Summary
Apicomplexan parasites utilize a unique form of “gliding motility” to traverse across substrates, migrate through tissues, and invade into and finally egress from their vertebrate host cells. Parasite gliding relies on the tread milling of surface adhesins linked to short actin filaments that are translocated rearward by a stationary small myosin motor. New details reveal mechanistic insight into the coordinated release and processing of adhesins, the complexity of adhesin-substrate interactions, the regulation of the actin-myosin motor complex, and the formation of a novel junction at the host-parasite interface. These activities are carefully orchestrated to provide an efficient process for motility that is essential for parasite survival. The parasite-specific nature of many of these steps reveals several essential points that may be targeted for intervention.
Introduction
Apicomplexan parasites infect a wide range of vertebrate hosts including humans where they are responsible for severe diseases such as malaria, toxoplasmosis, and cryptosporidiosis. Apicomplexans are unified by a specialized cytoskeleton [1], apically-directed secretory organelles, and a unique form of motility called “gliding” [2]. Unlike motility mechanisms based on cilia, flagella, or crawling, gliding operates by translocation of adhesive proteins along the cell surface in process akin to moving cargo along a conveyor belt. Apical discharge of adhesive proteins is coupled to their reward translocation by an actin-myosin motor and ultimate cleavage from the surface by intramembrane proteases called rhomboids [2]. This system has been most extensively studied in T. gondii due to its facility for cell biological, biochemical, and genetic studies, although a similar process is conserved in other apicomplexans, including Gregarines, which are common endoparasites of invertebrates [2]. Gliding is essential for the active invasion of host cells by T. gondii and is responsible for tissue migration and hence is a key component of virulence [3]. Gliding motility is also essential in the biology of Plasmodium sporozoites both for entry into the salivary glands in the mosquito insect host and in the vertebrate host where it plays important roles in migration through the skin as well as traversal though and invasion into hepatocytes [4]. Although Plasmodium merozoites do not display gliding motility on substrates, they nonetheless enter red blood cells by a similar active invasion process [5].
Due to their small size and corresponding low Reynolds number, the movement of parasites is dominated by viscous forces and they are not able to drift using inertial motion, but rather must continually crawl along the substrate as they burrow into tissues and cells. In doing so, they face several fundamental challenges including control of directionality, adhesion to the substrate (i.e. traction), power generation, and release from the substratum to allow forward movement. The complex machinery that governs gliding is largely unique to apicomplexans, although many underlying core aspects are shared among many systems, such as cytoskeletal components, adhesive domains, and proteases. Identifying those essential and unique aspects of the parasite machinery that governs gliding is the first step to validating potential targets for improved therapeutic intervention. This review summarizes recent work focused on defining the molecular mechanisms of key steps in gliding motility by apicomplexans.
Setting course: directional discharge and gaining traction
Discharge of adhesive proteins from apical storage organelles called micronemes is stimulated by contact with host cells and this process is regulated by increases in intracellular calcium within the parasite [6] (Fig. 1). Microneme secretion is also essential for gliding motility, a process that depends on contact with the substratum and leaves characteristic “trails” consisting of shed surface proteins (Fig. 1A). Gliding is controlled by secretion of microneme proteins and gents that alter calcium fluctuations in the cytosol, and hence stimulate microneme discharge, enhance gliding, while chelation of intracellular calcium blocks parasite motility [6]. Recent studies indicate that calcium-dependent protein kinases are key mediators of signaling in apicomplexans [7]. This family of serine threonine (S/T) kinases is uniquely expanded in apicomplexans, and is related to kinases found in ciliate and plants but distinct from those found in animal cells [7]. The recently solved X-ray crystal structures of CDPK1 from T. gondii and C. parvum reveal interesting structural rearrangements that accompany activation [8,9]. CDPKs are unique in having an N-terminal S/T kinase domain fused to a C-terminal calmodulin like domain. In their inactive state, the four EF-hands comprise an elongated inhibitory domain that contacts the kinase domain [8,9]. Upon binding to calcium, the EF hand domains undergo a massive rearrangement to fold back on the opposite face of the kinase domain, thus exposing the substrate binding site and nucleotide pocket [8,9].
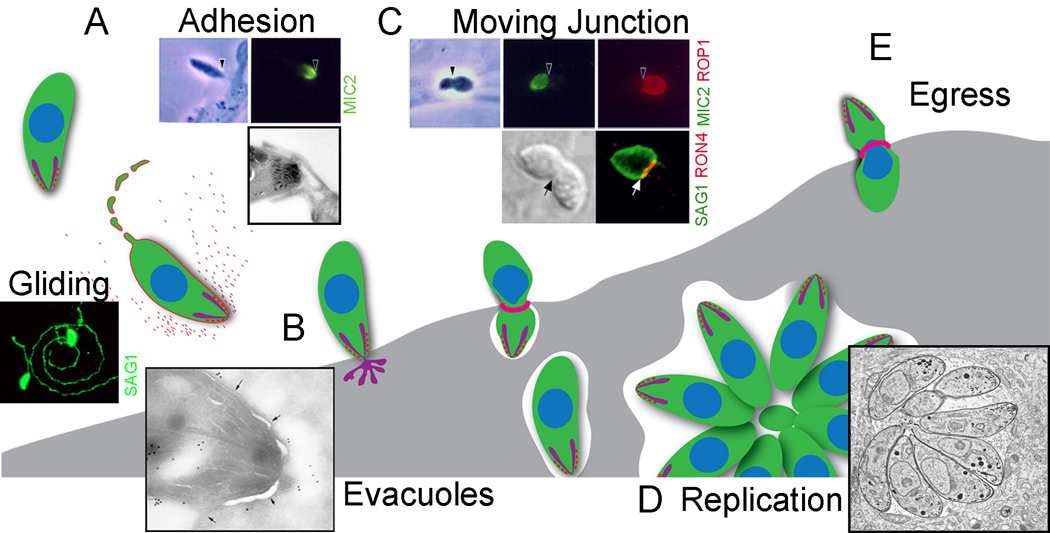
Figure 1.
Schematic depiction of intracellular cycle of T. gondii tachyzoites, which are representative of the invasive stages of most apicomplexans. A) Microneme secretion is required for gliding motility, cell attachment, and invasion, which are all powered by the parasite’s actin-myosin cytoskeleton. Gliding results in deposits of trails comprised of surface membrane proteins on the substratum (SAG1). During the initial contact with host cells, microneme secretion is stimulated by increases in intracellular calcium causing release of adhesive proteins at the apical end of the cell (i.e. MIC2). B) During invasion, discharge of rhoptry proteins results in formation of small vesicles within the host cell cytoplasm known as evacuoles [55]. In the electron micrograph at the lower left, the vacuole membrane is marked by arrows, while immunogold staining detects ROP1. C) Following formation of a tight junction, mediated by rhoptry neck proteins (i.e. RON4)(red ring), the parasite squeezes into the parasitophorous vacuole, which forms by invagination of the host cell membrane. The contents of rhoptries also decorate the PV membrane and fill the lumen as shown by immunofluorescence staining (i.e. ROP1 in the central top panel). D) Intracellular replication occurs by binary fission the parasite to form a rosette of daughter cells within the vacuole. E) The parasite actively egresses from the host cell, a process triggered by elevated calcium. Egress also requires microneme secretion, and is powered by the actin-myosin cytoskeleton, much the same as cell invasion. (Graphic drawing provided by S. Lourido, electron micrographs provided by W.L. Beatty, remaining panels adapted with permission from [2,18,27,55]).
Although apicomplexans contain a diverse array of CDPKs, preliminary evidence suggest that they are not redundant but rather control distinct aspects of the biology [7]. For example, genetic suppression of TgCDPK1 specifically blocks microneme secretion, leading to disruption of gliding motility, cell invasion, and egress [10]. Fortuitously, CPDK1 in T. gondii and C. parvum contain a small residue (Gly) at the so called gatekeeper position in the nucleotide binding pocket, rendering them naturally sensitive to analogs of pyrazolopyrimidines (PP1) [8–10]. Mutation of this small gatekeeper to a large bulky residue (Met) does not alter enzyme kinetics but confers resistance to PP1 analogs [10]. Allelic replacement of such mutants in T. gondii transfers resistance to PP1 analogs, which normally block cell invasion in vitro, thus validating that TgCDPK1 is the primary target of PP1 analogs [10]. Because nearly all mammalian kinases contain large gatekeeper residues, they are naturally resistant to PP1 analogs. Hence this chemical scaffold may provide a lead for developing improved inhibitors that block CDPK1 function and hence prevent infection. Another member of this family (i.e. CDPK3 in T. gondii or the orthologue known as CDPK1 in Plasmodium) may phosphorylate and hence regulate the myosin motor complex involved in motility [11], and a small molecular inhibitor of PfCDPK1 has also been described [12], although its specificity in vivo has yet to be demonstrated.
Among the proteins discharged from micronemes are the TRAP family of adhesins, which contain an integrin A domain and a variable number of thrombospondin TSR-1 domains [13]. Genetic disruption of TRAP in Plasmodium berghei, and suppression of the orthologue called MIC2 in T. gondii, impairs gliding motility and cell invasion, and several TRAP-related proteins have also been shown to be important for gliding motility and/or tissue migration in Plasmodium sporozoites [13]. Combined with evidence that these adhesins are translocated reward during gliding and cell invasion, these findings have lead to the prevailing model that gliding motility relies on treadmilling of adhesins, akin to a conveyor belt. Gliding motility of Plasmodium sporozoites in vitro occurs primarily in a circular pattern, although it shows much more complex patterns in vivo [14]. Likewise, tachyzoites of T. gondii undergo both circular and helical gliding, only the later of which leads to cell invasion [15].
Recent studies using reflection-interference or traction-force microscopy reveal new features of gliding motility that were not captured by previous techniques. Specifically, Plasmodium sporozoites were shown to move by a process of adhesion, release, and forward motion during which discrete portions of the crescent-shaped parasite change their contact points with the substratum [16]. Strong adhesion sites at the front and rear of the parasite are associated with initial attachment and breakage of these sites results in rapid forward motion [16]. Tractional forces at the poles orient with the direction of movement, although forces along the middle of the sporozoite run perpendicular to the axis of movement, likely reflecting attachment [16]. These studies also reveal important roles for TRAP in forming and breaking adhesion sites at the poles. Importantly, trap−/− parasites are still able to undergo pendulum movement and translocate beads along the cell surface, indicting that additional adhesins also contribute to actin-based motility [16]. Finally, perturbation of F-actin levels revealed that the cytoskeleton is involved in forming adhesive contacts with the substrate, in addition to its previously postulated role in translocation of adhesin-receptor complexes [16]. Thus far, such quantitative microscopy studies have focused on circular gliding sporozoites and it will be of interest to extend this approach to the more complex behaviors seen in vivo. Such methods would also be useful for analyzing mutants with altered motility. For example, a conditional knockout of mic2 in T. gondii shifts to predominately circular gliding, and consequently is impaired in invasion [17]. These patterns suggests that greater tractional forces are necessary to perform helical gliding and cell invasion, compared to the more simple circular pattern.
Anchorage: junction formation and entry
Following contact with the host cell, a second set of apical secretory organelles called rhoptries are discharged, injecting their contents into the forming vacuole and also into the host cell cytosol, where they for small vesicles known as “evacuoles” [18] (Fig. 1B). Rhoptries contain a diverse array of proteins including a polymorphic family of kinases, which are though to alter host cell functions [19,20]. During invasion of apicomplexans, a tight junction forms between the parasite and host membranes and is visible as a prominent constriction as the parasite squeezes into the host cell, stretching the host plasma membrane to form the vacuole (Fig. 1C). Although this interface has been called the “moving junction”, this is a misnomer as the junction itself remains fixed with respect to the host cell and substratum, while the parasite migrates past this point of anchorage. The process of invasion by T. gondii is rapid, occurring much faster than phagocytosis, and is accompanied by exclusion of many host proteins based both on membrane fluidity and interactions with the cytoskeleton [21,22]. Although early studies stressed the essential nature of parasite actin filaments in invasion [23], recent studies indicate that host cell actin may also participate in apicomplexan entry [24]. A variety of perturbations that alter actin dynamics, and hence reduce assembly of F-actin in host cells, where shown to decrease the invasion of T. gondii tachyzoites and P. berghei sporozoites into their respective host cells [24]. Interpretation of these findings is complicated by the fact that all of the conditions used to perturb assembly of new actin filaments also affect the steady state distribution between F and G actin. Hence, although these results have been interpreted to indicate that actin polymerization occurs at the site of entry, they could equally be explained by a requirement for a stable F-actin cytoskeleton in the host cell in order to provide anchorage of the moving junction.
Although the moving junction has been recognized morphologically for many decades, the proteins that contribute to this interface were only recently identified. Analysis of the proteins of the secretory organelles called rhoptries revealed a class of proteins found in the neck of this bulb-like organelle, and these have been called RONs, for rhoptry neck proteins [25]. Remarkably, several of these RON proteins are found in a complex along with the microneme protein AMA1, and these concentrate at the moving junction, forming a ring at the site of invasion [26,27] (Fig. 1C). Following the discovery of RON proteins in T. gondii, a similar complex was shown to participate in invasion of Plasmodium [28]. In T. gondii several RON proteins are inserted into the host cell plasma membrane and partially exposed to the host cell cytoplasm, suggesting one or more of them adopts a transmembrane topology [29]. The wide host cell range and efficient invasion mechanism of apicomplexan parasites may result from its unique ability to place its own receptor into the host cell, thus providing a point of anchorage for invasion. It is highly likely that members of the RON-junctional complex also mediate attachment to the host cytoskeleton as discussed above. Consistent with this, heterologous expression of RON8 results in a subcortical localization in mammalian cells [30]. Defining the mechanism by which RON proteins are inserted across the host cell plasma membrane, and elucidating the role of individual members of this complex remains a high priority for future work on apicomplexan invasion.
Power: the actin-myosin motor
Gliding motility is powered by class XIV myosins, a family unique to and conserved within apicomplexans and closely related taxa [31]. The myosin motor complex is anchored within the space between the inner membrane complex and the plasma membrane and is comprised of a small myosin motor, a calmodulin-like regulatory light chain (TgMLC), and two anchoring proteins known as GAP45 and GAP50 [32]. Following the description of this motor complex in T. gondii, a similar complex was described in Plasmodium, where recent structural data reveal that the light chain wraps around the short neck region of the myosin motor [33]; presumably in position to regulate its activity, although the mechanism by which this occurs is not yet defined. Post-translational modification of TgMLC in response to treatment with a small molecule that inhibits motility and hence blocks cell invasion, demonstrates the potential to exploit these unique and essential motor complexes to prevent parasite infection [34].
Gliding motility in apicomplexans also depends on polymerization of F-actin and yet paradoxically the majority of actin exists in small complexes rather than stable filaments [35,36]. Apicomplexans are also unique in lacking many actin-regulatory proteins, including the Arp23 complex, which normally induces polymerization of branches by polymerization at the pointed filament end [37,38]. Instead, apicomplexans contain formins, which complete with capping protein and direct actin polymerization at the barbed end [39]. Formins are expressed by invasive stages of apicomplexans where they are found peripherally distributed beneath the cell membrane, with a concentration at the apical pole and the moving junction [40]. Whether formins act primarily at the junction remains unclear and current studies have not established if they are essential to the process of motility. Additionally, although the activities of several parasite formins have been studied in vitro using heterologous actins [40], their interactions with natural substrates have not been characterized. Although formins remain the most likely candidates for directing assembly of new actin filaments in apicomplexans, their role in this process is as yet incompletely described.
Linking the motor complex to the extracellular adhesins also requires a bridging function, which has been ascribed to the F-actin binding protein aldolase, an abundant glycolytic enzyme [41]. Studies initially conducted in T. gondii revealed binding of aldolase to the cytoplasmic tail of MIC2 [41] and this was also subsequently demonstrated for the Plasmodium orthologue TRAP [42]. The role of aldolase in bridging adhesin-cytoskeletal interactions has recently been tested in vivo using transgenic parasites that express various mutants of aldolase in T. gondii [43]. Mapping the requirements of binding in vitro revealed a positive charged ridge lining a pocket in aldolase that is occupied by the acidic tails of MIC2 [43] and TRAP [44]. Mutations in these residues in T. gondii aldolase abrogated tail binding in vitro while minimally disrupting enzyme activity, thus allowing these two functions to be separated. Expression of various mutants of aldolase in vivo under a system where the wild type allele was suppressed revealed an essential role for aldolase in energy production and also in mediated binding to the MIC2 cytoplasmic tail [43]. The fact that the tail-binding pocket lies adjacent to but outwith the enzyme substrate-binding pocket suggests that specific inhibitors that bock adhesin-tail binding might provide selective inhibition of parasite motility and cell invasion.
Release: shedding of surface adhesins
Gliding motility and invasion of host cells depends on the rearward translocation of adhesins and this process culminates in shedding from the surface and accumulation of trails on the substrate [2]. Shedding occurs by proteolysis within the membrane spanning domain and this is accomplished by one of several rhomboid proteins found in apicomplexans [45]. Rhomboids are a broadly distributed family of serine proteins that perform a diverse variety of functions in development and signaling [46]. The major surface rhomboid expressed by merozoites of Plasmodium is called ROM4, and in vitro studies indicate it has a broad substrate specificity and can process a variety of adhesins [47]. Processing of EBA-175, an important ligand for binding to red blood cells, is mediated by PfROM4, and mutants of the transmembrane region that prevent processing of EBA-175 are unable to support parasite viability, indicating this step is essential to the life cycle [48]. Tachyzoites of T. gondii express two major surface rhomboids TgROM4, which is uniformly distributed and TgROM5, which has a posterior concentration [49]. The posterior localization of TgROM5 suggests that it is primarily responsible for processing surface ahdhesins as they complete their reward translocation. Consistent with this, TgROM5 has a broad specificity and is highly active in vitro, while no activity has been demonstrated for TgROM4 using similar assays [49,50]. Somewhat surprisingly, genetic suppression of TgROM4 recently revealed that this protease also participates in processing of surface adhesins including MIC2 and AMA1 in T. gondii [51]. Suppression of TgROM4 reduced processing of surface adhesins such as MIC2, resulting in the uniform accumulation on the cell surface. As a result the normal polarity of adhesins is disrupted and parasites attach laterally to the host cell, but are impaired in cell invasion [51]. Gliding motility is also disrupted and parasites remain stuck by their posterior end and twirl unproductively, yet are unable to make forward progress across the substrate [51]. These findings indicate that TgROM4 is responsible for maintaining an apical-posterior gradient of adhesins, and this is necessary for directional motility and cell invasion. Similar studies underway with TgROM5 should reveal if it in fact plays a role in terminal processing of adhesins at the back of the cell, a step that is likely critical at a later stage in invasion.
Egress: Invading from within
Time lapse video microscopy studies reveal that invasion of host cells by T. gondii is rapid event that occurs without dramatic reorganization of the host cell membrane or cytoskeleton [15]. Within the safe haven provided by the parasitophorous vacuole, the parasite replicates by binary fission, giving rise to daughter cells that form a srosette within the host cell (Fig. 1D). Cell division is not dependent on actin polymerzation, but rather occurs by a novel process called endodyogeny, in which daughter cells are formed de novo from within each parasite [1]. Parasites exit host cells by a similar actin-based motility process, either due to natural triggers or in response to agonists that perturb intracellular calcium [6,15]. During natural egress, accumulation of the plant hormone abscisic acid leads to a rise in cADPR and release of calcium from intracellular stores, thus stimulating egress [52]. In exiting the cell, the process of invasion is essential repeated, but in this case the parasite first crosses the parasitophorous vacuole membrane and then the host cell membrane (Fig. 1E). Importantly, the topology of this event is reversed from invasion and hence the parasite must recognize different receptors to complete this transaction. Discharge of micronemes and rhoptries also accompanies egress, and the formation of a tight junction consisting of RON proteins also accompanies crossing of membranes during exit form the cell [27]. One of the early events in egress is the release of a microneme protein bearing a membrane attack complex (MAC-perforin) domain that results in permeation of the parasitophorous vacuole membrane followed by activation of motility and egress [53]. As with invasion, egress in T. gondii is under the control of CPDK1, which controls microneme release [10]. Egress in Plasmodium merozoites from infected red blood cells appears to be less dependent on elevated calcium as a trigger, but rather relies on an elaborate cascade of proteases that are active with in the parasitophorous vacuole [54].
Conclusions and future directions
Despite recent progress, our current models for how gliding motility works does not adequately account for the physical or mechanical requirements or fully explain the role of adhesive proteins, sites of anchorage, or regulation of the actin-myosin motor. Among the unanswered questions are: 1) are adhesive proteins clustered into multimeric complexes and if so, what is the composition and functional consequences of this? 2) Do adhesive complexes signal outside-in to direct assembly of the motor complex either by initiating or stabilizing actin-myosin complexes? 3) What is the force generating capacity of an individual adhesion-motor complexes and how many such complexes are needed to drive different sorts of forward motility? 4) How are actin dynamics and the myosin motor regulated? 5) Does formation of the junction rely on a specific membrane composition, how are these proteins transferred across the host cell membrane, and how are they anchored to the host cell cytoskeleton? Addressing these important questions will require new methods for quantitative microscopy, biochemical and biophysical methods for evaluating the roles of protein complexes, and genetic and cell biological approaches for analyzing a variety of mutants. In addition to providing fundamental insight into a novel means of cell locomotion, such studies may identify essential and druggable targets that might be exploited for specific and selective inhibition of apicomplexan infections.
Acknowledgements
I am grateful to current and past members of my laboratory and other colleagues for helpful comments, thank S. Louirdo and W.L. Beatty for assistance with illustrations, and regret not being able to cite many sources due to space limitations. Supported by NIH grants AI073155 and AI034036.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics in publishing: general statement
The author complies with the Ethics in Publishing.
Conflicts of interest
The author has no conflicts of interest.
References and recommended reading
Papers of particular interest have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Morrissette NS, Sibley LD. Cytoskeleton of apicomplexan parasites. Microbiol. Mol. Biol. Rev. 2002;66:21–38. doi: 10.1128/MMBR.66.1.21-38.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sibley LD. Invasion strategies of intracellular parasites. Science. 2004;304:248–253. doi: 10.1126/science.1094717. [DOI] [PubMed] [Google Scholar]
- 3.Barragan A, Sibley LD. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J. Exp. Med. 2002;195:1625–1633. doi: 10.1084/jem.20020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ejigiri I, Sinnis P. Plasmodium sporozoite-host interactions from the dermis to the hepatocyte. Curr Opin Microbiol. 2009;12:401–407. doi: 10.1016/j.mib.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Nagamune K, Moreno SN, Chini EN, Sibley LD. Calcium regulation and signaling in apicomplexan parasites. Subcell. Biochem. 2008;47:70–81. doi: 10.1007/978-0-387-78267-6_5. [DOI] [PubMed] [Google Scholar]
- 7.Billker O, Lourido S, Sibley LD. Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe. 2009;5:612–622. doi: 10.1016/j.chom.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ojo KK, Larson ET, Keyloun KR, Castaneda LJ, DeRoucher AE, KInampudi KK, Kim JE, Arakaki TL, Murphy RC, Zhang L, et al. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat. Struct. Molec. Biol. 2010;17:602–607. doi: 10.1038/nsmb.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wernimont AK, Artz JD, Finerty P, Lin Y, Amani M, Allali-Hassani A, senisterra G, Vedadi M, Tempel W, Mackenzie F, et al. Structures of apicomplexan calcium-dependent protein kinases reveal mechanism of activation by calcium. Nat. Struct. Molec. Biol. 2010;17:596–601. doi: 10.1038/nsmb.1795. The above two papers provide the first complete structures of any calcium-dependent protein kinase and reveal important structural features about their regulation and activation.
- 10. Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010;465:359–362. doi: 10.1038/nature09022. Molecular and chemical genetic experiments demonstrate that CDPK1 controls microneme secretion in T. gondii and that repression of this kinase impair gliding motility, cell invasion and egress. Furthermore, the unique ATP-binding pocket of CDPK1 is exploited for the use of selective inhibitors, also discussed in ref 8, 9.
- 11.Green JL, Rees-Channer RR, Howell SA, Martin SR, Knuepfer E, Taylor HM, Grainger M, Holder AA. The motor complex of Plasmodium falciparum: phosphorylation by a calcium-dependent protein kinase. J. Biol. Chem. 2008;283:30980–30989. doi: 10.1074/jbc.M803129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato N, Sakata T, Breton G, Le Roch KG, Nagle A, Andersen C, Bursulaya B, Henson K, Johnson J, Kumar KA, et al. Gene expression signatures and small-molecule compounds link a protein kinase to Plasmodium falciparum motility. Nat. Chem. Biol. 2008;4:347–356. doi: 10.1038/nchembio.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacroix C, Menard R. TRAP-like protein of Plasmodium sporozoites: linking gliding motility to host-cell traversal. Trends Parasitol. 2008;24:431–434. doi: 10.1016/j.pt.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Amino R, Giovannini D, Thiberge S, Gueirard P, Boisson B, Dubremetz JF, Prevost MC, Ishino T, Yuda M, Menard R. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe. 2008;3:88–96. doi: 10.1016/j.chom.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Håkansson S, Morisaki H, Heuser JE, Sibley LD. Time-lapse video microscopy of gliding motility in Toxoplasma gondii reveals a novel, biphasic mechanism of cell locomotion. Mol. Biol. Cell. 1999;10:3539–3547. doi: 10.1091/mbc.10.11.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Munter S, Sabass B, Selhuber-Unkel C, Kudryashev M, Hegge S, Engel U, Spatz JP, Matuschewski K, Schwarz US, Frischknecht F. Plasmodium sporozoite motility is modulated by the turnover of discrete adhesion sites. Cell Host Microbe. 2009;6:551–562. doi: 10.1016/j.chom.2009.11.007. The use of reflection-interference or traction-force microscopy reveal novel aspects of gliding motility that involve complex interactions between the cytoskeleton, surface adhesins and the substratum.
- 17.Huynh MH, Carruthers VB. Toxoplasma MIC2 is a major determinant of invasion and virulence. PloS Pathog. 2006;2:753–762. doi: 10.1371/journal.ppat.0020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Håkansson S, Charron AJ, Sibley LD. Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. Embo J. 2001;20:3132–3144. doi: 10.1093/emboj/20.12.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boothroyd JC, Dubremetz JF. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat. Rev. Microbiol. 2008;6:79–88. doi: 10.1038/nrmicro1800. [DOI] [PubMed] [Google Scholar]
- 20.Bradley PJ, Sibley LD. Rhoptries: an arsenal of secreted virulence factors. Curr. Opin. Microbiol. 2007;10:582–587. doi: 10.1016/j.mib.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charron AJ, Sibley LD. Molecular partitioning during host cell penetration by Toxoplasma gondii. Traffic. 2004;5:855–867. doi: 10.1111/j.1600-0854.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- 22.Mordue DG, Desai N, Dustin M, Sibley LD. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. Journal of Experimental Medicine. 1999;190:1783–1792. doi: 10.1084/jem.190.12.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobrowolski JM, Sibley LD. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84:933–939. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- 24. Gonzalez V, Combe A, David V, Malmquist NA, Delorme V, Leroy C, Blazquez S, Menard R, Tardieux I. Host cell entry by apicomplexa parasites requires actin polymerization in the host cell. Cell Host Microbe. 2009;5:259–272. doi: 10.1016/j.chom.2009.01.011. Although previous studies demonstrated the requirement of parasite actin in cell invasion, this study reveals a role for F-actin in the host cell in facilitating this process.
- 25.Bradley PJ, Ward C, Cheng SJ, Alexander DL, Coller S, Coombs GH, Dunn JD, Ferguson DJ, Sanderson SJ, Wastling JM, et al. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in T. gondii. J. Biol. Chem. 2005;280:34245–34258. doi: 10.1074/jbc.M504158200. [DOI] [PubMed] [Google Scholar]
- 26.Lebrun M, Michelin A, El Hajj H, Poncet J, Bradley PJ, Vial HJ, Dubremetz JF. The rhoptry neck protein RON4 relocalizes at the moving junction during Toxoplasma gondii invasion. Cell. Micro. 2005;7:1823–1833. doi: 10.1111/j.1462-5822.2005.00646.x. [DOI] [PubMed] [Google Scholar]
- 27.Alexander DL, Mital J, Ward GE, Bradley PJ, Boothroyd JC. Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog. 2005;1:137–149. doi: 10.1371/journal.ppat.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richard D, Macraild CA, Riglar DT, Chan JA, Foley M, Baum J, Ralph SA, Norton RS, Cowman AF. Interaction between plasmodium falciparum apical membrane antigen 1 and the Rhoptry neck protein complex defines a key step in the erythrocyte invasion process of malaria parasites. J Biol Chem. doi: 10.1074/jbc.M109.080770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Besteiro S, Michelin A, Poncet J, Dubremetz J, Lebrun M. Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS Path. 2009;5 doi: 10.1371/journal.ppat.1000309. e1000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Straub KW, Cheng SJ, Sohn CS, Bradley PJ. Novel components of the apicomplexan moving junction reveal conserved and coccidia-restricted elements. Cell Microbiol. 2009;11:590–603. doi: 10.1111/j.1462-5822.2008.01276.x. The above two studies demonstrate that parasite proteins secreted from the rhoptry neck (so called RONS) are translocated into the host cell membrane, where they presumably act as a receptor for attachment and facilitate parasite invasion.
- 31.Foth BJ, Goedecke MC, Soldati D. New insights into myosin evolution and classification. Proc Natl Acad Sci (USA) 2006;103:3681–3686. doi: 10.1073/pnas.0506307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaskins E, Gilk S, DeVore N, Mann T, Ward GE, Beckers C. Identification of the membrane receptor of a class XIV myosin Toxoplasma gondii. J Cell Biol. 2004;165:383–393. doi: 10.1083/jcb.200311137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosch J, Turley S, Roach CM, Daly TM, Bergman LW, Hol WG. The closed MTIP-myosin A-tail complex from the malaria parasite invasion machinery. J. Mol. Biol. 2007;372:77–88. doi: 10.1016/j.jmb.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heaslip AT, Leung JM, Carey KL, Catti F, Warshaw DM, Westwood NJ, Ballif BA, Ward GE. A small-molecule inhibitor of T. gondii motility induces the posttranslational modification of myosin light chain-1 and inhibits myosin motor activity. PLoS Pathog. 6 doi: 10.1371/journal.ppat.1000720. e1000720. The use of small molecules inhibitors that modify the myosin light chain and block motility provide support for the essential nature of the motor complex and demonstrate the potential for selective chemical inhibition.
- 35.Sahoo N, Beatty WL, Heuser JE, Sept D, Sibley LD. Unusual kinetic and structural properties control rapid assembly and turnover of actin in the parasite Toxoplasma gondii. Mol. Biol. Cell. 2006;17:895–906. doi: 10.1091/mbc.E05-06-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz S, Grainger M, Howell SA, Calder LJ, Gaeb M, Pinder JC, Holder AA, Veigel C. Malaria parasite actin filaments are very short. J. Mol. Biol. 2005;349:113–125. doi: 10.1016/j.jmb.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 37.Gordon JL, Sibley LD. Comparative genome analysis reveals a conserved family of actin-like proteins in apicomplexan parasites. BMC Genomics. 2005;6:e179. doi: 10.1186/1471-2164-6-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schüler H, Matuschewski K. Regulation of apicomplexan microfilament dynamics by minimal set of actin-binding proteins. Traffic. 2006;7:1433–1439. doi: 10.1111/j.1600-0854.2006.00484.x. [DOI] [PubMed] [Google Scholar]
- 39.Goode BL, Eck MJ. Mechansim and function of formins in the control of actin assembly. Annu. Rev. Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 40.Baum J, Tonkin CJ, Paul AS, Rug M, Smith BJ, Gould SB, Richard D, Pollard TD, Cowman AF. A malaria parasite formin regulates actin polymerization and localizes to the parasite-erythrocyte moving junction during invasion. Cell Host Microbe. 2008;3:188–198. doi: 10.1016/j.chom.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Jewett TJ, Sibley LD. Aldolase forms a bridge between cell surface adhesins and the actin cytoskeleton in apicomplexan parasites. Molec. Cell. 2003;11:885–894. doi: 10.1016/s1097-2765(03)00113-8. [DOI] [PubMed] [Google Scholar]
- 42.Buscaglia CA, Coppens I, Hol WGJ, Nussenzweig V. Site of interaction between aldolase and thrombospondin-related anonymous protein in Plasmodium. Mol. Biol. Cell. 2003;14:4947–4957. doi: 10.1091/mbc.E03-06-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Starnes GL, Coincon M, Sygusch J, Sibley LD. Aldolase is essential for energy production and bridging adhesin-actin cytoskeletal interactions during parasite invasion of host cells. Cell Host Microbe. 2009;5:353–364. doi: 10.1016/j.chom.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosch J, Buscaglia CA, Krumm B, Ingason BP, Lucas R, Roach C, Cardozo T, Nussenzweig V, Hol WG. Aldolase provides an unusual binding site for thrombospondin-related anonymous protein in the invasion machinery of the malaria parasite. Proc. Nat. Acad. Sci. (USA) 2007;104:7015–7020. doi: 10.1073/pnas.0605301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dowse T, Soldati D. Rhomboid-like proteins in Apicomplexa: phylogeny and nomenclature. Trends Parastiol. 2005;35:747–756. doi: 10.1016/j.pt.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Urban S. Rhomboid proteins: conserved membrane proteases with divergent biological functions. Genes and Development. 2006;20:3054–3068. doi: 10.1101/gad.1488606. [DOI] [PubMed] [Google Scholar]
- 47.Baker RP, Wijetilaka R, Urban S. Two Plasmodium rhomboid proteases preferentially cleave different adhesins implicated in all invasive stages of malaria. PloS Path. 2006;2:e113. doi: 10.1371/journal.ppat.0020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Donnell RA, Hackett F, Howell SA, Treeck M, Struck N, Krnajski Z, Withers-Martinez C, Gilberger TW, Blackman MJ. Intramembrane proteolysis mediates shedding of a key adhesin during erythrocyte invasion by the malaria parasite. J. Cell Biol. 2006;174:1023–1033. doi: 10.1083/jcb.200604136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brossier F, Jewett TJ, Sibley LD, Urban S. A spatially-localized rhomboid protease cleaves cell surface adhesins essential for invasion by Toxoplasma. Proc. Natl. Acad. Sci. (USA) 2005;102:4146–4151. doi: 10.1073/pnas.0407918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dowse TJ, Pascall JC, Brown KD, Soldati D. Apicomplexan rhomboids have a potential role in microneme protein cleavage during host cell invasion. Intl. J. Parasitol. 2005;35:747–756. doi: 10.1016/j.ijpara.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Buguliskis JS, Brossier F, Shuman J, Sibley LD. Rhomboid 4 (ROM4) affects the processing of surface adhesins and facilitates host cell invasion by Toxoplasma gondii. PLoS Pathog. 6 doi: 10.1371/journal.ppat.1000858. e1000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nagamune K, Hicks LM, Fux B, Broissier F, Chini EN, Sibley LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature. 2008;451:207–211. doi: 10.1038/nature06478. The conservation of a plant-like pathway for absisic acid biosynthesis is shown to control elevated calcium signals leading to natural egress of intracellular T. gondii. Inhibition of this pathway blocks egress and leads to differentiation to the chronic state.
- 53. Kafsack BF, Pena JD, Coppens I, Ravindran S, Boothroyd JC, Carruthers VB. Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science. 2009;323:530–533. doi: 10.1126/science.1165740. Genetic depletion of a perforin-like protein that participates in rupture of the parasite-containing membrane reveals that egress is activated by microneme secretion from intracellular T. gondii parasites.
- 54.Blackman MJ. Malaria proteases and host cell egress: an emerging casade. Cell. Microbiol. 2008;10:1925–1934. doi: 10.1111/j.1462-5822.2008.01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]