Abstract
Objective(s)
To study posttherapy chemoradiation hysterectomy histology with long-term survival in bulky stage 1B cervical cancer patients.
Study Design
Gynecologic Oncology Group protocols #71 and #123 enrolled 464 patients randomly allocated to pelvic radiation (75Gy,n=291) plus hysterectomy (RTH) or to pelvic radiation (75Gy) and cisplatin (40mg/m2,n=176) plus hysterectomy (RTCH). Risk of progression and death were evaluated by posttherapy hysterectomy response (good: <10% viable; poor: ≥10% viable).
Results
Median survivor follow-up was 112 months. Relative risks of disease progression and death were 0.656 (95% confidence interval(CI)=0.472–0.912) and 0.638 (95%CI=0.449–0.908), favoring RTCH. Good response patients (345 [74%]) had similar 10-year overall survival (OS) and progression-free survival (PFS) after RTH or RTCH (P >0.47). Poor response patients after RTCH had superior OS (P=0.046) and PFS (P=0.084). Extrapelvic recurrences occurred more often in poor response patients.
Conclusions
Posttherapy viable residual disease < 10% was associated with reduced risk of progression and cancer-related death.
Keywords: cervix cancer, hysterectomy histopathology, chemoradiation
INTRODUCTION
The hypothesis that radiation plus single agent chemotherapy reduces pelvic recurrence has been tested in patients with bulky stage IB invasive cervical carcinomas.1–3 A rationale for such an approach relied on the premise that chemoradiation-induced cytoreduction of cervical cancer would facilitate a less radical adjuvant hysterectomy, thus perhaps decreasing perioperative and postoperative morbidity. Pre-surgical chemoradiation also was assumed to treat occult micrometastases, and thereby, would lessen the risk of extrapelvic metastatic disease progression. While clinical outcomes data ultimately did not support routine extrafascial hysterectomy after chemoradiation,1–3 an extrafascial hysterectomy after chemoradiation would provide an in vivo proof-of-principle biological measure of chemoradiation-induced cytoreduction of cervical cancer.
Pathological necrosis remains the most reliable method whereby an objective assessment of chemoradiation sensitivity is made,1–6 although 3-month posttherapy 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG CT/PET) has emerged as an early surrogate for chemoradiation response.7, 8 But before metabolic surrogates can be evaluated rigorously, an assessment of the likelihood of residual disease after chemoradiation is needed. The Gynecologic Oncology Group (GOG) has conducted two phase III randomized clinical trials in patients with bulky stage IB invasive cervical carcinomas allowing in vivo determination of residual disease after radiation and chemoradiation. The purpose of this retrospective study is to test the hypothesis that a radiation plus chemotherapy survival benefit in patients with bulky stage IB invasive cervical carcinomas is manifest by decreasing posttherapy residual disease.
MATERIALS and METHODS
GOG clinical trial #71 randomized patients by central telephone allocation to pelvic radiation alone or pelvic radiation plus hysterectomy.1 GOG clinical trial #123 randomized patients by central telephone allocation to radiation plus hysterectomy or chemoradiation plus hysterectomy.2, 3 In these trials, 464 patients with bulky invasive carcinomas of the cervix were entered (Table 1). Patients with cervical cancer recurrence, prior malignancies, or those not candidates for radiation were ineligible. Extraperitoneal surgical staging of lymph nodes was optional, but prior to enrollment para-aortic lymph nodes were required to be negative for metastatic disease by either extraperitoneal surgical staging or computed tomography. Written informed consent consistent with institutional, state, and federal regulations and each treating institution’s local institutional review board approval was obtained before conducting protocol treatments.
TABLE 1.
Patient Characteristics
| -Treatment Type- | |||
|---|---|---|---|
| RTH (n=289) | RTCH (n=175) | ||
| Characteristic | Value | n(%) | n(%) |
| Age (yr) | <=35 | 57 ( 19.7) | 40 ( 22.9) |
| 36 – 45 | 110 ( 38.1) | 85 ( 48.6) | |
| 46 – 55 | 76 ( 26.3) | 30 ( 17.1) | |
| 56 – 65 | 30 ( 10.4) | 9 ( 5.1) | |
| > 65 | 16 ( 5.5) | 11 ( 6.3) | |
| Race | Black | 70 ( 24.2) | 36 ( 20.6) |
| Hispanic | 22 ( 7.6) | 22 ( 12.6) | |
| White | 166 ( 57.4) | 107 ( 61.1) | |
| Other | 10 ( 3.5) | 9 ( 5.1) | |
| Unknown | 21 ( 7.3) | 1 ( 0.6) | |
| Performance status | 0 | 233 ( 80.6) | 148 ( 84.6) |
| 1 | 49 ( 17.0) | 26 ( 14.9) | |
| 2 | 7 ( 2.4) | 1 ( 0.6) | |
| Tumor size (cm) | <=4.0 | 29 ( 10.0) | 14 ( 8.0) |
| 4.1 – 6.0 | 171 ( 59.2) | 104 ( 59.4) | |
| 6.1 – 8.0 | 81 ( 28.0) | 48 ( 27.4) | |
| >=8.1 | 7 ( 2.4) | 9 ( 5.1) | |
| Not reported | 1 ( 0.3) | 0 (0.0) | |
| Tumor grade | 1 | 21 ( 7.3) | 14 ( 8.0) |
| 2 | 185 ( 64.0) | 94 ( 53.7) | |
| 3 | 77 ( 26.6) | 66 ( 37.7) | |
| Not graded | 6 ( 2.1) | 1 ( 0.6) | |
| Tumor type | Barrel | 139 ( 48.1) | 80 ( 45.7) |
| Exophytic | 150 ( 51.9) | 94 ( 53.7) | |
| Not reported | 0 ( 0.0) | 1 ( 0.6) | |
| Cell type | Adenocarcinoma, NS | 21 ( 7.3) | 9 ( 5.1) |
| Adeno-squamous carcinoma | 16 ( 5.5) | 16 ( 9.1) | |
| Squamous cell carcinoma | 242 ( 83.7) | 140 ( 80.0) | |
| Other | 10 ( 3.5) | 10 ( 5.7) | |
Radiation Therapy
On these trials, patients would receive external-beam radiation five days a week using opposed antero-posterior or four-field radiation treatment fields encompassing the uterine cervix and bilateral iliac and low common iliac lymph nodes.1, 2 On GOG protocol #71, patients were to receive 45 Gy to the pelvic mid-plane halfway between the L4–L5 vertebral body interspace and lower one-third of the obturator foramina at a daily fraction of 1.8 Gy. On GOG protocol #123, patients were to receive 45 Gy to the pelvic mid-plane of fields extending at least 3cm beyond the known extent of disease at a daily fraction of 1.8 to 2.0 Gy. Following external-beam treatment in both studies, patients were to undergo one or two low-dose rate intracavitary brachytherapy using tandem and ovoid applicators to a dose of 30.0 Gy to point A. An optional parametrial boost would have been delivered to bring the point B dose to 55.0 Gy. Complete blood counts and platelet counts were to be obtained weekly.
Chemotherapy
Only patients randomly allocated to chemotherapy during radiation on GOG protocol #123 were to receive chemotherapy.2, 3 Cisplatin (40mg/m2), not to exceed 70 mg total per week, was to be administered intravenously once per week during external-beam radiation for a maximum of six weekly cycles.
Surgery
All entered patients were to undergo standardized total extrafascial hysterectomy, two to six weeks (GOG #71) or six to eight weeks (GOG #123) after completion of all radiation including brachytherapy.
Statistical Analysis
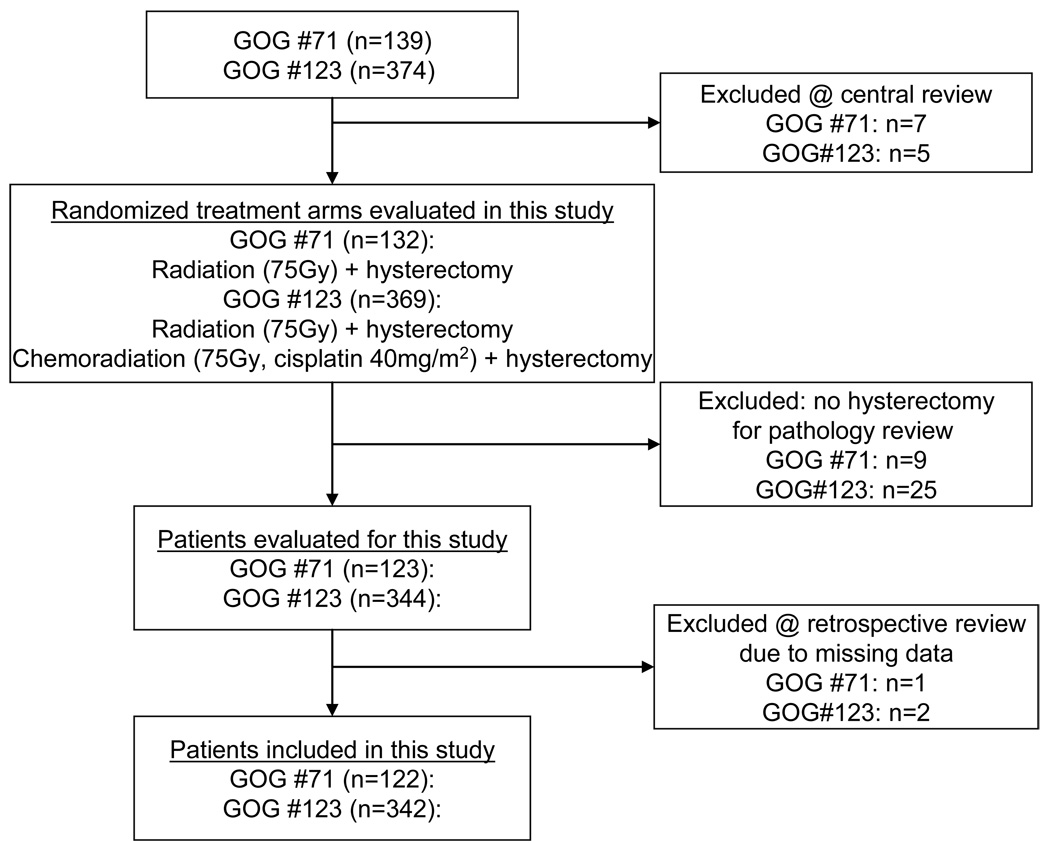
Patient clinical and follow-up data were abstracted from patient charts maintained at the GOG Statistical and Data Center (Buffalo, NY) where data has been closed since October, 2003 for these clinical trials. The design of this retrospective study included the desire to evaluate histopathological tumor response on long-term survival outcomes after radiation or cisplatin chemoradiation. For this study, median follow-ups were 128 months for GOG #71 and 118 months for GOG#123. For the current retrospective analysis, 464 patients were analyzed following an intent-to-treat method (Fig. 1).7 Three originally-reported patients do not contribute to the current statistical analyses due to missing data.
Figure 1.
STROBE diagram for progress through stages of analysis of randomized control trials Gynecologic Oncology Group (GOG) protocols #71 and #123.
This study retrospectively compared the original documented histopathology tumor response as assessed by central review of at least two GOG pathologists blinded to patient clinical, tumor, and treatment outcome variables by randomized treatment groups: either radiation plus hysterectomy (n=289) or cisplatin chemoradiation plus hysterectomy (n=175). The GOG Pathology Committee reviewed pathology from extrafascial hysterectomy specimens, including any persistent cancer in the cervix or adjacent tissues to assure uniform pathologic review. The original pathological review consisted of lesion size, location, thickness, histological grade, and presence of angiolymphatic invasion. To supplement the pathological review, the surgeon was required to include in the operative note clinical findings such as observed macroscopic disease of the cervix. For the purposes of this report, hysterectomy specimen histopathologies were scored (1) as having a good histopathological tumor response if less than 10 percent of observed cancer cells appeared viable, or (2) as having a poor histopathological tumor response if greater than or equal to 10 percent of observed cancer cells appeared viable. A proportion of 10 percent or greater chosen for poor histopathological response was based on the association of 10 percent or greater viable tumor presence and high local and systemic relapse rate seen in pediatric osteogenic sarcoma management.9–11 In most cases of microscopic residual disease (160 of 174, 92%), the amount of viable remnant tumor was obvious (>10% vital or nearly all vital) to the original GOG reviewers and an indication of the percentage of viable tumor was recorded in the original GOG histopathological assessment. These assessments by the original GOG reviewers were scored for good and poor histopathological response (CK). For 12 non-obvious cases, review of GOG pathologist original hand-written pathological assessment, including indications for tumor viability, and review of surgical records were done (CK) to classify histopathological tumor response. For two non-obvious cases, a GOG pathologist (FAK) blinded to treatment and outcome provided an independent review of histopathology for proportion of viable remnant tumor.
For each group, survival was determined from date of study entry to date of cancer-related or all-cause death or date last seen. Progression-free survival (PFS) was defined as date of study entry to date of physical or radiographic evidence of recurrent cervical cancer or death. Product-Limit estimates were calculated according to Kaplan–Meier method and differences in PFS and overall survival (OS) were assessed utilizing the log-rank test.12–14 The Cox model was used to adjust for prognostic factors and to estimate hazard ratios (and 95% confidence interval) of PFS and OS.15 All P values reported were two-sided and P <0.05 was interpreted as statistically significant.
RESULTS
GOG protocol #71 enrolled 122 eligible patients on the radiation and hysterectomy (RTH) treatment arm that underwent hysterectomy and had histopathology available for review. GOG protocol #123 enrolled 167 eligible patients on the RTH arm and 175 eligible patients on the radiation plus cisplatin chemotherapy and hysterectomy (RTCH) arm who underwent hysterectomy and had histopathology available for review. For the 464 evaluated patients in this study, median age was 43 years (range 21–78 yrs) for the RTH cohort and 40 years (range 21–81 yrs) for the RTCH cohort. Patient characteristics appear in Table 1. In this study, the RTH cohort was older (P =0.024) and had a higher non-Caucasian proportion (P =0.002). An increased proportion of high tumor grade cancers was observed in the RTCH cohort (P =0.047). Cohorts were balanced for patient performance status, tumor size, tumor morphology type, and tumor cell type (Table 1). The median total radiation treatment time was 49 days for the RTCH cohort and 49 days for the RTH cohort.
Survival and Recurrence
At the time of this report, there are 50 (17%) of 289 RTH and 16 (9%) of 175 RTCH patients whose disease has recurred locally in the pelvis, cervix, or vagina (Table 2). There are 54 (19%) of 289 RTH and 23 (13%) of 175 RTCH patients with extrapelvic recurrences (Table 2). There have been 90 (31%) versus 30 (17%) cancer-related deaths and 108 (37%) versus 45 (26%) all-cause deaths in the RTH and RTCH cohorts, respectively. Cervical cancer-related deaths comprised the majority (78%) of observed deaths on the GOG #71 and #123 clinical trials.
TABLE 2.
Recurrence by histopathological response
| Good Response | Poor Response | ||
|---|---|---|---|
| Group | Type of recurrence | N (%) | N (%) |
| RTH | Local | 12 ( 5.8) | 30 (35.7) |
| Distant | 23 (11.2) | 23 (27.4) | |
| Combined | 1 ( 0.5) | 7 ( 8.3) | |
| No evidence of disease | 169 (82.4) | 24 (28.6) | |
| RTCH | Local | 5 (3.6) | 8 (22.9) |
| Distant | 11 (7.9) | 9 (25.7) | |
| Combined | 1 (0.7) | 2 ( 5.7) | |
| No evidence of disease | 123 (87.9) | 16 (45.7) | |
| RTH +RTCH (entire cohort) | Local | 17 (4.9) | 38 (31.9) |
| Distant | 34 (9.9) | 32 (26.9) | |
| Combined | 2 ( 0.6) | 9 ( 7.6) | |
| No evidence of disease | 292 (84.6) | 40 (33.6) | |
Percentages are in parentheses. Because of rounding, not all percentages total 100.
Recurrences classified as local if first detected in the pelvis, cervix, or vagina; as distant if first detected outside the pelvis; as combined if first detected at sites both within and outside the pelvis.
When adjusting for patient age, performance status, and tumor size, the relative risk of progression of disease was 0.656 (95% Confidence Interval [CI] 0.472–0.912, P =0.012), favoring RTCH. Figure 1 depicts the cumulative incidence of local relapse for the two treatment groups, which plateaus 24 months after treatment. At five years, the strictly local relapse incidence is 14 percent for the RTH group versus 8 percent for the RTCH group. The relative risk of death (RTCH vs. RTH group) was 0.638 (95% CI 0.45–0.91, P =0.013), with 71 percent of patients receiving RTCH and 61 percent of patients receiving RTH estimated to be alive at ten years (P =0.017).
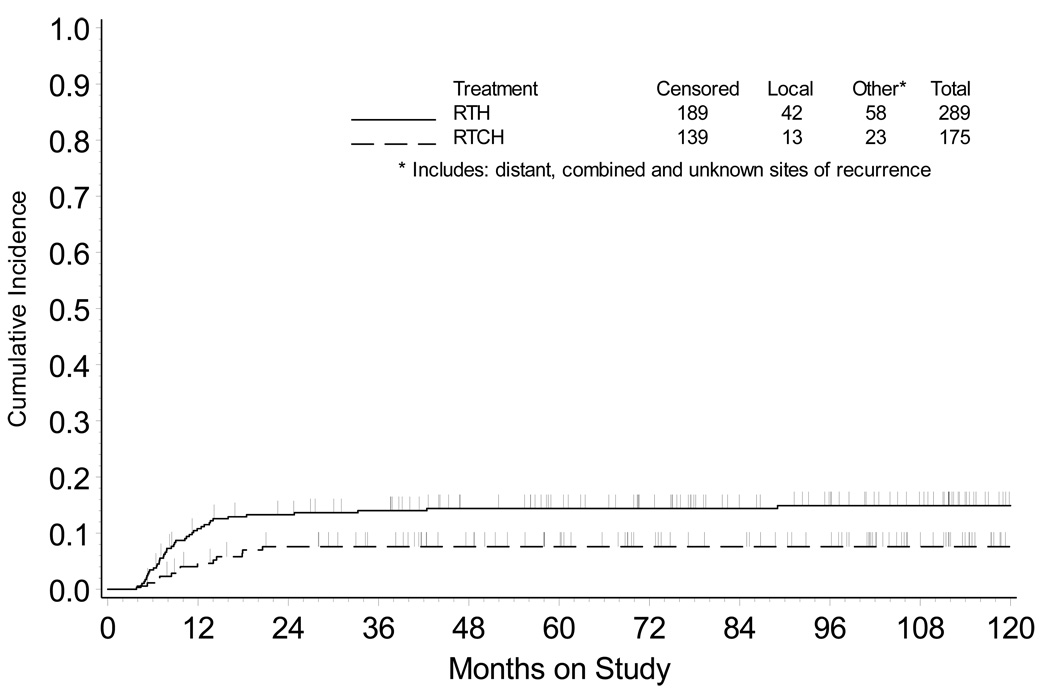
Response evaluation criteria in solid tumors are widely accepted,16, 17 and in cervical cancer are often assessed by serial pelvic examination with cervical cytology and/or computed tomography or 18F-FDG CT/PET imaging beginning three months after the completion of therapy.8, 18 These strategies may not recognize true tumor histopathological response to treatment due to subjectivity in clinical response assessment,16, 19 radiation-related effects on cervical cytology,20 and interpretive difficulties of 18F-FDG CT/PET partial metabolic response.8, 18 In the current study, patient hysterectomy cervical cancer tumor responses were dichotomized into good (i.e., <10% viable tumor) or poor (i.e., ≥10% viable tumor) histopathological responses. Fourteen of 174 patient hysterectomy cervical cancer tumor responses underwent second review for non-obvious findings regarding the percentage of viable tumor remain posttherapy. A significantly higher proportion of patients achieved a good histopathological response after RTCH (140 [80%] of 175) as compared to RTH (205 [71%] of 289, P =0.037). Cervical carcinoma cell-type was not associated with histopathological response (P =0.396). Median time to hysterectomy after radiation was 41 days (25–75% quartile: 33 to 52 days) for patients with a good histopathological response and 42 days (25–75% quartile: 34 to 52 days) for patients with a poor histopathological response. Table 2 shows the significant association between histopathological response and first site of recurrence, with significantly more local and distant recurrences noted overall in the poor histopathological response cohort after either RTH or RTCH therapy (P <0.001). The strictly local relapse incidence at five years is 6 percent after RTH versus 4 percent after RTCH when a good histopathological response is observed. The strict five-year local relapse incidence is 35 percent after RTH versus 23 percent after RTCH therapy when a poor histopathological response is observed (Fig. 2). Extrapelvic recurrences were significantly more common in patients with a poor (35%) versus good (11%) histopathological response (P <0.001).
Figure 2.
Cumulative incidence of local recurrence in the pelvis, cervix, or vagina by treatment group. RTH = radiation followed by hysterectomy; RTCH = chemoradiation followed by hysterectomy.
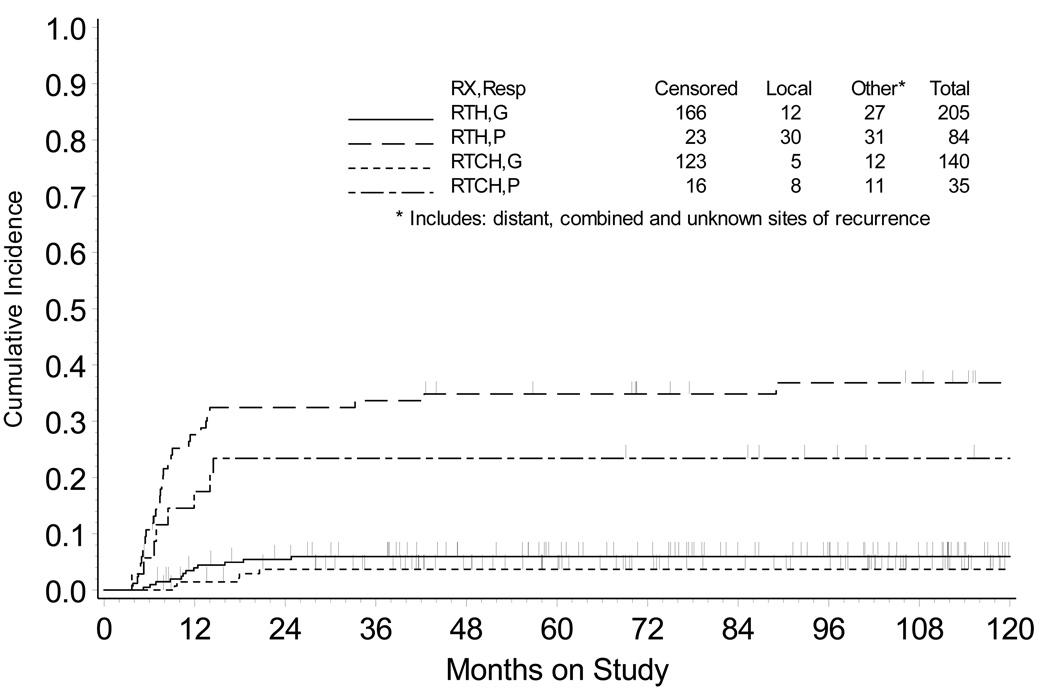
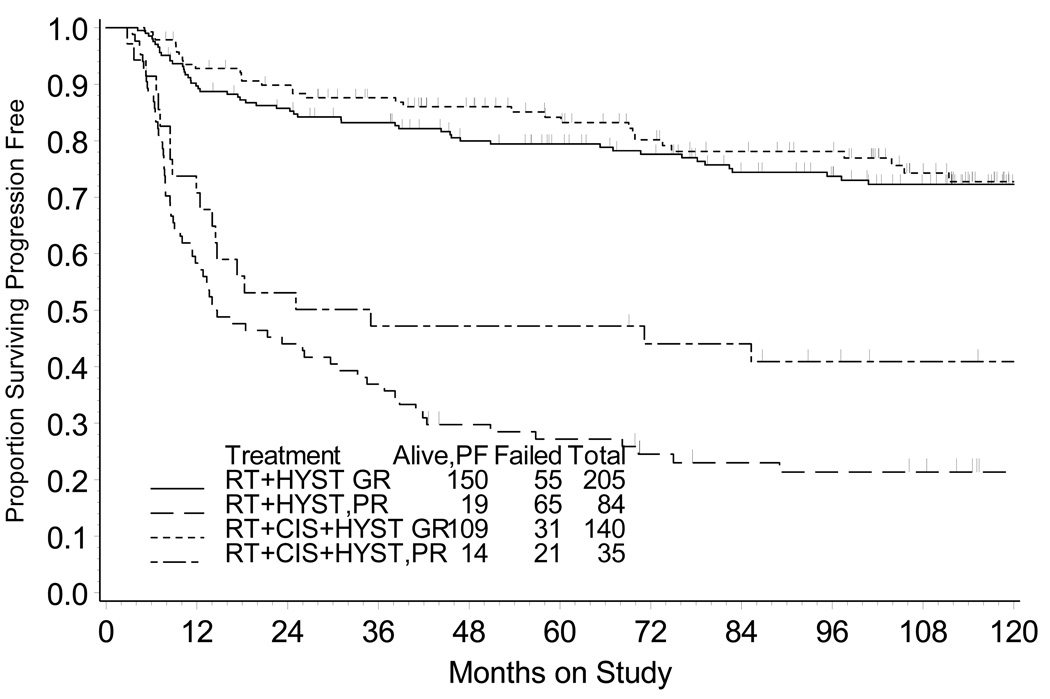
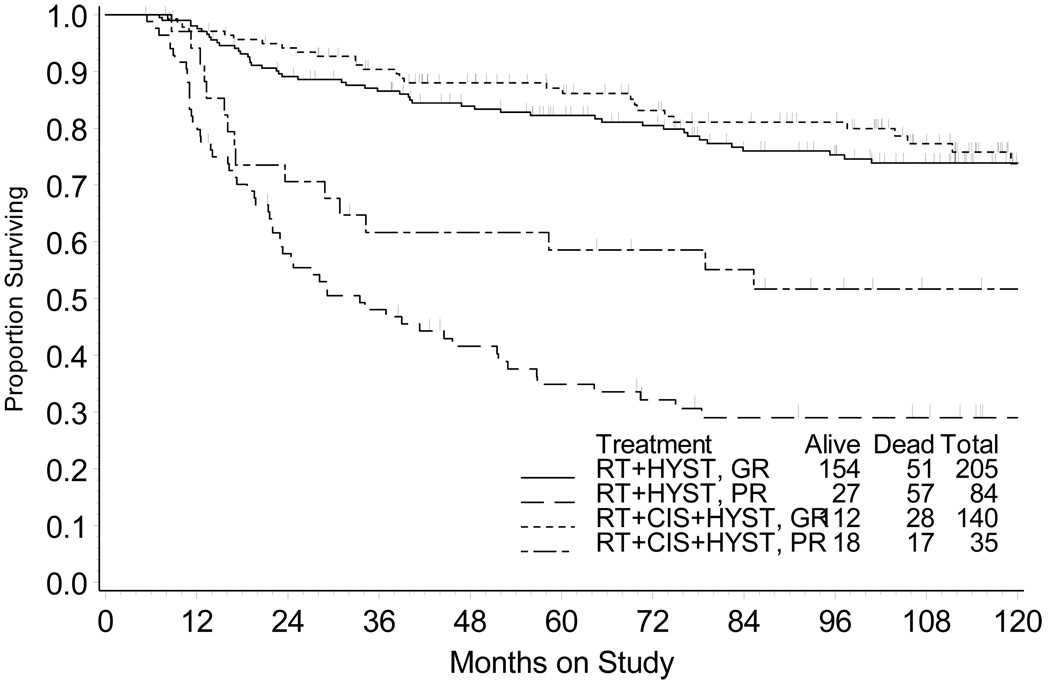
PFS (Fig. 3) and OS (Fig. 4) are depicted for those patients that therapy achieved a good versus poor histopathological response at the time of hysterectomy. Among patients with good histopathological tumor response, the relative risks of progression of disease and death (RTCH vs. RTH group) were 0.855 (95% CI = 0.550 – 1.330) and 0.844 (95% CI = 0.531 – 1.340), respectively. Patients with a good histopathological tumor response had similar outcome (10-year PFS P =0.488 and OS P =0.471) after RTH or RTCH (Table 3). For patients with poor histopathological tumor response, a lower risk for progression of disease (Hazard ratio [HR] = 0.647, 95% CI = [0.395 – 1.061]) and death (HR = 0.575, 95% CI = [0.334 – 0.990]) was observed after RTCH (vs. after RTH). RTCH was associated with higher OS (P =0.046) and PFS (P =0.084) among those patients whose hysterectomy demonstrated a poor histopathological tumor response (Table 3).
Figure 3.
Cumulative incidence of local recurrence in the pelvis, cervix, or vagina by treatment group and by histopathological response. RTH = radiation followed by hysterectomy; RTCH = chemoradiation followed by hysterectomy; G = good histopathological response (<10% viable cells); P = poor histopathological response (≥10% viable cells).
Figure 4.
Progression-free survival by treatment group and by histopathological response. RTH = radiation followed by hysterectomy; RTCH = chemoradiation followed by hysterectomy; GR = good histopathological response (<10% viable cells); PR = poor histopathological response (≥10% viable cells).
TABLE 3.
Outcome by histopathological response
| Good Response | Poor Response | ||
|---|---|---|---|
| Group | Outcome | % | % |
| RTH | 5-Year PFS | 81 | 27 |
| 5-Year OS | 82 | 34 | |
| 10-Year PFS | 72 | 22 | |
| 10-Year OS | 74 | 29 | |
| RTCH | 5-Year PFS | 85 | 48 |
| 5-Year OS | 87 | 58 | |
| 10-Year PFS | 72 | 41 | |
| 10-Year OS | 76 | 52 | |
COMMENT
In our analyses of patients with bulky stage IB invasive cervical carcinomas, chemoradiation significantly increased posttherapy good (<10% viable tumor) histopathological response and was associated with improved survival, when compared with radiation alone. Moreover, when histopathological response was poor (≥ 10% viable tumor), a reduction in relative risk of progression of disease and cancer-related death was apparent after chemoradiation.
In this study, we reviewed the histopathological response of cervical cancer to treatment of radiation alone and radiation plus cisplatin chemotherapy in the GOG trials #71 and #123 patient populations because the patients had hysterectomy specimens in which histopathological response could be reliably measured and treated patients had highest likelihood of long-term survival. Chemoradiation provided substantial reductions in five-year cumulative pelvic recurrences (Fig. 2), with the benefit most pronounced among patients with a poor (≥10% viable tumor) histopathological response (Fig. 3).
Extrapelvic recurrences of cervical cancer occurred significantly more often in patients whose therapy achieved a poor hysterectomy histopathological response (Table 2). Considering that radiation plus chemotherapy provides repeated drug dosing to occult micrometastases, a reduced number of extrapelvic metastases is not unexpected after RTCH, as compared to RTH where no chemotherapy agent is given to modify this risk. However, among patients with a poor histopathological response, chemotherapy provided only a 4 percent reduction in the proportion of patients having an extrapelvic recurrence. As a consequence, chemoradiation also was associated with improved cancer-related survival, with gains most substantial among patients with a poor (≥10% viable tumor) histopathological response (Figs. 4, 5). Our findings are consistent with prior chemoradiation cooperative group clinical trials showing improved cancer-related survival outcomes after radiation plus chemotherapy.21–25
Figure 5.
Overall survival by treatment group and by histopathological response. RTH = radiation followed by hysterectomy; RTCH = chemoradiation followed by hysterectomy; GR = good histopathological response (<10% viable cells); PR = poor histopathological response (≥10% viable cells).
Such a distinction between good versus poor histopathological responders and the marginal benefit of cisplatin alone for extrapelvic disease control in identified poor responders emphasizes the important need of identifying biologic chemotherapeutics that have both radiosensitizing and chemosensitizing properties. This provocative distinction may lie in inherent differences in the molecular makeup of each tumor, such as pretreatment cervical cancer tumor overexpression of ribonculeotide reductase (the rate-limiting enzyme for on-demand synthesis of deoxyribonucleotides needed for ionizing radiation and chemotherapy DNA-damage repair). Current translational science efforts are focused on identifying the molecular signaling footprint of radiation plus chemotherapy that will predict for response or resistance to such combination therapy.26, 27 A phase 1 clinical trial of radiation and cisplatin chemotherapy plus a ribonucleotide reductase inhibitor has shown early promise in significantly reducing posttherapy residual disease of the cervix and no extrapelvic disease progression through median 18-month patient follow-up.27
Strengths of our updated study of GOG trials #71 and #123 include a mostly contemporary para-aortic node-negative cervical cancer study population with long-term follow-up and diverse demographics. Administered radiation dose and duration were compliant with recommended prescription parameters listed in each clinical trial. After completion of all radiation therapy, surgery followed standardized total extrafascial hysterectomy. Histopathological assessment of tumor response to treatment was centralized with two or more GOG pathologists blinded to treatment and patient outcome reviewing histopathological response. Thereby, these data are broadly applicable for the management of cervical cancer.
This report could be stronger if posttherapy proportion of viable residual disease assessment was assigned at initial pathological quality assurance evaluation. For the original manuscripts,1, 2 histopathological treatment response was classified as gross residual disease, microscopic residual disease, or no viable disease (negative). In this retrospective review, gross residual disease was scored a poor histopathological response and no viable tumor (negative) was scored a good histopathological response. Although initial GOG two pathologist peer-review quality assurance was provided, 174 cases of microscopic residual disease were re-reviewed, at a time remote from the original tumor response assessment, to dichotomize patients into good or poor histopathological response. These classifications are subject to interpretive bias. Also, the timing of extrafascial hysterectomies performed on these trials ranged between two to eight weeks after completion of all radiation therapy, introducing histopathological variability in observed visual tumor regression. Lastly, contemporary molecular evaluation of whether radiation plus chemotherapy improves radiobiological effect (e.g., ribonucleotide reductase or molecular markers of hypoxia) cannot be answered in these data.
This update of GOG protocols #71 and #123 suggests a distinction between good and poor histopathological responders after radiation plus cisplatin chemotherapy or after radiation therapy alone. The GOG continues to pursue clinical trials to better define the optimal regimen for radiation and chemotherapy that achieves complete histopathological response without undue acute and late patient morbidity.
ACKNOWLEDGEMENTS
This study was supported in part by a grant (K12 CA076917) to CK from the National Institutes of Health and the Case Comprehensive Cancer Center, and also by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517). The following Gynecologic Oncology Group member institutions participated in this study: University of Alabama at Birmingham, Abington Memorial Hospital, University of Rochester Medical Center, University of Minnesota Medical School, University of California at Los Angeles, University of Pennsylvania Cancer Center, University of Miami School of Medicine, Milton S. Hershey Medical Center, Georgetown University Hospital, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, Albany Medical College, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke's Medical Center, Stanford University Medical Center, University of Kentucky and Eastern Virginia Medical School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Keys HM, Bundy BN, Stehman FB, Okagaki T, Gallup DG, Burnett AF, et al. Radiation therapy with and witout extrafascial hysterectomy for bulky stage IB cervical carcinoma: a randomized trial of the Gynecologic Oncology Group. Gynecol Oncol. 2003;89:343–353. doi: 10.1016/s0090-8258(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 2.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 3.Stehman FB, Ali S, Keys H, Muderspach LI, Chafe WE, Gallup DG, et al. Radiation therapy with or without weekly cisplatin for bulky stage IB cervical carcinoma: follow-up of A Gynecologic Oncology Group trial. Am J Obstet Gynecol. 2007;197(503):e1–e6. doi: 10.1016/j.ajog.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 5.Benedetti-Panici P, Greggi S, Colombo A, Amoroso M, Smaniotto D, Giannarelli D, et al. Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced squamous cell cancer: results from the Italian multicenter randomized study. J Clin Oncol. 2002;20:179–188. doi: 10.1200/JCO.2002.20.1.179. [DOI] [PubMed] [Google Scholar]
- 6.Zannoni GF, Vellone VG, Carbone A. Morphological effects of radiochemotherapy on cervical carcinoma: a morphological study of 50 cases of hysterectomy specimens after neoadjuvant treatment. Int J Gynecol Pathol. 2008;27:274–281. doi: 10.1097/PGP.0b013e31815b1263. [DOI] [PubMed] [Google Scholar]
- 7.Grigsby PW, Siegel BA, Dehdashti F, Mutch DG. Posttherapy surveillance monitoring of cervical cancer by FDG-PET. Int J Radiat Oncol Biol Phys. 2003;55:907–913. doi: 10.1016/s0360-3016(02)04287-6. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, et al. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314:111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- 9.Eilber FC, Rosen G, Eckardt J, Forscher C, Nelson SD, Selch M, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203–3209. doi: 10.1200/JCO.2001.19.13.3203. [DOI] [PubMed] [Google Scholar]
- 10.Picci P, Sangiorgi L, Rougraff BT, Neff JR, Casadei R, Campanacci M. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12:2699–2705. doi: 10.1200/JCO.1994.12.12.2699. [DOI] [PubMed] [Google Scholar]
- 11.Zunino JH, Johnston JO. Prognostic value of histologic tumor necrosis assessment in osteogenic sarcoma of bone. Am J Orthop. 2000;29:369–372. [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. The Logrank test. BMJ. 2004;328:1073. doi: 10.1136/bmj.328.7447.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowley J. A note on some recent likelihoods leading to the log rank test. Biometrika. 1974;61:533–538. [Google Scholar]
- 14.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Cox D. Regression models and life tables (with discussion) Journal of the Royal Statistical Society. 1972;34:187–220. [Google Scholar]
- 16.Therasse P, Arbuck S, Eisenhauer E, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Instl. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Hillman SL, An MW, O’Connell MJ, Goldberg RM, Schaefer P, Buckner JC, et al. Evaluation of the optimal number of lesions needed for tumor evaluation using the response evaluation criteria in solid tumors: a north central cancer treatment group investigation. J Clin Oncol. 2009;27:3205–3210. doi: 10.1200/JCO.2008.18.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammerstsma AA, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–1066. [PubMed] [Google Scholar]
- 19.Suzuki C, Jaconson H, Hatschek T, Torkzad MR, Bodén K, Eriksson-Alm Y, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics. 2008;28:329–344. doi: 10.1148/rg.282075068. [DOI] [PubMed] [Google Scholar]
- 20.Shield P, Daunter B, Wright R. Post-irradiation cytology of cervical cancer patients. Cytopathology. 1992;3:167–182. doi: 10.1111/j.1365-2303.1992.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 21.Rose PG, Ali S, Watkins E, Thigpen JT, Deppe G, Clarke-Pearson DL, et al. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:2804–2810. doi: 10.1200/JCO.2006.09.4532. [DOI] [PubMed] [Google Scholar]
- 22.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of Radiation Therapy Oncology Group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 23.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 24.Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 25.Lanciano R, Calkins A, Bundy BN, Parham G, Lucci JA, 3rd, Moore DH, et al. Randomized comparison of weekly cisplatin or protracted venous infusion of fluorouracil in combination with pelvic radiation in advanced cervix cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2005;23:8289–8295. doi: 10.1200/JCO.2004.00.0497. [DOI] [PubMed] [Google Scholar]
- 26.Kunos C, Chiu S, Pink J, Kinsella T. Modulating radiation resistance in cervical cancer by inhibiting ribonucleotide reductase with Triapine® (3-aminopyridine-2-carboxaldehyde thiosemicarbazone) Radiation Res. 2009;172:666–676. doi: 10.1667/RR1858.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunos C, Waggoner S, Von Gruenigen V, et al. Phase I trial of intravenous 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) in combination with pelvic radiation therapy and weekly cisplatin chemotherapy for locally advanced cervical cancer. Clin Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-09-2469. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]