Abstract
Objective
We tested the hypothesis that long-term resistance exercise combined with intradialytic oral nutrition (IDON) supplementation will improve markers of muscle mass and strength further compared to IDON alone in chronic hemodialysis (CHD) patients.
Design
Randomized controlled trial.
Setting
Outpatient Dialysis Unit at an academic center.
Main outcome measure
Lean body mass (LBM). Muscle strength and other nutritional parameters were measured as secondary outcomes.
Patients
Thirty-two participants (age 43±13 yrs, 21 male) on CHD
Design
Subjects were randomly assigned to IDON plus resistance exercise (NS+EX) or IDON (NS) alone for 6 months. IDON consisted of a lactose-free formula consisting of protein, carbohydrate and fat. Three sets of 12 repetitions of leg-press were completed prior to each dialysis session in the NS+EX arm.
Results
22 out of 32 participants completed the 6-month intervention. There were no statistically significant differences between the study interventions with respect to changes in LBM and body weight when comparing NS+EX to NS. There were also no statistically significant differences in any of the secondary outcomes measured in the study. Body weight (80.3±16.6 kg, 81.1±17.5 kg and 80.9±18.2 kg at baseline, month 3 and month 6, respectively, P=0.02) and 1-Repetition Maximum (468±148 lb, 535±144 lb, 552±142 lb, respectively, P=0.001) increased statistically significantly during the study for all patients combined.
Conclusion
This study did not show further benefits of additional resistance exercise on long-term somatic protein accretion above and beyond nutritional supplementation alone. When both treatments groups were combined, body weight and muscle strength improved during the study.
Keywords: hemodialysis, protein-energy wasting, resistance exercise, nutrition supplementation
Introduction
Protein energy wasting (PEW) in advanced chronic kidney disease is highly prevalent and represents an important target for improving hospitalization events and death risk in this patient population 1-5.Previous studies showed that intradialytic nutritional supplementation, administered parenterally or orally, prevent hemodialysis (HD)-associated protein catabolism and result in a protein anabolic response, at least in the acute setting6-9. A recent trial (FINEs) further extended the beneficial effects of nutritional supplementation to a longer-term in chronic hemodialysis (CHD) patients with apparent PEW 10.
A potential strategy to augment the anabolic effects of nutritional supplementation is concomitant exposure to resistance exercise around the time of administration of nutritional supplementation. Short-term studies in healthy subjects and CHD patients showed that post-exercise net muscle protein accretion is increased with oral nutrition supplement when compared to exercise or oral supplement alone 11-13. Further, recent long-term studies indicated a limited beneficial effect of long-term exercise alone on muscle protein accretion in CHD patients 14-17. There are no studies that examined the long-term effects of resistance exercise combined with intradialytic oral nutritional supplementation (IDON) on muscle protein accretion in CHD patients. In this study, we hypothesized that the combination of resistance exercise with IDON would lead to a higher accretion in lean body mass (LBM) when compared to IDON alone in CHD patients. In order to test this hypothesis, we performed a randomized clinical trial (RCT) in 32 CHD patients over a 6-month period.
Materials and Methods
Study Participants
Participants were recruited from the Vanderbilt University Medical Center (VUMC) Outpatient Dialysis Unit. Inclusion criteria included patients who were 18 years of age or older, had been on CHD for more than 3 months, and were delivered an adequate dose of dialysis (double pool Kt/V≥1.2) on a thrice-weekly HD program using a biocompatible HD membrane (Optiflux®, Fresenius USA, Lexington, MA). Patients with active inflammatory or infectious disease, pregnancy, hospitalization within 1 month prior to the study, and those not capable of exercise due to cardiovascular disease or osteoarthritis were excluded from the study. The Institutional Review Board of Vanderbilt University approved the study protocol and written informed consent was obtained from all study participants.
Study Design
This was a 6-month prospective, randomized, open label, parallel arm clinical trial (clinicaltrials.gov #NCT00179218). After obtaining written informed consent, subjects underwent two comprehensive baseline assessments, which included testing for body composition, muscle strength and nutrition status. Body composition was measured by Dual Energy X-ray Absorptiometry (DEXA), Bioelectrical Impedance (BIA), and anthropometric measures, as previously described18, 19. One-Repetition Maximum (1-RM) testing using a pneumatic leg press was done to measure muscle strength. Subjective global assessment based on history and physical examination according to the 7-scale method was also recorded20. Dietary protein and energy intake, normalized by the body weight from DEXA (DPI and DEI), were collected by a trained registered dietitian for two 24-hour diet recalls (one from a HD day; one from a non-HD day) and analyzed using the Nutrition Data System for Research (NDR-S) software version 5.0 (University of Minnesota, Minneapolis, MN). A fasting blood sample was obtained to examine serum concentrations of total protein, albumin, prealbumin, C-reactive protein (CRP) and creatinine. All measurements were done at a specialized chemistry laboratory (RenaLab, Richland, MS). Serum albumin, prealbumin and CRP were analyzed using bromcresol green technique, an antigen-antibody complex assay and nephelometric analysis, respectively.
The baseline assessments were done 4 weeks apart, during which subjects were asked to keep all physical activity and energy intake similar to their normal schedule. Once the 2nd baseline assessments were completed, subjects were randomly assigned to either nutrition supplementation alone (NS) or nutrition supplementation plus resistance exercise (NS+EX) using a computer-generated randomization schema. All subjects were exposed to body composition, muscle strength and nutritional assessment at month 3 and month 6 during the study.
Nutritional Supplementation
All study participants were provided nutritional supplementation during the study at each dialysis treatment, three days per week for six months. The supplement used was Nepro®, a lactose-free formula that is specially designed for CHD patients (Abbott Laboratories, Indianapolis, IN). Study subjects were prescribed 2 cans to be consumed during their routine dialysis sessions. Each supplement dose (2 cans) contained 480 ml and 960 kilocalories (132.8 kcal from protein, 412.8 kcal from carbohydrates, and 412.8 kcal from fat). Some participants who were not able to tolerate the full amount of the NS initially, was allowed to consume one can during dialysis, with the other can taken at home on the same day until full tolerance was achieved. At the end of the study, the compliance with the supplement dose was similar at 76% and 87% for NS+EX and NS groups, respectively.Weekly visits were completed by study personnel with each subject to evaluate tolerance and compliance to the supplement and to restock additional supplement.
Resistance Exercise
Subjects randomized to receive exercise (NS+EX) performed the prescribed resistance exercise, under supervision of study personnel, within 30 minutes prior to each dialysis session and ingestion of at least one can of Nepro®. A pneumatic leg press machine (Keiser®, Fresno, CA) was used, mainly focused at exercising the quadriceps, hamstring, and gluteus muscles. Subjects sat on the leg press machine with their feet placed on a platform, their legs at a 90-degree angle, and were instructed to push the platform forward, leaving their knees slightly bent. For the first month, exercise was set at approximately 70% of the subject’s 1-RM established at the baseline control visits. An initial leg press weight approximately equal to the participant’s body weight was used. Additional weight (~25-50 lb) was added at each repetition until the participant could no longer push the platform. Once the 1-RM was determined, 70% of this weight was used for participants in the NS+EX arm performing 3 sets of 12 repetitions prior to each dialysis session. At the month 3 and month 6 assessments, 1-RM was repeated in all subjects to evaluate progress and determine a new 1-RM for those in the NS+EX arm.
Statistical Analysis
Data are presented as proportions for categorical variables and mean ± standard deviation (SD) for continuous variables. Patients’ one time data were compared by using the chi-square test for categorical variables, and by using the Mann-Whitney U test for continuous variables. Body composition data, biochemistry and other nutrition parameters including dietary records were compared between groups at baseline, month 3 and month 6 using Mann-Whitney U tests at each time point. Changes over time were also compared between groups using a general linear models (GLM)analysis of variance, with bootstrap covariance accounting for correlation among repeated measures within a patient. The baseline value of the outcome variables was adjusted as a model covariate. The effect of treatment at each of the two time points was assessed only when the global test was rejected to avoid inflation of type I error. Residuals were assessed graphically for normality and transformation on the dependent variable was done to correct non-normal residuals if needed. Sensitivity analysis was performed with adjustment of patients’ age, gender and body mass index (BMI) in the model. We further assessed the short-term treatment effect at month 3 of follow-up using similar models. In addition, change in outcome variables over time was also assessed adjusting treatment effect. All analyses were performed with R-software version 2.7.2 (www.r-project.org) and a 2-sided significance level of at least 0.05 was required to reject the null hypothesis.
The primary outcome of the study was total LBM (kg) measured by DEXA. A sample size of 36 patients provided at least 98% power to detect a difference of 2.5 kg of LBM (e.g. a first condition mean, m1, of 50 kg and a second condition mean, m2, of 52.5 kg), assuming a standard deviation of differences of 2.5, using an unpaired t-test approach with a 0.05 two-sided significance level. With a drop-out rate of approximately 30%, i.e. 22 patients completing the study, the same assumptions as described above would provide power of 90%.
Results
Baseline Characteristics
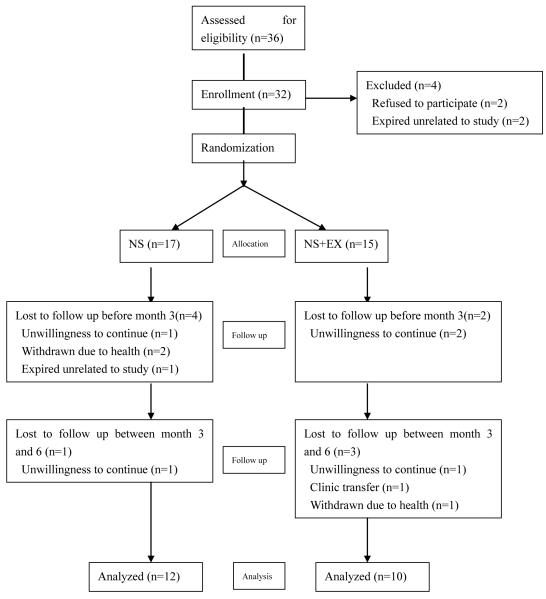
A total of 36 subjects were consented to participate in the study, 32 were randomized, and 22 completed the 6-month study: 12 in the NS group and 10 in the NS+EX group. A flow chart depicting subject allocation and drop-outs throughout the study period is shown in Fig 1. Baseline characteristics of the 32 randomized subjects are shown in Table 1. In general, the study cohort had a preponderance of males (66%), African Americans (72%), younger age (43 ± 13 years) and less than usual diabetics (19%). The mean BMI (28.4 ± 6.3kg/m2) was consistent with the general CHD population in the United States. Mean serum albumin was indicative of a relatively well preserved nutritional status. No statistically significant differences were observed between groups in clinical or demographic parameters and biochemistry data.
Fig 1.
Trial flow chart (NS, nutrition supplementation; NS+EX, nutrition supplementation plus resistance exercise)
Table 1.
Baseline patient characteristics
| Total (n=32) |
NS+EX (n=15) |
NS (n=17) |
P | |
|---|---|---|---|---|
| Gender | 0.53 | |||
| Male | 21 (66) | 9 (60) | 12 (71) | |
| Female | 11 (34) | 6 (40) | 5 (29) | |
| Race | 0.74 | |||
| African American | 23 (72) | 10 (66) | 13 (76) | |
| Asian | 1 (3) | 1 (7) | 0 (0) | |
| Caucasian | 6 (19) | 3 (20) | 3 (18) | |
| Hispanic | 2 (6) | 1 (7) | 1 (6) | |
| Age (y) | 43.2±13.1 | 46.5±12.1 | 40.2±13.5 | 0.17 |
| BMI (kg/m2) | 28.4±6.3 | 27.5±6.3 | 29.1±6.4 | 0.45 |
| Etiology of ESRD | 0.78 | |||
| Diabetes | 6 (19) | 3 (20) | 3 (18) | |
| HTN | 19 (59) | 8 (53) | 11 (65) | |
| Other | 7 (22) | 4 (27) | 3 (18) | |
| Other variables | ||||
| Hemoglobin (g/dL) | 12.1±1.5 | 12.0±1.4 | 12.2±1.7 | 0.66 |
| Albumin (mg/dL) | 41.2±2.9 | 40.7±2.8 | 41.8±3.0 | 0.40 |
| Creatinine (mg/dL) | 10.1±2.5 | 9.3±2.5 | 10.9±2.3 | 0.09 |
| CRP (mg/L) | 4.0(1.9~13.1) | 4.3(1.9~13.3) | 3.9(1.0~12.0) | 0.52 |
Note: Values are mean ± SE,median (lower~upper quartile) or absolute numbers with percentages.
Abbreviation: NS+EX, nutrition supplementation plus exercise; NS, nutrition supplementation; BMI, body mass index; ESRD, end-stage renal disease; HTN, hypertension; CRP, C-reactive protein
Compliance
The rate of achieving the consumption of expected cans for Nepro was not significantly different between groups, 76% and 87% respectively in NS+EX and NS. All the subject in NS+EX group completed 3 sets of 12 repetitions in each exercise session except for 2 patients missing 1 set in one session. The actual leg press weight achieved was 71%, 75% and 82% of 1RM at baseline, month 3 and month 6 respectively.
Body Composition
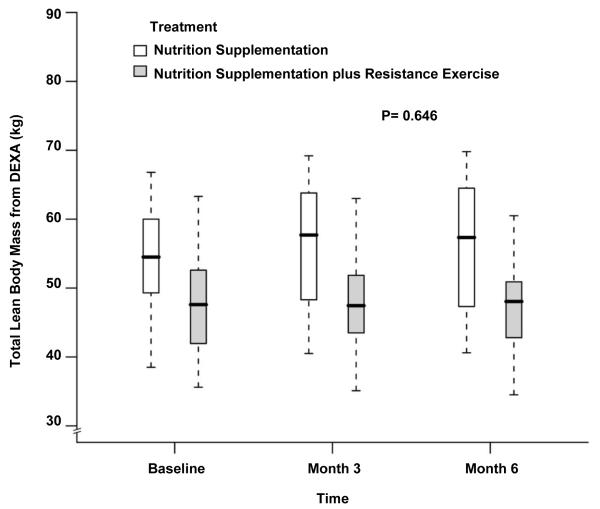
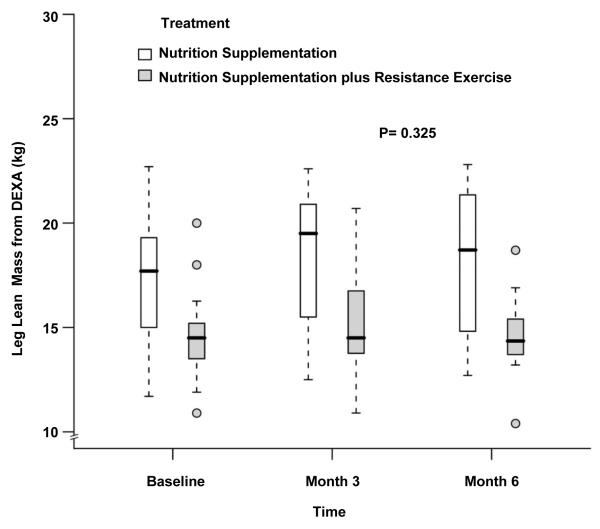
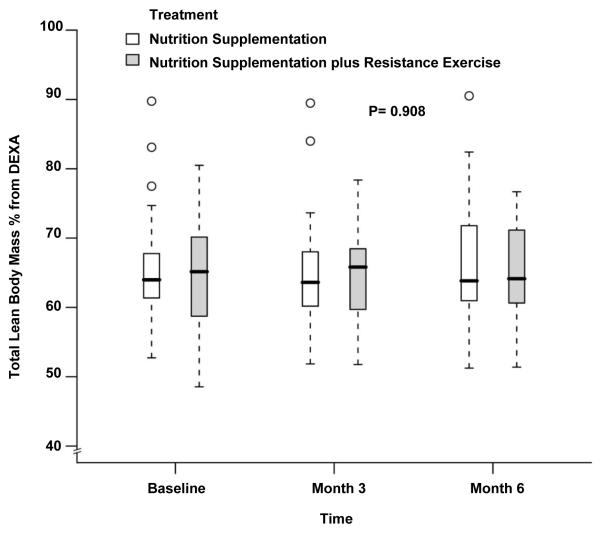
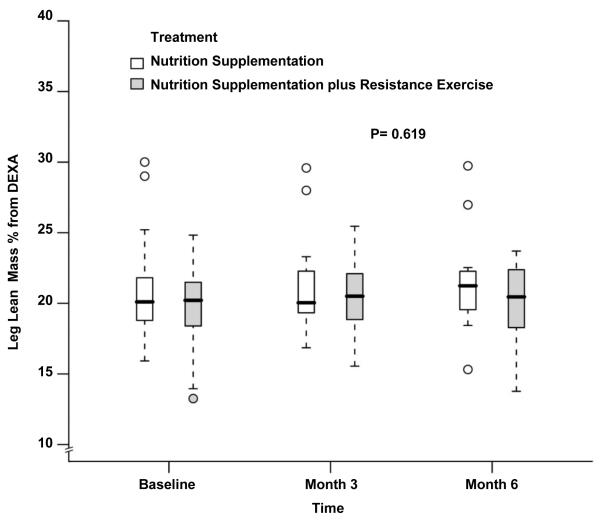
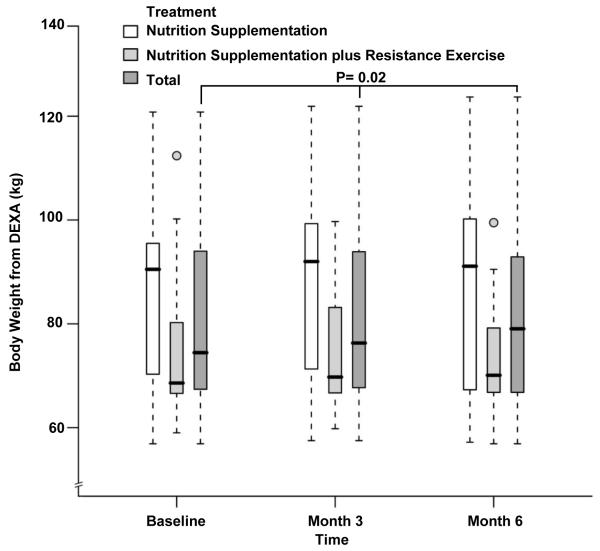
The body composition measurements during the study period are depicted in Table 2. The LBM at baseline was significantly higher in the NS group which continued to be the case at month 3 and month 6 (48.1±7.7kg vs 54.3±8.4kg at baseline, 48.2±7.8kg vs 55.7±9.1kg at month 3 and 47.4±7.1kg vs 56.2±9.9kg at month 6 for NS+EX and NS, P= 0.03, 0.05 and 0.05 respectively). There was no difference when comparing changes over time between groups after adjusting for baseline LBM, age, gender and BMI (P= 0.79 by 3 months, P=0.65 by 6 months, Figure 2). Similar to whole-body LBM, leg LBM also showed no differences over time between groups (14.7±2.2kg vs 17.4±3.2kg at baseline, 15.1±2.7kg vs 18.2±3.3kg at month 3 and 14.6±2.2kg vs 18.2±3.7kg at month 6 for NS+EX and NS; P= 0.44 by 3 months, P=0.33 by 6 months, Figure 3). No statistically or numerically significant differences were observed between study groups during the study for whole-body LBM by % of body weight (Figure 4) and leg LBM by % of body weight (Figure 5). While there were no differences between groups, there was a statistically significant increase over time in body weight for the whole cohort after adjusting for treatment effect (80.3±16.6 kg at baseline, 81.1±17.5 kg at month 3, and 80.9±18.2 kg at month 6, P=0.02, Figure 6).
Table 2.
Body composition measured by anthropometrics, BIA and DEXA and muscular strength at the follow-up times point during the study period
| Total | NS+EX | NS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 month (n=32) |
3 month (n=26) |
6 month (n=22) |
0 month (n=15) |
3 month (n=13) |
6 month (n=10) |
0 month (n=17) |
3 month (n=13) |
6 month (n=12) |
|
| DEXA | |||||||||
| Weight (kg) | 80.3±16.6 | 81.1±17.5 | 80.9±18.2 | 75.8±15.1 | 74.9±12.9 | 74.6±12.7 | 84.2±17.3 | 86.9±19.6 | 86.2±20.7 |
| FM (kg) | 26.3±12.3 | 26.4±12.1 | 26.3±12.8 | 25.3±12.1 | 24.2±9.5 | 24.7±10.5 | 27.2±12.7 | 28.5±14.2 | 27.6±14.8 |
| FM (%) | 32.3±10.8 | 32.2±10.7 | 32.1±10.6 | 32.9±10.7 | 32.9±8.2 | 33.5±9.0 | 31.7±11.1 | 31.6±12.8 | 31.0±12.0 |
| LBM (kg) | 51.4±8.5 | 52.1±9.2 | 52.2±9.7 | 48.1±7.7 | 48.2±7.8 | 47.4±7.1 | 54.3±8.4* | 55.7±9.1Δ | 56.2±9.9○ |
| LBM (%) | 65.1±9.7 | 65.3±9.3 | 65.6±9.7 | 64.3±9.4 | 64.8±7.6 | 64.2±8.4 | 65.8±10.3 | 65.7±11.0 | 66.8±10.9 |
| Leg LBM (kg) |
16.1±3.1 | 16.7±3.4 | 16.6±3.5 | 14.7±2.2 | 15.1±2.7 | 14.6±2.2 | 17.4±3.2* | 18.2±3.3Δ | 18.2±3.7○ |
| Leg LBM (%) |
20.4±3.8 | 20.9±3.4 | 20.8±3.6 | 19.7±3.4 | 20.3±2.9 | 19.9±3.4 | 21.1±4.0 | 21.4±3.7 | 21.6±3.8 |
| BMI (kg/m2) | 28.4±6.3 | 28.2±5.9 | 28.2±5.8 | 27.5±6.3 | 26.2±4.0 | 26.8±4.3 | 29.1±6.4 | 30.0±7.0 | 29.3±6.8 |
| Waist/hip ratio | 0.93±0.07 | 0.94±0.06 | 0.94±0.08 | 0.92±0.05 | 0.92±0.03 | 0.91±0.06 | 0.95±0.09 | 0.95±0.07 | 0.96±0.09 |
| FM% from BIA |
29.1±13.6 | 28.2±11.8 | 27.4±10.7 | 30.1±14.9 | 28.4±12.5 | 29.4±13.2 | 28.1±12.7 | 27.9±11.5 | 25.8±8.3 |
| FM% from Anthropometry |
20.3±9.2 | 19.1±9.2 | 18.4±7.9 | 21.2±9.5 | 19.0±9.2 | 19.9±8.0 | 19.4±8.7 | 19.1±9.7 | 17.1±8.0 |
| 1-RM (lb) | 468±148 | 535±144 | 552±142 | 459±117 | 536±126 | 582±147 | 475±175 | 534±165 | 527±139 |
Note: Values are mean ± SE.
Abbreviation: NS+EX,nutrition supplementation plus exercise; NS, nutritionsupplementation; DEXA, dual energy X-ray absorptiometry; FM, fat mass;fat mass as percentage of body weight; LBM, lean body mass; LBM%, lean body mass as percentage of body weight; leg LBM%, leg lean body percentage of body weight; BMI, body mass index; BIA, bioelectrical impedance analysis; 1-RM, one-repetition maximum
P<0.05, compared to NS+EX group at baseline;
P<0.05, compared to NS+EX group at 3rd month;
P<0.05, compared to NS+EX group at 6th month.
Fig 2.
Box and whisker plot (box represents the interquartile range, whiskers extend to the most extreme data point which is no more than 1.5 times the interquartile range from the box, and circles beyond the whiskers are extreme values, the line within the box represents the median) of the total lean body mass as measured by dual-energy x-ray absorptiometry at baseline, 3 month and 6 month of follow up. □, nutrition supplementation group; ■ nutrition supplementation plus resistance exercise group. P value comparing the two treatments over time was obtained from the general linear model with bootstrap covariance accounting for correlated measures within a subject.
Fig 3.
Box and whisker plot (box represents the interquartile range, whiskers extend to the most extreme data point which is no more than 1.5 times the interquartile range from the box, and circles beyond the whiskers are extreme values, the line within the box represents the median) of the leg lean mass by dual-energy x-ray absorptiometry at baseline, 3 month and 6 month of follow up. □, nutrition supplementation group; ■ nutrition supplementation plus resistance exercise group. P value comparing the two treatments over time was obtained from the general linear model with bootstrap covariance accounting for correlated measures within a subject.
Fig 4.
Box and whisker plot (box represents the interquartile range, whiskers extend to the most extreme data point which is no more than 1.5 times the interquartile range from the box, and circles beyond the whiskers are extreme values, the line within the box represents the median) of the lean body mass as percentage of body weight measured by dual-energy x-ray absorptiometry at baseline, 3 month and 6 month of follow up. □, nutrition supplementation group; ■ nutrition supplementation plus resistance exercise group. P value comparing the two treatments over time was obtained from the general linear model with bootstrap covariance accounting for correlated measures within a subject.
Fig 5.
Box and whisker plot (box represents the interquartile range, whiskers extend to the most extreme data point which is no more than 1.5 times the interquartile range from the box, and circles beyond the whiskers are extreme values, the line within the box represents the median) of the leg lean mass as percentage of body weight measured by dual-energy x-ray absorptiometry at baseline, 3 month and 6 month of follow up. □, nutrition supplementation group; ■ nutrition supplementation plus resistance exercise group. P value comparing the two treatments over time was obtained from the general linear model with bootstrap covariance accounting for correlated measures within a subject.
Fig 6.
Box and whisker plot (box represents the interquartile range, whiskers extend to the most extreme data point which is no more than 1.5 times the interquartile range from the box, and circles beyond the whiskers are extreme values, the line within the box represents the median) of body weight measured by dual-energy x-ray absorptiometry (DEXA) at baseline, 3 month and 6 month of follow up. □, nutrition supplementation group; ■ nutrition supplementation plus resistance exercise group; ■ total. P value showing the changes over time in the whole cohort adjusted for treatment effect was obtained from the general linear model with bootstrap covariance accounting for correlated measures within a subject.
Muscle Strength
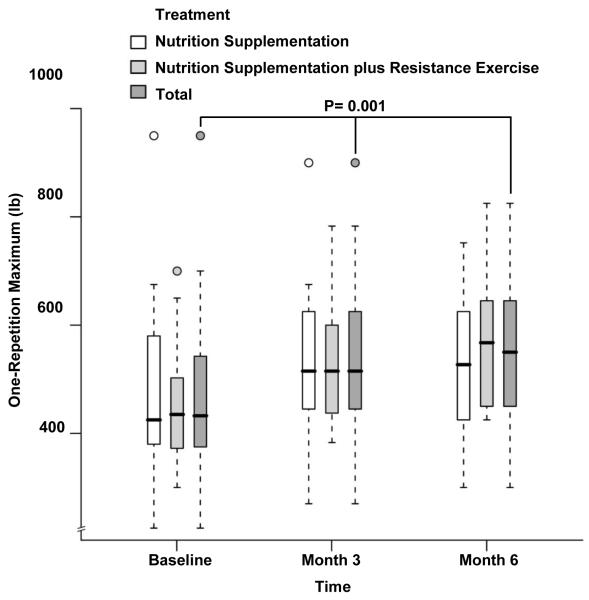
There were no statistically significant differences between groups at baseline, month 3 or month 6 with respect to 1-RM (459 ±117 lb vs 475±175 lb at baseline, 536±126 lb vs 534±165 lb at month 3 and 582±147 lb vs 527±139 lb at month 6 for NS+EX and NS, respectively; Table 2 Figure 7). Small differences in favor of NS+EX were observed in the changes of 1-RM over time between groups (P= 0.07 by month 3, P=0.12 by month 6). When the overall time effect was evaluated for the whole cohort, there was a statistically significant increase over time in 1-RM after adjusting for treatment effect (468±148 lb at baseline, 535±144 lb at month 3 and 552±142 lb at month 6, P=0.001, Table 2, Figure 7)
Fig 7.
Box and whisker plot (box represents the interquartile range, whiskers extend to the most extreme data point which is no more than 1.5 times the interquartile range from the box, and circles beyond the whiskers are extreme values, the line within the box represents the median) of one-repetition maximum at baseline, 3 month and 6 month of follow up. □, nutrition supplementation group; ■ nutrition supplementation plus resistance exercise group; ■ total. P value showing the changes over time in the whole cohort adjusted for treatment effect was obtained from the general linear model with bootstrap covariance accounting for correlated measures within a subject.
Biochemical Parameters, SGA, and dietary recall
The biochemical, nutritional parameters, and SGA measurements during the study period are depicted in Table 3 Only glucose levels at baseline were significantly different between groups (P=0.02). There were no statistically significant differences in study groups at baseline, month 3 or month 6 in other biochemical variables and SGA, nor were there any significant differences when comparing changes over time. Similarly, DPI and DEI were not different when comparing study groups at baseline, month 3 and month 6, as well as no significant changes over time were observed.
Table 3.
Biochemistry and nutrition parameters at the follow-up time points during the study period
| NS+EX | NS | |||||
|---|---|---|---|---|---|---|
| 0 month (n=15) |
3month (n=13) |
6 month (n=10) |
0 month (n=17) |
3 month (n-=13) |
6 month (n=12) |
|
| SGA = 7 | 13 (87) | 11 (85) | 9 (90) | 14 (82) | 11 (85) | 10 (83) |
| CRP (mg/L) | 4.3(1.9~13.3) | 3.7(2.3~12.3) | 2.6 (1.3~8.3) | 3.9(1.0~12.0) | 4.8(1.9~11.7) | 6.9 (5.9~12.4) |
| Hemoglobin (g/dL) | 12.0±1.4 | 12.6±1.8 | 11.4±1.9 | 12.2±1.7 | 12.4±1.1 | 12.2±1.8 |
| Total protein(g/dL) | 7.3±0.6 | 7.3±0.7 | 7.3±0.8 | 7.3±0.4 | 7.4±0.7 | 7.2±0.5 |
| Albumin(mg/dL) | 40.7±2.8 | 41.3±3.5 | 41.5±4.4 | 41.8±3.0 | 42.9±3.3 | 42.1±2.2 |
| Pre-albumin(mg/dL) | 40.0±10.7 | 40.3±11.7 | 41.7±12.4 | 38.2±9.5 | 42.5±9.9 | 41.7±7.2 |
| Creatinine(mg/dL) | 9.3±2.5 | 10.3±2.2 | 10.2±2.5 | 10.9±2.3 | 11.5±2.1 | 11.7±2.5 |
| Glucose (mg/dl) | 131.0±72.9 | 111.7±44.0 | 115.9±47.1 | 105.6±58.1* | 88.0±10.8 | 91±9.6 |
| Bicarbonate (mmol/l) | 26.1±5.2 | 25.9±6.0 | 27.6±9.2 | 25.4±6.9 | 24.4±5.7 | 24.4±7.9 |
| Cholesterol (mmol/l) | 163.8±33.5 | 167.3±46.2 | 168.5±24.9 | 166.8±50.4 | 174.8±48.5 | 173.7±42.6 |
| DPI (g/kg/day) | 0.8±0.2 | 1.3±0.5 | 1.0±0.3 | 0.8±0.3 | 1.0±0.4 | 1.1±0.4 |
| DEI (kcal/kg/day) | 23.8±7.4 | 27.5±6.4 | 26.5±7.1 | 22.0±8.5 | 26.5±10.6 | 27.6±11.9 |
Note: Values are mean ± SE, median(lower~upper quartile) or absolute numbers with percentages.
Abbreviation : NS+EX, nutrition supplementation plus exercise; NS, nutrition supplementation; SGA,subjective global assessment;CRP, C-reactive protein; DPI,dietary protein intake; DEI,dietary energy intake.
P<0.05, compared to NS+EX group at baseline.
Discussion
This prospective randomized open-label clinical trial was undertaken to test the hypothesis that addition of resistance exercise would enhance the protein anabolic effects of IDON on body composition and visceral protein mass over a period of 6 months. Our rationale was based on encouraging data indicating that one bout of intradialytic parenteral or oral nutrition provides protein anabolic effects (measured by protein kinetic studies) and that resistance exercise augments these acute anabolic effects even further 13, 21. Despite increases in body weight and 1-repetition maximum test for the entire study population, we did not detect any discernible additional beneficial effect of resistance exercise over 3 or 6 months on body composition or markers of visceral protein concentrations compared to nutrition supplementation alone.
Despite the proven efficacy of resistance exercise as an anabolic intervention in otherwise healthy elderly and certain chronic disease states 22, 23, recent studies in CHD patients have not been encouraging in terms of long-term improvements in markers of muscle mass. Several recent studies reported a lack of significant effects with 12 to 24-weeks of resistance exercise on LBM 14-17. An overlapping aspect of these studies is the lack of any attempt to increase nutritional intake in the study patients, especially around the time of exercise. Multiple studies demonstrated that resistance exercise combined with oral nutritional supplementation facilitates muscle uptake of amino acids and muscle protein accretion in healthy subjects, which is believed to be due to increased blood flow to the muscle as well as enhanced insulin signaling in the cellular level24-27. In accordance with these data, several short-term studies in CHD patients showed that net muscle protein accretion and albumin synthesis are increased with nutritional supplementation combined with exercise when compared to supplementation alone13, 28, 29. Of note, these beneficial effects of combined intervention were above and beyond of what nutritional supplementation provided alone. Accordingly, the principal aim of our study was to determine if resistance exercise can augment the long-term benefits of nutrition supplementation in HD patients. Despite the encouraging results from short-term protein homeostasis studies, we were unable to show any additional long-term benefits of resistance exercise compared to nutritional supplementation alone.
While speculative, there may be several reasons why addition of resistance exercise failed to augment protein mass in our study. First, the subjects enrolled in our study were younger than the general dialysis population in the U.S. and in relatively good nutritional status. Therefore, interventions aimed at ameliorating muscle mass loss could not be detected as much as in the elderly or subjects with obvious muscle wasting 22, 23. The rationale for recruitment for such subjects in our study was the limited exercise capacity of the elderly and lack of a gold standard for loss of muscle mass in CHD patients. While an older and more debilitated cohort would have been preferable, we were limited by our inclusion and exclusion criteria and the intervention administered in this study for recruitment of such subjects. This is a common limitation of published studies using this strategy including a very recent publication with results similar to ours. 16, 30, 31 A second reason for lack of clear benefits can be attributed to the sensitivity and precision of DEXA to evaluate changes in muscle mass, as suggested by a study in which increases in quadriceps muscle area was observed by MRI despite no changes in DEXA 14. Further, Castaneda et al showed that resistance exercise significantly increased type I and type II muscle fiber hypertrophy detected via muscle biopsies despite a non-significant change in mid-thigh muscle CSA as evaluated by CT scan 32. Finally, it is possible that the intensity and duration of exercise in our study was not adequate to induce a significant change in muscle homeostasis despite our best efforts to prescribe a progressive leg-press intervention. Of note, the intensity of exercise was progressively increased according to the month 3 and month 6 assessments and the actual leg press weight achieved was beyond what was originally predicted. Moreover, although 1-RM increased in all subjects combined, there was a trend for a higher 1RM in the NS+EX group, suggesting an efficacy of exercise on muscle strength, albeit inadequate. Similar to our results, Flakoll et al showed that specialized nutritional supplementation alone can improve leg muscle strength without resistance exercise 33. The overall improvement in the 1-RM during the study period suggests that the exercise regimen provided in this study either falls short of improving leg strength above and beyond of nutritional supplementation alone or that the effect is too small to be detected by our assessment tools.
The results of this study have important implications. Despite no any obvious additional benefit of resistance exercise, there was a statistically significant increase in body weight and muscle strength for the whole cohort over time, similar to the previous long-term studies regarding nutrition supplementation10, 34-36 or resistance exercise17 in CHD patients. Given the strong epidemiological data showing improved survival with increasing body weight in CHD patients37, 38, the interventions applied in this study are of obvious clinical benefit 39, 40. On the other hand, if benefits of resistance exercise are to be tested further in CHD patients, either a more aggressive treatment regimen is necessary or outcome measures other than body composition should be considered.
This study has several strengths. To our knowledge, this is the first long-term trial which examines the potential additive effects of resistance exercise on IDON in CHD patients. The patients were closely monitored with good compliance and were thoroughly examined. In spite of the strengths of the present study, results should be interpreted in view of its limitations as well, including the relatively younger CHD population with good nutritional status and whether or not this might have limited the ability to detect clear benefits. In addition, in spite of the adequate power estimation, the relatively small sample size with 32% withdrawal of subjects during the study period prevented a robust comparison of differences in outcomes secondary to our interventions. Finally, we elected not to include a control group with no intervention, a decision based on ethical considerations since a plethora of studies indicate the beneficial effects of nutritional supplementation. Similarly, a group with resistance exercise alone was not included based on the studies indicating a lack of effect of exercise alone14-17.
In conclusion, we showed that a 6-month nutrition supplementation regimen increases body weight and 1-RM but the addition of resistance exercise to this regimen fails to show any extra long-term effects on muscle protein accretion as measured by DEXA. More precise and sensitive techniques such as CT, MRI or muscle biopsy could provide more information on this aspect. At this point, the addition of resistance exercise to intradialytic nutrition supplementation, although practical, cannot be recommended until further studies confirm its benefits on muscle mass and muscle strength in CHD patients. Additional benefits of resistance exercise not explored in this study such as physical functioning, long-term protein turnover, and cardiovascular outcomes should also be explored in future studies.
Acknowledgements
The authors would like to express their appreciation to the patients and staff of Vanderbilt University Medical Center, Outpatient Dialysis Unit for their participation in the study. This study is supported in part by National Institutes of Health Grants R01-DK45604, K24-DK62849 and Diabetes Research Training Center Grant DK-20593 from the National Institute of Diabetes, Digestive and Kidney Diseases and the Clinical Translational Science Award 1UL-1RR024975 from the National Center for Research Resources. Dr. Jie Dong was supported in part by grants from Chinese Society of Nephrology and International Society of Peritoneal Dialysis. Mary B. Sundell was supported in part by a grant from National Kidney Foundation, Council of Renal Nutrition. The excellent technical assistance of Phyllis Egbert, Cindy Booker, Suzan Vaughan, Feng Sha, Mu Zheng, Wanda Snead, and the nursing staff on the Vanderbilt General Clinical Research Center is appreciated.
Footnotes
Conflict of interest statement The authors declare no conflict of interest with regard to involvement with commercial entities that supply nutritional supplements. Dr. Lara. B. Pupim is an employee of Mitsubishi Pharmaceuticals and declares no conflict of interest with regards to this study.
References
- 1.Pupim LB, Ikizler TA. Assessment and monitoring of uremic malnutrition. J Ren Nutr. 2004 Jan;14(1):6–19. doi: 10.1053/j.jrn.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Ikizler TA, Wingard RL, Harvell J, Shyr Y, Hakim RM. Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: a prospective study. Kidney Int. 1999 May;55(5):1945–1951. doi: 10.1046/j.1523-1755.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 3.Kopple JD. Effect of nutrition on morbidity and mortality in maintenance dialysis patients. Am J Kidney Dis. 1994;24:1002–1009. doi: 10.1016/s0272-6386(12)81075-4. [DOI] [PubMed] [Google Scholar]
- 4.Owen WF, Jr., Lew NL, Liu Y, Lowrie EG, Lazarus JM. The urea reduction ratio and serum albumin concentrations as predictors of mortality in patients undergoing hemodialysis. New England Journal of Medicine. 1993;329(14):1001–1006. doi: 10.1056/NEJM199309303291404. [DOI] [PubMed] [Google Scholar]
- 5.Desmeules S, Levesque R, Jaussent I, Leray-Moragues H, Chalabi L, Canaud B. Creatinine index and lean body mass are excellent predictors of long-term survival in haemodiafiltration patients. Nephrol Dial Transplant. 2004 May;19(5):1182–1189. doi: 10.1093/ndt/gfh016. [DOI] [PubMed] [Google Scholar]
- 6.Pupim LB, Flakoll PJ, Brouillette JR, Levenhagen DK, Hakim RM, Ikizler TA. Intradialytic parenteral nutrition improves protein and energy homeostasis in chronic hemodialysis patients. J Clin Invest. 2002;110:483–492. doi: 10.1172/JCI15449. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veeneman JM, Kingma HA, Boer TS, et al. Protein intake during hemodialysis maintains a positive whole body protein balance in chronic hemodialysis patients. Am J Physiol Endocrinol Metab. 2003 May;284(5):E954–965. doi: 10.1152/ajpendo.00264.2002. [DOI] [PubMed] [Google Scholar]
- 8.Pupim LB, Majchrzak KM, Flakoll PJ, Ikizler TA. Intradialytic oral nutrition improves protein homeostasis in chronic hemodialysis patients with deranged nutritional status. J Am Soc Nephrol. 2006 Nov;17(11):3149–3157. doi: 10.1681/ASN.2006040413. [DOI] [PubMed] [Google Scholar]
- 9.Cano N. Nutritional supplementation in adult patients on hemodialysis. J Ren Nutr. 2007 Jan;17(1):103–105. doi: 10.1053/j.jrn.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Cano NJ, Fouque D, Roth H, et al. Intradialytic parenteral nutrition does not improve survival in malnourished hemodialysis patients: a 2-year multicenter, prospective, randomized study. J Am Soc Nephrol. 2007 Sep;18(9):2583–2591. doi: 10.1681/ASN.2007020184. [DOI] [PubMed] [Google Scholar]
- 11.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997 Jul;273(1 Pt 1):E122–129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 12.Gautsch TA, Anthony JC, Kimball SR, Paul GL, Layman DK, Jefferson LS. Availability of eIF4E regulates skeletal muscle protein synthesis during recovery from exercise. Am J Physiol. 1998 Feb;274(2 Pt 1):C406–414. doi: 10.1152/ajpcell.1998.274.2.C406. [DOI] [PubMed] [Google Scholar]
- 13.Majchrzak KM, Pupim LB, Flakoll PJ, Ikizler TA. Resistance exercise augments the acute anabolic effects of intradialytic oral nutritional supplementation. Nephrol Dial Transplant. 2008 Apr;23(4):1362–1369. doi: 10.1093/ndt/gfm773. [DOI] [PubMed] [Google Scholar]
- 14.Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J Am Soc Nephrol. 2006 Aug;17(8):2307–2314. doi: 10.1681/ASN.2006010034. [DOI] [PubMed] [Google Scholar]
- 15.Cheema B, Abas H, Smith B, et al. Randomized controlled trial of intradialytic resistance training to target muscle wasting in ESRD: the Progressive Exercise for Anabolism in Kidney Disease (PEAK) study. Am J Kidney Dis. 2007 Oct;50(4):574–584. doi: 10.1053/j.ajkd.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Kopple JD, Wang H, Casaburi R, et al. Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. J Am Soc Nephrol. 2007 Nov;18(11):2975–2986. doi: 10.1681/ASN.2006070794. [DOI] [PubMed] [Google Scholar]
- 17.Cheema B, Abas H, Smith B, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007 May;18(5):1594–1601. doi: 10.1681/ASN.2006121329. [DOI] [PubMed] [Google Scholar]
- 18.Majchrzak KM, Pupim LB, Sundell M, Ikizler TA. Body composition and physical activity in end-stage renal disease. J Ren Nutr. 2007 May;17(3):196–204. doi: 10.1053/j.jrn.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trirogoff ML, Shintani A, Himmelfarb J, Ikizler TA. Body mass index and fat mass are the primary correlates of insulin resistance in nondiabetic stage 3-4 chronic kidney disease patients. Am J Clin Nutr. 2007 Dec;86(6):1642–1648. doi: 10.1093/ajcn/86.5.1642. [DOI] [PubMed] [Google Scholar]
- 20.Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1996 Feb;7(2):198–207. doi: 10.1681/ASN.V72198. [DOI] [PubMed] [Google Scholar]
- 21.Pupim LB, Flakoll PJ, Levenhagen DK, Ikizler TA. Exercise augments the acute anabolic effects of intradialytic parenteral nutrition in chronic hemodialysis patients. Am J Physiol Endocrinol Metab. 2004 Apr;286(4):E589–597. doi: 10.1152/ajpendo.00384.2003. [DOI] [PubMed] [Google Scholar]
- 22.Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol. 2009 Jun;106(6):2040–2048. doi: 10.1152/japplphysiol.91551.2008. [DOI] [PubMed] [Google Scholar]
- 23.Zinna EM, Yarasheski KE. Exercise treatment to counteract protein wasting of chronic diseases. Curr Opin Clin Nutr Metab Care. 2003 Jan;6(1):87–93. doi: 10.1097/00075197-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Levenhagen DK, Carr C, Carlson MG, Maron DJ, Borel MJ, Flakoll PJ. Postexercise protein intake enhances whole-body and leg protein accretion in humans. Med Sci Sports Exerc. 2002 May;34(5):828–837. doi: 10.1097/00005768-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Levenhagen DK, Gresham JD, Carlson MG, Maron DJ, Borel MJ, Flakoll PJ. Postexercise nutrient intake timing in humans is critical to recovery of leg glucose and protein homeostasis. Am J Physiol Endocrinol Metab. 2001 Jun;280(6):E982–993. doi: 10.1152/ajpendo.2001.280.6.E982. [DOI] [PubMed] [Google Scholar]
- 26.Tipton KD, Wolfe RR. Exercise-induced changes in protein metabolism. Acta Physiol Scand. 1998 Mar;162(3):377–387. doi: 10.1046/j.1365-201X.1998.00306.x. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe RR. Effects of amino acid intake on anabolic processes. Can J Appl Physiol. 2001;26(Suppl):S220–227. doi: 10.1139/h2001-056. [DOI] [PubMed] [Google Scholar]
- 28.Pupim LB, Flakoll PJ, Ikizler TA. Exercise improves albumin fractional synthetic rate in chronic hemodialysis patients. Eur J Clin Nutr. 2006 Dec 20; doi: 10.1038/sj.ejcn.1602578. [DOI] [PubMed] [Google Scholar]
- 29.Pupim LB, Flakoll PJ, Levenhagen DK, Ikizler TA. Exercise Augments the Acute Anabolic Effects of Intradialytic Parenteral Nutrition in Chronic Hemodialysis Patients. Am J Physiol Endocrinol Metab. 2004 April;286(4):E589–597. doi: 10.1152/ajpendo.00384.2003. [DOI] [PubMed] [Google Scholar]
- 30.Headley S, Germain M, Mailloux P, et al. Resistance training improves strength and functional measures in patients with end-stage renal disease. Am J Kidney Dis. 2002 Aug;40(2):355–364. doi: 10.1053/ajkd.2002.34520. [DOI] [PubMed] [Google Scholar]
- 31.Koh KP, Fassett RG, Sharman JE, Coombes JS, Williams AD. Effect of Intradialytic Versus Home-Based Aerobic Exercise Training on Physical Function and Vascular Parameters in Hemodialysis Patients: A Randomized Pilot Study. Am J Kidney Dis. 2009 Nov 20; doi: 10.1053/j.ajkd.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 32.Castaneda C, Gordon PL, Uhlin KL, et al. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. A randomized, controlled trial. Ann Intern Med. 2001 Dec 4;135(11):965–976. doi: 10.7326/0003-4819-135-11-200112040-00008. [DOI] [PubMed] [Google Scholar]
- 33.Flakoll P, Sharp R, Baier S, Levenhagen D, Carr C, Nissen S. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition. 2004 May;20(5):445–451. doi: 10.1016/j.nut.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Sharma M, Rao M, Jacob S, Jacob CK. A controlled trial of intermittent enteral nutrient supplementation in maintenance hemodialysis patients. J Ren Nutr. 2002 Oct;12(4):229–237. doi: 10.1053/jren.2002.35300. [DOI] [PubMed] [Google Scholar]
- 35.Cano N, Labastie-Coeyrehourq J, Lacombe P, et al. Perdialytic parenteral nutrition with lipids and amino acids in malnourished hemodialysis patients. Am J Clin Nutr. 1990 Oct;52(4):726–730. doi: 10.1093/ajcn/52.4.726. [DOI] [PubMed] [Google Scholar]
- 36.Mortelmans AK, Duym P, Vandenbroucke J, et al. Intradialytic parenteral nutrition in malnourished hemodialysis patients: a prospective long-term study. JPEN J Parenter Enteral Nutr. 1999 Mar-Apr;23(2):90–95. doi: 10.1177/014860719902300290. [DOI] [PubMed] [Google Scholar]
- 37.Hakim RM, Lowrie E. Obesity and mortality in ESRD: is it good to be fat? Kidney Int. 1999 Apr;55(4):1580–1581. doi: 10.1046/j.1523-1755.1999.00453.x. [DOI] [PubMed] [Google Scholar]
- 38.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005 Mar;81(3):543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 39.Ikizler TA. Resolved: being fat is good for dialysis patients: the Godzilla effect: pro. J Am Soc Nephrol. 2008 Jun;19(6):1059–1062. doi: 10.1681/ASN.2007090983. [DOI] [PubMed] [Google Scholar]
- 40.Zoccali C. The obesity epidemics in ESRD: from wasting to waist? Nephrol Dial Transplant. 2009 Feb;24(2):376–380. doi: 10.1093/ndt/gfn589. [DOI] [PubMed] [Google Scholar]