Abstract
We evaluated two nonionic surfactants, one hydrophobic (Brij 30) and one hydrophilic (C12E8), for their ability to enhance the biodegradation of polycyclic aromatic hydrocarbons (PAHs) in contaminated soil after it had been treated in an aerobic bioreactor. The effects of each surfactant were evaluated at doses corresponding to equilibrium aqueous-phase concentrations well above the surfactant’s critical micelle concentration (CMC), slightly above the CMC, and below the CMC. The concentrations of all 3- and 4-ring PAHs were significantly lower in the soil amended with Brij 30 at the two lower doses compared to controls, whereas removal of only the 3-ring PAHs was significantly enhanced at the highest Brij 30 dose. In contrast, C12E8 did not enhance PAH removal at any dose. In the absence of surfactant, <5% of any PAH desorbed from the soil over an 18-d period. Brij 30 addition at the lowest dose significantly increased the desorption of most PAHs, whereas the addition of C12E8 at the lowest dose actually decreased the desorption of all PAHs. These findings suggest that the effects of the two surfactants on PAH biodegradation could be explained by their effects on PAH bioavailability. Overall, this study demonstrates that the properties of the surfactant and its dose relative to the corresponding aqueous-phase concentration are important factors in designing systems for surfactant-enhanced bioremediation of PAH-contaminated soils in which PAH bioavailability is limited.
Introduction
Bioremediation has attracted great interest for treatment of soils and sediments contaminated with polycyclic aromatic hydrocarbons (PAHs). However, bioremediation can be limited by the bioavailability of soil-bound PAHs due to their low aqueous solubility and strong sorption to soil, which is exacerbated by the long aging of contaminants in field-contaminated soils (1, 2). Strategies to improve bioavailability can enhance the biodegradation of PAHs (3, 4), although the biodegradation of high-molecular-weight (HMW) PAHs can be limited by factors other than bioavailability as well (5, 6).
The use of surfactants has been proposed as a strategy to improve the bioavailability of hydrophobic organic compounds (HOCs) in soil (7–11). Surfactants can greatly enhance the apparent solubility of an otherwise poorly soluble HOC by partitioning of the HOC into micelles, which occurs only if the aqueous-phase concentration of the surfactant is above its critical micelle concentration (CMC). Micellar solubilization of PAHs from field-contaminated soils by surfactant addition has been evaluated in several previous studies (12–14). The extensive sorption of surfactants to soil, however, leads to a substantially higher surfactant dose to reach the CMC in a soil/water system than in the absence of soil (9, 15–18). In addition, surfactants can have several deleterious effects on biodegradation processes, including toxicity due to disruption of the bacterial cell membrane (19); accumulation of inhibitory products of incomplete metabolism due to the substantial increase of substrate availability by solubilization (20, 21); and competitive degradation of surfactant and contaminants (8, 9, 22, 23).
There has been limited previous work on the effects of surfactants on desorption or biodegradation of PAHs in field-contaminated soil (for the purposes of subsequent discussion, we distinguish “solubilization” as PAH partitioning into micelles and “desorption” as a mechanism of PAH release from soil that occurs in the absence of micelles). Yeom et al. (12) studied the kinetics of PAH release from a field-contaminated soil upon surfactant addition and suggested that the enhancement of PAH release occurred by both micellar solubilization and increased matrix diffusivity of PAHs. Others have reported that surfactant addition had either no effect or a negative effect on biodegradation of PAHs in field-contaminated soil (7, 9, 10, 24). There has been even less work on surfactant addition at doses corresponding to aqueous-phase surfactant concentrations below the CMC, where solubilization becomes insignificant. Grasso et al. (13) observed that desorption of PAHs from field-contaminated soil was negligible when the aqueous-phase concentration of a nonionic surfactant was below or close to the CMC, which is in contrast to the results obtained by Yeom et al. (12). It is important to consider the potential efficacy of adding surfactants at such low doses because of the cost implications in field applications.
The objective of the present study was to compare two nonionic, ethoxylated surfactants of differing hydrophobicity (Brij 30, which is relatively hydrophobic, and C12E8, which is relatively hydrophilic) with respect to desorption and biodegradation of the residual PAHs in the effluent from a slurry-phase bioreactor treating soil from a former manufactured gas plant (MGP) site. Each surfactant was evaluated at doses corresponding to aqueous-phase concentrations above and below the CMC. We focused on surfactant addition after biological treatment because surfactant addition should be necessary only when biodegradation is limited by mass transfer. To our knowledge, such a two-stage strategy (conventional biological treatment followed by surfactant addition and further treatment) has not been evaluated previously with a field-contaminated soil.
Materials and Methods
Chemicals
Tenax TA® (60/80 mesh) was obtained from Alltech (Deerfield, IL). Brij® 30 (polyoxyethylene (4) lauryl ether) and C12E8 (octaethylene glycol mono n-dodecyl ether) were purchased from Sigma-Aldrich (St. Louis, Missouri). The two surfactants have a common hydrocarbon chain length, differing only in the length of the polyethoxylate moiety. The hydrophile/lipophile balance (HLB) value of Brij 30 is 9.7 and the HLB of C12E8 is 13.1. The CMC of both Brij 30 and C12E8 in water is 10 mg/L, as measured by following surface tension as a function of concentration.
Samples
The soil used in this study was the effluent slurry from a laboratory-scale, aerobic bioreactor used to treat contaminated soil from a former MGP site in Charlotte, NC. Operation of the reactor is described elsewhere (25). Concentrations of PAHs in the feed (untreated) soil and the effluent slurry samples are shown in the Supporting Information (Table S1). The organic matter and black carbon content of the untreated soil (feed to the bioreactor) were each 10% (wt:wt) as determined by thermogravimetric methods (26). The total extractable organics were 1.1% in the untreated soil and 0.9% in the bioreactor slurry.
Sorption of Surfactant to Soil and Solubilization of PAHs
About 2 g of centrifuged slurry from the reactor was weighed into each of 16 centrifuge tubes. Surfactant at each of eight different doses was added to the centrifuge tubes in duplicate. The soil was resuspended in phosphate buffer (15 mM, pH 7.0) to make a slurry with a solids content of 10% (wt:wt). All tubes were shaken for 48 h, which was observed in preliminary experiments to be sufficient to reach sorption equilibrium (data not shown). After equilibration, each tube was centrifuged again and the supernatant was collected and syringe-filtered through a 0.8 μm pore-size polycarbonate filter. The surface tension of the filtrate was analyzed by a Du Nouy tensiometer (CSC Scientific Co., INC. Fairfax, VA) after necessary dilutions were made to get a final surface tension corresponding to a concentration below the CMC. The concentration of surfactant was calculated by using a calibration curve of surface tension vs. surfactant concentration. A 2-mL aliquot of the filtrate for each sample was diluted with acetonitrile to a final volume of 10 mL, and PAH concentrations were analyzed by HPLC as described previously (25).
Biodegradation Experiments
The effects of surfactant addition on PAH removal from freshly sampled reactor slurry were evaluated under five conditions: reactor slurry without surfactant addition, reactor slurry amended with surfactant at a low, medium, or high dose, and an inhibited control with surfactant added at the high dose. The doses selected for the two surfactants are listed in Table 1. For each condition, 6.35 mL of reactor slurry with a solids content of 10% was added into each of ten 40-mL glass centrifuge tubes. After centrifugation, a predetermined amount of surfactant stock solution in phosphate buffer corresponding to the selected dose was added to the soil pellet. Inhibited controls were prepared by adding 0.5 mL sodium azide solution (4.15 mg/mL) to each tube. The volume in each tube was brought up with fresh phosphate buffer to 6.35 mL to form a slurry of 10% solids. All 50 centrifuge tubes were closed by caps with Teflon-lined silicone septa and were shaken at 200 rpm at room temperature. The tubes were opened for 5 min every day to let in oxygen required for biodegradation. For each condition, five tubes were sacrificed at day 4 for PAH concentration analysis in both the liquid and solid phases. After the tubes were centrifuged at 3000 rpm for 20 min, the supernatant was decanted into a clean tube and filtered, and both the filtered supernatant and solid phase were analyzed for PAHs. The remaining five tubes of each condition were sacrificed at day 18 following the same procedure.
Table 1.
Doses of the two nonionic surfactants used in this study (mg surfactant/g dry soil) and their equilibrium aqueous-phase concentrations.
| Estimated Aqueous-Phase Concentrationa |
||
|---|---|---|
| Dose (mg/g) | (mg/L) | (×CMC) |
| Brij 30 | ||
| 0 | 0 | 0 |
| 5 | 8 | 0.8 |
| 20 | 24 | 2.4 |
| 50 | ≈1000 | ≈97 |
| C12E8 | ||
| 0 | 0 | 0 |
| 2 | 8 | 0.8 |
| 10 | 38 | 3.8 |
| 25 | ≈860 | ≈86 |
Estimated from data shown in Figure 1.
A multiple comparison of the final PAH concentrations under each condition was performed with Tukey’s test (27), using ProStat 4.02 (Poly Software International, Pearl River, NY).
PAH Desorption Experiments
Desorption of PAHs from the soil was measured using Tenax beads as an infinite sink for the desorbed PAHs (25); measurements in the presence of surfactant were evaluated at the low dose only because PAH micellar solubilization would have been insignificant at that dose. A 15-mL aliquot of reactor slurry was added into each of 24 glass centrifuge tubes. After centrifugation, a predetermined amount of surfactant stock solution was added to the soil pellet in 12 of the tubes. The other 12 tubes were not amended with surfactant to serve as controls. To inhibit PAH biodegradation, all 24 samples were amended with 0.5 mL sodium azide solution (4.15 mg/mL). The volume of the mixture in each tube was supplemented with phosphate buffer to obtain a slurry of 10% solids. All samples were closed by caps with Teflon-lined silicone septa and were shaken at 200 rpm at room temperature for 48 h to allow for sorption of the surfactant to the soil. Each sample was then amended with 0.08 g Tenax beads and shaking was resumed. At days 1, 4, 8 and 18 after adding Tenax beads, three tubes with surfactant and three tubes without surfactant were sacrificed for the removal and extraction of Tenax beads as described elsewhere (25) to quantify the cumulatively desorbed PAH mass.
Effect of Brij 30 Addition to the Original Contaminated Soil
A slurry of the original contaminated soil was prepared in buffer in the same manner used to feed the reactor. This slurry was centrifuged at 3000 rpm for 20 min. After removing aliquots of the soil pellet for PAH analysis and moisture content determination, an amount of slurry corresponding to 1 g dry weight of the centrifuged soil was added to each of eight glass centrifuge tubes. A 5 mg dose of Brij 30 was added to four of the tubes and the other four tubes were prepared as controls. Phosphate buffer was added to each tube to bring the volume up to 10 mL, and the tubes were incubated and shaken as described above. At day 18, all samples were subjected to solvent extraction for residual PAH concentration analysis.
Results
Surfactant Sorption and PAH Solubilization
The sorption of nonionic surfactants to soil usually follows the Langmuir isotherm (16, 28), which was observed for the sorption of both Brij 30 and C12E8 to the soil used in this study (Figure S1 in the Supporting Information). From regression of the Langmuir equation against the data, the maximum concentration of surfactant sorbed to the soil was 39 mg/g for Brij 30 and 16 mg/g for C12E8. Stronger sorption to the soil of Brij 30 than C12E8 is consistent with their respective HLB values.
At surfactant doses beyond that corresponding to maximum sorption to the soil, the aqueous-phase surfactant concentration and PAH solubilization both increased linearly with increasing dose (Figure 1). The data in Figure 1 were used to select a high, medium and low surfactant dose for the subsequent biodegradation experiments, corresponding to equilibrium aqueous-phase concentrations well above the CMC, slightly above the CMC, and below the CMC (Table 1).
Figure 1.
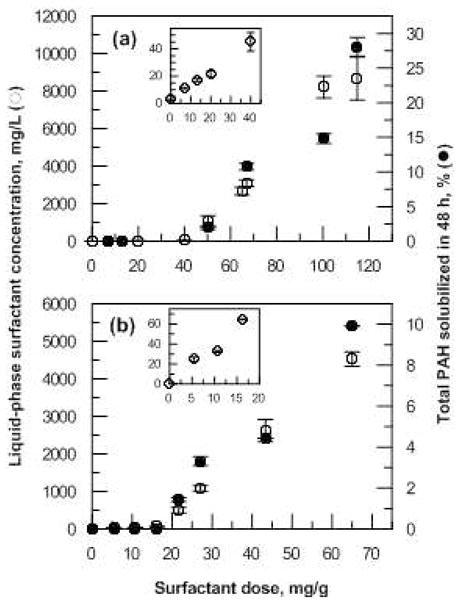
Micellar solubilization of PAHs and corresponding liquid-phase surfactant concentration as a function of surfactant dose added to treated soil from a slurry-phase bioreactor for (a) Brij 30 and (b) C12E8. Insets show liquid-phase surfactant concentration data at low surfactant doses.
Biodegradation Experiments
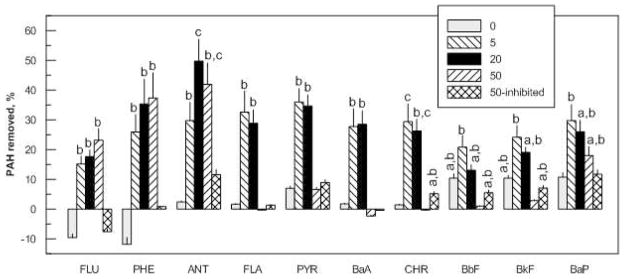
Biodegradation of residual PAHs in the treated soil from a slurry-phase bioreactor was evaluated at each of the selected doses for each surfactant. Incubation with Brij 30 for 18 d at all three doses improved the biodegradation of all 3-ring PAHs (Figure 2). The fractions of fluorene and phenanthrene removed were not significantly different for the three doses tested. For anthracene, the greatest biodegradation was observed with the medium dose, which corresponded to an aqueous-phase Brij 30 concentration of approximately twice the CMC. For the 4-ring PAHs, only the two lower doses of Brij 30 improved biodegradation, and the extents of improvement were similar between doses for a given PAH. Unlike the situation for the 3-ring PAHs, Brij 30 addition at the highest dose (corresponding to an aqueous-phase concentration of 100 times the CMC) did not improve the biodegradation of the 4-ring PAHs. Brij 30 addition generally did not improve the biodegradation of the 5- or 6-ring PAHs at any dose, except the lowest dose significantly increased benzo[a]pyrene (BaP) removal. The extent of removal without surfactant addition was not significantly different than for the inhibited control for any PAH, implying that PAH removal had already reached the maximum extent in the bioreactor.
Figure 2.
Effects of the surfactant Brij 30 on biodegradation of PAHs in treated soil from a slurry-phase bioreactor after incubation for 18 d. The legend indicates surfactant dose in mg/g dry soil, and “inhibited” refers to controls to which sodium azide was added. The lowest surfactant dose tested (5 mg/g) corresponded to an aqueous-phase concentration of Brij 30 below its CMC. The other two doses corresponded to Brij 30 concentrations slightly above (20 mg/g) or well above (50 mg/g) the CMC. PAH removal is relative to the initial concentration in the treated soil from the bioreactor. Final PAH concentrations in the soil for the various surfactant doses were compared using Tukey’s method; conditions for which there was not a significant difference (p > 0.05) are assigned the same letter. Bars for which no letter is shown are implicitly designated “a”. No significant differences among conditions were observed for naphthalene, dibenz[a,h]anthracene, and benzo[g,h,i]perylene (not shown). Data represent means and standard deviations of five replicates except for the 20 mg/g dose, for which there were four replicates. Abbreviations of PAHs are defined in Table S1 of the Supporting Information.
None of the PAHs was detected in the liquid phase of the reactor slurry without Brij 30 addition or with Brij 30 addition at the two lower doses (data not shown). Significant amounts of PAHs were present in the liquid phase at the high dose at the end of the 18-d incubation period (data not shown), indicating that some of the solubilized PAHs had not been biodegraded.
Although the addition of Brij 30 improved the biodegradation of many PAHs in the reactor slurry, the addition of C12E8 at all three doses had no effect on PAH biodegradation (Figure S2 in Supporting Information).
PAH Desorption
The lowest dose of Brij 30 used in the biodegradation experiment (5 mg/g) was below the dose required to reach the CMC in the liquid phase. Micellar solubilization of PAHs would, therefore, be insignificant at this dose. Because the lowest dose led to significant PAH removal, we evaluated its effect on PAH desorption from the soil. The amount of PAH desorbed from the soil amended with Brij 30 after 18 d of incubation was significantly greater than that from the soil without Brij 30 addition for all of the PAHs except naphthalene (Figure 3). In the presence of Brij 30, 15–20% of fluorene, phenanthrene, anthracene, fluoranthene, and pyrene desorbed over the 18-d incubation period.
Figure 3.
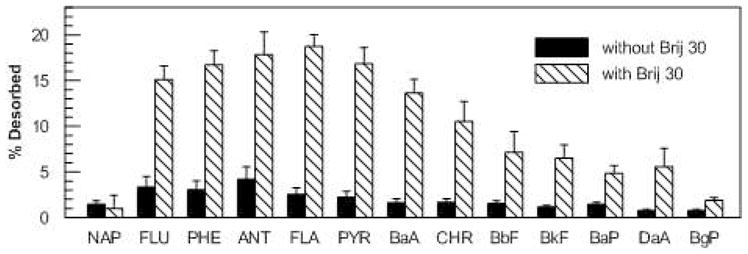
Cumulative desorption of PAHs from bioreactor-treated soil after 18 d in the absence of surfactant or in the presence of Brij 30 at a dose of 5 mg/g. Bars represent means and standard deviations of triplicates for incubations with Brij 30 and the mean and range of duplicates for incubations without Brij 30.
In contrast to results with Brij 30, less desorption was observed in the presence of C12E8 at the lowest dose than in its absence (not shown). No desorption was observed for naphthalene, acenaphthene, fluorene, anthracene, dibenzo[a,h]anthracene and indeno[c,d]pyrene, and < 2% of the initial mass desorbed over the 18-d incubation period for all of the other PAHs in the presence of C12E8.
Brij 30 addition to feed soil
Because Brij 30 enhanced the biodegradation of the residual PAHs from the bioreactor slurry at a dose as low as 5 mg/g, we wanted to know whether applying the same dose to the original contaminated soil (the feed soil for the bioreactor) for the same incubation time (18 d) would have a similar effect. The addition of 5 mg/g Brij 30 to the feed soil did not enhance PAH biodegradation compared to the controls without surfactant (Figure S3 in Supporting Information).
Discussion
For applications of surfactants in contaminated soil, sorption of the surfactant will directly influence the dose required to achieve appreciable solubilization of hydrophobic contaminants (15). Micellar solubilization can increase the rates of biodegradation of HOCs (29), but the effects of surfactants at doses insufficient to achieve contaminant solubilization have been less well-studied. It is important to understand such effects, because minimizing the surfactant dose that improves bioremediation will minimize the cost of treatment.
The hydrophobic, nonionic surfactant Brij 30 improved the biodegradation of the 3- and 4-ring PAHs in field-contaminated soil when added at the low and medium doses (5 and 20 mg/g, respectively), even though micellar solubilization of the PAHs would have been negligible at these doses (Figure 1). In contrast, the more hydrophilic surfactant C12E8 had no effect on PAH biodegradation, regardless of whether the added dose would have led to PAH solubilization.
The addition of Brij 30 at the highest dose (50 mg/g), at which the PAHs would have been solubilized (Figure 1), did not improve the degradation of the 4-ring PAHs. However, biodegradation of the 3-ring PAHs increased when Brij 30 was applied at the highest dose (Figure 2) and biodegradation of the 4-ring compounds was enhanced at the two lower doses. It is possible that partitioning of the 4-ring compounds into surfactant micelles influenced the kinetics of biodegradation (30), or that the increased PAH bioavailability caused by their solubilization led to metabolic intermediates or byproducts that could be toxic (20, 21, 31). For the latter mechanism, however, we would have expected inhibition of the 3-ring compounds as well. In a parallel study reported separately (32), Brij 30 at the highest dose reduced the concentrations of several groups of PAH-degrading bacteria, including known pyrene degraders, in the soil slurry relative to controls and to microcosms incubated with the two lower surfactant doses. It is possible, therefore, that the lack of influence on 4-ring PAH degradation by Brij 30 at the highest dose was related to its influence on the organisms able to degrade those compounds.
The effects of each surfactant on PAH biodegradation is consistent with their effects on PAH desorption, which was measured at the lowest surfactant dose only (i.e., under conditions in which partitioning into micelles would not have prevailed). Brij 30 increased PAH desorption (Figure 3), which is consistent with results from an earlier study on the effects of Brij 30 on desorption of phenanthrene from contaminated soil from an MGP site (12). In contrast, C12E8 inhibited PAH desorption. The minimal desorption (<5%) of residual PAHs in the reactor slurry in the absence of Brij 30 is consistent with previous desorption experiments on the same slurry (25), and suggests that the most readily bioavailable fractions of the PAHs had been removed during treatment in the bioreactor. In a comprehensive study on rates of PAH desorption from contaminated soil and sediment from MGP sites, Loehr et al (33) observed that the rapidly desorbing fraction corresponding to the most bioavailable fraction of PAHs typically desorbs within 7 to 12 d; our desorption measurements were carried out for 18 d. It appears, therefore, that Brij 30 enhanced PAH biodegradation at the two lowest doses primarily by enhancing their bioavailability to PAH-degrading bacteria in the soil slurry. Allan et al. (34) came to a similar conclusion for the effects of cyclodextrin on PAH biodegradation in field-contaminated soils from MGP sites.
The lowest dose of Brij 30 enhanced the desorption of the 5- and 6-ring PAHs (Figure 3) but did not significantly improve their biodegradation under the same conditions relative to inhibited controls and controls with no surfactant (Figure 2). There are several explanations for this result, including inherently slow rates of degradation of these compounds, insufficient numbers of organisms able to degrade them, or competitive inhibition of their degradation by the lower-molecular-weight PAHs (5). It is not possible from the available data to distinguish among these explanations. If biodegradation of the 5- and 6-ring compounds were limited by kinetics, then a longer incubation period might have increased their degradation. Longer batch incubation is likely to lead to extensive surfactant biodegradation, although measurements of surface tension in dilutions of the aqueous phase at the highest Brij 30 dose indicated that the residual surfactant concentration was relatively high after 18 d (data not shown).
Brij 30 and C12E8 clearly had different effects on PAH desorption from the solid phase in the soil slurry, which we attribute to differences in their hydrophobicity and, correspondingly, differences in their association with various sorptive compartments in the solid phase. The matrix with which PAHs associate in field-contaminated soils and sediments is complex, and cannot be reproduced in soils to which contaminants are spiked (1, 34, 35). Forms of black carbon such as soot, coal, and lampblack have been recognized as significant materials in soil and sediment to which PAHs sorb strongly (36–38), as has plant-derived organic matter (39). However, it has also been recognized that complex nonaqueous-phase liquids (NAPLs) and semi-solids such as coal tar, oil tar, and creosote are important matrix components in PAH-contaminated soils and sediments (40–46). Pitch, which appears to be an aggregate of highly weathered coal tar and inert materials, was also reported recently to be a significant sorbent of PAHs in contaminated sediment from MGP sites and is measured in the same fraction as black carbon (47).
The association of nonionic surfactants with specific sorptive compartments in field-contaminated soil or sediment has not been well-studied. It appears that hydrophilic, ethoxylated surfactants such as Triton X-100 have a higher affinity for clay minerals than for natural organic matter (18, 48–50). Triton X-100 has an HLB value of 13.5 (51), comparable to that of the C12E8 surfactant used in this study. Furthermore, sorption of hydrophilic surfactants to soil at doses that maintain aqueous-phase surfactant concentrations near or below the CMC will actually increase the sorption of hydrophobic contaminants to the soil (18, 48, 52, 53). If the behavior of hydrophilic ethoxylated surfactants in soil observed in earlier studies can be extended to the field-contaminated soil used in the present study, it might explain the decrease in PAH desorption caused by C12E8.
The mechanism by which the more hydrophobic surfactant Brij 30 enhanced PAH desorption is unclear. Brij 30 sorbed more strongly to the soil than did C12E8, which is consistent with the relationship between the HLB values of nonionic surfactants and their sorption to soil from an MGP site observed in an earlier study (12). We assume that Brij 30 is likely to interact with hydrophobic compartments in field-contaminated soil, such as black carbon, NAPLs (51), and pitch. Surfactants that associate with NAPLs in contact with soil influence their wetting behavior and correspondingly help release the NAPLs from the soil, even when the aqueous-phase surfactant concentration is below the CMC (54–56). If coal tar, for example, were released from soil aggregates this way, it would have more surface area for contaminant desorption or dissolution, or for direct contact with microorganisms. It is also possible that a surfactant such as Brij 30 could influence the diffusivity of PAHs in coal tar, which has been suggested to control the rate of PAH mass transfer into water (12, 57).
Our strategy in this study was to add surfactants to the soil after treatment in a bioreactor, because we hypothesize that surfactant addition will be beneficial only when contaminant bioavailability is limited. This hypothesis was supported by the finding that Brij 30 did not improve PAH degradation in the feed soil under the same conditions in which it did so in the treated slurry from the bioreactor. Adding surfactant as a second stage of treatment after a main biological treatment stage has some other potential benefits as well: the chances of selecting surfactant degraders over PAH degraders (22, 23) are reduced; the required surfactant concentration might be lower because contaminant concentrations would be lower; and the risk of accumulating inhibitory byproducts is lower if the readily bioavailable substrate concentration is lower.
The effects of Brij 30 addition on PAH biodegradation were similar for all PAHs at surfactant doses of 5 and 20 mg/g, but we did not attempt to optimize either the surfactant dose or the incubation time. However, the findings of this study could provide insights into designing surfactant-enhanced bioremediation systems at relatively low surfactant doses.
Supplementary Material
Acknowledgments
We thank Dr. Wei Sun of the UNC Department of Biostatistics for assistance with statistical analysis. This work was supported by the National Institute of Environmental Health Sciences’ Superfund Research Program (5 P42 ES05948).
Footnotes
Supporting Information Available
This information is available free of charge via the Internet at http://pubs.acs.org
References
- 1.terLaak TL, Barendregt A, Hermens JLM. Freely dissolved pore water concentrations and sorption coefficients of PAHs in spiked, aged, and field-contaminated soils. Environ Sci Technol. 2006;40(7):2184–2190. doi: 10.1021/es0524548. [DOI] [PubMed] [Google Scholar]
- 2.Chai Y, Kochetkov A, Reible DD. Desorption resistance of polycyclic aromatic hydrocarbons and duration of exposure. Environ Toxicol Chem. 2006;25(11):2827–2833. doi: 10.1897/05-750r.1. [DOI] [PubMed] [Google Scholar]
- 3.White JC, Alexander M, Pignatello JJ. Enhancing the bioavailability of organic compounds sequestered in soil and aquifer solids. Environ Toxicol Chem. 1999;18(2):182–187. [Google Scholar]
- 4.Kim HS, Weber WJ., Jr Optimizing contaminant desorption and bioavailability in dense slurry systems. 2. PAH bioavailability and rates of degradation. Environ Sci Technol. 2005;39(7):2274–2279. doi: 10.1021/es049564j. [DOI] [PubMed] [Google Scholar]
- 5.Aitken MD, Long TC. Biotransformation, biodegradation and bioremediation of polycyclic aromatic hydrocarbons. In: Singh A, Ward OP, editors. Soil Biology, Volume 2: Biodegradation and Bioremediation. Springer-Verlag: Heidelberg, Germany; 2004. pp. 83–124. [Google Scholar]
- 6.Cornelissen G, Rigterink H, Ferdinandy MMA, Van Noort PCM. Rapidly desorbing fractions of PAHs in contaminated sediments as a predictor of the extent of bioremediation. Environ Sci Technol. 1998;32(7):966–970. [Google Scholar]
- 7.Tiehm A, Stieber M, Werner P, Frimmel FH. Surfactant-enhanced mobilization and biodegradation of polycyclic aromatic hydrocarbons in manufactured gas plant soil. Environ Sci Technol. 1997;31(9):2570–2576. [Google Scholar]
- 8.Makkar RS, Rockne KJ. Comparison of synthetic surfactants and biosurfactants in enhancing biodegradation of polycyclic aromatic hydrocarbons. Environ Toxicol Chem. 2003;22(10):2280–2292. doi: 10.1897/02-472. [DOI] [PubMed] [Google Scholar]
- 9.Kim HS, Weber WJ. Polycyclic aromatic hydrocarbon behavior in bioactive soil slurry reactors amended with a nonionic surfactant. Environ Toxicol Chem. 2005;24(2):268–276. doi: 10.1897/04-219r.1. [DOI] [PubMed] [Google Scholar]
- 10.Lei L, Khodadoust AP, Suidan MT, Tabak HH. Biodegradation of sediment-bound PAHs in field-contaminated sediment. Water Res. 2005;39(2–3):349–361. doi: 10.1016/j.watres.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Calvo C, Manzanera M, Silva-Castro GA, Uad I, González-López J. Application of bioemulsifiers in soil oil bioremediation processes. Future prospects. Sci Total Environ. 2009;407(12):3634–3640. doi: 10.1016/j.scitotenv.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Yeom IT, Ghosh MM, Cox CD. Kinetic aspects of surfactant solubilization of soil-bound polycyclic aromatic hydrocarbons. Environ Sci Technol. 1996;30:1589–1595. [Google Scholar]
- 13.Grasso D, Subramaniam K, Pignatello JJ, Yang Y, Ratte D. Micellar desorption of polynuclear aromatic hydrocarbons from contaminated soil. Colloid Surf A-Physicochem Eng Asp. 2001;194(1–3):65–74. [Google Scholar]
- 14.Torres LG, Rojas N, Bautista G, Iturbe R. Effect of temperature, and surfactant's HLB and dose over the TPH-diesel biodegradation process in aged soils. Process Biochem. 2005;40(10):3296–3302. [Google Scholar]
- 15.Liu Z, Edwards DA, Luthy RG. Sorption of non-ionic surfactants onto soil. Water Res. 1992;26(10):1337–1345. [Google Scholar]
- 16.Walters GW, Aitken MD. Surfactant-enhanced solubilization and anaerobic biodegradation of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)-ethane (DDT) in contaminated soil. Water Environ Res. 2001;73(1):15–23. doi: 10.2175/106143001x138633. [DOI] [PubMed] [Google Scholar]
- 17.Yang K, Zhu L, Xing B. Enhanced soil washing of phenanthrene by mixed solutions of TX100 and SDBS. Environ Sci Technol. 2006;40(13):4274–4280. doi: 10.1021/es060122c. [DOI] [PubMed] [Google Scholar]
- 18.Wang P, Keller AA. Particle-size dependent sorption and desorption of pesticides within a water-soil-nonionic surfactant system. Environ Sci Technol. 2008;42(9):3381–3387. doi: 10.1021/es702732g. [DOI] [PubMed] [Google Scholar]
- 19.Rosen MJ, Li F, Morrall SW, Versteeg DJ. The relationship between the interfacial properties of surfactants and their toxicity to aquatic organisms. Environ Sci Technol. 2001;35(5):954–959. doi: 10.1021/es0015141. [DOI] [PubMed] [Google Scholar]
- 20.Auger RL, Jacobson AM, Domach MM. Effect of nonionic surfactant addition on bacterial metabolism of naphthalene: assessment of toxicity and overflow metabolism potential. J Hazard Mater. 1995;43(3):263–272. [Google Scholar]
- 21.Willumsen PA, Arvin E. Kinetics of degradation of surfactant-solubilized fluoranthene by a Sphingomonas paucimobilis. Environ Sci Technol. 1999;33(15):2571–2578. [Google Scholar]
- 22.Tiehm A. Degradation of polycyclic aromatic hydrocarbons in the presence of synthetic surfactants. Appl Environ Microbiol. 1994;60(1):258–263. doi: 10.1128/aem.60.1.258-263.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HS, Weber WJ., Jr Preferential surfactant utilization by a PAH-degrading strain: effects on micellar solubilization phenomena. Environ Sci Technol. 2003;37(16):3574–3580. doi: 10.1021/es0210493. [DOI] [PubMed] [Google Scholar]
- 24.Deschênes L, Lafrance P, Villeneuve JP, Samson R. Adding sodium dodecyl sulfate and Pseudomonas aeruginosa UG2 biosurfactants inhibits polycyclic aromatic hydrocarbon biodegradation in a weathered creosote-contaminated soil. Appl Microbiol Biotechnol. 1996;46(5–6):638–646. doi: 10.1007/s002530050874. [DOI] [PubMed] [Google Scholar]
- 25.Zhu H, Roper JC, Pfaender FK, Aitken MD. Effects of anaerobic incubation on the desorption of polycyclic aromatic hydrocarbons from contaminated soils. Environ Toxicol Chem. 2008;27(4):837–844. doi: 10.1897/07-166.1. [DOI] [PubMed] [Google Scholar]
- 26.Gustafsson Ö, Haghseta F, Chan C, MacFarlane J, Gschwend PM. Quantification of the dilute sedimentary soot phase: implications for PAH speciation and bioavailability. Environ Sci Technol. 1997;31 (1):203–209. [Google Scholar]
- 27.Walpole RE, Myers RH. Probability and Statistics for Engineers and Scientists. 4. MacMillan Publishing Co; New York: 1989. [Google Scholar]
- 28.Tadros TF. Applied Surfactants: Principles and Applications. Wiley and Sons; New York, NY: 2005. [Google Scholar]
- 29.Grimberg SJ, Stringfellow WT, Aitken MD. Quantifying the biodegradation of phenanthrene by Pseudomonas stutzeri P16 in the presence of a nonionic surfactant. Appl Environ Microbiol. 1996;62(7):2387–2392. doi: 10.1128/aem.62.7.2387-2392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimberg SJ, Aitken MD. Biodegradation kinetics of phenanthrene solubilized in surfactant micelles. In: Hinchee RE, Vogel CM, Brockman FJ, editors. Microbial Processes for Bioremediation. Battelle Press; Columbus, OH: 1995. pp. 59–66. [Google Scholar]
- 31.Kolomytseva MP, Randazzo D, Baskunov BP, Scozzafava A, Briganti F, Golovleva LA. Role of surfactants in optimizing fluorene assimilation and intermediate formation by Rhodococcus rhodochrous VKM B-2469. Bioresour Technol. 2009;100(2):839–844. doi: 10.1016/j.biortech.2008.06.059. [DOI] [PubMed] [Google Scholar]
- 32.Zhu H, Aitken MD. Effects of nonionic surfactant addition on populations of polycyclic aromatic hydrocarbon-degrading bacteria in contaminated soil. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loehr RC, Lamar MR, Poppendieck DG. A protocol to estimate the release of anthropogenic hydrocarbons from contaminated soils. Environ Toxicol Chem. 2003;22(9):2202–2208. doi: 10.1897/02-463. [DOI] [PubMed] [Google Scholar]
- 34.Allan IJ, Semple KT, Hare R, Reid BJ. Cyclodextrin enhanced biodegradation of polycyclic aromatic hydrocarbons and phenols in contaminated soil slurries. Environ Sci Technol. 2007;41(15):5498–5504. doi: 10.1021/es0704939. [DOI] [PubMed] [Google Scholar]
- 35.Hawthorne SB, Grabanski CB, Miller DJ. Measured partitioning coefficients for parent and alkyl polycyclic aromatic hydrocarbons in 114 historically contaminated sediments: Part 1. Koc values. Environ Toxicol Chem. 2006;25(11):2901–2911. doi: 10.1897/06-115r.1. [DOI] [PubMed] [Google Scholar]
- 36.Accardi-Dey A, Gschwend PM. Reinterpreting literature sorption data considering both absorption into organic carbon and adsorption onto black carbon. Environ Sci Technol. 2003;37(1):99–106. doi: 10.1021/es020569v. [DOI] [PubMed] [Google Scholar]
- 37.Hong L, Ghosh U, Mahajan T, Zare RN, Luthy RG. PAH sorption mechanism and partitioning behavior in lampblack-impacted soils from former oil-gas plant sites. Environ Sci Technol. 2003;37(16):3625–3634. doi: 10.1021/es0262683. [DOI] [PubMed] [Google Scholar]
- 38.Cornelissen G, Gustafsson Ö, Bucheli TD, Jonker MTO, Koelmans AA, van Noort PCM. Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ Sci Technol. 2005;39(18):6881–6895. doi: 10.1021/es050191b. [DOI] [PubMed] [Google Scholar]
- 39.Rockne KJ, Shor LM, Young LY, Taghon GL, Kosson DS. Distributed sequestration and release of PAHs in weathered sediment: the role of sediment structure and organic carbon properties. Environ Sci Technol. 2002;36(12):2636–2644. doi: 10.1021/es015652h. [DOI] [PubMed] [Google Scholar]
- 40.Karimi-Lotfabad S, Gray MR. Characterization of contaminated soils using confocal laser scanning microscopy and cryogenic-scanning electron microscopy. Environ Sci Technol. 2000;34(16):3408–3414. [Google Scholar]
- 41.Jonker MTO, Sinke AJC, Brils JM, Koelmans AA. Sorption of polycyclic aromatic hydrocarbons to oil contaminated sediment: unresolved complex? Environ Sci Technol. 2003;37(22):5197–5203. doi: 10.1021/es0300564. [DOI] [PubMed] [Google Scholar]
- 42.Hawthorne SB, Grabanski CB, Miller DJ. Measured partition coefficients for parent and alkyl polycyclic aromatic hydrocarbons in 114 historically contaminated sediments: Part 2. Testing the KOCKBC two carbon-type model. Environ Toxicol Chem. 2007;26(12):2505–2516. doi: 10.1897/07-087.1. [DOI] [PubMed] [Google Scholar]
- 43.Hong L, Luthy RG. Availability of polycyclic aromatic hydrocarbons from lampblack-impacted soils at former oil-gas plant sites in California, USA. Environ Toxicol Chem. 2007;26(3):394–405. doi: 10.1897/06-200r.1. [DOI] [PubMed] [Google Scholar]
- 44.Vermeulen J, van Gool MPM, Mentink GH, Joziasse J, Bruning H, Rulkens WH, Grotenhuis JTC. Biochemical ripening of dredged sediments. Part 2. Degradation of polycyclic aromatic hydrocarbons and total petroleum hydrocarbons in slurried and consolidated sediments. Environ Toxicol Chem. 2007;26(12):2540–2549. doi: 10.1897/06-546.1. [DOI] [PubMed] [Google Scholar]
- 45.Ahn S, Werner D, Luthy RG. Modeling PAH mass transfer in a slurry of contaminated soil or sediment amended with organic sorbents. Water Res. 2008;42(12):2931–2942. doi: 10.1016/j.watres.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Birak PS, Miller CT. Dense non-aqueous phase liquids at former manufactured gas plants: challenges to modeling and remediation. J Contam Hydrol. 2009;105(3–4):81–98. doi: 10.1016/j.jconhyd.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khalil MF, Ghosh U, Kreitinger JP. Role of weathered coal tar pitch in the partitioning of polycyclic aromatic hydrocarbons in manufactured gas plant site sediments. Environ Sci Technol. 2006;40(18):5681–5687. doi: 10.1021/es0607032. [DOI] [PubMed] [Google Scholar]
- 48.Lee JF, Liao PM, Kuo CC, Yang HT, Chiou CT. Influence of a nonionic surfactant (Triton X-100) on contaminant distribution between water and several soil solids. J Colloid Interface Sci. 2000;229 (2):445–452. doi: 10.1006/jcis.2000.7039. [DOI] [PubMed] [Google Scholar]
- 49.Salloum MJ, Dudas MJ, McGill WB, Murphy SM. Surfactant sorption to soil and geologic samples with varying mineralogical and chemical properties. Environ Toxicol Chem. 2000;19(10) doi: 10.1016/s0045-6535(00)00513-0. [DOI] [PubMed] [Google Scholar]
- 50.Zhu L, Yang K, Lou B, Yuan B. A multi-component statistic analysis for the influence of sediment/soil composition on the sorption of a nonionic surfactant (Triton X-100) onto natural sediments/soils. Water Res. 2003;37(19):4792–4800. doi: 10.1016/S0043-1354(03)00428-7. [DOI] [PubMed] [Google Scholar]
- 51.Bernardez LA, Ghoshal S. Selective solubilization of polycyclic aromatic hydrocarbons from multicomponent nonaqueous-phase liquids into nonionic surfactant micelles. Environ Sci Technol. 2004;38(22):5878–5887. doi: 10.1021/es0497429. [DOI] [PubMed] [Google Scholar]
- 52.Edwards DA, Adeel Z, Luthy RG. Distribution of nonionic surfactant and phenanthrene in a sediment/aqueous system. Environ Sci Technol. 1994;28:1550–1560. doi: 10.1021/es00057a027. [DOI] [PubMed] [Google Scholar]
- 53.Sun S, Inskeep WP, Boyd SA. Sorption of nonioinc organic compounds in soil-water systems containing a micelle-forming surfactant. Environ Sci Technol. 1995;29:903–913. doi: 10.1021/es00004a010. [DOI] [PubMed] [Google Scholar]
- 54.Deitsch JJ, Smith JA. Effect of Triton X-100 on the rate of trichloroethene desorption from soil to water. Environ Sci Technol. 1995;29(4):1069–1080. doi: 10.1021/es00004a029. [DOI] [PubMed] [Google Scholar]
- 55.Deshpande S, Shiau BJ, Wade D, Sabatini DA, Harwell JH. Surfactant selection for enhancing ex situ soil washing. Water Res. 1999;33(2):351–360. [Google Scholar]
- 56.Dong J, Chowdhry B, Leharne S. Investigation of the wetting behavior of coal tar in three phase systems and its modification by poloxamine block copolymeric surfactants. Environ Sci Technol. 2004;38 (2):594–602. doi: 10.1021/es026426q. [DOI] [PubMed] [Google Scholar]
- 57.Benhabib K, Simonnot M, Sardin M. PAHs and organic matter partitioning and mass transfer from coal tar particles to water. Environ Sci Technol. 2006;40(19):6038–6043. doi: 10.1021/es0600431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.