Abstract
Growth arrest-specific gene 6 (GAS6) promotes growth and cell survival during tissue repair and development in different organs, including the liver. However, the specific role of GAS6 in liver ischemia/reperfusion (I/R) injury has not been previously addressed. Here, we report an early increase in serum GAS6 levels following I/R exposure. Moreover, unlike wild type mice, Gas6-/- mice were highly sensitive to partial hepatic I/R, with 90% of mice dying within 12 hours of reperfusion due to massive hepatocellular injury. I/R induced early hepatic AKT phosphorylation in wild type but not in Gas6-/- mice, without significant changes in JNK phosphorylation or nuclear NF-κB translocation, whereas hepatic IL-1β and TNF mRNA levels were higher in Gas6-/- mice compared to wild type mice. In line with the in vivo data, in vitro studies indicated that GAS6 induced AKT phosphorylation in primary mouse hepatocytes protecting them from hypoxia-induced cell death, while GAS6 diminished lipopolysaccharide (LPS)-induced cytokine expression (IL-1β and TNF) in murine macrophages. Finally, in vivo recombinant GAS6 treatment not only rescued GAS6 knockout mice from I/R-induced severe liver damage, but also attenuated hepatic damage in wild type mice following I/R. In conclusion, our data uncover GAS6 as a new player in liver I/R injury, emerging as a potential therapeutic target to reduce post-ischemic hepatic damage.
Introduction
Growth arrest-specific gene 6 (GAS6) product and its tyrosine kinase TAM receptors (Tyro3, Axl and Mer) have been involved in growth and survival processes during tissue repair and development (1, 2). GAS6 is a vitamin K-dependent protein, which has a high structural homology with the natural anticoagulant protein S, sharing the same modular composition and 40% sequence identity. Despite these common features between GAS6 and the anticoagulant protein S, their biological role is clearly differentiated, with GAS6 being mainly involved in cell protection and tissue formation with a minor participation in the coagulation cascade (3, 4).
The low concentration of GAS6 in plasma and its specific pattern of tissue expression suggest a unique function of GAS6 among vitamin-K dependent proteins. In the liver, GAS6 is mainly expressed in Kupffer cells with levels below those observed in other tissues such as lung, kidney or heart (3). However, after specific liver injury other hepatic cell types may participate in its production. For instance, GAS6 produced by hepatic stellate cells together with its receptor Axl participate in the signaling involved in the wound healing response to liver injury by CCl4, and oval cells induce GAS6 production after hepatectomy (5-7). In addition, increased plasma levels of GAS6 have been reported in different pathologies, such as cancer (8-10), acute coronary syndrome (11), pulmonary embolism (12), acute pancreatitis (13) or severe sepsis (14, 15), both in patients as well as in experimental models. In particular, GAS6 expression correlates with disease severity in patients with septic shock, especially in relation to renal and hepatic dysfunction (15). However, the role of GAS6 in hepatocyte signaling and liver injury after ischemia/reperfusion (I/R) has not been previously reported to the best of our knowledge.
GAS6 and its signaling through TAM receptors (Mer, Axl and Tyro3) have been proposed not only as a protective pathway in several cell types, including endothelial and epithelial cells, neurons and fibroblasts (16-19), but also as a molecular device to modulate cytokine secretion. For instance, mice deficient in TAM receptors or with mutated Mer displayed high susceptibility to endotoxic shock, with monocytes showing increased TNF secretion after LPS challenge (20). Moreover, recent data in monocytes/macrophages have reported that exogenous GAS6 reduced LPS-induced TNF and IL-1 stimulation via Mer but not Axl or Tyro3 signaling (21). Therefore, our aim was to address the role of GAS6 during hepatic I/R injury and the potential mechanisms involved. Our results showed that plasma GAS6 levels increased early during hepatic I/R concomitant with decreased hepatic GAS6 mRNA content, preceding major liver injury. Exogenous GAS6 induces AKT phosphorylation, protecting primary hepatocytes from hypoxia. In addition, partial I/R was lethal in GAS6 knockout mice due to massive hepatocellular injury, an event that was abrogated by recombinant GAS6 i.v. injection. Overall, these findings indicate that GAS6 protects against liver I/R injury, emerging as a potential novel target in diverse clinical settings where hepatic I/R damage occurs such as liver transplantation, hemorrhagic shock or liver surgery.
Experimental Procedures
Partial Hepatic Ischemia and Treatments
The experimental animal protocol was approved by the IDIBAPS Animal Care and Use Committee. Wild type C57BL/6 and Gas6-/- male mice, backcrossed into the C57BL/6 background (8-12 weeks), were generated and propagated as previously characterized (22). Hepatic partial warm ischemia was performed for 90 minutes (23), as detailed in Supplementary Methods. Mice were pretreated 15-20 minutes prior to surgery with recombinant GAS6 protein (0.5-10.0 μg/mice, R&D, St. Louis, MO) or with an equal volume of the vehicle (PBS solution). To follow animal survival, mice were monitored every 12 hours during one week.
Cell Culture and hypoxia exposure
Primary hepatocytes, obtained from mouse liver by collagenase digestion and cultured on collagen-coated plates (23), were routinely grown at 37 °C and 5% CO2 in DMEM:F12 medium with 10% FBS under normoxic atmosphere or exposed to hypoxia (1% O2 and 5% CO2) as previously described (24, 25, Supplementary Methods). In some experiments, conditioned media from GAS6-expressing HEK293 cells (100 ng GAS6/ml) or from control HEK293 pcDNA3-transfected cells was added to cultured mouse hepatocytes (26). Cell survival was measured by MTT assay and trypan blue exclusion.
Western Blotting and nuclear extracts preparation
Cell and nuclear extracts were prepared as previously described (27) and protein levels analyzed with specific antibodies (Supplementary Methods).
Statistical analyses
Results were expressed as mean ± standard deviation with the number of individual experiments detailed in Figure legends. Statistical significance was established by One-way ANOVA followed by Dunnett's and Tukey-Kramer as post-hoc tests. Animal survival was evaluated using the Kaplan-Meier method and compared using the log rank test.
Results
I/R decreases hepatic GAS6 mRNA levels while increasing the presence of GAS6 in serum
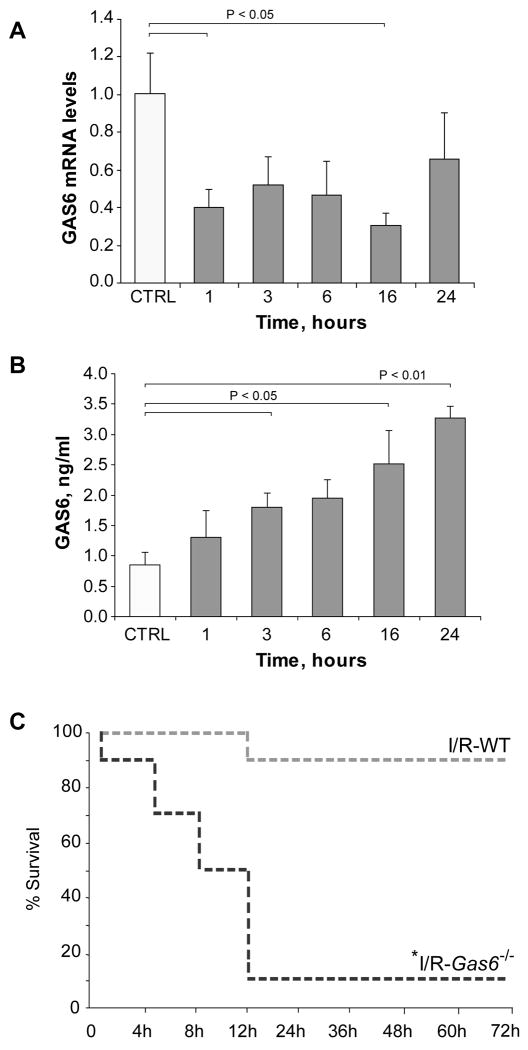
We evaluated if I/R modulates hepatic GAS6 homeostasis in wild type C57BL/6 mice subjected to partial ischemia for 90 minutes, assessing the content of GAS6 mRNA and GAS6 levels in serum after different times of reperfusion. As shown, GAS6 mRNA levels determined in liver biopsies fell early after reperfusion, with levels remaining below control up to 16 hours post-reperfusion (Fig. 1A). In contrast, ELISA analyses of serum indicated a time-dependent increase in the levels of GAS6 detected as soon as 3 hours post-reperfusion, remaining above control for 24 hours after reperfusion (Fig. 1B). Although this model of partial I/R typically results in maximal liver damage between 4-8 hours post-reperfusion, increased serum ALT levels were already detected as soon as 1 hour after reperfusion (659±284 U/ml), coinciding with the decrease in hepatic GAS6 mRNA levels and the initiation of the progressive increase observed in GAS6 serum levels. Thus, GAS6 homeostasis is regulated during hepatic I/R.
Figure 1. Hepatic GAS6 regulation and susceptibility of Gas6-/- mice to partial hepatic I/R.
A, GAS6 mRNA levels in liver biopsies from mice subjected to 90 minutes of liver ischemia and different times of reperfusion were measured by real time PCR, using β-actin as a control (n = 3). B, Serum samples from ischemic mice were collected after different times of reperfusion and GAS6 protein content measured using an ELISA kit. C, Wild-type or Gas6-/- mice were subjected to liver ischemia during 90 minutes and monitored for animal survival for 7 days. No changes in animal living status were observed after day three. *, P=0.008 vs. wild-type I/R-exposed mice by log rank test, n=10 in each group.
Partial hepatic I/R is lethal in GAS6 knockout mice due to massive hepatocellular damage and proinflammatory state
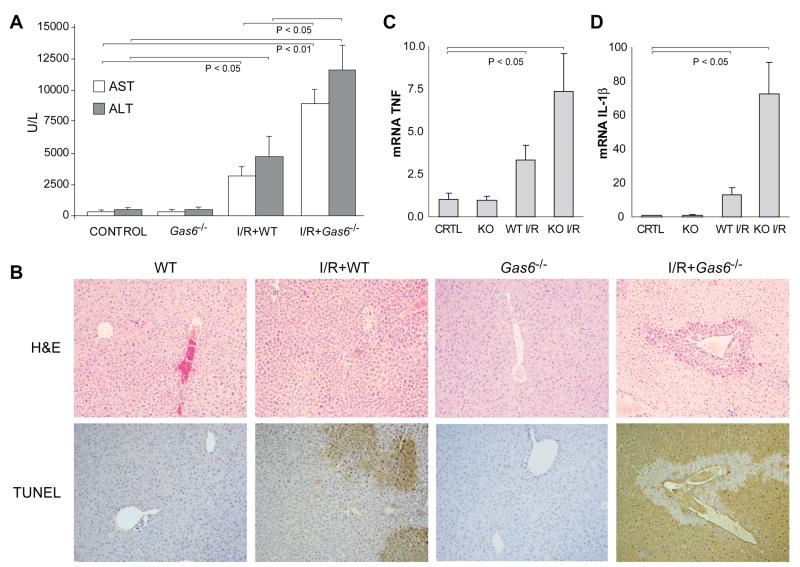
The model of partial hepatic I/R follows a typical time dependent pattern characterized by an initial tissue damage, which then resolves by 24-48 hours due to liver regeneration. In order to critically assess the role of GAS6 during I/R-mediated liver injury, we generated Gas6-/- mice as previously described (20), which exhibit abnormalities in platelet function, erythropoiesis and endothelial activation but normal liver morphology and TAM receptors expression. Interestingly, 9 out of 10 Gas6-/- mice died within the first 12 hours of reperfusion, with 50% of animals dying before 8 hours post-reperfusion. In contrast, 90% of wild type mice survived partial I/R (Fig. 1C), in line with our previous studies (23). The cause of death in Gas6-/- mice during hepatic I/R was most likely related to liver failure due to massive hepatocellular damage, as serum transaminase levels 6 hours post-reperfusion were dramatically elevated in GAS6-deficient mice compared to wild type mice (Fig. 2A). Moreover, this outcome mirrored histology findings revealing a severe deterioration of the liver parenchyma after I/R exposure in GAS6-deficient mice with respect to wild type mice (Fig. 2B). In addition, parallel liver sections from Gas6-/- mice undergoing I/R displayed extensive cell death detected by TUNEL staining affecting areas all over the hepatic parenchyma, which contrasts with the confined TUNEL-positive areas observed in wild type mice (Fig. 2B). Finally, we examined whether GAS6 regulates inflammatory mediators during I/R as previously proposed (20-21). Interestingly, TNF and IL-1β mRNA levels were markedly elevated in the null mice compared to wild type animals exposed to I/R (Fig. 2C, D) after 6 hours of reperfusion, suggesting that GAS6 counterbalances the inflammatory response after hepatic ischemia. Thus, overall these findings clearly demonstrate that the lack of GAS6 sensitizes the liver to I/R, resulting in massive hepatic destruction incompatible with life.
Figure 2. GAS6-deficient mice display severe liver damage after I/R exposure.
A, Wild-type or Gas6-/- mice were subjected to liver ischemia during 90 minutes and transaminases in serum samples were measured after 6 hours of reperfusion (n = 3-4). B, Representative images of H&E and TUNEL staining of liver biopsies from I/R-treated WT and Gas6-/- mice after 6 hours of reperfusion. C and D, mRNA levels of pro-inflammatory cytokines (TNF and IL-1β) were detected by qRT-PCR in liver samples after 6 hours of reperfusion (n = 6).
GAS6-deficient mice exhibit defective hepatic I/R-induced Mer signaling and AKT phosphorylation
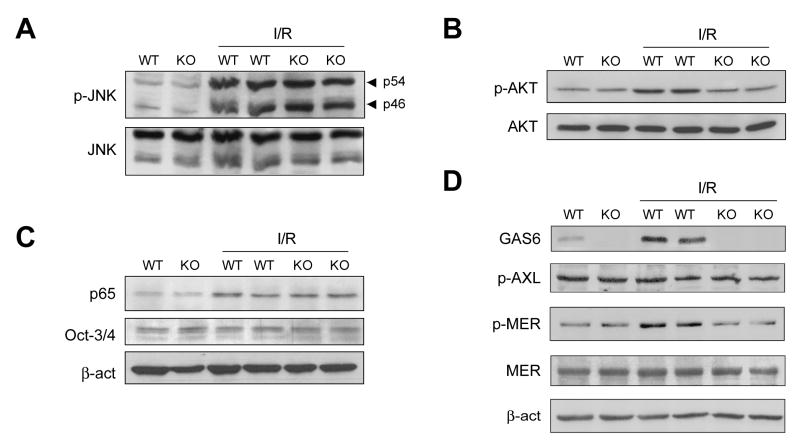
Although massive liver injury in GAS6-null mice was evident 3-6 hours post reperfusion, a significant increase in ALT levels was already detected as soon as 1 hour after ischemia compared to wild type mice (Gas6-/- mice 1835±893 U/ml vs. wild type mice 695±248 U/ml). Therefore, we next evaluated early changes in hepatic signaling responsible for the hepatic sensitization to I/R observed in Gas6-/- mice, focusing particularly in protective/apoptotic pathways. Since JNK activation has been shown to contribute to hepatic I/R injury, we analyzed the phosphorylation state of JNK 60 minutes post-reperfusion. The robust phosphorylation of JNK p46 and p54 isoforms observed after reperfusion was comparable in the liver of both wild type and GAS6 null mice (Fig 3A), discarding this particular pathway in the sensitization of GAS6 knockout mice to hepatic I/R. Since AKT regulates cell survival, we examined the phosphorylation state of AKT during hepatic I/R. In contrast to JNK activation, hepatic AKT phosphorylation was clearly reduced in Gas6-/- mice compared to I/R-exposed wild type mice (Fig. 3B). In addition to AKT, NF-κB regulates hepatocellular susceptibility to I/R (28) and hence we compared the extent of activation of NF-κB in wild type and GAS6-deficient mice. In contrast to AKT, the translocation of p65 to hepatic nuclear extracts after 60 minutes of reperfusion was similar in wild type and GAS6 null mice (Fig. 3C).
Figure 3. Impaired hepatic AKT activation and Mer phosphorylation in GAS6-deficient mice during I/R.
Wild-type or Gas6-/- mice were subjected to hepatic ischemia for 90 minutes and liver protein extracts were obtained for western analysis after 60 minutes of reperfusion. A, JNK activation, B, AKT phosphorylation, C, NF-κB nuclear p65 translocation, and D, GAS6 and phosphorylation of Axl and Mer were analyzed. Representative blots of two independent experiments are shown.
Since GAS6 serum concentration increases after I/R, we evaluated if ischemia stimulates GAS6 signaling through activation of TAM receptors. First, GAS6 protein levels augmented in liver extracts from wild type-I/R exposed animals (Figure 3D) and, as expected, these changes were undetectable in GAS6-deficient mice. Axl and Mer are TAM receptors located in liver cells that are phosphorylated after GAS6 binding. Therefore, we decided to verify their participation in I/R-induced TAM signaling. While no changes in Axl activation were evident after I/R, an increase in Mer phosphorylation was detected in wild type mice exposed to I/R, but this response was blunted in GAS-6 knockout mice (Figure 3D). Hence, our data indicate that GAS6 levels increase in the liver after I/R inducing Mer-dependent signaling and AKT phosphorylation, independently of NF-κB activation. The lack of these events in GAS6-knockout mice may contribute to their susceptibility to hepatic I/R injury.
GAS6 supplementation protects primary mouse hepatocytes from hypoxia-induced cell death
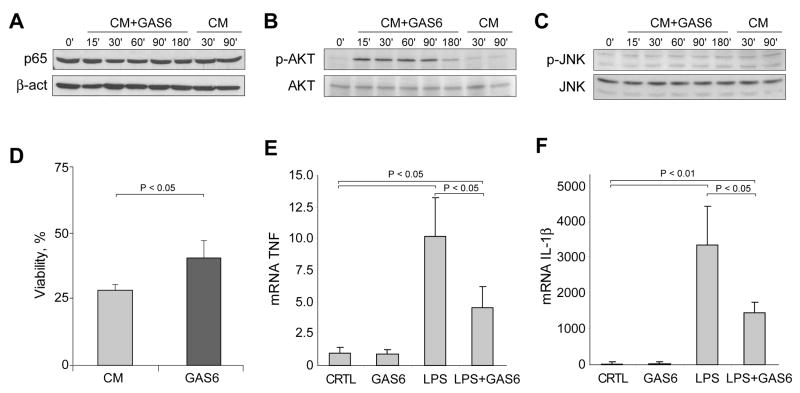
In view of the preceding findings, we extended the in vivo observations to cultured hepatocytes and examined whether exogenous administration of GAS6 directly regulates AKT phosphorylation and hypoxia susceptibility. First, we analyzed NF-κB activation after the addition of preconditioned media from GAS6-overexpressing HEK293 cells to primary mouse hepatocytes. As seen, GAS6 supplementation did not change the presence of p65 nuclear levels in cultured mouse hepatocytes (Fig. 4A). However, a marked increase in AKT phosphorylation was detected after the addition of GAS6-containing medium. As soon as 15 minutes after administration of GAS6 conditioned medium, primary hepatocytes displayed a robust AKT phosphorylation (Fig. 4B). Moreover, in accordance with the in vivo findings, no changes in JNK activation were observed after hepatocyte incubation with conditioned medium containing GAS6 (Fig. 4C). These findings confirm that parenchymal cells are targets of GAS6 that translates in AKT phosphorylation regardless of p65 nuclear translocation, supporting a similar mechanism in vivo following I/R.
Figure 4. GAS6 induces AKT activation in primary mouse hepatocytes and protects against oxygen deprivation.
A, Cultured mouse hepatocytes were treated with medium enriched in GAS6 (CM+GAS6) or control medium (CM) and cell extracts were prepared for western analysis at different times. A, NF-κB nuclear translocation, B, AKT phosphorylation, and C, JNK activation were analyzed. Representative blots of three independent experiments are shown. D, Cell viability of primary hepatocytes exposed for 24 hours to hypoxia (1% O2) in the presence or absence of GAS6-enriched medium (n = 3). E and F, the effect of GAS6 preincubation (20 minutes) on cytokine production (TNF and IL-1β) of RAW264.7 cells treated with LPS (50 ng/ml) was analyzed after 2 hours (n = 4).
To verify if the signaling effects induced by GAS6 administration could have any protective effect against oxygen deprivation, primary mouse hepatocytes exposed to hypoxia (1% O2) were preincubated with conditioned medium containing or not GAS6. First, we verified if hypoxia activated HIF-1α, a known target of oxygen deprivation. Consistent with previous findings (24), the nuclear levels of HIF-1α increased in hepatocytes cultured at 1% O2 (not shown). Interestingly, GAS6 supplementation protected cultured hepatocytes against hypoxia-induced cell death (25±4% survival in control cells vs. 40±5% in GAS6 supplemented cells) (Fig. 4D). Thus, these in vitro findings further confirm the link between GAS6 and AKT signaling observed in vivo during I/R, and establish a potential protective mechanism against hypoxia. Therefore, our work suggests that defective AKT activation in Gas6-/- mice may contribute to the sensitivity of the liver to I/R. The identification of other intracellular mechanisms which may play a relevant role in the signaling triggered by GAS6 downstream of AKT in hepatic I/R deserves further investigation. In this regard, GAS6 has been shown to activate FOXO1a in cultured endothelial cells (29).
Moreover, as GAS6 has been shown to reduce LPS-induced inflammatory cytokine release in human monocytes (21) or in murine Sertoli cells (30), and since the LPS/TLR pathway is increasingly recognized as an important contributing mechanism in I/R-induced liver injury (32), we next decided to verify if this mechanism can also modulate the response of murine macrophages after LPS challenging. As shown, RAW264.7 macrophages highly increased TNF and IL-1β mRNAs after LPS treatment and this response was significantly reduced by GAS6 (Figure 4E). Hence, these findings indicate that the intrahepatic increase of GAS6 after I/R restrains the overgeneration of inflammatory cytokines, and that the lack of this pathway in the absence of GAS6 further contributes to the sensitization to I/R-induced liver damage.
Recombinant GAS6 protects GAS6-deficient mice against liver I/R damage
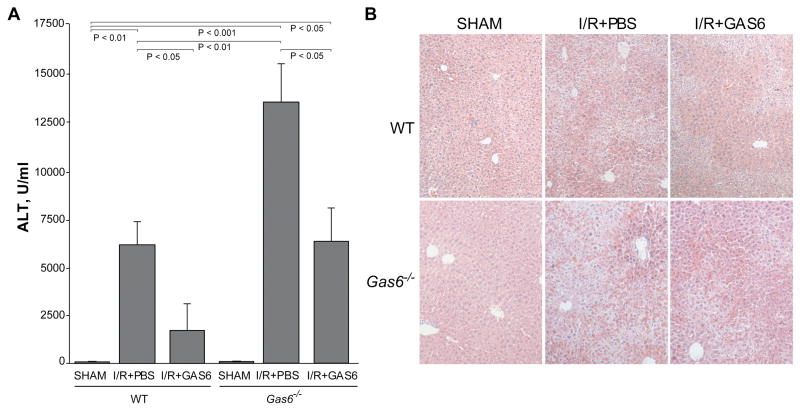
We next evaluated whether the severe liver injury of Gas6-/- mice after I/R could be prevented by recombinant GAS6 administration. GAS6-deficient mice were intravenously injected with a commercial mouse recombinant protein (5 μg/mice) before being subjected to partial ischemia. Remarkably, Gas6-/- mice that received recombinant GAS6 protein 15-20 minutes before ischemia displayed reduced liver damage that was comparable to the injury seen in wild type mice as reflected by the lower ALT/AST concentration detected in serum (Fig. 5A and Suppl. Fig. 1). Of note, doses of recombinant GAS6 above 5 μg/mice (up to 10 μg/mice) exerted a similar protective effect against I/R (not shown), and GAS even at doses ten times lower (0.5 μg/mice) was able to induce liver protection, but to a lesser extent (Suppl. Fig. 2). In parallel with the transaminase levels, liver biopsies from GAS6-injected knockout mice displayed preserved parenchymal architecture and organization with lesser areas of hepatocellular damage as seen by H&E staining (Fig. 5B). Moreover, TNF and IL-1β expression after I/R were repressed at mRNA levels by GAS6 administration both to wild-type and null mice (Suppl. Fig. 3). Thus, these results confirm that the sensitivity of Gas6-/- mice to hepatic I/R injury was due to the lack of expression of GAS6 and not due to other previously unnoticed phenotypic changes. Importantly, when recombinant GAS6 was administered to wild type mice, preservation of liver structure and significant prevention of hepatic injury after I/R was observed in GAS6-injected wild type mice, as measured by transaminase levels and H&E (Fig. 5A, B). These results are indicative that not only GAS6 deficiency sensitizes to I/R-induced liver damage, but also that the elevation of serum GAS6 levels is a protective strategy against liver I/R injury.
Figure 5. GAS6 administration attenuates hepatic I/R injury in Gas6-/- mice and wild-type mice.
GAS6 or vehicle (PBS) was intravenously injected to wild-type or Gas6-/- mice subjected to 90 minutes of liver ischemia. A, After 6 hours of reperfusion ALT serum transaminases were measured in blood samples (n = 3-6). B, Representative H&E staining of liver biopsies obtained after sham-operation or 6 hours of reperfusion from wild-type or Gas6-/- mice that received i.v. injection of GAS6 solution (5 μg/mouse) or vehicle (PBS).
Discussion
Our findings disclose a novel role for GAS6 in the hepatocellular defense from hypoxia and I/R, expanding the biological facets described for this atypical member of the vitamin K-dependent family with negligible anticoagulant function but recognized involvement in cancer, inflammation and in the regulation of liver regeneration and fibrosis (1, 2, 5, 6). Specifically, we show that GAS6 is dispensable for the activation of JNK and NF-κB during hepatic I/R, but required for efficient AKT phosphorylation in hepatocytes, in agreement with previous findings reported in other cell types (3). In addition to the increased susceptibility to hepatic I/R injury, GAS6 null mice exhibit enhanced expression of IL-1β and TNF, suggesting that GAS6 may mediate suppression of liver inflammation during I/R. These findings are in line with previously reported data in human and murine monocytes/macropages (20, 21, 32) or Sertoli cells (30), via regulation of TAM receptor signaling (1). Together, our findings indicate that the susceptibility to hepatic I/R injury in the absence of GAS6 reflects the combination of the inability to turn on survival pathways dependent on AKT activation and the overstimulation of inflammatory cytokines. Interestingly, this hepatoprotective role displayed by GAS6 against I/R contrasts with findings in mouse heart subjected to warm ischemia, where GAS6 promoted graft destruction by enhancing interactions between endothelial cells, platelets and leukocytes (33), exemplifying the importance of organ-dependent mechanisms in GAS6 signaling.
The outcome of GAS6 null mice following hepatic I/R is reversible upon addition of GAS6, suggesting the maintenance of TAM receptors in the null mice and the functional interaction of GAS6 with TAM receptors. Among TAM receptors, most of the protective effects of GAS6 described in different cell types are due to the binding of GAS6 to Axl (34-37). In contrast to these findings, Axl seems to play a minor role in hepatic I/R as increased Axl phosphorylation was not detected after I/R exposure. Rather, Mer becomes phosphorylated in wild type mice following I/R, and this response is blunted in GAS6 knockout mice. Of note, recent data in differentiated monocytes and macrophages have shown that GAS6 inhibited TNF and IL-1β stimulation in response to LPS via Mer activation (21). Moreover, the binding of GAS6 to Mer but not to Axl or Tyro3 resulted in the activation of AKT via GSK3β phosphorylation, which in turn prevented NF-κB activation. Together, these data and the present study expand the biological impact of GAS6-Mer interaction eliciting a combined anti-inflammatory and survival pathway to protect against hepatic I/R. Although we did not examine the involvement of GSK3β in the protective action of GAS6 in hepatic I/R, we did observe that GAS6 is dispensable for NF-κB activation in normothermic I/R.
Consistent with a protective role for GAS6 during hepatic I/R, the serum levels of GAS6 increase early after I/R, which paralleled the upregulation of GAS6 in hepatic extracts. Recent findings in liver regeneration and chemical-induced liver damage indicated a predominant expression of GAS6 in Kupffer cells and hepatic stellate cells (5, 6). Although we did not estimate the relative contribute of these putative sources of GAS6 during I/R, GAS6 reproduced the anti-inflammatory effect in downregulating TNF and IL-1β in RAW264.7 macrophages, a surrogate cell line for Kupffer cells. In this scenario, it would be tempting to speculate that GAS6 derived from hepatic macrophages initiates a paracrine signaling event via Mer in hepatocytes to activate protective and anti-inflammatory pathways of relevance in hepatic I/R.
In summary, our work identifies GAS6 as a survival factor released during hepatic I/R damage, which protects the hepatocyte from oxygen deprivation and reduces inflammatory cytokine production. Quite interestingly, we observe that GAS6 not only rescued null mice from I/R-mediated liver injury but it also proved useful in protecting wild type mice against hepatic I/R damage. Given the broad implications of hepatic I/R injury, GAS6 emerges as a novel pharmacological therapy of potential relevance in different clinical settings.
Supplementary Material
Acknowledgments
The technical assistance of Susana Nuñez and Anghara Menendez is highly appreciated.
Grant support: The work presented was supported by the CIBEREHD and by grants FIS09/00056, FIS07/0193, SAF2009-11417, SAF2008-02199, SAF2006-06780, SAF2004-07539 and BFU2007-61699/BFI from the Instituto de Salud Carlos III and Ministry of Science and Innovation from Spain, and grant P50 AA 11999 from the Research Center for Liver and Pancreatic Diseases, funded by the US National Institute on Alcohol Abuse and Alcoholism.
References
- 1.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellido-Martin L, de Frutos PG. Vitamin K-dependent actions of Gas6. Vitam Horm. 2008;78:185–209. doi: 10.1016/S0083-6729(07)00009-X. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Fernandez L, Bellido-Martin L, Garcia de Frutos P. Growth arrest-specific gene 6 (GAS6). An outline of its role in haemostasis and inflammation. Thromb Haemost. 2008;100:604–610. doi: 10.1160/th08-04-0253. [DOI] [PubMed] [Google Scholar]
- 5.Couchie D, Lafdil F, Martin-Garcia N, Laperche Y, Zafrani ES, Mavier P. Expression and role of Gas6 protein and of its receptor Axl in hepatic regeneration from oval cells in the rat. Gastroenterology. 2005;129:1633–1642. doi: 10.1053/j.gastro.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Lafdil F, Chobert MN, Couchie D, Brouillet A, Zafrani ES, Mavier P, Laperche Y. Induction of Gas6 protein in CCl4-induced rat liver injury and anti-apoptotic effect on hepatic stellate cells. Hepatology. 2006;44:228–239. doi: 10.1002/hep.21237. [DOI] [PubMed] [Google Scholar]
- 7.Lafdil F, Chobert MN, Deveaux V, Zafrani ES, Mavier P, Nakano T, Laperche Y, et al. Growth arrest-specific protein 6 deficiency impairs liver tissue repair after acute toxic hepatitis in mice. J Hepatol. 2009;51:55–66. doi: 10.1016/j.jhep.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Gustafsson A, Martuszewska D, Johansson M, Ekman C, Hafizi S, Ljungberg B, Dahlback B. Differential expression of Axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin Cancer Res. 2009;15:4742–4749. doi: 10.1158/1078-0432.CCR-08-2514. [DOI] [PubMed] [Google Scholar]
- 9.Hutterer M, Knyazev P, Abate A, Reschke M, Maier H, Stefanova N, Knyazeva T, et al. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14:130–138. doi: 10.1158/1078-0432.CCR-07-0862. [DOI] [PubMed] [Google Scholar]
- 10.Loges S, Schmidt T, Tjwa M, Van Geyte K, Lievens D, Lutgens E, Vanhoutte D, et al. Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood. 2009 doi: 10.1182/blood-2009-06-228684. [DOI] [PubMed] [Google Scholar]
- 11.Jiang L, Liu CY, Yang QF, Wang P, Zhang W. Plasma level of growth arrest-specific 6 (GAS6) protein and genetic variations in the GAS6 gene in patients with acute coronary syndrome. Am J Clin Pathol. 2009;131:738–743. doi: 10.1309/AJCP3CX3AUVRBHCF. [DOI] [PubMed] [Google Scholar]
- 12.Sainaghi PP, Alciato F, Carnieletto S, Castello L, Bergamasco L, Sola D, Bongo AS, et al. Gas6 evaluation in patients with acute dyspnea due to suspected pulmonary embolism. Respir Med. 2009;103:589–594. doi: 10.1016/j.rmed.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Uehara S, Handa H, Gotoh K, Tomita H, Sennshuu M. Plasma concentrations of growth arrest-specific protein 6 and protein S in patients with acute pancreatitis. J Gastroenterol Hepatol. 2009;24:1567–1573. doi: 10.1111/j.1440-1746.2009.05875.x. [DOI] [PubMed] [Google Scholar]
- 14.Borgel D, Clauser S, Bornstain C, Bieche I, Bissery A, Remones V, Fagon JY, et al. Elevated growth-arrest-specific protein 6 plasma levels in patients with severe sepsis. Crit Care Med. 2006;34:219–222. doi: 10.1097/01.ccm.0000195014.56254.8a. [DOI] [PubMed] [Google Scholar]
- 15.Gibot S, Massin F, Cravoisy A, Dupays R, Barraud D, Nace L, Bollaert PE. Growth arrest-specific protein 6 plasma concentrations during septic shock. Crit Care. 2007;11:R8. doi: 10.1186/cc5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellosta P, Zhang Q, Goff SP, Basilico C. Signaling through the ARK tyrosine kinase receptor protects from apoptosis in the absence of growth stimulation. Oncogene. 1997;15:2387–2397. doi: 10.1038/sj.onc.1201419. [DOI] [PubMed] [Google Scholar]
- 17.Goruppi S, Ruaro E, Varnum B, Schneider C. Requirement of phosphatidylinositol 3-kinase-dependent pathway and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17:4442–4453. doi: 10.1128/mcb.17.8.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasanbasic I, Cuerquis J, Varnum B, Blostein MD. Intracellular signaling pathways involved in Gas6-Axl-mediated survival of endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H1207–1213. doi: 10.1152/ajpheart.00020.2004. [DOI] [PubMed] [Google Scholar]
- 19.Valverde P, Obin MS, Taylor A. Role of Gas6/Axl signaling in lens epithelial cell proliferation and survival. Exp Eye Res. 2004;78:27–37. doi: 10.1016/j.exer.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 21.Alciato F, Sainaghi PP, Sola D, Castello L, Avanzi GC. TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J Leukoc Biol. 2010;87:869–875. doi: 10.1189/jlb.0909610. [DOI] [PubMed] [Google Scholar]
- 22.Angelillo-Scherrer A, de Frutos P, Aparicio C, Melis E, Savi P, Lupu F, Arnout J, et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7:215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 23.Llacuna L, Mari M, Garcia-Ruiz C, Fernandez-Checa JC, Morales A. Critical role of acidic sphingomyelinase in murine hepatic ischemia-reperfusion injury. Hepatology. 2006;44:561–572. doi: 10.1002/hep.21285. [DOI] [PubMed] [Google Scholar]
- 24.Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer Res. 2007;67:7368–7377. doi: 10.1158/0008-5472.CAN-07-0515. [DOI] [PubMed] [Google Scholar]
- 25.Lluis JM, Llacuna L, von Montfort C, Barcena C, Enrich C, Morales A, Fernandez-Checa JC. GD3 synthase overexpression sensitizes hepatocarcinoma cells to hypoxia and reduces tumor growth by suppressing the cSrc/NF-kappaB survival pathway. PLoS One. 2009;4:e8059. doi: 10.1371/journal.pone.0008059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyberg P, Dahlback B, Garcia de Frutos P. The SHBG-like region of protein S is crucial for factor V-dependent APC-cofactor function. FEBS Lett. 1998;433:28–32. doi: 10.1016/s0014-5793(98)00877-1. [DOI] [PubMed] [Google Scholar]
- 27.Morales A, Miranda M, Sanchez-Reyes A, Colell A, Biete A, Fernandez-Checa JC. Transcriptional regulation of the heavy subunit chain of gamma-glutamylcysteine synthetase by ionizing radiation. FEBS Lett. 1998;427:15–20. doi: 10.1016/s0014-5793(98)00381-0. [DOI] [PubMed] [Google Scholar]
- 28.Llacuna L, Mari M, Lluis JM, Garcia-Ruiz C, Fernandez-Checa JC, Morales A. Reactive oxygen species mediate liver injury through parenchymal nuclear factor-kappaB inactivation in prolonged ischemia/reperfusion. Am J Pathol. 2009;174:1776–1785. doi: 10.2353/ajpath.2009.080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganopolsky JG, Abid MR, Aird WC, Blostein MD. GAS6-induced signaling in human endothelial cells is mediated by FOXO1a. J Thromb Haemost. 2008;6:1804–1811. doi: 10.1111/j.1538-7836.2008.03114.x. [DOI] [PubMed] [Google Scholar]
- 30.Sun B, Qi N, Shang T, Wu H, Deng T, Han D. Sertoli cell-initiated testicular innate immune response through toll-like receptor-3 activation is negatively regulated by Tyro3, Axl, and mer receptors. Endocrinology. 2010;151:2886–2897. doi: 10.1210/en.2009-1498. [DOI] [PubMed] [Google Scholar]
- 31.Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32:4–16. doi: 10.1097/SHK.0b013e318193e333. [DOI] [PubMed] [Google Scholar]
- 32.Sharif MN, Sosic D, Rothlin CV, Kelly E, Lemke G, Olson EN, Ivashkiv LB. Twist mediates suppression of inflammation by type I IFNs and Axl. J Exp Med. 2006;203:1891–1901. doi: 10.1084/jem.20051725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tjwa M, Bellido-Martin L, Lin Y, Lutgens E, Plaisance S, Bono F, Delesque-Touchard N, et al. Gas6 promotes inflammation by enhancing interactions between endothelial cells, platelets, and leukocytes. Blood. 2008;111:4096–4105. doi: 10.1182/blood-2007-05-089565. [DOI] [PubMed] [Google Scholar]
- 34.Sainaghi PP, Castello L, Bergamasco L, Galletti M, Bellosta P, Avanzi GC. Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. J Cell Physiol. 2005;204:36–44. doi: 10.1002/jcp.20265. [DOI] [PubMed] [Google Scholar]
- 35.Sawabu T, Seno H, Kawashima T, Fukuda A, Uenoyama Y, Kawada M, Kanda N, et al. Growth arrest-specific gene 6 and Axl signaling enhances gastric cancer cell survival via Akt pathway. Mol Carcinog. 2007;46:155–164. doi: 10.1002/mc.20211. [DOI] [PubMed] [Google Scholar]
- 36.Shankar SL, O'Guin K, Kim M, Varnum B, Lemke G, Brosnan CF, Shafit-Zagardo B. Gas6/Axl signaling activates the phosphatidylinositol 3-kinase/Akt1 survival pathway to protect oligodendrocytes from tumor necrosis factor alpha-induced apoptosis. J Neurosci. 2006;26:5638–5648. doi: 10.1523/JNEUROSCI.5063-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varnum BC, Young C, Elliott G, Garcia A, Bartley TD, Fridell YW, Hunt RW, et al. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature. 1995;373:623–626. doi: 10.1038/373623a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.