Abstract
The risk of endometrial cancer (EC) subsequent to a diagnosis of colorectal cancer in women with a germline mutation in a mismatch repair gene (Lynch syndrome or HNPCC) is unknown. We estimated the risk of EC following a diagnosis of CRC for women with Lynch syndrome.
A retrospective cohort study was performed on women diagnosed with CRC with a germline mutation in a mismatch repair gene (Lynch Syndrome cases), and women with microsatellite stable CRC who were not known to carry a germline mutation (non-Lynch cases), identified from the Colon Cancer Family Registry. The incidence of EC following CRC was estimated and compared for women with and without Lynch syndrome, using adjusted hazards ratios calculated for time at risk among each group.
One hundred and twelve women with Lynch syndrome and a previous diagnosis of CRC were compared with 908 women without Lynch and with a microsatellite stable CRC diagnosis. The estimated ten year cumulative risk of EC subsequent to CRC was 23.4% (95% CI: 15–36%) for Lynch syndrome women compared to 1.6% (95% CI: 0.7–3.8%) for non-Lynch women. After adjusting for ascertainment, age at diagnosis and diagnosis of other cancers, risk of subsequent diagnosis with EC was elevated 6-fold in women with Lynch syndrome compared to non-Lynch women (HR 6.2; 95% CI 2.2–17.3; p=0.001).
Approximately one quarter of women diagnosed with Lynch syndrome-associated CRC developed EC within 10 years. This supports the sentinel cancer concept and suggests that active and early management is important for these women.
Introduction
Lynch syndrome (also known as hereditary non-polyposis colon cancer, HNPCC) is caused by a germline mutation in one of several DNA mismatch repair (MMR) genes, MLH1, MSH2, MSH6, or PMS2. Carriers of MMR gene mutations are at increased risk of cancers of the colon, endometrium, ovary, upper urologic tract, stomach, small bowel, biliary/pancreas, skin and brain.1 In individuals who carry such mutations, inactivation of the remaining normal allele in a cell results in dysfunctional or absent DNA mismatch repair. These unrepaired mismatches typically occur in regions of repetitive nucleotide sequences, commonly referred to as microsatellites. The consequent phenotype, referred to as microsatellite instability (MSI), is the signature change in Lynch syndrome-associated cancers.2, 3 Detection of a deleterious germline mutation in a particular MMR gene in a case of cancer with MSI unequivocally establishes the diagnosis of Lynch syndrome, with MLH1 and MSH2 sequence variants accounting for approximately 80 per cent of all mismatch repair gene mutations.4
Early age of cancer diagnosis and multiplicity of cancers are considered hallmarks of Lynch syndrome. A meta-analysis5 of data from three population-based studies,6–8 and previous clinic-based work,9 estimated that the risk of colorectal cancer for MLH1 and MSH2 carriers to age 70 years was 53% for males, 33% for females, and for endometrial cancer was 44%. While the increased risk of endometrial cancer in women with Lynch syndrome is well established, the risk of endometrial cancer after Lynch-associated colorectal cancer is unknown. Possible reasons for a change in endometrial cancer risk after colorectal cancer diagnosis include, but are not limited to: effects of radio- or chemotherapy for the colorectal cancer, increased surveillance, weight change, and changes in exposures to environmental risk factors. A number of attempts have been made to address this question, but the relationship could not be estimated with sufficient precision due to the limited number of cases analyzed or the design of the original study.10–12 If women with Lynch syndrome-related colorectal cancer have an increased risk for subsequent primary gynaecological cancer, they could be advised to undertake interventions such as screening13, 14 and/or risk-reducing surgery to prevent subsequent primary cancers.
Hence, the aim of this study was to estimate the risk of endometrial cancer subsequent to a diagnosis of colorectal cancer in women with Lynch Syndrome. To achieve this aim we analysed cancer risk using a large cohort of MMR mutation carriers from the international Colon Cancer Family Registry (Colon CFR).15
Materials and Methods
Subjects
Subjects for this retrospective cohort study were participants from the Colon CFR. The Colon CFR consists of data and biospecimens from clinic- and population-based colorectal cancer cases and controls as well as their families. Details of recruitment methods have been described previously.15 Briefly, clinic-based ascertainment was as follows: individuals with colorectal cancer were selected from multiple-case colorectal or Lynch syndrome cancer families attending cancer family clinics in the USA (Mayo Clinic, Rochester, Minnesota; and Cleveland), Australia (Melbourne, Adelaide, Perth, Brisbane and Sydney), and New Zealand (Auckland). For population-based ascertainment, probands were defined as incident colorectal cancer cases identified by population-based cancer registries in the USA (Puget Sound, Washington; the State of Minnesota; Los Angeles County, California; Arizona; Colorado; New Hampshire; parts of North Carolina; and Hawaii), Australia (Victoria) and Canada (Ontario). Probands were asked for permission for the study to contact selected relatives for participation.
For probands and participating relatives, information on demography, personal and family history of cancer, cancer screening, lifestyle factors, cancer surgery and gynecological surgery was obtained by interview, questionnaire, or extraction from clinical records. Efforts were made to verify reported cancer diagnoses using family reporting, pathology reports, medical records and death certificates. All probands and participating relatives were asked to provide a blood sample for DNA analysis and for consent to retrieve archived colorectal cancer tissue. Women with both CRC and endometrial cancer were not more likely to be eligible for the study, and were not more likely to be tested for MMR mutations than women with CRC only.
Participants were followed-up and re-interviewed approximately four to five years from the date of the baseline interview. They were also asked to report any new cancers or deaths in relatives including those who had participated at baseline and were unable to participate in the follow-up interview or who had died.
Colorectal cancer tumour testing for MSI and germline testing for MMR gene mutations
Colorectal cancers were tested for MSI using a panel of 10 markers as previously described.16 If one or more markers tested displayed a difference between normal tissue and tumour tissue, the tumour was defined as MSI; otherwise the tumour was classified as microsatellite stable (MSS). Germline mutation testing was conducted for three mismatch repair genes (MLH1, MSH2, MSH6) by DNA sequence analysis, and large insertion deletions were screened by using Multiplex Ligation-Dependent Probe Amplification.17 A deleterious mutation was defined as a variant predicted to result in a stop codon, a frameshift mutation, a large insertion or deletion, or a missense mutation judged to be deleterious. All relatives of identified mutation carriers who provided a DNA sample were subsequently tested for the same variant.
Inclusion criteria
For this study, Lynch syndrome cases were defined as females with a previous diagnosis of colorectal cancer who had a documented deleterious germline mutation in MLH1, MSH2 or MSH6. Non-Lynch cases were defined as females with a previous diagnosis of colorectal cancer that was MSS and not known to have a deleterious mutation in MLH1, MSH2 or MSH6. Cases were excluded if: the date of diagnosis of colorectal cancer or endometrial cancer, age at diagnosis, age at death or age at hysterectomy were missing or incomplete; data had only been provided by proxy; or if endometrial cancer was diagnosed, or a hysterectomy occurred, before the first diagnosis of colorectal cancer. We also excluded any MSI cases for whom testing for mismatch repair gene mutations was not available. Details of the selection process for this analysis are shown in Table 1.
Table 1.
| Lyncha | Non-Lynchb | |
|---|---|---|
| CRC cases of the Colon CFR | 197 | 1,416 |
| Exclusions | ||
| Epidemiological data provided by proxy | 1 | 27 |
| Date or age at CRC diagnosis missing or incompatible | 4 | 0 |
| Endometrial cancer diagnosed prior to first diagnosis of CRC | 27 | 22 |
| Age at hysterectomy missing | 8 | 70 |
| Hysterectomy prior to first CRC diagnosis | 45 | 389 |
| Final study groups | 112 | 908 |
Lynch = Known carrier of germline mutation in MLH1, MSH2 or MSH6.
Non-Lynch = Microsatellite stable (MSS) and no known germline mutation in MLH1, MSH2 or MSH6
Statistical Analysis
Chi-square tests (or Fisher’s exact test when cell sizes were small) were used to assess differences between women with Lynch syndrome and those without for a number of key demographic and clinical characteristics (as shown in Table 2). Among women with Lynch syndrome, chi-square tests were also conducted to determine whether differences in the mutated mismatch repair genes involved were statistically significant.
Table 2.
Cohort characteristics by study group
| Lynch Syndrome | Non-Lynch group | ||||
|---|---|---|---|---|---|
| n | % | n | % | p | |
| Total cohort | 112 | 100.0 | 908 | 100.0 | |
| Colon CFR centera | <0.001 | ||||
| Ontario | 8 | 7.1 | 234 | 25.8 | |
| Los Angeles | 17 | 15.2 | 94 | 10.4 | |
| Australia | 59 | 52.7 | 139 | 15.3 | |
| Hawaii | 4 | 3.6 | 31 | 3.4 | |
| Mayo Foundation | 21 | 18.8 | 142 | 15.6 | |
| Seattle | 3 | 2.7 | 268 | 29.5 | |
| Ascertainment type | <0.001 | ||||
| Population-based | 33 | 29.5 | 829 | 91.3 | |
| Clinic-based | 79 | 70.5 | 79 | 8.7 | |
| Age at first colorectal cancer diagnosis | <0.001 | ||||
| 15–39 years | 57 | 50.9 | 102 | 11.2 | |
| 40–49 years | 36 | 32.1 | 232 | 25.6 | |
| 50–59 years | 14 | 12.5 | 230 | 25.3 | |
| 60 years and over | 5 | 4.5 | 344 | 37.9 | |
| Median age at first colorectal cancer diagnosis | 39 years (IQR = 34 yrs –45 yrs) | 54 years (IQR = 46 yrs – 65 yrs) | <0.001 | ||
| Number of other cancer diagnoses | <0.001 | ||||
| None | 77 | 68.8 | 781 | 86.0 | |
| One | 20 | 17.9 | 103 | 11.4 | |
| Two or more | 15 | 13.4 | 24 | 2.6 | |
| Timing of other cancer diagnosesb | |||||
| Prior to first colorectal cancer | |||||
| Ovarian cancer | 0 | 0.0 | 4 | 0.3 | 1.000 |
| Other cancer (excluding endometrial) | 2 | 1.8 | 68 | 7.5 | 0.040 |
| Subsequent to first colorectal cancer | |||||
| Endometrial cancer | 22 | 19.6 | 8 | 0.9 | <0.001 |
| Ovarian cancer | 2 | 1.8 | 3 | 0.3 | 0.096 |
| Other cancers | 19c | 17.0 | 53 | 5.8 | <0.001 |
| Procedures/Medicationb | |||||
| Hysterectomy | 50 | 44.6 | 37 | 4.1 | <0.001 |
| Tamoxifen | 1 | 0.9 | 15 | 1.7 | 0.836 |
| Median follow-up after initial colorectal cancer diagnosis | 10.7 years (IQR = 6.1 yrs–23.1 yrs) | 6.1 years (IQR = 4.9 yrs–6.9 yrs) | <0.001 | ||
| Median time at risk for endometrial cancer | 6.3 years (IQR = 3.7 yrs–12.3 yrs) | 6.0 years (IQR = 4.4 yrs–6.8 yrs) | 0.011 | ||
IQR = inter-quartile range
Ontario (Cancer Care Ontario, recruitment from Ontario, Canda), Los Angeles (University of Southern California consortium, recruitment from Los Angeles County, California; Cleveland, Ohio; Arizona; Colorado; New Hampshire; and parts of North Carolina), Australasia (University of Melbourne, recruitment from Victoria, New South Wales, South Australia and Western Australia, Australia, and Auckland, New Zealand), Hawaii (University of Hawaii, recruitment from Hawaii), Mayo Clinic (Mayo Clinic, recruitment from Rochester, Minnesota), Seattle (Fred Hutchinson Cancer Research Centre, recruitment from Puget Sound are, Washington).
Categories do not add to total cohort
Other cancers diagnosed in the Lynch group include 7 melanomas, 3 breast cancers, 3 kidney cancers, 2 cancers of the vulva, 2 bladder cancers, 1 stomach cancer, 1 cancer of the small intestine, 1 liver cancer, 1 lung cancer and 1 cancer of other female genital organs (NOS).
The principal outcome of interest was endometrial cancer. For each participant, follow-up time was calculated by subtracting the date of first colorectal cancer diagnosis from the date of death or last known date alive. Time at risk was derived from follow-up time by truncating at date of diagnosis of endometrial cancer or censoring at date of hysterectomy if either of these events occurred. If only year and month were known for any relevant date, then the 15th of the month was used as an approximation. If only year was known, then 30th June was used as an approximation.
Median values were calculated for age at diagnosis of first colorectal cancer, follow-up time and time at risk for the Lynch and non-Lynch groups, and differences in the distributions were evaluated using the non-parametric Wilcoxon-Mann-Whitney test. Directly age-standardised incidence rates for endometrial cancer following colorectal cancer were calculated for both the Lynch and non-Lynch groups, based on the American Standard Population (year 2000) and using broad age categories (<50 years, 50-59 years and 60 years and over).
Kaplan-Meier curves were used to assess differences in the cumulative risk of being diagnosed with endometrial cancer following colorectal cancer between the Lynch and non-Lynch groups, with a log-rank test used to test for equality of the curves. The analysis was restricted to 10 years time at risk because of insufficient data to calculate reliable estimates beyond that time.
A Cox proportional hazards model was fitted to time at risk to calculate hazard ratios for the development of endometrial cancer among cases with Lynch syndrome compared to the non-Lynch group. To account for familial clustering, a robust variance estimation approach was used. The model was adjusted for age group at time of first diagnosis with colorectal cancer (under 50 years versus 50 years and older) as well as presence of cancers other than colorectal cancer or endometrial cancer and ascertainment type (population-based versus clinic-based). Explanatory variables were tested individually and collectively for proportionality before being included in the model.
All analyses were performed using the SAS statistical package, version 9.1 for Windows (SAS Institute Inc., Cary, NC).
Results
Characteristics of the cohort are summarised in Table 2. There were 112 women with a previous diagnosis of colorectal cancer who had Lynch syndrome (Lynch group) and 908 women with a previous diagnosis of colorectal cancer who did not have Lynch syndrome (non-Lynch group). Approximately one-third (32.1%,n=36) of the women with Lynch syndrome and 1.7% (n=15) of the non-Lynch women had at least one other relative included in this analysis. Relative to the non-Lynch cases, the Lynch group were younger when first diagnosed with colorectal cancer, with a median age of 39 years compared to 54 years (p<0.001). Few participants reported using tamoxifen - only one of the Lynch group (0.9%) and 15 (1.7%) from the non-Lynch group.
After a diagnosis of colorectal cancer, almost half of the cases (44.6%) with Lynch syndrome had a hysterectomy, compared to only 4.1% of the non-Lynch group (p<0.001). The reasons for those hysterectomies were not stated. None of the 50 cases in the Lynch group who had a hysterectomy died within five years of follow-up. Among the 62 Lynch group cases that did not have a hysterectomy, six (9.7%) had died within five years. About 1 in 5 of the women in the Lynch group were subsequently diagnosed with endometrial cancer (n=22, 19.6%) compared to 0.9% (n=8) in the non-Lynch group (p<0.001). Of the 30 reported endometrial cancer diagnoses in both groups, we were able to verify 23 (76.7%) by either pathology reports or medical records.
Two of 112 (1.8%) cases of the Lynch group developed ovarian cancer subsequent to colorectal cancer compared to three of 908 (0.3%) in the non-Lynch group. The proportion of cases diagnosed with cancers other than endometrial or ovarian cancer subsequent to colorectal cancer was significantly higher in the Lynch group (17.0% versus 5.8%; p<0.001).
There were a total of 1,059 person years at risk among the Lynch group and 5,383 person years at risk among the non-Lynch group. Although differences in the distribution of time at risk for endometrial cancer between Lynch and non-Lynch CRC cases were statistically significant (p=0.011), the median time at risk was similar for the two groups (6.3 years and 6.0 years respectively).
For the Lynch group there was no significant difference (p=0.284) in the mismatch repair gene that was mutated for those who were subsequently diagnosed with endometrial cancer and those who were not (Table 3). The majority of the deleterious mutations occurred in either the MLH1 gene (50.0% for both those with and without endometrial cancer) or the MSH2 gene (40.9% for those with endometrial cancer and 47.8% for those without subsequent endometrial cancer). In the group of mutation carriers who developed endometrial cancer two women (9.1%) had a MSH6 mutation compared to two women in the group who did not (2.2%).
Table 3.
Subsequent diagnosis of endometrial cancer by type of deleterious mutation for colorectal cancer cases with Lynch Syndrome
| Mismatch repair gene with deleterious mutation | Subsequent Endometrial Cancer | No Subsequent Endometrial Cancer | |||
|---|---|---|---|---|---|
| n | % | n | % | p | |
| MLH1 | 11 | 50.0 | 45 | 50.0 | |
| MSH2 | 9 | 40.9 | 43 | 47.8 | 0.284 |
| MSH6 | 2 | 9.1 | 2 | 2.2 | |
Crude incidence rates of endometrial cancer following colorectal cancer were 20.8 cases per 1,000 woman years at risk for women in the Lynch group and 1.5 cases per 1,000 woman years at risk for women in the non-Lynch group. Corresponding age standardised rates were significantly higher in the Lynch group with 18.6 cases per 1,000 person years at risk (95% confidence interval (CI) = 11.7 – 28.2) in contrast to 1.1 cases per 1,000 person years at risk (95% CI = 0.5 – 2.1) respectively (p<0.001).
The 10-year cumulative risk of endometrial cancer following colorectal cancer in the Lynch group was 23.4% (95% CI = 14.5% – 36.3%) compared to the corresponding risk of 1.6% (95% CI = 0.7% – 3.8%; p<0.001) for the non-Lynch group (see Figure 1).
Figure 1.
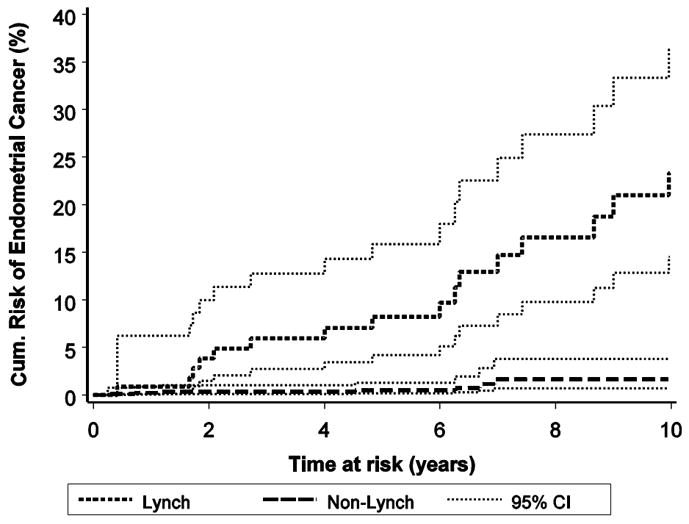
After adjusting for age at colorectal cancer diagnosis, presence of cancers other than colorectal or endometrial cancer and ascertainment type (Table 4), it was estimated that women in the Lynch group were about six times more likely to be diagnosed with endometrial cancer following colorectal cancer (hazard ratio = 6.2; 95% CI = 2.2 – 17.7, p=0.001) .
Table 4.
Adjusted hazard ratios for endometrial cancer following colorectal cancer
| n | Adjusted Hazard Ratio | 95% CI | p-value | |
|---|---|---|---|---|
| Lynch Syndrome | ||||
| No | 908 | 1.00 | (baseline) | |
| Yes | 112 | 6.24 | (2.20–17.73) | 0.001 |
| Age at colorectal cancer diagnosis | ||||
| 15–49 years | 427 | 0.85 | (0.32–2.29) | 0.753 |
| 50 years and over | 593 | 1.00 | (baseline) | |
| Other cancera | ||||
| No | 876 | 1.00 | (baseline) | |
| Yes | 144 | 2.63 | (1.16–5.94) | 0.020 |
| Ascertainment type | ||||
| Population-based | 862 | 1.00 | (baseline) | |
| Clinic-based | 158 | 3.18 | (1.24–8.20) | 0.017 |
Other cancers exclude colorectal cancer and endometrial cancer
Discussion
We found that for every 100 women diagnosed with Lynch syndrome-associated colorectal cancer, around 23 (95% CI = 15–36) will be diagnosed with endometrial cancer within 10 years if they do not have a hysterectomy. This compares to less than 2 women being diagnosed with endometrial cancer for every 100 women who are diagnosed with colorectal cancer who have not inherited a mutation in a mismatch repair gene, although the non-Lynch group available for our study were somewhat younger on average than is generally the case.
The concept of a “sentinel” cancer suggests that Lynch carriers are at increased risk of developing subsequent primary cancers following an initial (sentinel) cancer diagnosis compared to non-Lynch carriers. Two other research groups suggested that the risk of a second primary cancer in Lynch carriers is 25% and 50% by 10 and 15 years, respectively, after diagnosis of a first cancer.18, 19 In a series of 117 women with dual primary (endometrial and colon) cancers from 223 Amsterdam families, 16 women had synchronous cancers.20 Of the remaining 101 women, 52 developed an endometrial or ovarian cancer first whilst 49 cases were diagnosed with colorectal cancer first and had a second primary cancer subsequently. Hence, colorectal cancer can be seen as a sentinel event in approximately half of women with Lynch syndrome affected with multiple malignancies, and endometrial cancer represents the sentinel cancer in the remainder, a finding consistent with the present report. Our data confirm the sentinel cancer concept; the risk of subsequent endometrial cancer in cases with Lynch Syndrome on the occasion of a colorectal cancer event is a significant increase over what would be expected in unselected cases.
Clinically, the concept of sentinel cancer is potentially very helpful not only for affected individuals but also for their relatives. The proportion of women with endometrial cancer diagnosed at or before the age of 50 years who carry germline mutations in a mismatch repair gene varies between nine and 18%.21, 22 Current evidence supports colorectal screening in those cases. In one observational analysis, screened cases had more than a 60% reduction of colorectal cancer incidence compared to unscreened cases.23 Data from the United Kingdom suggested a 72% reduction in mortality attributed to screening colonoscopy for individuals with a strong family history of colorectal cancer,24 and women diagnosed with Lynch syndrome-related endometrial cancer are advised to have regular colorectal and other screening post-operatively.
Until recently, no population-based data were available regarding the risk of endometrial cancer following a Lynch syndrome-associated colorectal cancer diagnosis. Current recommendations are based on the extrapolation of evidence from various contexts. This is the first study of sufficient size to quantify the risk of endometrial cancer following Lynch syndrome-related colorectal cancer, thus supporting early and active management in those young women.
Recent reports have addressed the efficacy of endometrial cancer screening in Lynch carriers. In a series of 35 premenopausal and six postmenopausal women who were MMR gene mutation carriers or who fulfilled the Amsterdam criteria and mismatch gene mutation status was unknown, three premalignant lesions were detected within a total of 197 patient years at risk.25 In addition, one patient was diagnosed with interval endometrial cancer based on her symptoms. Generally it is believed that endometrial sampling/biopsy is superior to transvaginal ultrasound alone for endometrial cancer surveillance in premenopausal women.13, 14 In postmenopausal women, the threshold for endometrial thickness on transvaginal ultrasound has been analysed several times and ultrasound might be equivalent to endometrial sampling for that group of patients.26, 27 In a multi-institutional retrospective cohort analysis, Schmeler et al investigated 315 women with documented germline MMR mutations.28 Sixty-one women had undergone prophylactic risk-reducing hysterectomy and 47 women had undergone prophylactic bilateral salpingo-oophorectomy. These women were matched with mutation-positive women who had not undergone prophylactic surgery. The prophylactic surgery group experienced no occurrences of endometrial or ovarian cancer, comparing favourably with 33% endometrial cancer and five percent ovarian cancer in the control group. The results from this study suggest that prophylactic hysterectomy with bilateral salpingo-oophorectomy is effective in preventing gynaecological cancer in women with Lynch syndrome.
While 100% of endometrial cancer could be prevented by prophylactic surgery, this may or may not translate into survival benefits. Given that the incidence of ovarian cancer is consistently low in Lynch syndrome series,29, 30 and given the normally excellent prognosis of clinically diagnosed endometrial cancer,31 the actual survival benefits may be modest at best. To date, only case reports32–34 but no data from cohort series or clinical trials are available on the use of a levonorgestrel intrauterine device and its potential ability to prevent endometrial cancer.
Clinically, one of the major research questions is: “Should women diagnosed with Lynch-associated colorectal cancer require specific management addressing this risk?” Several outcome parameters are relevant to this important question. Our study focused on the incidence rates of endometrial cancer and we demonstrate a high risk of developing endometrial cancer after Lynch-associated colorectal cancer but only five cases of ovarian cancer were diagnosed . Two of those five women had Lynch Syndrome and three were non-Lynch. Unfortunately, we are unable to answer whether screening or prophylactic, risk-reducing hysterectomy will improve survival.
Because the recruitment to the Colon CFR was weighted towards early-onset colorectal cancer,15 the majority of women in this series (96% for Lynch patients and 62% for non-Lynch) were diagnosed with colorectal cancer before the age of 60 years. Inference of these findings to cases diagnosed at a relatively young age is therefore straightforward, but a potential limitation may be the inability to infer our findings to cases diagnosed at an older age.
Another potential limitation of this study relates to the Colon CFR recruitment process. Although no clinic-based families were ascertained because of a case of endometrial cancer following a colorectal cancer, some families may have been recruited due to endometrial cancer occurring in a relative; hence for the estimate of increased risk over non-Lynch women, we adjusted for ascertainment type. However this recruitment strategy is unlikely to have resulted in biased estimates as women with both CRC and endometrial cancer were not more likely to be eligible for the study, and were not more likely to be tested for MMR mutations than women with CRC only. Less than a third of all cases completed their baseline interview within one year of being diagnosed with colorectal cancer, meaning that those who died within the first year were less likely to be recruited to the study than those who survived longer. It is unclear what effect excluding these women would have on the calculation of cumulative risk of endometrial cancer.
In summary, our paper suggests that about one quarter of cases who were diagnosed with Lynch syndrome-associated colorectal cancer who do not have a hysterectomy will develop endometrial cancer within 10 years. Given the extent of that risk, identification of these patients and proactive management is justified to reduce the burden of this cancer. Management options include risk-reducing, prophylactic total hysterectomy and bilateral salpingo-oophorectomy28 or regular screening with endometrial sampling on a yearly basis for premenopausal women or transvaginal ultrasound examinations for postmenopausal women.
Acknowledgments
Research Support: None for this project. The Colon CFR work was supported by the National Cancer Institute, National Institutes of Health under RFA #CA-95-011 and through cooperative agreements with the members of the Colon Cancer Family Registry (CFR) and principal investigators.
Footnotes
Prior study presentations: None
Contributor Information
Andreas Obermair, The University of Queensland and the Royal Brisbane and Women’s Hospital, Butterfield Street, Herston QLD 4006, School of Medicine, University of Queensland, Herston Q 4006, Australia.
Danny R. Youlden, Viertel Centre for Research in Cancer Control, Cancer Council Queensland, Brisbane, Queensland, Australia
Joanne P. Young, Familial Cancer Laboratory, QIMR, Herston Q 4006, Australia, School of Medicine, University of Queensland, Herston Q 4006, Australia
Noralane M. Lindor, Departments of Medical Genetics, Mayo Clinic, Rochester, Minnesota, USA
John A. Baron, Dartmouth Medical School, Hanover, New Hampshire, USA
Polly Newcomb, Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA.
Susan Parry, Department of Gastroenterology and Hepatology, Middlemore Hospital, Auckland, New Zealand.
John L. Hopper, Centre for Molecular, Environmental, Genetic and Analytic Epidemiology, Melbourne School of Population Health, The University of Melbourne, Parkville Victoria, 3010, Australia
Robert Haile, Department of Preventive Medicine, University of Southern California, Keck School of Medicine, Los Angeles, California, USA.
Mark A. Jenkins, Centre for Molecular, Environmental, Genetic and Analytic Epidemiology, Melbourne School of Population Health, The University of Melbourne, Parkville Victoria, 3010, Australia
References
- 1.Lynch HT, Boland CR, Gong G, Shaw TG, Lynch PM, Fodde R, Lynch JF, de la Chapelle A. Phenotypic and genotypic heterogeneity in the Lynch syndrome: diagnostic, surveillance and management implications. Eur J Hum Genet. 2006;14:390–402. doi: 10.1038/sj.ejhg.5201584. [DOI] [PubMed] [Google Scholar]
- 2.Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, Mecklin JP, Jarvinen H, Powell SM, Jen J, Hamilton SR, et al. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–6. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 3.Peltomaki P, Lothe RA, Aaltonen LA, Pylkkanen L, Nystrom-Lahti M, Seruca R, David L, Holm R, Ryberg D, Haugen A, et al. Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res. 1993;53:5853–5. [PubMed] [Google Scholar]
- 4.Woods MO, Williams P, Careen A, Edwards L, Bartlett S, McLaughlin JR, Younghusband HB. A new variant database for mismatch repair genes associated with Lynch syndrome. Hum Mutat. 2007;28:669–73. doi: 10.1002/humu.20502. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Wang W, Lee S, Nafa K, Lee J, Romans K, Watson P, Gruber SB, Euhus D, Kinzler KW, Jass J, Gallinger S, et al. Prediction of germline mutations and cancer risk in the Lynch syndrome. JAMA. 2006;296:1479–87. doi: 10.1001/jama.296.12.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunlop MG, Farrington SM, Carothers AD, Wyllie AH, Sharp L, Burn J, Liu B, Kinzler KW, Vogelstein B. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105–10. doi: 10.1093/hmg/6.1.105. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins MA, Baglietto L, Dowty JG, Van Vliet CM, Smith L, Mead LJ, Macrae FA, St John DJ, Jass JR, Giles GG, Hopper JL, Southey MC. Cancer risks for mismatch repair gene mutation carriers: a population-based early onset case-family study. Clin Gastroenterol Hepatol. 2006;4:489–98. doi: 10.1016/j.cgh.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Hampel H, Stephens JA, Pukkala E, Sankila R, Aaltonen LA, Mecklin JP, de la Chapelle A. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology. 2005;129:415–21. doi: 10.1016/j.gastro.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491–6. doi: 10.1136/jmg.2004.024299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arrigoni A, Sprujevnik T, Alvisi V, Rossi A, Ricci G, Pennazio M, Spandre M, Cavallero M, Bertone A, Foco A, Rossini FP. Clinical identification and long-term surveillance of 22 hereditary non-polyposis colon cancer Italian families. Eur J Gastroenterol Hepatol. 2005;17:213–9. doi: 10.1097/00042737-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Boilesen AE, Bisgaard ML, Bernstein I. Risk of gynecologic cancers in Danish hereditary non-polyposis colorectal cancer families. Acta Obstet Gynecol Scand. 2008;87:1129–35. doi: 10.1080/00016340802443806. [DOI] [PubMed] [Google Scholar]
- 12.Kastrinos F, Stoffel EM, Balmana J, Steyerberg EW, Mercado R, Syngal S. Phenotype comparison of MLH1 and MSH2 mutation carriers in a cohort of 1,914 individuals undergoing clinical genetic testing in the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2044–51. doi: 10.1158/1055-9965.EPI-08-0301. [DOI] [PubMed] [Google Scholar]
- 13.Gerritzen LH, Hoogerbrugge N, Oei AL, Nagengast FM, van Ham MA, Massuger LF, de Hullu JA. Improvement of endometrial biopsy over transvaginal ultrasound alone for endometrial surveillance in women with Lynch syndrome. Fam Cancer. 2009;8:391–7. doi: 10.1007/s10689-009-9252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renkonen-Sinisalo L, Butzow R, Leminen A, Lehtovirta P, Mecklin JP, Jarvinen HJ. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int J Cancer. 2007;120:821–4. doi: 10.1002/ijc.22446. [DOI] [PubMed] [Google Scholar]
- 15.Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, Hall D, Hopper JL, Jass J, Le Marchand L, Limburg P, Lindor N, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2331–43. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 16.Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, Walsh-Vockley C, Petersen GM, Walsh MD, Leggett BA, Young JP, Barker MA, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–8. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 17.Baudhuin LM, Mai M, French AJ, Kruckeberg KE, Swanson RL, Winters JL, Courteau LK, Thibodeau SN. Analysis of hMLH1 and hMSH2 gene dosage alterations in hereditary nonpolyposis colorectal cancer patients by novel methods. J Mol Diagn. 2005;7:226–35. doi: 10.1016/S1525-1578(10)60549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch HT, Harris RE, Lynch PM, Guirgis HA, Lynch JF, Bardawil WA. Role of heredity in multiple primary cancer. Cancer. 1977;40:1849–54. doi: 10.1002/1097-0142(197710)40:4+<1849::aid-cncr2820400813>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 19.Mecklin JP, Jarvinen HJ. Clinical features of colorectal carcinoma in cancer family syndrome. Dis Colon Rectum. 1986;29:160–4. doi: 10.1007/BF02555012. [DOI] [PubMed] [Google Scholar]
- 20.Lu KH, Dinh M, Kohlmann W, Watson P, Green J, Syngal S, Bandipalliam P, Chen LM, Allen B, Conrad P, Terdiman J, Sun C, et al. Gynecologic cancer as a "sentinel cancer" for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol. 2005;105:569–74. doi: 10.1097/01.AOG.0000154885.44002.ae. [DOI] [PubMed] [Google Scholar]
- 21.Walsh MD, Cummings MC, Buchanan DD, Dambacher WM, Arnold S, McKeone D, Byrnes R, Barker MA, Leggett BA, Gattas M, Jass JR, Spurdle AB, et al. Molecular, pathologic, and clinical features of early-onset endometrial cancer: identifying presumptive Lynch syndrome patients. Clin Cancer Res. 2008;14:1692–700. doi: 10.1158/1078-0432.CCR-07-1849. [DOI] [PubMed] [Google Scholar]
- 22.Lu KH, Schorge JO, Rodabaugh KJ, Daniels MS, Sun CC, Soliman PT, White KG, Luthra R, Gershenson DM, Broaddus RR. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25:5158–64. doi: 10.1200/JCO.2007.10.8597. [DOI] [PubMed] [Google Scholar]
- 23.Jarvinen HJ, Mecklin JP, Sistonen P. Screening reduces colorectal cancer rate in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 1995;108:1405–11. doi: 10.1016/0016-5085(95)90688-6. [DOI] [PubMed] [Google Scholar]
- 24.Dove-Edwin I, Sasieni P, Adams J, Thomas HJ. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. BMJ. 2005;331:1047–52. doi: 10.1136/bmj.38606.794560.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rijcken FE, Mourits MJ, Kleibeuker JH, Hollema H, van der Zee AG. Gynecologic screening in hereditary nonpolyposis colorectal cancer. Gynecol Oncol. 2003;91:74–80. doi: 10.1016/s0090-8258(03)00371-8. [DOI] [PubMed] [Google Scholar]
- 26.Nasri MN, Coast GJ. Correlation of ultrasound findings and endometrial histopathology in postmenopausal women. Br J Obstet Gynaecol. 1989;96:1333–8. doi: 10.1111/j.1471-0528.1989.tb03233.x. [DOI] [PubMed] [Google Scholar]
- 27.Nasri MN, Shepherd JH, Setchell ME, Lowe DG, Chard T. Sonographic depiction of postmenopausal endometrium with transabdominal and transvaginal scanning. Ultrasound Obstet Gynecol. 1991;1:279–83. doi: 10.1046/j.1469-0705.1991.01040279.x. [DOI] [PubMed] [Google Scholar]
- 28.Schmeler KM, Lynch HT, Chen LM, Munsell MF, Soliman PT, Clark MB, Daniels MS, White KG, Boyd-Rogers SG, Conrad PG, Yang KY, Rubin MM, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–9. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 29.Watson P, Vasen HF, Mecklin JP, Bernstein I, Aarnio M, Jarvinen HJ, Myrhoj T, Sunde L, Wijnen JT, Lynch HT. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. 2008;123:444–9. doi: 10.1002/ijc.23508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrow E, Robinson L, Alduaij W, Shenton A, Clancy T, Lalloo F, Hill J, Evans DG. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet. 2009;75:141–9. doi: 10.1111/j.1399-0004.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 31.Ries L, Eisner M, Kosary C, Hankey B, Miller B, Clegg L, Mariotto A, Feuer E, Edwards Be. SEER Cancer Statistics Review, 1975–2002. National Cancer Institute; 2005. [Google Scholar]
- 32.Sinha A, Nwosu EC. Endometrial polyp and the levonorgestrel intrauterine system--a case report and literature review. J Obstet Gynaecol. 2002;22:695. doi: 10.1080/014436102762062448. [DOI] [PubMed] [Google Scholar]
- 33.Giannopoulos T, Butler-Manuel S, Tailor A. Levonorgestrel-releasing intrauterine system (LNG-IUS) as a therapy for endometrial carcinoma. Gynecol Oncol. 2004;95:762–4. doi: 10.1016/j.ygyno.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Wildemeersch D, Dhont M. Treatment of nonatypical and atypical endometrial hyperplasia with a levonorgestrel-releasing intrauterine system. Am J Obstet Gynecol. 2003;188:1297–8. doi: 10.1067/mob.2003.346. [DOI] [PubMed] [Google Scholar]


