Abstract
Objective
Docosahexaenoic acid (DHA) is a dietary fatty acid with neuroprotective properties. We hypothesized that DHA treatment after hypoxia-ischemia (HI) would improve function and reduce brain volume loss in a perinatal rat model.
Study design
Seven-day-old Wistar rat pups from 7 litters (N=84) underwent right carotid ligation, followed by 8% O2 for 90 minutes. Fifteen minutes after HI, pups were divided into 3 treatment groups (intraperitoneal injections of DHA 1, 2.5 or 5 mg/kg) and 2 control groups (25% albumin or saline). At 14 days, rats underwent vibrissae-stimulated forepaw placing testing, and bilateral regional volumes were calculated for cortex, striatum, hippocampus, and hemisphere.
Results
Post HI treatment with DHA significantly improved vibrissae forepaw placing (complete responses: 8.5±2 treatment vs. 7.4±2 controls; normal=10; p = 0.032, t-test). Post injury DHA treatment did not attenuate brain volume loss in any region.
Conclusion
Post-hypoxia-ischemia DHA treatment significantly improves functional outcome.
Keywords: Docosahexaenoic Acid, Hypoxia ischemia, neuroprotection, perinatal rescue therapy
Background
In a recent systematic review, 14% of cases of cerebral palsy among non-anomalous term infants were associated with intrapartum hypoxia-ischemia.1 Neonatal hypoxic-ischemic encephalopathy (HIE) complicates approximately 2-9 per 1000 live births.1-3 Although the majority of infants with mild HIE will develop normally, approximately one-third to one-half of infants with moderate HIE and the vast majority of surviving infants with severe HIE will ultimately be found to have neurodevelopmental disability.4,5 Many recent investigations have focused on the pre-clinical development of strategies to rescue the immature brain after a hypoxic-ischemic insult. Perinatal hypoxic-ischemic brain injury involves an acute injury phase followed by a reperfusion phase with a six to eight hour therapeutic window during which there is potential for attenuation of perinatal brain injury.6,7 Investigators have studied a number of agents with post-HI neuroprotective properties, including allopurinol, magnesium sulfate, simvastatin, erythropoietin, isofluorane, topiramate, and matrix metalloproteinase inhibition.6,8 Most of these neuroprotective modalities target a particular stage of the brain injury cascade, but therapeutic use of several of these agents is limited by safety or toxicity considerations.6,8 Of measures designed for post-hypoxia “rescue” treatment, only therapeutic hypothermia has been tested in large randomized controlled trials and has entered into neonatal clinical practice.9,10 [NNT=7].
Because of its well-established safety record and its ability to readily cross the blood-brain barrier, docosahexaenoic acid (DHA) is an especially promising neuroprotective agent.11 DHA is a long chain polyunsaturated fatty acid available in the diet. Along with its major metabolite, Neuroprotectin D1, DHA has been shown to have neuroprotective properties in animal models of adult brain and spinal cord injury.12-16 DHA may simultaneously attenuate several stages of the brain injury cascade including bioactive lipid mediators, free radicals, inflammatory cytokines and apoptosis.11, 12, 17 We have previously demonstrated that DHA pretreatment improves function and reduces brain volume loss in a rodent model of perinatal hypoxia-ischemia.18 We have chosen to use the Rice Vannucci model, a highly characterized model of perinatal hypoxemia that is well suited for assessment of neurodevelopmental outcomes and neuropathology.19 For our studies to date, we have chosen a regimen that incorporates DHA complexed to albumin as originally described by Belayev et al in an adult stroke model.11 The objective of the current investigation is to test whether “rescue” treatment with DHA after hypoxia-ischemia will improve neurologic function and reduce volume loss in a rodent model simulating perinatal hypoxia-ischemia.
Materials and Methods
Preparation of DHA-Albumin Complex
DHA (Sigma, St. Louis, Mo, Cat#: D2534 as cis-4,7,10,13,16,19-DHA) was complexed to human albumin by incubating 4mL of human serum albumin 25% (Baxter, Deerfield, IL) with 4mg of DHA to yield a final concentration of DHA 25mg/25μL. Each vial was aliquoted in 1-mg/mL samples and kept under nitrogen in a -20°C freezer. Nitrogen was reapplied to the vials weekly.
Animals
Postnatal day 7 (P7) Wistar rats were obtained in litters adjusted to equal sex distribution (Charles River Laboratories, Portage, MI). Animals were treated in accordance with protocols approved by our University Committee on the Use and Care of Animals in research. Pups were housed with the dam and littermates throughout the duration of the experiments.
To test the effect of post hypoxia-ischemia DHA treatment, seven day old (P7) Wistar rat pups from 7 litters (N=84) were randomly divided into three treatment groups and two control groups. Treatment groups received intraperitoneal (IP) injections of DHA 1mg/kg, 2.5 mg/kg or 5 mg/kg as DHA-albumin complex. Control groups received a similar volume of 25% albumin or normal saline (NaCl). There were at least two pups (one male and one female) in each litter from each of the three treatment groups and each of the two control groups. Because each litter contained 12 animals, each litter included four animals from one of the five groups, which varied from litter to litter to balance out the sample size among treatment groups across all litters.
P7 pups anesthetized with isoflourane (induction at 3.5%, maintenance at 1.5%) underwent right common carotid artery double ligation and division through a ventral neck incision. Pups were returned to their dams and recovered for 1.5 hours at 37°C. After carotid ligation and recovery, HI was induced by placing the pups in 500mL jars partially submerged in a water bath at 36°C in 8% oxygen for 90 minutes to induce unilateral cerebral HI. In this model, unilateral carotid ligation alone or 90 minute hypoxia exposure alone do not produce detectible brain injury.20 However, unilateral carotid ligation followed by timed hypoxia exposure induces ipsilateral cerebral ischemia during the hypoxia exposure followed by reperfusion immediately after return to room air.21 Fifteen minutes after HI, the pups received control or treatment IP injections. Once normal activity was resumed, the pups were returned to the dam where they remained until P14. Pups were weighed before HI on P7 and subsequently on P14.
Vibrissae Stimulated Forepaw Placing Test
On P14, we evaluated sensorimotor outcome using the vibrissae-stimulated forepaw placing test, as previously described.18 This test is a quantifiable functional measure of injury to the contralateral sensorimotor cortex or striatum.22 Briefly, stimulation of the vibrissae (whiskers) on a surface edge results in extension of the forepaw on the same side as the simulated whiskers to reach the stimulating surface, in a complete response. At P14, the typical response contralateral to the non-lesioned hemisphere is immediate extension of the forepaw to contact the stimulating surface in 10 out of 10 trials. In a partial response, the forepaw is incompletely extended without contacting the stimulating surface. A weighted vibrissae score to incorporate data from both complete and partial responses was calculated using the formula [partial contacts + 2 * (complete contacts)].
Histopathology
Severity of brain injury was evaluated on P14 by calculating volume of tissue with intact staining using ImageJ software (U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/), in regularly spaced cresyl violet stained coronal sections, as previously described.18 Volumes were calculated from hemispheric and regional (cortex, striatum, hippocampus, and “other”) area measurements in regularly spaced frozen coronal 20 μm sections. Volume was estimated by summing areas and multiplying by the distance between sections. The “other” region incorporated all remaining intact tissue in the hemisphere other than cortex, striatum or hippocampus and included thalamus, septum, fimbria-fornix, corpus callosum, and other major white matter tracts. Thus hemisphere area in each section was the sum of cortex, striatum, hippocampus and “other”.
Statistical Analysis
A linear mixed models analysis of variance was used to evaluate differences in percent damage for hemisphere and each brain region among groups. We used litter as a random effect, treatment and sex as fixed effects, and evaluated treatment by sex interaction. A similar linear mixed model was used to compare forepaw placing successes among treatments. Post hoc comparisons of treatment group means were carried out using the Fischer Protected least significant difference (PLSD) test. The simplest model containing only treatment as a predictor with litter as a random effect was obtained. Our sample size (16-17/group) was chosen both based on our prior experience with DHA treatment prior to HI and on a sample size calculation in which 16 subjects/group is sufficient to detect a between-group difference of one standard deviation with a power of 0.8 and alpha of 0.05.
Results
Survival/Morbidity
Of the eighty-four pups that underwent the HI procedures, 77 survived (91%) during the experiments. In one litter, after seven days of housing, the dam appeared unwell. Within this litter, five of the 12 pups had died by P14; the remainder appeared unwell and unusually small. Because of concerns regarding dam and litter health, statistical analysis did not include this litter. Two pups from another litter died between P7 and P14, and were excluded from analysis of pathology and function (one received albumin, the other received NS). Final data analysis included 70 animals.
Body Weights
There were no differences in initial weight or weight change from P7 to P14 among the three treatment groups or between treatment groups and controls.
Vibrissae Stimulated Forepaw Placing Test
Post HI treatment with DHA improved the contralateral (left) vibrissae forepaw placing response on P14 (p = 0.035, ANOVA, Figure 1). By post-hoc testing, functional outcome with the DHA 2.5 mg/kg dose was superior to control groups and DHA 1mg/kg. Comparing pooled treatment groups to pooled control groups revealed an overall beneficial effect of DHA treatment (weighted scores: 17.4±3.5 treatment vs. 15.4±3.6 controls; normal function=20, p = 0.032, t-test; complete responses: 8.5±2 treatment vs. 7.4 ± 2 controls, p = 0.032, t-test). There was no effect of sex on vibrissae response. No litter effects on forepaw placing responses were seen. Right vibrissae stimulation consistently elicited correct right paw placement 10 out of 10 trials in all groups.
Figure 1. DHA Treatment after hypoxia ischemia attenuates forepaw placing deficits among hypoxic ischemic pups.
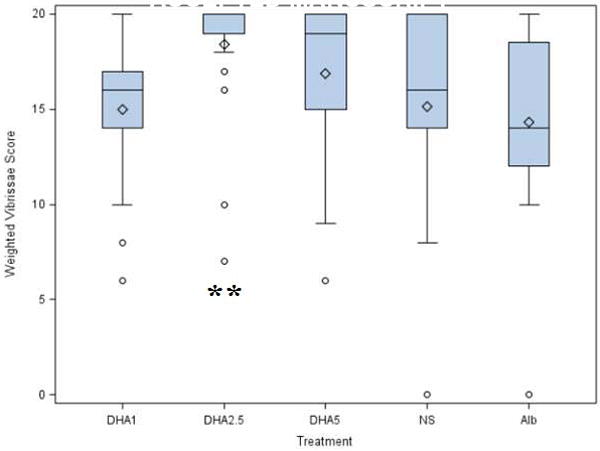
Vibrissae-stimulated forepaw placing (10 trials/side) was evaluated on postnatal-day-14 (P14) in pups that underwent right carotid ligation followed by 90 minutes of hypoxia at 8% oxygen followed by administration of treatment (DHA 1mg/kg, 2.5mg/kg or 5mg/kg) or control (albumin, normal saline (NS)) on P7. The y-axis represents the weighted response score (see Methods; best possible score is 20). Boxes represent interquartile range; horizontal bars represent medians. Whiskers extend to data points no more than 1.5 width of box. There is an overall beneficial effect of DHA treatment when comparing pooled treatment (3 DHA doses) with pooled control groups (NaCl and Albumin (p = 0.035, t-test). ** Vibrissae scores are significantly better with DHA 2.5 mg/kg than 1 mg/kg or either control group (p = 0.032, Tukey Kramer). Because there is minimal variability in treatment groups, the circles represent the artificial distinction of outlier and stars represent extreme values.
Histopathology
Ninety minutes of HI resulted in moderately severe right hemisphere damage as demonstrated by the NaCl group who had striatal and hippocampal atrophy, cortical thinning and/or cystic infarction [see Table 1]. No differences in hemisphere or regional volumes were detected when comparing the three treatment groups and the control groups [see Table 1]. Neither sex or litter effects nor interactions were detected.
Table 1.
Comparison of regional tissue volumes among groups, by region
| Injection group * | n | (L) Striatum | (R) Striatum | (L) Cortex | (R) Cortex | (L) “ Other” | (R) “ Other” | (L) Hippocampus | (R) Hippocampus | (L) Hemisphere | (R) Hemisphere |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DHA5 | 12 | 18.2±1.2 | 13.1±1.3 | 53.6±7.7 | 35.4±7.5 | 61.7±9.2 | 41.9±9.1 | 6.4±1.6 | 3.3±1.6 | 139.9±18.8 | 93.7±18.9 |
| DHA2.5 | 16 | 18.9±1.3 | 12.6±1.2 | 57.9±7.3 | 37.8±7.2 | 65.0±9.1 | 43.1±9.0 | 7.6±1.6 | 4.0±1.6 | 149.4±18.7 | 97.5±18.7 |
| DHA1 | 14 | 17.3±1.3 | 11.3±1.3 | 52.8±7.3 | 32.4±7.4 | 60.6±9.0 | 43.1±9.1 | 6.5±1.6 | 3.1±1.6 | 137.2±18.8 | 88.9±18.8 |
| Albumin | 13 | 19.3±1.2 | 11.9±1.3 | 58.1±7.4 | 32.7±7.4 | 64.9±9.1 | 40.5±9.1 | 7.5±1.6 | 3.5±1.6 | 149.8±18.8 | 88.6±18.8 |
| NS | 15 | 17.8±1.3 | 12.8±1.3 | 51.8±7.3 | 33.6±7.3 | 51.6±9.1 | 42.8±9.1 | 5.2±1.6 | 3.5±1.6 | 126.4±18.7 | 92.7±18.8 |
Animals received I.P. injections of DHA 1 mg/kg, 2.5 mg/kg, 5 mg/kg (see Methods) or an equivalent volume of 25% Albumin or normal saline (NS) immediately following 90 minutes right cerebral hypoxia-ischemia on postnatal day 7 (P7).
(L)=left, (R)=right
Values are mean ± standard deviation regional volume on P14, in mm3
“Other” refers to any intact standing tissue other than hippocampus, cortex, or striatum
Discussion
The identification of intervention strategies to achieve improved function is a central concern in obstetrical practice. Post-ischemic neuroprotective interventions may be of more therapeutic value than pre-ischemic treatments, given the difficulty to prospectively identify which fetuses are at risk for antenatal or intrapartum HI. The objective of our study was to examine whether treatment with DHA immediately after cerebral hypoxia-ischemia would improve functional outcome and reduce brain volume loss in neonatal rats. Post-injury DHA treatment significantly improved contralateral sensorimotor function compared to controls, as demonstrated with the vibrissae forepaw placing response.
We and others have previously reported that DHA for neuroprotection appears to have an inverted U-shaped dose-response curve.18 11, 23 In our current study, the 2.5 mg/kg dose was more effective than the 1 mg/kg and the 5 mg/kg dose in preserving function, although the difference between the 2.5 and 5 mg doses was not statistically significant. This dose-response characteristic has also been reported by Belayev, et al, who in 2 sets of studies in a rat model of adult experimental stroke, found that low and moderate doses of DHA were more neuroprotective than higher DHA doses.11, 23 These investigators speculated that at higher doses, DHA may serve as a substrate for lipid peroxidation and may thus exert neurotoxic effects.11
In our model, although functional outcome was improved, post injury DHA treatment did not attenuate brain volume loss. This result differs from the findings of Belayev et al, who in an adult rat model of DHA post-treatment after experimental stroke noted both functional and histologic improvement with low and medium but not high dose DHA post-treatment.23 In Belayev’s recent study, DHA was not bound to albumin. It is possible that our use of DHA complexed to albumin reduced the neuroprotective efficacy of DHA by reducing the concentration of DHA reaching the brain, as DHA may be tightly bound to albumin.23
It is also possible that repetitive post-ischemic dosing with DHA would have been necessary to attenuate brain volume loss. Our laboratory has previously tested another neuroprotective agent, Topiramate, in a perinatal hypoxia-ischemia model.24 In that study we found that a single post-ischemic topiramate dose did not reduce brain volume loss compared to controls. However, other investigators have demonstrated robust reduction of brain volume loss with repeated topiramate dosing.25
Several authors have reported discordance between histologic brain injury assessment and functional outcome. Weber et al. demonstrated that in an adult rat model of experimental stroke, lesion volume did not correlate with functional deficit.26 Similarly, in a recently-described precocial spiny mouse model of perinatal asphyxia, Hutton et al. reported that asphyxiated neonatal mice exhibited significant functional deficits in the absence of measurable brain volume loss.27 As Weber noted, functional outcome will ultimately be the most meaningful measure of therapeutic success of treatment of neurologic diseases.26
Potential mechanisms whereby DHA may improve function even in the absence of reduced volume loss include increased neural cell membrane fluidity and permeability,28, 29 and alterations in neurotransmitter signaling. 30,31 Neuroprotectin D1, a metabolite of DHA, counteracts proinflammatory responses in cerebral ischemia-reperfusion.13 Although we did not evaluate the tissue inflammatory response in this study, it is possible that a DHA-initiated reduction in brain tissue inflammation might have beneficial effects on function.
A limitation of our study is that this model of focal cerebral ischemia may not perfectly replicate the global events experienced in human perinatal hypoxia-ischemia.27 Additionally, evaluation on P14 assesses short term outcome; we have not yet assessed the long-term durability of these beneficial effects, nor have we explored the molecular mechanisms leading to the observed benefits.
In aggregate, our series of experiments with DHA treatment before or after perinatal HI has consistently demonstrated improvement in short-term function after hypoxia-ischemia in a perinatal rat model, although effects on histopathology have been inconsistent.18 Combining the results of the present experiments and our previous studies, we have demonstrated that DHA pretreatment improves functional outcomes after HI alone18 and after HI potentiated by LPS.32 We have demonstrated that DHA improves functional outcome when given after HI during the evolution of brain injury. There is clearly discordance between functional and structural evidence of benefit. However, in terms of translation to human studies, the outcome of most clinical interest and significance would be function, not neuropathology.
Further studies are needed to test the long-term persistence of the functional benefits of DHA treatment after HI. Likewise, studies are needed to determine whether both neuropathology and function may be improved with repetitive DHA dosing. It also remains to be established whether DHA post-treatment may augment the beneficial effects of hypothermia, the current standard of care for term or near term infants with hypoxic-ischemic encephalopathy. These questions need to be answered before DHA can be introduced into human perinatal trials.
Acknowledgments
The authors thank Edward D. Rothman, PhD, and Kathleen B. Welch, MPH, MS, at the University of Michigan Center for Statistical Consultation and Research for statistical advice and assistance.
This work was supported through the University of Michigan and the NIH/NCRR CTSA grant UL1 RR024986.
Footnotes
This paper was presented as a poster at The Society for Maternal Fetal Medicine (SMFM) 2010 Annual Meeting in Chicago, Illinois February 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199:587–95. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 2.Wu YW, Backstrand KH, Zhao S, Fullerton HJ, Johnston SC. Declining diagnosis of birth asphyxia in California: 1991-2000. Pediatrics. 2004;114:1584–90. doi: 10.1542/peds.2004-0708. [DOI] [PubMed] [Google Scholar]
- 3.Thornberg E, Thiringer K, Odeback A, Milsom I. Birth asphyxia: Incidence, clinical course and outcome in a Swedish population. Acta Pædiatr. 1995;84:927. doi: 10.1111/j.1651-2227.1995.tb13794.x. [DOI] [PubMed] [Google Scholar]
- 4.de Vries LS, Cowan FM. Evolving understanding of hypoxic-ischemic encephalopathy in the term infant. Semin Pediatr Neurol. 2009;16:216–25. doi: 10.1016/j.spen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Ambalavanan N, Carlo W, Shankaran S, Bann C, Emrich S, Higgins R, et al. Predicting outcomes of neonates diagnosed with hypoxemic-ischemic encephalopathy. Pediatrics. 2006;118:2084. doi: 10.1542/peds.2006-1591. [DOI] [PubMed] [Google Scholar]
- 6.van Bel F, Groenendaal F. Long-term pharmacologic neuroprotection after birth asphyxia: Where do we stand? Neonatology. 2008;94:203. doi: 10.1159/000143723. [DOI] [PubMed] [Google Scholar]
- 7.Grow J, Barks JDE. Pathogenesis of hypoxic-ischemic cerebral injury in the term infant: Current concepts. Clin Perinatol. 2002;29:585–602. doi: 10.1016/s0095-5108(02)00059-3. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez FF, Ferriero DM. Neuroprotection in the newborn infant. Clin Perinatol. 2009;36:859–80. doi: 10.1016/j.clp.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulzke SM, Rao S, Patole SK. A systematic review of cooling for neuroprotection in neonates with hypoxic ischemic encephalopathy - are we there yet? BMC Pediatr. 2007;7:30. doi: 10.1186/1471-2431-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. The Cochrane database of systematic reviews. 2007:CD003311. doi: 10.1002/14651858.CD003311.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Belayev L, Marcheselli VL, Khoutorova L, Rodriguez de Turco EB, Busto R, Ginsberg MD, et al. Docosahexaenoic acid complexed to albumin elicits high-grade ischemic neuroprotection. Stroke. 2005;36:118–23. doi: 10.1161/01.STR.0000149620.74770.2e. [DOI] [PubMed] [Google Scholar]
- 12.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10:136–41. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- 13.Bazan NG. Lipid signaling in neural plasticity, brain repair, and neuroprotection. Mol Neurobiol. 2005;32:89–103. doi: 10.1385/MN:32:1:089. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–6. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serhan CN, Arita M, Hong S, Gotlinger K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39:1125. doi: 10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- 16.Huang WL, King VR, Curran OE, Dyall SC, Ward RE, Lal N, et al. A combination of intravenous and dietary docosahexaenoic acid significantly improves outcome after spinal cord injury. Brain. 2007;130:3004–19. doi: 10.1093/brain/awm223. [DOI] [PubMed] [Google Scholar]
- 17.Yavin E. Versatile roles of docosahexaenoic acid in the prenatal brain: From pro- and anti-oxidant features to regulation of gene expression. Prostaglandins, Leukot Essent Fatty Acids. 2006;75:203–11. doi: 10.1016/j.plefa.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Berman DR, Mozurkewich E, Liu Y, Barks J. Docosahexaenoic acid pretreatment confers neuroprotection in a rat model of perinatal cerebral hypoxia-ischemia. Am J Obstet Gynecol. 2009;200:305.e1–6. doi: 10.1016/j.ajog.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yager JY, Ashwal S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr Neurol. 2009;40:156–67. doi: 10.1016/j.pediatrneurol.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–41. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 21.Vannucci R, Vannucci S. Perinatal hypoxic-ischemic brain damage: Evolution of an animal model. Dev Neurosci. 2005;27:81–6. doi: 10.1159/000085978. [DOI] [PubMed] [Google Scholar]
- 22.Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRX. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belayev L, Khoutorova L, Atkins K, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke. 2009;40:3121–6. doi: 10.1161/STROKEAHA.109.555979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Barks JD, Xu G, Silverstein FS. Topiramate extends the therapeutic window for hypothermia-mediated neuroprotection after stroke in neonatal rats. Stroke. 2004;35:1460–5. doi: 10.1161/01.STR.0000128029.50221.fa. [DOI] [PubMed] [Google Scholar]
- 25.Noh M, Kim SK, Sun W, Park SK, Choi HC, Lim JH, et al. Neuroprotective effect of topiramate on hypoxic ischemic brain injury in neonatal rats. Exp Neurol. 2006;201:470–8. doi: 10.1016/j.expneurol.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 26.Weber R, Ramos-Cabrer P, Justicia C, Wiedermann D, Strecker C, Sprenger C, et al. Early prediction of functional recovery after experimental stroke: Functional magnetic resonance imaging, electrophysiology, and behavioral testing in rats. J Neurosci. 2008;28:1022–9. doi: 10.1523/JNEUROSCI.4147-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutton L, Ratnayake U, Shields A, Walker D. Neuropathology and functional deficits in a model of birth asphyxia in the precocial spiny mouse (Acomys Cahirinus) Dev Neurosci. 2009;31:523–35. doi: 10.1159/000251907. [DOI] [PubMed] [Google Scholar]
- 28.Stillwell W, Wassall SR. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 29.Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr. 2007;137:855–9. doi: 10.1093/jn/137.4.855. [DOI] [PubMed] [Google Scholar]
- 30.Schuchardt JP, Huss M, Stauss-Grabo M, Hahn A. Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur J Pediatr. 2010;169:149–64. doi: 10.1007/s00431-009-1035-8. [DOI] [PubMed] [Google Scholar]
- 31.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, et al. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–21. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berman D, Liu Y, Barks J, Mozurkewich E. Docosahexaenoic acid confers neuroprotection in a rat model of perinatal hypoxia-ischemia potentiated by Escherichia coli lipopolysaccharide-induced systemic inflammation. Am J Obstet Gynecol. 2010 Mar 29; doi: 10.1016/j.ajog.2010.01.076. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


