Abstract
Immune mediated liver injury in hepatitis is due to activated T cells producing interferon-γ (IFN-γ). It is important to identify negative feedback immune mechanisms that can regulate T cell activity. In this study, we demonstrate that Th1-mediated liver inflammation can induce the accumulation of myeloid derived suppressor cells (MDSC), pleiomorphic cells capable of modulating T cell mediated immunity, that heretofore have been studied almost exclusively in the context of tumor-associated inflammation. Mice deficient in the gene encoding transforming growth factor-β1 (Tgfb1−/− mice) acutely develop liver necroinflammation caused by IFN-γ-producing CD4+ T cells. Liver Th1 cell accumulation was accompanied by myeloid cells expressing CD11b and Gr1, phenotypic hallmarks of MDSC. Isolated Tgfb1−/− liver CD11b+Gr1+ cells were functional MDSC, readily suppressing T cell proliferation in vitro. Pharmacologic inhibitors of inducible nitric oxide synthase (iNOS) completely eliminated suppressor function. Suppressor function and the production of nitric oxide (NO) were dependent upon cell-cell contact between MDSC and T cells, and upon IFN-γ, and were specifically associated with the “monocytic” CD11b+Ly6G−Ly6Chi subset of liver Tgfb1−/− CD11b+ cells. The rapid accumulation of CD11b+Gr1+ cells in Tgfb1−/− liver was abrogated when mice were either depleted of CD4+ T cells or rendered unable to produce IFN-γ, showing that Th1 activity induces MDSC accumulation.
Conclusion
Th1 liver inflammation mobilizes an MDSC response that, through the production of NO, can inhibit T cell proliferation. We propose that MDSC serve an important negative feedback function in liver immune homeostasis, and that insufficient or inappropriate activity of this cell population may contribute to inflammatory liver pathology.
Keywords: Interferon-γ, autoimmune hepatitis, nitric oxide synthase, T cell, Tgfb1 knockout
T cells are the proximal agents of parenchymal liver damage in inflammatory liver diseases such as autoimmune hepatitis (AIH) and viral hepatitis. In AIH, CD4+ T cells infiltrate liver parenchyma (1) and release hepatotoxic cytokines such as IFN-γ and TNF-α (2, 3). IFN-γ expression by ex vivo cultured T cells strongly correlates with disease activity (4), implicating type 1 T cell responses in hepatocellular damage. In hepatitis C virus (HCV) infection, liver pathology results from the activity of T cells producing IFN-γ within liver parenchyma, since HCV is not cytopathic (5–7). IFN-γ is essential for parenchymal damage in mouse models of T cell mediated liver injury, including Concanavalin A -induced liver injury (8), and spontaneous liver injury in BALB/c TGF-β1 knockout mice (9). A common theme, therefore, in immune mediated liver injury is pathology associated with activated T cells producing IFN-γ.
Given the potential for liver damage by activated Th1 cells, it is important to identify mechanisms that regulate their activity. A variety of liver resident cells participate in the regulation of T cells, including Treg, dendritic cells, Kupffer cells, NK cells, NKT cells, stellate cells, and liver sinusoidal epithelial cells (10). Whether regulatory immunocytes accumulate in liver in response to activated T cells is not known. Such cells may represent an important negative feedback mechanism mitigating pathology mediated by T cell activation. It is reasonable to postulate that inflammatory pathology in liver is attributable both to aberrant activation of T cells and to a deficit in appropriate counter-regulatory mechanisms.
Studies emerging from the field of tumor immunity show that tumor-associated inflammation induces the development and accumulation of myeloid-lineage cells with immunomodulatory activity. Termed myeloid derived suppressor cells (MDSC), these pleiomorphic cells are capable of suppressing T cell proliferation and subjugating T cell mediated immunity (11, 12). MDSC comprise a heterogenous group of myeloid cells, employing a variety of mechanisms to inhibit T cell responses. Murine MDSC are operationally defined as CD11b+Gr1+ myeloid cells that suppress T cell proliferation (11, 12). While MDSC have been most extensively described in the context of tumors, recent studies show their involvement in inflammatory responses not associated with tumors (13, 14). MDSC home to liver in tumor-bearing mice (15), and hepatocellular carcinoma, like other solid tumors, exhibit associated populations of MDSC (16, 17), but little is otherwise known about MDSC in liver, particularly in inflammatory pathology. Here, we demonstrate in the BALB/c TGF-β1 knockout mouse model that Th1 cells, through release of IFN-γ, drive accumulation in liver of an MDSC population that can effectively inhibit T cell proliferation through a mechanism involving expression of inducible nitric oxide synthase (iNOS) and the production of nitric oxide (NO).
Materials and Methods
Mice
Mice were bred at Dartmouth Medical School according to AAALAC practices. BALB/c-background Tgfb1−/− mice, Ifng−/− Tgfb1−/− mice, and Rag1−/− Tgfb1−/− mice were genotyped as described (9, 18, 19). Depletion of CD4+ T cells and Gr1+ cells used intraperitoneal injections of anti-CD4 and anti-Gr1 (Clone: RB6-8C5, BioXCell), respectively, initiated at postnatal day 5 as described (18). Flow cytometry at day 11 confirmed depletion efficiencies of > 95%.
Liver cell subset isolation
Following cardiac perfusion with PBS, livers were aseptically removed and mechanically disrupted between sterile frosted microscope slides. Cell suspensions were passed twice through 70 μM filters before cell isolation. Liver CD11b+ cells were isolated using anti-CD11b magnetic beads and positive selection columns (Miltenyi) per the manufacturer’s protocol. Gr1+ cells were isolated using PE-anti-Gr1 (RB6-8C5; eBioscience) and positive immunomagnetic separation using a PE selection kit (StemCell Technologies, Inc.). CD11b+Ly6GhiLy6Clo cells were isolated via positive selection employing biotinylated anti-Ly6G and anti-biotin magnetic microbeads (Miltenyi). CD11b+Ly6G−Ly6Chi cells were isolated by negative selection of Ly6G− cells followed by positive selection with biotinylated anti-Ly6C and anti-biotin magnetic microbeads. (Miltenyi). Flow cytometry verified that all cell isolations yielded > 90% pure populations.
Th1 effector cell development
Bead isolated-CD4+ T cells were cultured for three days with plate-bound anti-CD3ε 10.0 μg/ml), soluble anti-CD28 (1.0 μg/ml) (BD Biosciences) rIL-12 (10 ng/ml; Peprotech) and anti-IL-4 (10 μg/ml; NCI). Th1 effector development was confirmed by intracellular IFN-γ staining.
Flow Cytometry
Cells were incubated with Fc Block (anti-CD16/CD32; eBioscience) for 20 min at 4°C then washed twice. Cells were stained with antibodies to CD4, CD11b, Gr1, F4/80, PD-L1 (eBioscience, San Diego), Ly6G (Clone 1A8), Ly6C (Clone 1G7.G10), MHCII (BD Biosciences) or CD14 (Biolegend) and acquired on either BD FACSCalibur (BD Biosciences) or Accuri C6. Data analysis used FlowJo Ver.8.8.6 (Tree Star) software.
iNOS Detection
Cells obtained from suppression assay cultures at 48 hrs were surface stained as above, fixed and permeabilized (CytoFix/CytoPerm; BD Biosciences), stained with rabbit anti-mouse iNOS (BD Biosciences), followed by blocking with 10% normal donkey serum, and secondary staining with donkey anti-rabbit (Jackson Immuno). Surface-marker-appropriate isotype and intracellular staining with secondary antibody alone served as negative control. RAW 264.7 cells cultured 24 hours with LPS and IFN-γ served as positive control. Cells were acquired by flow cytometry.
Suppression Assays
Prior to inclusion in co-cultures, bead-isolated CD4+ or CD8+ T cells from wild-type mouse spleens were stained with 5.0 μM 5- (and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE; Invitrogen) for 10 minutes, quenched by washing twice in RPMI/10% FCS. Isolated Tgfb1+/− or Tgfb1−/− CD11b+ cells were added at 3.0 × 105 (“300K”) or 1.0 × 105 (“100K”) cells to co-cultures with CFSE-labeled purified CD4+ T cells or CD8+ T cells (@3.0 × 105 cells). Th1 cells were stimulated with plate-bound anti-CD3ε BD Biosciences) at 10.0 μg/ml, whereas cultures of isolated splenic T cells also included soluble anti-CD28 at 1.0 μg/ml (BD Biosciences). 1.0 × 106 DO11.10 mouse splenocytes were stimulated with 0.3 μM OVA323-339 peptide. Inhibitors were added at the start of culture as follows: 5.0 mM NG-Monomethyl-L-Arginine (L-NMMA) (Calbiochem), 5.0 mM NG-Monomethyl-D-Arginine (D-NMMA) (Calbiochem), 0.5 mM N6- (1-iminoethyl)-L-lysine (L-NIL) (Sigma), 1.0 mM N-Hydroxy-nor-Arginine (nor-NOHA) (Caymen), 0.2 mM 1-methyl-tryptophan (1-MT) (Sigma), 1000 U/ml Catalase (Sigma), 200 U/ml Superoxide Dismutase (SOD) (MP Biomedicals), 10 μg/ml anti-PD-L1 (CD274) (Clone 10F.9G2 Biolegend), 10 μg/ml anti-PD-1 (CD279) (Clone RMP1-14 Biolegend), 10 μg/ml anti-TGF-β1,2,3 (Clone:1D11 R&D Systems), 10μg/ml anti-IFNγ Clone 37895.11 R&D Systems), 20 μg/ml anti-IL10 (Clone JES5-2A5), 20 μg/ml anti-IL-10R/CD210 (Clone 1B1.3A). To assess contact dependence, assays used 0.2 μm transwell inserts (Costar), with Gr1+CD11b+ cells and responder T cells separated by membrane. Cells were cultured in standard media for 72 hours and analyzed by flow cytometry for CFSE dilution.
Measurements of nitrite and IFN-γ
NO production was determined by measuring nitrite (20). IFN-γ protein levels in plasma and in supernatants were determined by ELISA (BD Biosciences).
Histology
Liver H&E staining was as described (21). Isolated CD11b+ cells were analyzed for cell morphology following cytospin centrifugation and Wright-Giemsa staining.
Statistics
Student’s t test was employed using GraphPad Prism version 4.0. All bar graphs indicate mean ± S.D. Statistical significance is defined as p ≤ 0.05.
Results
Myeloid CD11b+Gr1+ cells rapidly accumulate in Tgfb1−/− mouse livers
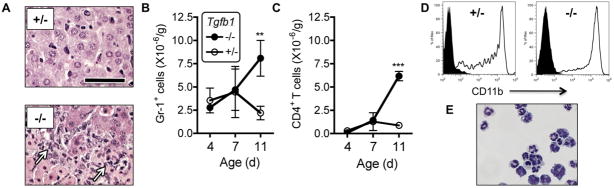
Tgfb1−/− mice rapidly develop acute liver necroinflammation (9) and a liver CD4+ T cell lymphocytosis (18). Liver damage requires CD4+ Th1 cells producing the cytokine IFN-γ 9, 18, 22). CD11b+ myeloid cells also are abundant in Tgfb1−/− liver (18), but have not been further studied heretofore. Histology confirmed the presence of cells with myeloid morphology in or apposed to necrotic areas (Fig. 1A). We assessed the kinetics of accumulation of Gr1+ myeloid cells by flow cytometry. At post-natal days 4 and 7, Gr1+ cell numbers were equivalent between Tgfb1−/− livers and healthy littermate Tgfb1+/− livers. At post-natal day 11, Gr1+ cells were ~3-fold more numerous in Tgfb1−/− livers (Fig. 1B). The rapid rise in Gr1+ cells closely paralleled the rise in CD4+ T cells (Fig. 1C). Gr1+ cells from 11 day old Tgfb1−/− liver strongly co-expressed CD11b (Fig. 1D), as did liver resident Gr1+ cells from littermate Tgfb1+/− mice (Fig. 1D). Tgfb1−/− liver CD11b+ cells were heterogeneous, with both granulocytic forms and monocytic forms, and representatives of various stages of lineage maturation (Fig. 1E).
Figure 1. Myeloid CD11b+Gr1+ cells accumulate in Tgfb1−/− livers.
(A) Abundant myeloid cells (arrows) are observed in liver from day 11 Tgfb1−/− mice (−/−) but not age-matched Tgfb1+/− mice (+/−). Bar = 50 μm. (B, C) CD4+ T cells and Gr1+ cells were counted after isolation from livers from Tgfb1−/− mice and Tgfb1+/− mice at 4, 7, and 11 days of age. n = 3 or more mice per group. (D) Isolated liver Gr1+ cells from Tgfb1−/− mice and Tgfb1+/− mice co-express CD11b. Solid: Isotype control. (E) After cytospin, Tgfb1−/− liver CD11b+ cells were stained with Wright-Giemsa. ** p < 0.01; *** p < 0.0001.
Tgfb1−/− liver Gr1+CD11b+ cells potently inhibit T cell proliferation
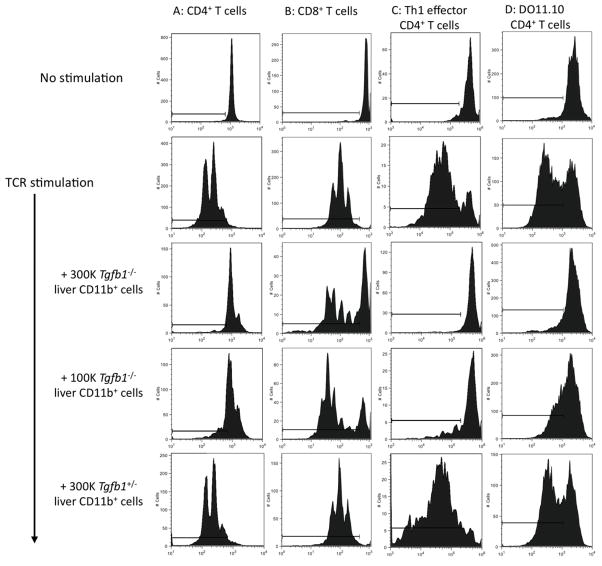
We tested the hypothesis that Tgfb1−/− liver Gr1+CD11b+ cells represent MDSC by specifically assessing their ability to suppress T cell proliferation. Wild-type splenic CD4+ T cells were CFSE-labeled and stimulated in vitro with anti-CD3/28. Tgfb1−/− liver CD11b+Gr1+ cells suppressed the proliferation of T cells completely when added at either 3- or 1- × 105 cells per well (Fig. 2A), and partially when added at 3 × 104 cells per well (data not shown). Control Tgfb1+/− liver CD11b+Gr1+ cells had no effect. Tgfb1−/− liver Gr1+CD11b+ cells also suppressed proliferation of CD8+ T cells (Fig. 2B), and of effector Th1 cells (Fig. 2C), the cell type chiefly responsible for necroinflammation in the Tgfb1−/− mouse. Suppression was also observed with T cell stimulation mediated by cognate antigen, as Tgfb1−/− liver CD11b+Gr1+ cells suppressed APC/OVA-induced proliferation of DO11.10 CD4+ T cells (Fig. 2D). Control Tgfb1+/− liver CD11b+Gr1+ cells had no suppressor effects in any assay.
Figure 2. Tgfb1−/− liver CD11b+Gr1+ cells suppress T cell proliferation.
CFSE-labeled T cells were stimulated in vitro. Some cultures included liver CD11b+Gr1+ cells from Tgfb1−/− mice or Tgfb1+/− mice. (A) CD4+ T cells or (B) CD8+ T cells were stimulated with anti-CD3ε/anti-CD28. (C) CFSE-labeled Th1 effector cells were stimulated with anti-CD3ε. (D) DO11.10 splenocytes were stimulated with OVA. Cells harvested at 72 h were analyzed by flow cytometry. Gates were established using unstimulated CFSE-stained cells. Data represent 3 or more independent experiments.
Thus, Tgfb1−/− liver CD11b+Gr1+ cells are functional MDSC that strongly suppress TCR-mediated T cell proliferation. The lack of similar activity in control Tgfb1+/− liver CD11b+Gr1+ cells demonstrates that the suppressor function is specific to inflamed liver, and not a general property of liver-resident CD11b+Gr1+ cells. Tgfb1−/− liver CD11b+Gr1+ cells exhibited higher expression of F4/80, CD11c, CD14, MHC class II, and PD-L1 (Supplemental Fig. 1) supporting the conclusion that Tgfb1−/− liver CD11b+Gr1+ cells are distinct from control liver-resident CD11b+Gr1+ cells.
Tgfb1−/− liver MDSC suppression of T cell proliferation is dependent on NO, IFN-γ, and cell-cell contact
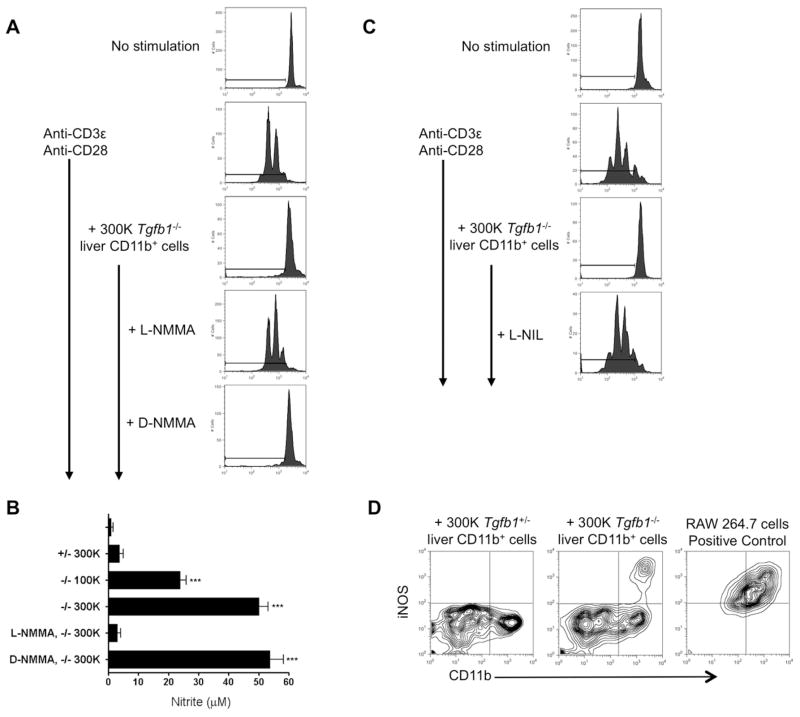
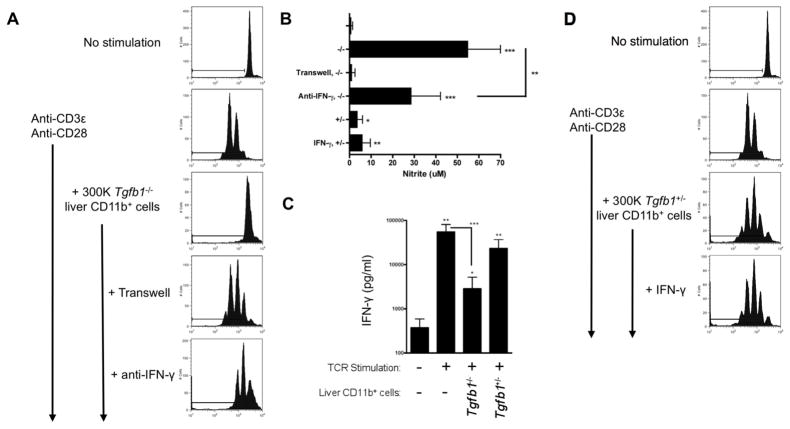
To assess the mechanism(s) of suppression, we carried out the suppression assay as before, blocking specific pathways individually. Specific inhibitors of arginase, indoleamine 2,3-dioxygenase (IDO), reactive oxygen species (ROS), PD-L1/PD-1, TGF-β, and IL-10 had no effect on Tgfb1−/− liver MDSC suppressor function (Table 1 and data not shown). L-NMMA, an inhibitor of NO synthases, completely eliminated suppressor function, whereas the inactive enantiomer D-NMMA had no effect (Fig. 3A; Table 1). Supporting these findings, nitrite levels in culture supernatants were significantly increased when Tgfb1−/− liver MDSC were co-cultured with stimulated T cells, but not when control CD11b+Gr1+ cells were used (Fig. 3B); as expected, nitrite production was suppressible by L-NMMA but not D-NMMA. L-NMMA inhibits all three isoforms of NO synthase (iNOS, nNOS and eNOS). The iNOS-specific inhibitor L-NIL; like L-NMMA, abrogated suppression (Fig. 3C; Table 1). Flow cytometry confirmed iNOS expression in a subset of Tgfb1−/− liver CD11b+ cells, but not in Tgfb1+/− liver CD11b+ cells (Fig. 3D). Suppression was not observed when MDSC and T cells were physically separated by a transwell membrane, indicating that cell-cell contact is required (Fig. 4A). mAb neutralization of IFN-γ in vitro partly inhibited suppression (Fig. 4A). Additional studies clarified that cell-cell contact and IFN-γ are required for NO production, since nitrite was undetectable in the transwell assay, and significantly reduced with anti-IFN-γ Fig. 4B). ELISA confirmed IFN-γ production in co-cultures, albeit lower than in cultures of T cells stimulated alone (Fig. 4C). Since substantial IFN-γ was produced in T cell -Tgfb1+/− liver CD11b+Gr1+ cell co-cultures (Fig. 4C) IFN-γ appears insufficient to confer MDSC activity on liver-resident Gr1+CD11b+ cells. Indeed, when IFN-γ was added exogenously, Tgfb1+/− liver CD11b+Gr1+ cells were unable to inhibit T cell proliferation (Fig. 4D), and NO production was not augmented (Fig. 4B). Thus, IFN-γ is necessary but not sufficient for MDSC activity.
Table 1.
Pathways assessed to evaluate the mechanistic basis for the T cell suppressive activity of liver Tgfb1−/− MDSC.
| Target Enzyme or Molecule | Inhibitor | Concentration | Effect on MDSC activity |
|---|---|---|---|
| Nitric oxide synthase (NOS) -1, -2, -3 | L-NG-monomethyl Arginine citrate (L-NMMA) | 5.0 mM | Blocks |
| NOS-2 | N6- (1-iminoethyl)-L-lysine (L-NIL) | 0.5 mM | Blocks |
| Arginase | N-hydroxy-nor-Arginine (nor-NOHA) | 1.0 mM | No effect |
| Indoleamine 2,3-dioxygenase (IDO) | 1-methyl-tryptophan (1-MT) | 0.2 mM | No effect |
| Reactive oxygen species (ROS) | Catalase | 1000 U/ml | No effect |
| Superoxide Dismutase (SOD) | 200 U/ml | No effect | |
| PD-L1/PD-1 | Blocking Anti-PD-L1 mAb | 10 μg/ml | No effect |
| Blocking Anti-PD-1 mAb | 10 μg/ml | No effect | |
| TGF-β | Blocking Anti-TGF-β-1, -2, -3 mAb | 10 μg/ml | No effect |
| IL-10/IL-10R | Blocking Anti-IL-10 mAb | 20 μg/ml | No effect |
| Blocking Anti-IL-10R mAb | 20 μg/ml | No effect |
Figure 3. Suppression requires the production of nitric oxide.
(A, C) CD4+ T cell - CD11b+ cell co-cultures were set up as in the legend to Figure 2; L-NMMA, D-NMMA, or L-NIL was added to some wells at the beginning of the assay. Flow plots represent at least 3 independent experiments. (B) Nitrite was measured in T cell -CD11b+ cell co-culture supernatants. *** p < 0.001. (D) Intracellular iNOS protein expression was determined by flow cytometry at 48 hrs. LPS/IFN-γ-treated RAW 264.7 cells served as positive control for iNOS staining.
Figure 4. Suppression requires cell-cell contact and IFN-γ.
(A) Co-cultures were set up as before. In some wells, T cells were separated from CD11b+ cells by a 0.2 μM transwell membrane; in others, neutralizing anti-IFN-γ was included. (B, C) Nitrite (B) and IFN-γ C) were measured in culture supernatants. * p < 0.05; ** p < 0.01; *** p < 0.001. (D) T cell - Tgfb1+/− liver CD11b+ cell co-cultures were established; some wells included exogenously added rIFN-γ. Data represent at least 3 independent experiments.
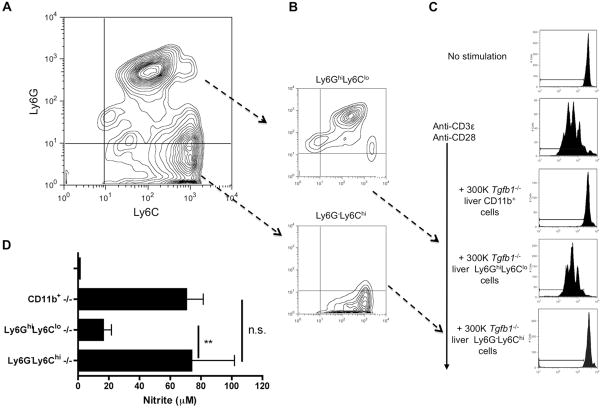
Suppressive activity is preferentially found in the monocytic CD11b+Ly6G−Ly6Chi subset of liver Tgfb1−/− CD11b+ cells
Anti-Gr1 recognizes two highly related cell surface proteins, Ly6C and Ly6G (23). Expression patterns of these cell surface proteins distinguish two major MDSC subsets, with the Ly6G−Ly6Chi phenotype characteristic of monocyte-like MDSC and the Ly6GhiLy6Clo phenotype characteristic of granulocyte-like MDSC (24). Most CD11b+ cells from Tgfb1−/− livers co-expressed Ly6C (Fig. 5A). Among CD11b+Ly6C+ cells, about 2/3rd were Ly6GhiLy6Clo, whereas the rest were Ly6G−Ly6Chi (Fig. 5A). After isolation of these subsets (Fig. 5B), we observed that suppressor activity resides exclusively in the “monocytic” CD11b+Ly6G−Ly6Chi cell population, with no activity found in the “granulocytic” CD11b+Ly6GhiLy6Clo cell population (Fig. 5C). NO production tracked with suppressor function (Fig. 5D).
Figure 5. Suppressor activity is in the Ly6G−Ly6Chi monocytic subset.
(A) Isolated Tgfb1−/− liver mononuclear cells were stained for CD11b, Ly6C, and Ly6G. Gated CD11b+ cells were analyzed for expression of Ly6C and Ly6G. (B) CD11b+ cells were fractionated into Ly6GhiLy6Clo and Ly6G−Ly6Chi cell populations, and (C) placed in co-culture with CFSE-labeled stimulated CD4+ T cells. (D) Nitrite was measured in supernatants. ** p < 0.01; n.s. p not significant.
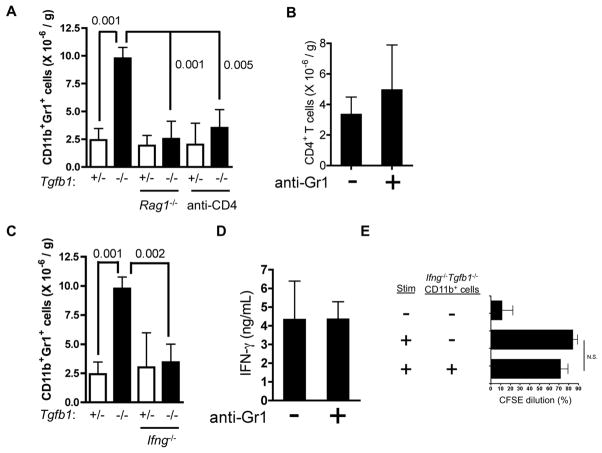
The accumulation of CD11b+Gr1+ cells in Tgfb1−/− liver is dependent on CD4+ T cells and IFN-γ
The rapid accumulation of MDSC parallels that of CD4+ T cells (Fig. 1). Therefore, we asked whether one cell type influences the accumulation of the other in vivo, by examining CD11b+Gr1+ cell accumulation at day 11 in livers of Tgfb1−/− mice rendered deficient either in all adaptive lymphocytes (Rag1−/−) or specifically in CD4+ T cells (anti-CD4 mAb). Neither Rag1−/− Tgfb1−/− mice nor anti-CD4-treated Tgfb1−/− mice exhibited an increase in liver CD11b+Gr1+ cells (Fig. 6A). Conversely, CD4+ T cells accumulated to high levels in Tgfb1−/− mice whether or not CD11b+Gr1+ cells had been depleted (anti-Gr1; Fig. 6B). Thus, CD4+ T cells are required for MDSC accumulation in Tgfb1−/− liver, whereas CD4+ T cells accumulate despite MDSC depletion.
Figure 6. Th1 cells drive MDSC accumulation in liver through IFN-γ.
(A-D) Th1 cells drive MDSC accumulation liver, not vice versa. (A, C) CD11b+Gr1+ cells, (B) CD4+ T cells, and (D) plasma IFN-γ were quantified on day 11 for mice of the genotypes (Tgfb1; Rag1; Ifng) and treatments (anti-CD4; anti-Gr1) indicated. n = 3 or more mice per group. Numbers represent p values for the comparisons indicated by lines. (B, D) Data are for Tgfb1−/− mice that were either untreated (“−“) or treated (“+”) with an anti-Gr1 mAb. (E) Liver CD11b+ cells from Ifng−/− Tgfb1−/− mice do not suppress T cell proliferation. Data aggregate four separate experiments.
We examined the role of IFN-γ in MDSC accumulation. Circulating plasma IFN-γ levels are highly elevated in Tgfb1−/− mice (22), IFN-γ is necessary for hepatocellular damage (9), and CD4+ T cells are the only significant source of IFN-γ in this model (22). Ifng−/− Tgfb1−/− mice exhibited normal liver CD11b+Gr1+ cell numbers (Fig. 6C). Conversely, depletion of CD11b+Gr1+ cells had no effect on plasma IFN-γ levels in Tgfb1−/− mice (Fig. 6D), which remained elevated. In addition, Ifng−/− Tgfb1−/− liver CD11b+ cells failed to suppress T cell proliferation in vitro (Fig. 6E). Thus, IFN-γ is essential both for the in vivo accumulation of CD11b+Gr1+ cells and for their in vitro suppressor function. Ifng−/− Tgfb1−/− livers and Ifng+/+Tgfb1−/− livers exhibit equivalent accumulation of CD4+ T cells (22), indicating that the effects of IFN-γ on MDSC accumulation are not attributable to an indirect effect of this cytokine on CD4+ T cell accumulation.
Discussion
In Tgfb1−/− mice, a murine model of acute Th1-mediated hepatocellular injury, CD11b+Gr1+ cells accumulate in liver in response to the production of IFN-γ from CD4+ T cells. Tgfb1−/− liver CD11b+Gr1+ cells are potent MDSC in vitro, producing NO to inhibit the proliferation of TCR-activated T cells. The production of IFN-γ is important for the development of the MDSC response at several junctures. First, IFN-γ is required for the accumulation of MDSC in liver, which does not occur in Ifng−/− Tgfb1−/− mice; second, IFN-γ is required for full MDSC suppressor function, as inclusion of a neutralizing anti-IFN-γ mAb in MDSC-T cell co-culture partially abrogates suppressor activity. These studies show that IFN-γ is necessary not only for hepatocellular injury but also for the development of the MDSC response. Thus, IFN-γ sits at a critical node of the liver immune response, responsible on the one hand for T cell mediated parenchymal damage and on the other for initiating an MDSC-mediated negative feedback pathway that can restrain T cell proliferation.
Murine liver schistosomiasis is a classic model of Th2 mediated inflammation, with granulomata forming around parasite eggs deposited in the liver (25, 26). Myeloid cells restrain granulomatous inflammation and fibrosis through activity of arginase (27), which acts by depleting T cells of the essential amino acid L-arginine. By contrast, inflammation and parenchymal damage in Tgfb1−/− mice is a “pure” Th1 phenomenon, dependent upon the Th1 cytokine IFN-γ and independent of the Th2 cytokine IL-4 (9). Thus, distinct types of inflammation induce distinct subsets of myeloid suppressor cells that act through subset-specific mechanisms. The association of iNOS with myeloid cells in Th1 responses and arginase with myeloid cells in Th2 responses is a recurring theme in inflammation (28), and the dichotomy appears applicable to liver inflammation as well. An important aspect of our work is the demonstration that Th1 cells themselves are responsible for the accumulation of MDSC in liver. Although it has been shown that IFN-γ can activate MDSC (29, 30), to our knowledge, this is the first demonstration that IFN-γ from CD4+ T cells can drive MDSC accumulation to a site of inflammation. How might IFN-γ effect MDSC accumulation? While IFN-γ might act directly, more likely IFN-γ acts indirectly, inducing other cells (e.g. hepatocytes, endothelial cells, Kuppfer cells) to secrete chemoattractants that in turn recruit MDSC. Previous work shows that MDSC accumulate at sites of inflammation in response to a number of inflammatory molecules. MDSC isolated from hepatocellular carcinoma (HCC) tumors in B6 mice express the chemokine receptor CCR2 and migrate in vitro in response to the ligand CCL2 (31). Directly implicating the importance of this pathway, murine CCR2−/− MDSC exhibit deficiencies in migration into HCC tumors (31), and, in another system, into the ovarian tumor microenvironment (32). The IL-1 response axis as well as proteins of the S100 family are important for MDSC accumulation in the tumor microenvironment (13, 33–35). Microarray analyses show that, at the mRNA level, in Tgfb1−/− liver, CCR2 and CCL2 are over-expressed ~10 fold (36), IL-1β is over-expressed 17-fold (36), and various S100-encoding mRNAs are over-expressed 2- to 11-fold (unpublished data), but we have not yet tested whether any of these pathways is important for MDSC accumulation.
As discussed, unrestrained auto-reactive Th1 responses in the liver likely contribute to the pathophysiologic basis of AIH, but the participation of cells of myeloid origin is currently unclear. It is known that populations of CD11b+ myeloid cells infiltrate the livers of AIH patients (1), but functional analyses of these cells are lacking. Longhi et al. recently characterized peripheral blood monocytes from AIH patients. Although surrogates for their intrahepatic counterparts, circulating monocytes from AIH patients -compared with circulating monocytes from healthy controls - are more numerous (with frequency correlating with AST), more spontaneously migratory, and express greater TLR4 and TNF-α (37). The authors suggested that “monocyte involvement in the liver damage [would] perpetuate the autoimmune attack.” However, this study did not examine iNOS expression or the production of NO, and did not test whether blood (or liver) monocytes from AIH patients are capable of inhibiting T cell proliferation in vitro. Therefore, we offer an alternative possibility, that the activated myeloid/monocytic cell population in AIH patients represents monocytic MDSC recruited by activated T cells producing IFN-γ, with the potential, perhaps unrealized or somehow blocked, to inhibit T cell mediated autoimmunity. Whether and how cells of myeloid origin participate in regulating inflammatory and/or autoimmune processes in the liver, and whether and how MDSC may fail in their suppressor function, are important research questions in AIH and other inflammatory liver diseases.
Supplementary Material
Acknowledgments
We thank Drs. Mary Jo Turk, Edward Usherwood, and Jose Conejo-Garcia (all at Dartmouth Medical School) for, respectively, the GK1.5 antibody, the IL-10/IL-10R neutralizing antibodies, and the use of the microscope and related software, and Beverly Gorham and Christine Kretowicz for mouse breeding.
Financial Support: This work was supported by National Institutes of Health Grants AI078195 (J. D. G.) and P20RR16437 from the COBRE Program of the National Center for Research Resources, as well as by a grant from the Hitchcock Foundation (J. D. G.). J. W. was supported by a Samuel A. Hamacher Autoimmune Hepatitis Postdoctoral Research Fellowship from the American Liver Foundation. J. G. C. was supported by National Institutes of Health Training Grant T32AI07363.
Abbreviations
- AIH
autoimmune hepatitis
- HCV
hepatitis C virus
- MDSC
myeloid derived suppressor cells
- iNOS
inducible nitric oxide synthase
- NO
nitric oxide
- CFSE
5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester
- IDO
indoleamine 2,3-dioxygenase
- ROS
reactive oxygen species
- L-NMMA
L-NG-monomethyl Arginine citrate
- D-NMMA
D-NG-monomethyl Arginine citrate
- L-NIL
N6- (1-iminoethyl)-L-lysine
- nor-NOHA
N-Hydroxy-nor-Arginine
- 1-MT
1-methyl-tryptophan
References
- 1.Senaldi G, Portmann B, Mowat AP, Mieli-Vergani G, Vergani D. Immunohistochemical features of the portal tract mononuclear cell infiltrate in chronic aggressive hepatitis. Arch Dis Child. 1992;67:1447–1453. doi: 10.1136/adc.67.12.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlaak JF, Lohr H, Gallati H, Meyer Zum Buschenfelde KH, Fleischer B. Analysis of the in vitro cytokine production by liver-infiltrating T cells of patients with autoimmune hepatitis. Clin Exp Immunol. 1993;94:168–173. doi: 10.1111/j.1365-2249.1993.tb05996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohr HF, Schlaak JF, Gerken G, Fleischer B, Dienes HP, Meyer Zum Buschenfelde KH. Phenotypical analysis and cytokine release of liver-infiltrating and peripheral blood T lymphocytes from patients with chronic hepatitis of different etiology. Liver. 1994;14:161–166. doi: 10.1111/j.1600-0676.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 4.Longhi MS, Hussain MJ, Bogdanos DP, Quaglia A, Mieli-Vergani G, Ma Y, Vergani D. Cytochrome P450IID6-specific CD8 T cell immune responses mirror disease activity in autoimmune hepatitis type 2. Hepatology. 2007;46:472–484. doi: 10.1002/hep.21658. [DOI] [PubMed] [Google Scholar]
- 5.Rosen HR. Hepatitis C pathogenesis: mechanisms of viral clearance and liver injury. Liver Transpl. 2003;9:S35–S43. doi: 10.1053/jlts.2003.50253. [DOI] [PubMed] [Google Scholar]
- 6.Sobue S, Nomura T, Ishikawa T, Ito S, Saso K, Ohara H, Joh T, et al. Th1/Th2 cytokine profiles and their relationship to clinical features in patients with chronic hepatitis C virus infection. J Gastroenterol. 2001;36:544–551. doi: 10.1007/s005350170057. [DOI] [PubMed] [Google Scholar]
- 7.Napoli J, Bishop GA, McGuinness PH, Painter DM, McCaughan GW. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology. 1996;24:759–765. doi: 10.1002/hep.510240402. [DOI] [PubMed] [Google Scholar]
- 8.Kusters S, Gantner F, Kunstle G, Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–471. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- 9.Gorham JD, Lin JT, Sung JL, Rudner LA, French MA. Genetic regulation of autoimmune disease: BALB/c background TGF-beta 1- deficient mice develop necroinflammatory IFN-gamma-dependent hepatitis. J Immunol. 2001;166:6413–6422. doi: 10.4049/jimmunol.166.10.6413. [DOI] [PubMed] [Google Scholar]
- 10.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 11.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 15.Ilkovitch D, Lopez DM. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 2009;69:5514–5521. doi: 10.1158/0008-5472.CAN-08-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudner LA, Lin JT, Park IK, Cates JM, Dyer DA, Franz DM, French MA, et al. Necroinflammatory liver disease in BALB/c background, TGF-beta 1-deficient mice requires CD4+ T cells. J Immunol. 2003;170:4785–4792. doi: 10.4049/jimmunol.170.9.4785. [DOI] [PubMed] [Google Scholar]
- 19.Lin JT, Kitzmiller TJ, Cates JMM, Gorham JD. MHC-independent genetic regulation of liver damage in a mouse model of autoimmune hepatocellular injury. Laboratory Investigation. 2005;85:550–561. doi: 10.1038/labinvest.3700246. [DOI] [PubMed] [Google Scholar]
- 20.Titheradge MA. The enzymatic measurement of nitrate and nitrite. Methods Mol Biol. 1998;100:83–91. doi: 10.1385/1-59259-749-1:83. [DOI] [PubMed] [Google Scholar]
- 21.Gorham JD, Lin JT, Sung JL, Rudner LA, French MA. Genetic regulation of autoimmune disease: BALB/c background TGF-beta 1-deficient mice develop necroinflammatory IFN-gamma-dependent hepatitis. J Immunol. 2001;166:6413–6422. doi: 10.4049/jimmunol.166.10.6413. [DOI] [PubMed] [Google Scholar]
- 22.Robinson RT, Wang J, Cripps JG, Milks MW, English KA, Pearson TA, Gorham JD. End-organ damage in a mouse model of fulminant liver inflammation requires CD4+ T cell production of IFN-gamma but is independent of Fas. J Immunol. 2009;182:3278–3284. doi: 10.4049/jimmunol.0803417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 24.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- 26.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 29.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 31.Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, Liu Y, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Hart KM, Bak SP, Alonso A, Berwin B. Phenotypic and functional delineation of murine CX(3)CR1 monocyte-derived cells in ovarian cancer. Neoplasia. 2009;11:564–573. doi: 10.1593/neo.09228. 561 p following 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milks MW, Cripps JG, Lin H, Wang J, Robinson RT, Sargent JL, Whitfield ML, et al. The role of Ifng in alterations in liver gene expression in a mouse model of fulminant autoimmune hepatitis. Liver Int. 2009 doi: 10.1111/j.1478-3231.2009.02028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longhi MS, Mitry RR, Samyn M, Scalori A, Hussain MJ, Quaglia A, Mieli-Vergani G, et al. Vigorous activation of monocytes in juvenile autoimmune liver disease escapes the control of regulatory T-cells. Hepatology. 2009;50:130–142. doi: 10.1002/hep.22914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.