-PCR analyses of matrix components in freshly isolated liver mesenchymal cells. F=fetal; A=adult. CD117=angioblasts (CD117+/VEGFr2+); CD31= endothelia; CD146= stellate cells. The cDNAs from different cell subpopulations from fetal or adult were reverse transcribed and subjected to qRT-PCR analyses for matrix molecules. The proteoglycans assayed included HS-PG2 (perlecan), GPYC (glypican) and SDC2 (syndecan 2); The collagens assayed included collagens 1 (C1A1), 3 (C3A1), 4 (C4A5), and 5 (C5A2); The basal adhesion molecules assayed were isoforms of laminin (LA4, LB2, LB3) and fibronectin (FN); Elastin (ELS) was also assayed. The data from the maximal value among the 6 cellular subpopulations for each matrix component were given a value of 100% and used to standardize data for the other cellular subpopulations. Thus, the amount of that matrix component produced by the other cell populations is given as a fraction of this maximum one. This allows one to see that forms of HS-PGs, laminins, elastin, and collagens type III and IV dominate in endothelial cell lineages, whereas all the collagens, proteoglycans, fibronectin and elastin dominate in the stellate ones. Bars indicate standard deviation, n=6.
Angioblasts were isolated from fetal liver cell suspensions by immunoselection for cells expressing CD117 and VEGFR2 (KDR). The percentage of sorted CD117+ KDR+ cells within the fetal liver samples was found to be about 0.5%. In culture, they appeared as aggregates demonstrating expression of CD117 (Fig. 1A); other antigens included CD133, NCAM, von Willebrand Factor (vWF) and weak or no levels of CD31 (PECAM). They give rise to mature endothelia that are CD31++, VEGFR+, vWF+ and ICAM1+, and with classic cobble stone-like clusters in monolayer cultures or tubes of cells if embedded into hyaluronan hydrogels or Matrigel.
2). Hepatic stellate cell (hHpSTC) lineage (Figs. 1, S3)
The hHpSTC precursors, are recognizable by their morphology as short (<10 μm), bipolar cells with their nucleus on one end and expressing CD146. They have very low levels of desmin, α-smooth muscle actin (ASMA), vitamin A and lipids. They were negative for glial fibrillary acidic protein (GFAP), were found at the edges of aggregates of angioblasts (arrowheads, Fig. 1A), and found separately from these clusters. They give rise to mature hHpSTCs, also called hepatic-specific pericytes, expressing CD146 strongly (11, 12). Freshly isolated hHpSTCs from adult liver cell suspensions were longer (∼15-20 μm) with their nuclei more centrally located than found in the precursors. The pericytes express elevated levels of ASMA, desmin, and GFAP, vitamin A and have high lipid content (not shown). When hHpSTCs are plated onto culture plastic and in KM supplemented with 5% FBS, they were activated into the cells with a myofibroblast phenotype emerging within 3-5 days of culture (Fig. S3). The cells were even longer (up to or greater than 50 μm in length), with a centrally located nucleus and expressing the highest levels observed of ICAM1, ASMA and desmin.
Expression of matrix Components in the Mesenchymal Cell Lineages
We surveyed the biological activities of numerous mesenchymal cell lines and primary cultures of mesenchymal cells as feeders (Supplement Table 4). We realized eventually that exposure to serum, even transiently, resulted in muting of the distinctions in paracrine signals produced by the different mesenchymal cell subpopulations and skewing of them towards biological activity typical for fibrosis or cirrhosis. Therefore, a serum-free medium for mesenchymal cells (MCM) was developed (Supplement, Fig. S1) enabling us to define feeder effects and the paracrine signals produced using freshly immunoselected mesenchymal cell subpopulations from fetal versus adult human liver under serum-free conditions in short-term cultures (up to two weeks).
Using this strategy, we determined that all the mesenchymal subpopulations produced multiple types of collagen, basal adhesion molecules, proteoglycans and elastin but at quite different levels (Fig 1E and Table 1). The angioblasts (CD117+/KDR+ or CD133+/KDR+) from fetal livers produced less matrix than any of the mesenchymal cell subpopulations tested; low levels of type III, IV and V collagens of which only type III was found detectable by immunohistochemistry; laminin A4 but not the other laminins or fibronectin; CS-PGs and low levels of syndecan of which only the CS-PG was detected by immunohistochemistry, and hyaluronans. Those from adult livers produced higher levels of syndecan, laminin A4, fibronectin and type IV collagen. Fetal liver-derived endothelia (CD31+) cells make all of the forms of HS-PGs, collagen type I, III and V but not IV; low levels of laminin B2 and fibronectin; and elastin. Adult liver-derived endothelial cells (CD31+) make high levels of HS-PG2 and syndecan, collagen type I and IV; Laminin A4 and B3, fibronectin and elastin. In summary, the matrix chemistry in angioblast and endothelial subpopulations is dominated by heparan sulfate proteoglycans and some but not all forms of laminin and with a significant increase in elastin with development.
Table 1. Extracellular Matrix Components found in Feeder Cells (data from Immunohistochemistry or biochemistry).
| Matrix Components | Angioblasts* | HUVEC Cells | hMSCs | STO Feeders | NPCs minus fibroblasts | CD31 or VEGFr+ | CD146+ (ASMA+) |
|---|---|---|---|---|---|---|---|
| Source of the cells for immunoselection | Human fetal liver | Human umbilical vein | Bone Marrow | Murine embryos | Human fetal liver | Human fetal and adult livers | |
| Collagen types Produced | Small amounts of type III | Type IV | Types I, III and IV | Types III, IV | Types I and IV collagen | Type III in fetal shifting to type IV in adults | Type I in fetal shifting to Types I and IV in adults |
| Proteoglycans /GAGs produced | CS-PGs, Hyaluronans | CS-PGs |
CS-PGs HS-PGs Hyaluronans |
CS-PGs | HS-PGs |
CS-PGs HS-PGs |
|
| Adhesion proteins and other proteins produced | Negligible levels of those assayed | Fibronectin | Fibronectin Laminins |
Fibronectin, Laminins | Low levels of fibronectin, high levels of laminin, elastin and increasing in adults | Low levels of fibronectin and Elastin in fetal and increasing to very high levels of these plus laminin in adults | |
| Response of hHpSCs when co-cultured with these mesenchymal cells | Self-replication | Slow lineage restriction (>36-48 hours) | Lineage restriction to hHBs and committed progenitors within ∼24 hours | Differentiation to mature parenchymal cells | |||
Note: (CD117+KDR+ or CD133+KDR+). The bolded letters are to indicate the matrix components found in particular abundance. The amount of staining for matrix components was least with the angioblasts and the maximum with the STO feeders and CD146+. NPCs minus fibroblasts= non-parenchymal cells that were depleted of human fibroblasts by magnetic immunoselection cells ASMA=alpha-smooth muscle actin (marker for stellate cells); n.d. =not done. Others have shown that the bone marrow-derived MSCs, in their undifferentiated state, produce type I and X collagen and forms of CS-PGs that include decorin and versican (30).
The stellate cell subpopulations produced the highest amounts of most of the matrix components analyzed; expressed low or negligible levels of laminins, strong expression of fibronectin and elastin and were high producers of all the collagens, especially type I collagen. As for the angioblast and endothelial cell subpopulations, the levels were highest in those from adult livers.
In Supplement Figure 7 are summarized the findings from changes in matrix components at varying stages of the lineage through to the mature hepatocytes and cholangiocytes and containing also information from the reports of others. In addition, the composition of the serum-free, hormonally defined media (HDM) for the different stages is shown and is that established in prior studies (9).
Strong dependence on mesenchymal companion cells
Rigorous purification of parenchymal cells away from their native mesenchymal cell partners resulted in loss of viability of the parenchymal cells, especially the stem cells and progenitors, as shown previously (2, 9). Co-culture of the hHpSCs with the different subpopulations of mesenchymal feeder cells elicited distinct biological responses. Those with angioblasts remained as stem cells, and those with precursors to hepatic stellate cells and endothelia became hepatoblasts, providing distinctive antigenic, biochemical and ultrastructural features for both parenchymal and mesenchymal cell populations (Figs. 2, 3). The hHpSCs/angioblasts partnership results in cells that are tightly bound to each other on their lateral borders through large numbers of tight junctions, desmosomes and interdigitated microvilli. Efforts to disperse the angioblasts/hHpSCs into single cells were not successful with the customary enzymes (e.g. trypsin, chymotrypsin, dispase, collagenases), resulting in rapid loss of cell viability. Mechanical passaging, as used for human embryonic stem (ES) cells in culture, resulted in reasonably efficient passaging of hHpSCs (13) and was used for the studies reported here.
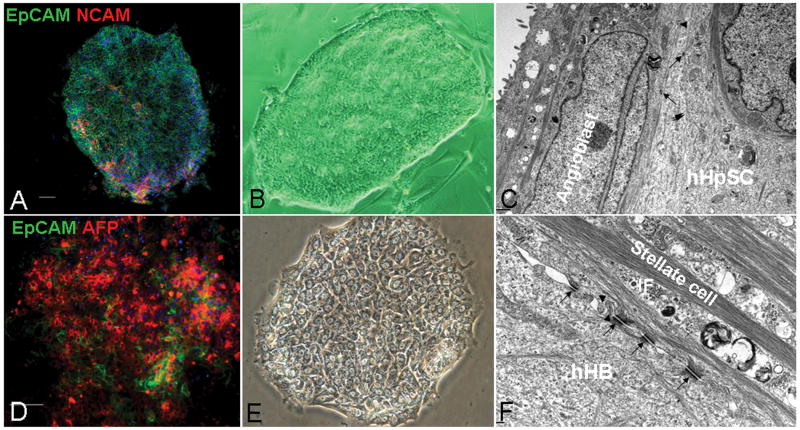
Figure 2. Colonies of hHpSCs (A-C) versus hHBs (D-E).
The hHpSCs express EpCAM (green), NCAM (red) (A), whereas the hHBs (D) express EpCAM (green) and AFP (red); nuclei stained with DAPI (blue), scale bar=100μm. Phase images of the colonies indicate that the small hHpSCs tightly bind to each other and have an elevated region at the periphery of the colony, at which the highest numbers of angioblasts are found. By contrast, the colonies of hHBs contain cords of parenchymal cells interspersed by clear channels, bile canaliculi. Ultrastructural image of the hHpSC colony (C) shows that the mesenchymal cells are tightly bound along their length to the hHpSCs; with few desmosomes and only small bundles of intermediate filaments (IF) in both the mesenchymal cells and the hHpSCs. By comparison, an ultrastructural image of the hHB colony (F) shows that the boundary between the hHBs and the mesenchymal cells is partially separated but held together with desmosomes that connect in the mesenchymal cells to large bundles of intermediate filaments. Arrows = desmosome; arrow head = microvilli.
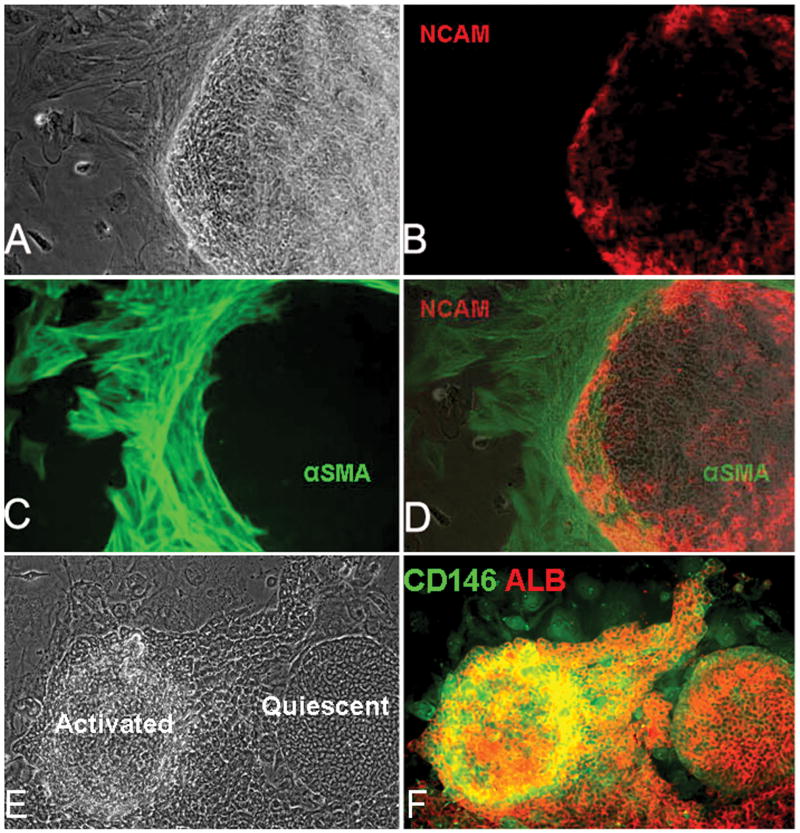
Figure 3. Colonies of hHpSCs with mesenchymal partners.
There are two types of mesenchymal cells in association with the hHpSCs: angioblasts within the colonies and stellate cell precursors at the periphery. A colony of hHpSCs shown in phase (A) and after staining for NCAM (B). NCAM (Red) is found on angioblasts (NCAM+, CD117+, vWF+) and also on the hHpSCs (2). The angioblasts are found scattered throughout the colony but especially at the periphery of the colonies. The stellate cell precursors (21) are found outside and surrounding the colonies and are readily identified by staining for ASMA (green). Magnification, 10×. (D) is a merged image of A, B and C showing the overlap in expression of NCAM and ASMA at the periphery of the stem cell colony. (E) shows a phase contrast image of two adjacent hHpSC colonies; one on the right is associated with angioblasts and is in a quiescent state, conditions under which the hHpSCs self-renew; the other colony is associated with hepatic stellate cell precursors (and also with endothelial cell precursors that are present but cannot be seen with the staining used) leading to an eruption of cords of hHBs extending from the hHpSCs colony, F shows the same colonies as in E stained for CD146 (yellow/green) and for albumin (red).
The hHBs/stellate cell/endothelial cell precursors partnership resulted in cells that were more loosely bound to each other as evidenced by both light microscopic and ultrastructural analyses. Transmission electron micrographic (TEM) observations confirmed that hHBs are distinct from hHpSCs in having striking increases in the number and size of desmosomes as well as of the intermediate filaments that terminated at the desmosomes in the mesenchymal cells and in the appearance of bile canaliculi. In parallel with morphological changes, the hHBs have an antigenic profile that overlaps with that of hHpSCs but with the distinctions of expressing ICAM-1, not NCAM, and expressing α-fetoprotein (AFP) and P450-A7 (data not shown). Activation of angioblasts to give rise to hHpSTCs and endothelial cell precursors was associated with dramatically elevated levels of CD146 (Fig 3), with elevated ASMA and desmin (data not shown), all correlating with the formation of cords of hHBs and committed progenitors from the colonies of the hHpSCs.
Later lineage stages of parenchymal cells are partnered either with endothelia (hepatocytes) or hepatic stellate cells/pericytes/myofibroblasts (cholangiocytes). The data from cultures of these epithelial-mesenchymal partnerships are not shown except in summary form in Fig S7 though we provide the data on the identified paracrine signals from those stages of mesenchymal cells.
Defined Mixes of Signals can replace the Feeders
The information obtained from analyses of the feeders was used to define sets of signals to elicit regulation of hHpSCs under feeder-free conditions. We demonstrate with 4 examples: 1) self-renewal; 2) lineage restriction into hepatoblasts; 3) differentiation to hepatocytes and 4) differentiation to cholangiocytes. (Summary is given in the Supplement Figs S5-S7)
1) Self-Renewal (Fig 4)
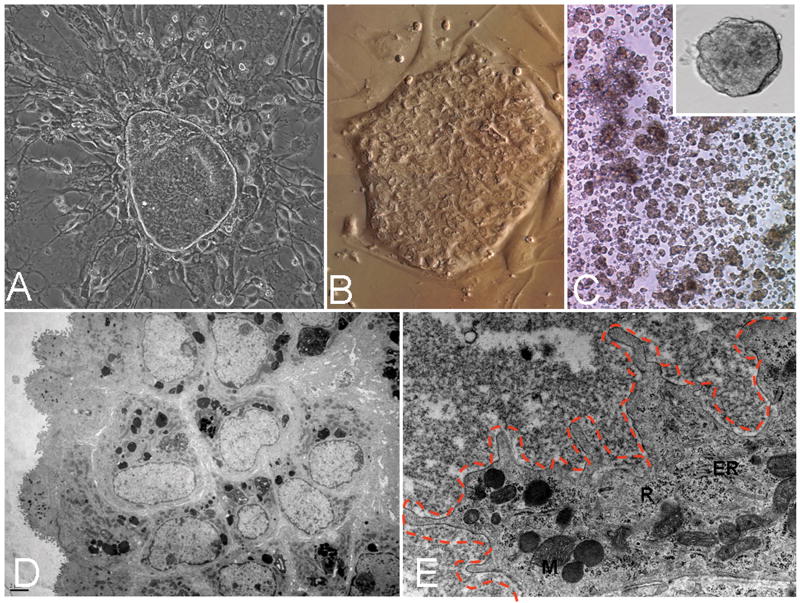
Figure 4. Conditions for Self-renewal.
HHpSCs self-replicated if maintained as monolayers on feeders of angioblasts (A) or on a substratum of purified type III collagen (B) or embedded into weakly cross-linked HA hydrogels in which the hHpSCs formed aggregates or balls of cells (C). Under all three conditions, the hHpSCs could self-renew for many weeks and at division rates of up to a division every 36 hours on culture plastic and as rapidly as every 20-24 hrs on type III collagen (13). TEM of the hHpSCs in the hydrogels (D and E) indicate aggregates of cells with an outer layer interfacing with the hydrogel through numerous microvilli which have been outlined in E with dashes. Cell diameters are about 7-9 μm, with a high nucleo-cytoplasmic ratio. At high magnification, the interface cells appear to have small number of undeveloped endoplasmic reticulum (ER), mitochondria (M), rough and free ribosomes (R) beneath the microvilli, characteristics of immature undifferentiated hepatic parenchymal cells.
Self-renewal occurs with angioblasts feeders, replaceable with KM and type III collagen and/or uncross-linked hyaluronans. These conditions resulted in hHpSC colonies with maintenance of the stem cell phenotype (positive for EpCAM, NCAM, CK19, weak levels of albumin, and negative for AFP, P450A7, urea synthesis and ICG uptake) for more than 2 months in culture and with the ability of the cells from those colonies to give rise to both hepatocytes and cholangiocytes if transferred to differentiation conditions.
Ultrastructural studies of the cells in uncross-linked hyaluronan hydrogels showed tightly aggregated hHpSCs enveloped by the mesenchymal cells and with quite distinctive desmosomes and tight junctions. At low magnification, the surface layer of cells forms an interface with the hydrogel characterized by numerous short microvilli. At higher magnification, the microvilli are shown to be irregular in size and spacing. Beneath the microvilli are clusters of mitochondria and free and bound ribosomes. This outer cell layer of mesenchymal cells envelopes a large aggregate of hHpSCs.
2) Lineage restriction to hepatoblasts (hHBs) (Fig. 5)
Figure 5. Lineage restriction to hHBs.
The hHpSCs lineage restrict to hHBs within 24 hours when grown on stellate cell precursor feeders (A, D and E). The feeders could be replaced with substrata of type IV collagen or laminin (B), or hyaluronans that are cross-linked (C). Combinations of these matrix components give the same result yielding cells with AFP expression (C), glycogen synthesis as evidenced with PAS staining (D), lack of ICG uptake and secretion in those cells expressing a hepatoblast phenotype (E). Crosslinked hyaluronan hydrogels also yield this result, indicating that mechanical forces conferred by the rigidity of the gel are factors in lineage restriction (D). Ultrastructural images of the hHBs (F) show that they are much more loosely connected to each other than are the hHpSCs, and the bile canaliculi (BC) are separated from the remainder of the intercellular space by tight junctions and sparse microvilli on the surface of the cells. The cells displayed oval-shaped nuclei, a paucity of cytoplasm, immature endoplasmic reticulum, mitochondria and ribosomes.
Feeders of stellate cell precursors or activated stellate cells caused the hHpSCs to lineage restrict to hHBs within 24 hours and to express AFP and glycogen. The feeders proved replaceable with KM and matrix components, ones produced by these cells, including type IV collagen and laminin or cross-linked hyaluronan hydrogels or combinations of these. The hHBs do not take up indocyanine green (ICG), though the cultures contained some committed progenitors that did demonstrate some uptake. The lineage restriction to hHBs was associated with a separation between the cells, the formation of bile canaliculi, an increase in the presence of desmosomes, and in the size of the bundles of intermediate filaments in the mesenchymal cells.
3) Differentiation to mature parenchyma
Required MKM described in Methods, all variables defined previously as critical for mature parenchymal cell metabolism (2) and also utilized in lineage restriction of ES cells to liver fates (6). However, the ability to drive the cells to the hepatocytic versus biliary pathways necessitated distinctions in both the hormonal constituents of the media and the matrix chemistry.
Differentiation selectively to hepatocytes occurred with feeders of mature endothelia (Fig. 6) replaceable with 3-D cultures in MKM-H and in HA hydrogels comprised of 60% type IV collagen. Partial effects were observed in monolayer cultures on matrix-coating plates. The cells grew in size to >18 μm, demonstrated cord-like morphology in the colonies with classic bile canaliculi, lost expression of EpCAM, NCAM and AFP, and acquired expression of albumin, glycogen storage, ICG uptake, and urea secretion. In ultrastructural studies, the cells acquired the classic hepatocyte features of large numbers of mitochondria, rough ER and Golgi complexes.
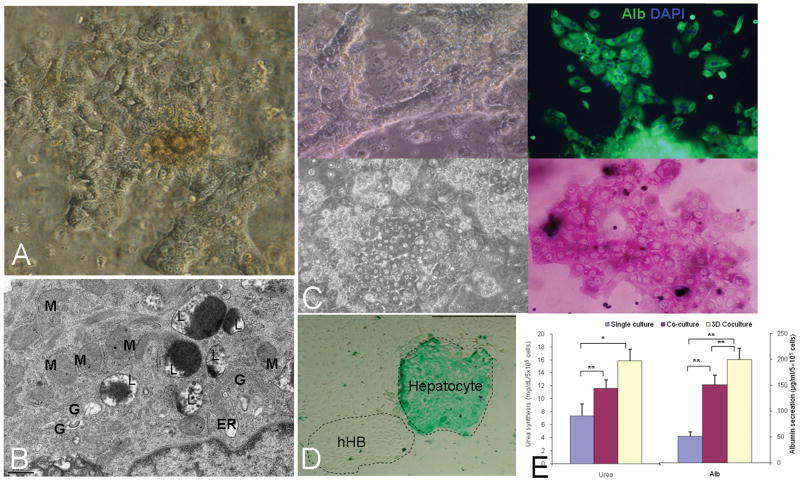
Figure 6. Conditions yielding mature Hepatocytes.
Full differentiation to hepatocytes occurs within 7-10 days if the cells are plated in MKM supplemented further with factors for hepatocytes (MKM-H) and with feeders of endothelia (A, B). The feeders can be replaced by embedding the cells into a mix of 40% hydrogels (HA or Matrigel) and 60% type IV collagen and laminin in combination with the MKM-H (C). The cells demonstrate strong albumin expression (C and E), glycogen (C), ICG uptake and excretion (D) and urea secretion (E), TEM of the hepatocytes showing typical microstructures, including large numbers of spherical, elongated mitochondria, Golgi complexes, lysosomes, and endoplasmic reticulum in the cytoplasm (B). Quantitative measures of albumin secretion and urea synthesis are shown in E. Student's t-test, n=3, data are means ± SEM. *, P<.05 and **, P<.01 for the comparisons. M=mitochondria, G=golgi; L=lysosomes; ER=endoplasmic reticulum.
Differentiation selectively to cholangiocytes occurs with feeders of mature stellate cells and myofibroblasts from adult livers. Feeder-free conditions that yielded equivalent results consisted of embedding the hHpSCs into hydrogels containing 60% type I collagen and 40% hyaluronans (or Matrigel) and using the MKM-C. The cells formed branches and ducts, especially when in 3-D cultures, and the cells within the ducts expressed secretin receptors and CK19 (Fig. 7).
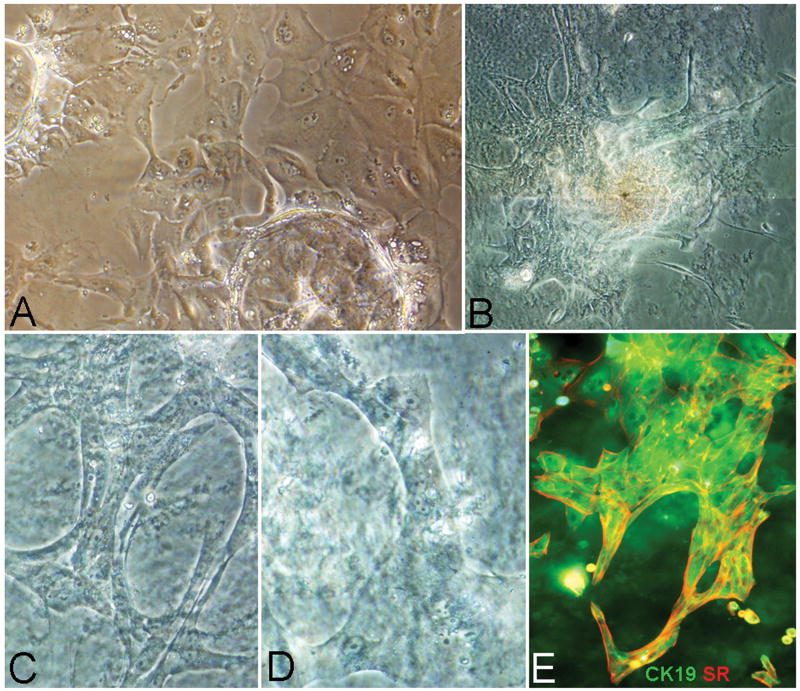
Figure 7. Conditions yielding mature Cholangiocytes.
Formation of bile duct structures within 7-10 days requires that the hHpSCs be cultured in MKM-C (MKM supplemented with VEGF, HGF) and plated onto mature stellate cells (pericytes) or myofibroblasts (A). The feeders can be replaced by embedding the cells into a mix of 60% type I collagen gel and 40% hydrogel (Hyaluronans or Matrigel) and in the HDM tailored for cholangiocytes (B-E). The cells form ramifying ducts and tubes that express cytokeratin 19 (green) and secretin receptor (red) (E) as well as other markers (e.g. aquaporins, ABAT, data not shown) for cholangiocytes. The image in B is a composite of multiple images to show a large colony within the hydrogel/matrix mixture. The images in C and D are enlargements of some of the ducts from the colony in B.
Discussion
Liver development is induced in a step-wise process with signals from cardiac mesoderm and then from subpopulations of mesenchymal cells (14). During liver organogenesis, endodermal cells are induced by cardiac mesoderm to differentiate into hHpSCs within the ventral endoderm. Subsequently, newly specified hepatic cells delaminate and migrate into the surrounding septum transversum mesenchyme and intermingle with endothelia which remain in contact with hepatic cells throughout development (14). Thus, Flk-1 (a receptor for VEGF, essential for endothelia to form) mutant mouse embryos, lacking endothelia, show initial hepatic induction but without the proliferation of hepatic cells into the surrounding septum transversum mesenchyme, indicating the importance of endothelia for liver organogenesis (15).
At the time of hepatic induction, septum transversum mesenchymal cells surround the developing cardiac region near the ventral foregut endoderm and are the source of inductive signals including fibroblast growth factors (FGFs) and bone morphogenetic proteins (BMPs), angiogenesis and involving intense hedgehog signaling, also a key regulator of murine and human hepatic progenitors throughout life (14). The liver is organized in physiological units that contain all developmental stages of the hepatic cells, and the stem cell niche in vivo has been shown to be the ductal plates in fetal and neonatal livers and the canals of Hering in pediatric and adult livers (8, 16). These niches contain type III collagen, hyaluronans, a form of laminin binding to α6β4 integrin (assumed to be laminin 5), and a novel form of chondroitin sulfate-proteoglycan (CS-PG) found to have minimal sulfation (8, 17, 18). By contrast, the in vivo microenvironment associated with the hHBs is comprised of type III, IV and V collagens, laminin isoforms binding to α3β1, CS-PGs with normal levels of sulfation and various forms of heparan sulfate-PGs (HS-PGs) (8, 17, 18). The matrix chemistry found in the Space of Disse (the space between differentiated hepatocytes and endothelium) forms a gradient going from the periportal region (zone 1) to pericentral region (zone 3) (19). The portal triads are dominated by fibrillar collagens (types I and III), forms of laminin (weak levels), vimentin, hyaluronans, and less sulfated forms of CS-PGs and HS-PGs transitioning in gradient fashion through the Space of Disse to a matrix chemistry around the central vein comprised of type IV and VI collagens (with weak expression of type III), syndecans 1 and 4, highly sulfated proteoglycans, especially heparin proteoglycans, and no hyaluronans or laminin. In addition, elastin is found generally throughout the acinus, as is collagen type I8, a form of heparan sulfate proteoglycan, both closely associated with the blood vessels.
The behavior of hHpSCs and feeders parallels that observed during liver development and that occurring between the parenchyma and mesenchymal cells in the Space of Disse (14). Our data on matrix components in immunoselected angioblasts from fetal livers, show that they produce low levels of collagens, of which only type III collagen was found by immunohistochemistry; one isoform of laminin (A4), elastin, hyaluronans, syndecan and CS-PG of which only CS-PG was detected by immunohistochemistry. Those from adult livers have higher levels of syndecan, type IV collagen, elevated levels of laminin A4 and fibronectin. The endothelial cells (CD31+) from fetal liver make all the forms tested of HS-PGs, low levels of type I, III and V and laminin B2. Those from adult livers expressed the highest levels observed of HS-PG2 and syndecan, collagen type I and IV, high levels of the laminins and some fibronectin and very high levels of elastin.
There are multiple stellate cell subpopulations. The stellate cell precursors appear to derive from angioblasts, as evidenced by the proximity of the precursors at the edges of the angioblast colonies and the sharing of markers such as VCAM-1, β3 integrin, and CD146 (20, 21) and, if serum is present transiently or permanently, the transition of primary cultures of immunoselected angioblasts to cultures dominated by activated stellate cells within a few days in culture. Although we cannot exclude culture selection for a pre-existing, initially minor subpopulation of activated stellate cells, we propose that the net sum of evidence implicates a lineage connection between angioblasts and stellate cells. Efforts are ongoing to assess this hypothesis.
The stellate cell/myofibroblast subpopulations are in a maturational lineage with overlapping but also distinct characteristics comprising the cell length or size, position of the nucleus, level of expression of CD146 and other markers (e.g. ASMA, desmin), extent of intermediate filaments and desmosomes, and the composition and levels of their matrix components. Those from fetal livers have characteristics as reported previously (21), and those from adult livers have a phenotype of pericytes or of myofibroblasts (11). Activation of either fetal or adult subpopulations occurred at high cell densities (confluence of the cells), following sustained serum exposure, or with rigidity of the substratum (e.g. highly cross-linked hyaluronan hydrogels) (22).
Mature stellate cells produced both network and fibrillar collagens (large amounts of type I collagen and lower levels of type III, IV and V collagen), large amounts of elastin and both HS-PGs and CS-PGs. The levels of all of these were the highest observed in the activated stellate cells/myofibroblasts obtained from adult livers. A primary biological activity of activated hHpSTCs is matrix synthesis and includes production of diverse collagen types (types I, III, IV, V) and multiple types of basal adhesion molecules (fibronectin, and laminin α1 and γ1 chains) (23). Disease states such as fibrosis and cirrhosis are associated with highly activated stellate cells that contribute to the scar tissue formation throughout the liver. Indeed mice defective in the lhx2 gene have early and inappropriate activation of stellate cells and “spontaneous” cirrhosis (24).
CS-PGs, as detected by the immunohistochemical assays were present in feeders derived from human fetal liver or the hHpSC colonies. They can form complexes with growth factors and chemokines, albeit more weakly than that found for HS-PGs (18, 25, 26). A recent report identified unique forms of CS-PGs with little to no sulfation present in stem cell niches, including liver (18). The liver's stem cell niche is dominated by hyaluronans and by forms of such CS-PGs that make a non-sulfated (or minimally sulfated) glycosaminoglycan (GAG) barrier minimizing the presentation of signals, that is of those bound to GAGs, to the stem cells. As the stem cells migrate out of the niche, they come into contact with GAGs and proteoglycans with more extensive sulfation and stably bound growth factors that are known to influence the stem cells either with respect to growth or with respect to differentiation to various mature cell fates (27).
The feeders with the most extensive effects on differentiation were those found to have the highest levels of heparan sulfate proteoglycans (HS-PGs), renowned for operating as high affinity chemical scaffolds for growth factors. HS-PGs have been purified and characterized from rodent livers by Gallagher and associates and from human livers by Linhardt and associates (27, 28). Maturation of liver parenchymal cells is induced by HS-PGs with higher degree of sulfation, especially O-sulfation, as is found in heparin chains, which in both humans and rodents, is associated with the most mature parenchymal cells in the liver(29).
The extent of differentiation correlated also with 3-dimensionality, the ratio of type I collagen to other collagen types, the ratio of fibronectin to laminin isoforms, the presence of proteoglycans with moderate to high levels of sulfation such as HS-PGs isoforms and the rigidity of the hydrogels. The least differentiated were in monolayer; intermediate levels were in type I collagen sandwich cultures that resulted in a mix of hepatocytic and biliary lineages; and the most differentiated were cells suspended 3-dimensionally into hydrogels with matrix components required for driving cells differentially towards hepatocytic or biliary fates. Preferential differentiation towards cholangiocytic fates occurred with conditions of higher rigidity (and higher levels of CS-PGs), whereas less rigidity and higher levels of HS-PGs or HP-PGs correlated with differentiation towards hepatocytic fates. The effects of CS-PGs versus HS-PGs are assumed to be due to their distinctions in growth factor binding. The relevance of mechanical forces on differentiation is now the focus of ongoing experiments.
Although the data presented here emphasized the role of the changes in matrix chemistry along with certain known soluble signals, we have identified more than a dozen soluble signals changing qualitatively and quantitatively with differentiation (Uronis J and Reid L, manuscript in preparation). Matrix molecules such as proteoglycans, and especially HS-PGs and HP-PGs, have many growth factor-binding sites determining growth factors' storage, release, conformation, stability, affinities for specific receptors, and other aspects of the signal transduction processes. Therefore completion of the ongoing studies to define the lineage dependent, soluble paracrine signals should allow future studies on mechanisms by which paracrine signalling, involving synergies between the soluble signals and the matrix components, dictates the cell responses.
In summary, the interdependency of parenchymal cells and their mesenchymal companions is a stringent constraint on stem cell and maturational lineage biology and has been mimicked by the use feeders. The uniformity of the cell population within a feeder cell line facilitates the analyses of cell-cell and cell-matrix interactions but ignores that mesenchymal cells mature coordinately with epithelia, a maturation associated with changes in the paracrine signaling. In addition, feeder cell lines stably maintained in animal serum have muted effects relative to those maintained serum-free and are a barrier for clinical programs, commercial, and research applications due to concerns of unidentified factors and pathogens in serum. Thus, the identification of the matrix and soluble signals that control the fate of stem cells is critical to translate the use of normal cells into the realm of reproducibility and effectiveness. Our success in generating cultures of stem cells with specific biological fates is possible by using specific paracrine signals (both matrix and soluble) and by the recognition that serum has to be eliminated to the extent possible. In addition, the ability to generate reproducibly uniform cultures of liver parenchymal cells maintained at a precise maturational lineage stage represents an important step for the development of safe stem cell-based therapy and drug development as well as model systems to analyze development.
Supplementary Material
Acknowledgments
We thank Lucendia English for technical assistance, Dr. Michael Chua and staffs of the UNC Michael Hooker Microscopy Core Facility, the UNC Cell Services, Dr. Victoria Madden, Microscopy Services Laboratory in Pathology and Laboratory Medicine in UNC and Histology Core Facility. The findings from some of these studies have been included in patent applications that belong to UNC.
Financial support: Funding derived from a sponsored research agreement (SRA) from Vesta Therapeutics and from NIH grants (AA014243, IP30-DK065933).
Abbreviations
- AFP
α-fetoprotein
- ALB
albumin
- ASMA
α-smooth muscle actin
- bFGF
basic fibroblast growth factor
- CD
common determinant
- CK
cytokeratin
- CS-PG
chondroitin sulfate proteoglycan
- EC
endothelial cell
- EGF
epidermal growth factor
- EpCAM
epithelial cell adhesion molecule
- FBS
fetal bovine serum
- FN
fibronectin
- HA
Hyaluronan
- HGF
hepatocyte growth factor
- HS-PG
heparan sulfate proteoglycan
- hHpSC
human hepatic stem cell
- hHB
human hepatoblast
- hHpSTC
human hepatic stellate cell
- ICG
Indocyanine green
- KM
Kubota's Medium, a serum-free, hormonally defined medium for progenitors
- MACS
magnetically activated cell sorting
- MSC
mesenchymal stem cell
- NCAM
neural cell adhesion molecule
- NPC
non-parenchymal cell
- PAS
Periodic Acid Schiff
- TEM
transmission electron microscopy
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
Footnotes
Disclosures: The authors declare there are no conflicts of interests.
References
- 1.Turner R, Lozoya O, Mendel G, Wang YF, Cardinale V, Gaudio E, Alpini G, et al. Hepatic stem cells and maturational lineage biology. Nature Reviews for Gastroenterology and Hepatolgy. 2010 doi: 10.1002/hep.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.*Schmelzer E, *Zhang L, *Bruce A, Wauthier E, Ludlow J, Yao H, Moss N, et al. Human hepatic stem cells from fetal and postnatal donors. Journal of Experimental Medicine. 2007;204:1973–1987. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]; *co-equal first authors;**co-equal senior authors
- 3.Schmelzer E, Triolo F, Turner ME, Thompson RL, Zeilinger K, Reid LM, Grideli B, et al. Three-dimensional perfusion bioreactor culture supports differentiation of human fetal liver cells. Tissue Engineering. 2010;16:115–121. doi: 10.1089/ten.TEA.2009.0569. [DOI] [PubMed] [Google Scholar]
- 4.Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258–264. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- 5.Hamazaki T, Iiboshi Y, Oka M, Papst PJ, Meacham AM, Zon LI, Terada N. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett. 2001;497:15–19. doi: 10.1016/s0014-5793(01)02423-1. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto H, Quinn G, Asari A, Yamanokuchi H, Teratani T, Terada M, Ochiya T. Differentiation of embryonic stem cells into hepatocytes: biological functions and therapeutic application. Hepatology. 2003;37:983–993. doi: 10.1053/jhep.2003.50202. [DOI] [PubMed] [Google Scholar]
- 7.Snykers S, D J, Rogiers V, Vanhaecke T. In Vitro Differentiation of Embryonic and Adult Stem Cells into Hepatocytes: State of the Art. Stem Cells. 2009;27:577–605. doi: 10.1634/stemcells.2008-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Theise N, Chua M, Reid LM. Human hepatic stem cells and hepatoblasts: Symmetry between Liver Development and Liver Regeneration. Hepatology. 2008;48:1598–1607. doi: 10.1002/hep.22516. [DOI] [PubMed] [Google Scholar]
- 9.Kubota H, Reid LM. Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigens. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12132–12137. doi: 10.1073/pnas.97.22.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmelzer E, Wauthier E, Reid LM. Phenotypes of pluripotent human hepatic progenitors. Stem Cell. 2006;24:1852–1858. doi: 10.1634/stemcells.2006-0036. [DOI] [PubMed] [Google Scholar]
- 11.Pinzani M. Hepatic stellate (ITO) cells: expanding roles for a liver-specific pericyte. Journal of Hepatology. 1995;22:700–706. doi: 10.1016/0168-8278(95)80227-4. [DOI] [PubMed] [Google Scholar]
- 12.Flores D. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histology and histopathology: cellular and molecular biology. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 13.McClelland R, Wauthier E, Zhang L, Barbier C, Melhem A, Schmelzer E, Reid LM. Ex vivo conditions for self-replication of human hepatic stem cells. Tissue Engineering. 2008;14:1–11. doi: 10.1089/ten.tec.2008.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemaigre F, Zaret KS. Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr Opin Genet Dev. 2004;14:582–590. doi: 10.1016/j.gde.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, Bernstein A, et al. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. CELL. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 16.Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, et al. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- 17.Couvelard A, Bringuier AF, Dauge MC, Nejjari M, Darai E, Benifla JL, Feldmann G, et al. Expression of integrins during liver organogenesis in humans. Hepatology. 1998;27:839–847. doi: 10.1002/hep.510270328. [DOI] [PubMed] [Google Scholar]
- 18.Hayes A, Tudor D, Nowell M, Caterson B, Hughes C. Unique forms of chondroitin sulfate proteoglycans in stem cell niches. Journal of Histochemistry and Cytochemistry. 2007;56:125–138. doi: 10.1369/jhc.7A7320.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Hernandez A, Amenta PS. The hepatic extracellular matrix. I. Components and distribution in normal liver. Virchows Arch A Pathol Anat Histopathol. 1993;423:1–11. doi: 10.1007/BF01606425. [DOI] [PubMed] [Google Scholar]
- 20.Bardin N, Anfosso F, Masse JM, Cramer E, Sabatier F, Le Bivic A, Sampol J, et al. Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesion. Blood. 2001;98:3677–3684. doi: 10.1182/blood.v98.13.3677. [DOI] [PubMed] [Google Scholar]
- 21.Kubota H, Yao H, Reid LM. Identification and characterization of vitamin A-storing cells in fetal liver. Stem Cell. 2007;25:2339–2349. doi: 10.1634/stemcells.2006-0316. [DOI] [PubMed] [Google Scholar]
- 22.Buniatian G, Hamprecht B, Gebhardt R. Glial fibrillary acidic protein as a marker of perisinusoidal stellate cells that can distinguish between the normal and myofibroblast-like phenotypes. Biology of the Cell. 1996;87:65–73. [PubMed] [Google Scholar]
- 23.Skrtic S, Wallenius K, Gressner AM, Jansson JO. Characterization of hepatocyte-derived mitogenic activity on hepatic stellate cells. Liver. 2000;20:157–164. doi: 10.1034/j.1600-0676.2000.020002157.x. [DOI] [PubMed] [Google Scholar]
- 24.Wandzioch E, Kolterud A, Friedman S, Carlsson L. Lhx2-/- mice develop liver fibrosis. Proceedings of the National Academy of Science (USA) 2004;101:16549–16554. doi: 10.1073/pnas.0404678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caterson B, Mahmoodian F, Sorrell J, Hardingham T, Bayliss M, Carney S, Ratcliffe A, et al. Modulation of native chondroitin sulphate structure in tissue development and in disease. Journal of Cell Science. 1990;97(Pt 3):411–417. doi: 10.1242/jcs.97.3.411. [DOI] [PubMed] [Google Scholar]
- 26.Lyon M. The isolation of membrane proteoglycans. Methods in Molecular Biology. 1993;19:243–251. doi: 10.1385/0-89603-236-1:243. [DOI] [PubMed] [Google Scholar]
- 27.Vongchan P, Warda M, Toyoda H, Toida T, Marks RM, Linhardt RJ. Structural characterization of human liver heparan sulfate. Biochim Biophys Acta. 2005;1721:1–8. doi: 10.1016/j.bbagen.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Pierce A, Lyon M, Hampson IN, Cowling GJ, Gallagher JT. Molecular cloning of the major cell surface heparan sulfate proteoglycan from rat liver. Journal of Biological Chemistry. 1992;267:3894–3900. [PubMed] [Google Scholar]
- 29.Spray DC, Fujita M, Saez JC, Choi H, Watanabe T, Hertzberg E, Rosenberg LC, et al. Proteoglycans and glycosaminoglycans induce gap junction synthesis and function in primary liver cultures. J Cell Biol. 1987;105:541–551. doi: 10.1083/jcb.105.1.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic Differentiation of Mesenchymal Stem Cells from Bone Marrow: Differentiation-Dependent Gene Expression of Matrix Components. Experimental Cell Research. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.