Abstract
Background
An extensive animal literature suggests that stress or excessive corticosteroid exposure is associated with changes in hippocampal function and memory. These findings are pertinent to psychiatric disorders with elevated cortisol, Cushing’s disease and the millions of patients receiving prescription corticosteroids. In animals, agents that decrease glutamate release attenuate the effects of corticosteroids on the hippocampus. Minimal data are available on preventing or reversing the effects of corticosteroids on the human hippocampus. We previously reported improvement in memory in corticosteroid-treated patients given lamotrigine. In this report, we examined the impact of lamotrigine on task-related hippocampal activation in patients taking prescription corticosteroids.
Methods
A total of 28 outpatients taking long-term oral prednisone for medical conditions, such as renal transplant rejection, were randomized to lamotrigine or placebo for 24 weeks. Hippocampal activation in response to a visual memory task was assessed with blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI).
Results
Consistent with a reduction in glutamate release, the right posterior hippocampus showed a significant decrease in task-related activation in the lamotrigine group as compared to the placebo group.
Limitations
The modest sample size and an assessment period of only 24 weeks are study limitations.
Conclusions
Between-group differences in hippocampal activation were observed. The results suggest that an agent that modulates glutamate may modify the effects of long-term corticosteroid exposure on the human hippocampus.
Keywords: functional magnetic resonance imaging, lamotrigine, prednisone, hippocampus
INTRODUCTION
Excessive corticosteroid exposure is associated with changes in memory and hippocampal structure in animal models (Magarinos et al., 1997; Uno et al., 1994; Vyas et al., 2002). In animals, agents that decrease glutamate release (Magarinos et al., 1996), enhance serotonin reuptake (Watanabe et al., 1992) or block the N-methyl-D-aspartate (NMDA) receptor (Magarinos and McEwen, 1995) attenuate corticosteroid effects on the hippocampus. A much smaller literature on the effects of corticosteroids on the human hippocampus is available. Acute administration of exogenous corticosteroids in humans is associated with reversible decline in declarative memory performance (de Quervain et al., 2000; Newcomer et al., 1999). Cushing’s disease is associated with memory impairment (Mauri et al., 1993) and hippocampal atrophy (Starkman et al., 1992) that is, at least partially, reversible with normalization of cortisol levels (Starkman et al., 1999; Starkman et al., 2003). We reported that patients receiving long-term prescription corticosteroid therapy had poorer declarative memory, decreased hippocampal volume and decreased temporal lobe levels of N-acetyl aspartate as compared to controls with similar medical histories but minimal corticosteroid exposure (Brown et al., 2004). However, a study examining patients taking corticosteroids at lower dosages and for shorter periods than in or report did not find significant reductions in hippocampal volume (Coluccia et al., 2008).
We also examined whether the corticosteroid-induced changes in the human hippocampus can be reversed with agents that decrease glutamate release or act as antagonists at the NMDA receptor. We reported a significantly greater improvement in declarative memory with memantine, an NMDA receptor antagonist, than with placebo in patients taking corticosteroids (Brown et al., 2008a). We also found significant improvement in declarative memory and differences in amygdala volume, but no significant changes in hippocampal volume, in corticosteroid-treated patients given lamotrigine, a glutamate release inhibitor, as compared to placebo (Brown et al., 2008b). We now report functional magnetic resonance imaging (fMRI) data from this trial to determine the impact of treatment with lamotrigine on task-related hippocampal activation.
METHODS
Participants and Study Medication
A total of 28 medically stable adult outpatients receiving chronic oral corticosteroid therapy (≥ 10 mg/day of prednisone equivalents for ≥ 6 months) participated in a 24 week randomized, double-blind, placebo-controlled trial of lamotrigine. All participants signed an IRB-approved informed consent form. The study was registered at clinicaltrials.gov. Lamotrigine or identical appearing placebo was initiated at 25 mg/day and titrated to a dose of 400 mg/day over 10 weeks using a fixed dosing schedule unless side effects required a slower titration or dose reduction. For additional information on the experimental procedures and patient sample please see the primary data analysis (Brown et al., 2008b).
fMRI procedures
Functional magnetic resonance images of the brain were obtained at baseline and week 24. MR images were acquired on a General Electric Horizon LX NV/i 1.5 Tesla scanner (General Electric Medical Systems, Milwaukee, WI) using the standard GE quadrature birdcage RF head coil. For the collection of fMRI data, a time series of 120 echo-planar image (EPI) volumes was acquired at 21 coronal slice locations through the whole brain. Echo-planar images were acquired with a single-shot gradient-recalled EPI pulse sequence (sequential slice acquisition; repetition time [TR] = 2000 ms; echo time [TE] = 45 ms; flip angle = 90°; matrix = 64×64; field of view [FOV] = 24 cm; slice thickness = 7 mm; slice-to-slice gap = 0.5 mm). High-resolution T1-weighted images of the entire brain (3D Spoiled Grass pulse sequence: TR = 30 ms; TE = 5 ms; flip angle = 45°; matrix = 256 × 256; FOV = 24 cm; slice thickness = 2.0 mm) were acquired during the same scan session for each participant.
fMRI activation task
A visual scene encoding task (Stern et al., 1996) was used to activate the hippocampus. This task was presented as a block design alternating between the visual scene encoding condition and a control condition. During each encoding block, participants viewed complex nature scenes that they were instructed to study carefully, as they would be asked to remember them later. During control condition, participants attended to a single familiar nature scene presented repeatedly at the same rate as the stimuli to be encoded. Prior to scanning, subjects were given instructions on the task and an opportunity to practice. None of the encoding pictures were shown during this training, but subjects were introduced to the familiar picture. Stimuli were back-projected onto a screen at the participants’ feet. This was viewed through a mirror attached to the head coil. Pictures were presented for 3 seconds and each block was 60 sec in length. Encoding occurred during the second and fourth block, while the control task was performed during the first and third.
MRI Data Analysis
Pre-processing
AFNI software (Cox, 1996) was used for the analysis of MRI data. To correct for motion, a three-dimensional volume registration algorithm was applied to each EPI dataset. One participant’s data were excluded due to excessive motion. Remaining data were examined for excessive signal values at individual time points and these outlier values (i.e., signal greater than four times the standard deviation for the entire signal time course) were replaced with the median value. Data were then spatially smoothed with a three-dimensional Gaussian filter (8 mm full-width at half maximum) to increase signal-to-noise.
Isolation of hippocampus region of interest for fMRI analyses
For each participant, hippocampal volumes were identified. Each hippocampal mask was resampled to match the lower resolution of the functional dataset (Li et al., 2002). Differential functional roles of the anterior and posterior hippocampus (Eichenbaum and Lipton, 2008; Peters et al., 2007) has been reported. Therefore, the resampled tracings were further edited to create separate anterior and posterior regions of interest (ROIs). To define the anterior hippocampus, the whole hippocampus tracing was edited to include only the slices that occupied the head of the hippocampus. For the posterior hippocampus, the whole hippocampus tracing was edited to include only the slices that occupied the tail of the hippocampus. This identification procedure was repeated for the right and left hemisphere for each participant.
Identification of activated regions
An ideal function reflecting the alternation between control and experimental conditions was created and used as a reference for cross-correlation with temporal fluctuations in MR signal from all brain voxels. The Least-Squares Fit coefficient, which estimates the proportion of ideal function that exists in each voxel’s time course signal data after removing the mean and linear trend, was used as the index of functional activation. Average fit coefficients within the separate anterior and posterior hippocampal ROIs were calculated and used to reflect the magnitude of activation in each region. Effect of lamotrigine treatment on activation in the hippocampal ROIs was investigated using four separate Group (placebo, lamotrigine) × Time (Pre-treatment, Post-treatment) mixed design ANOVAs.
RESULTS
Data at the week 24 neuroimaging assessment were available on 12 completers. Participants in lamotrigine and placebo groups did not differ significantly on baseline demographic characteristics. Those who discontinued participation did not differ significantly from completers on baseline demographic characteristics.
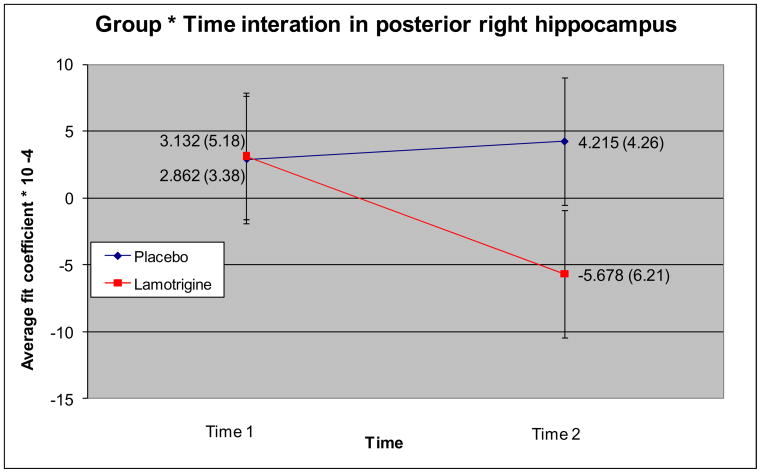
Hippocampal tracings are shown in Figure 1, and group MRI data are provided in Table 1. There was a significant Group * Time interaction for the posterior right hippocampus, F (1, 10) = 5.73, p = .038 (Figure 2). To rule out that age had an effect on the interaction, age was added as a covariate in an ANCOVA. Results suggested age could not have accounted for this effect, as the significance level of the interaction increased from p = .038 to p = .023 Follow-up comparisons showed a trend for an effect of time in the lamotrigine group, t (5) = 2.15, p = .084 while the effect of time in the placebo group was not significant, t (5) = −1.21, p = .28. Post hoc comparison showed a significant difference between groups at Time 2, t (10) = 3.21, p = .009, but no difference between the groups at Time 1, t (10) = −.11, p = .92.
Figure 1.
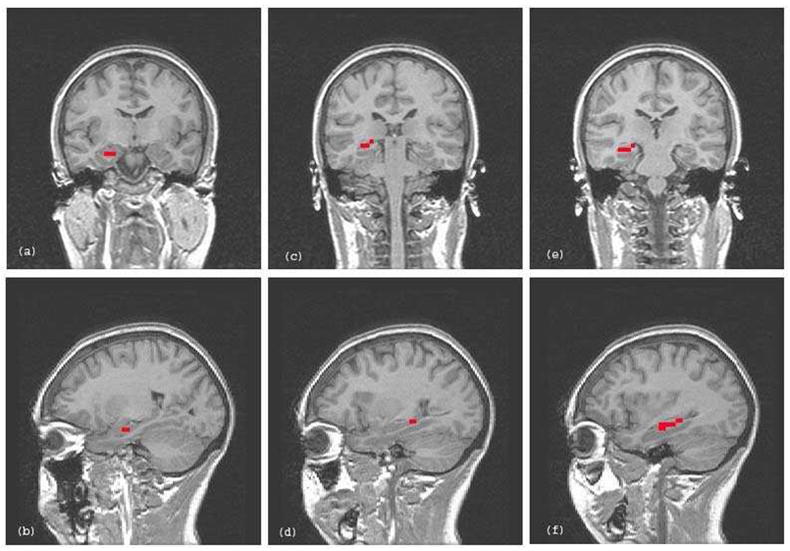
Examples of partial (i.e., anterior and posterior), and whole right hippocampus tracings from a single subject overlaid on representative coronal and sagittal slices. (a) and (b) = anterior right hippocampus tracing in coronal and sagittal planes; (c) and (d) = posterior right hippocampus tracing in coronal and sagittal planes; (e) and (f) = whole right hippocampus tracing in coronal and sagittal planes.
Table 1.
Whole, anterior and posterior hippocampus ROI mean and standard deviation (pre, post) average fit coefficient values* 10−4 for lamotrigine and placebo groups
| ROI | Group | Pre-treatment | Post-treatment | N |
|---|---|---|---|---|
| M (SD) | M (SD) | |||
| Left | Placebo | 2.26 (10.14) | −4.09 (6.74) | 6 |
| Lamotrigine | −2.27 (7.93) | −8.87 (4.73) | 6 | |
| Anterior left | Placebo | −2.53 (17.91) | −7.60 (10.90) | 6 |
| Lamotrigine | −8.10 (15.93) | −12.02 (9.72) | 6 | |
| Posterior left | Placebo | 3.45 (2.61) | −3.03 (12.74) | 6 |
| Lamotrigine | 1.21 (7.77) | −6.19 (10.65) | 6 | |
| Right | Placebo | 3.65 (4.07) | −3.44 (6.75) | 6 |
| Lamotrigine | −0.57 (0.87) | −6.02 (4.71) | 6 | |
| Posterior right | Placebo | 2.86 (3.38) | 4.21 (4.26) | 6 |
| Lamotrigine | 3.13 (5.18) | −5.68 (6.21) | 6 | |
| Anterior right | Placebo | 1.15 (10.19) | −9.21 (12.56) | 6 |
| Lamotrigine | −2.30 (2.13) | −6.39 (3.32) | 6 | |
Figure 2.
Directions of change in task-related activation from pre- to post-treatment in lamotrigine and placebo groups in the posterior right hippocampus.
DISCUSSION
This investigation demonstrated an effect of lamotrigine on fMRI activation in the posterior right hippocampus. We found a decrease in right posterior hippocampal activation in the lamotrigine group from pre- to post-treatment and a slight increase over time in the placebo group. No significant between-group differences were found in other hippocampal regions with reductions in activation observed in both treatment conditions.
Decreased task-related activation in the lamotrigine group is consistent with animal (Hyder et al., 2001; Kida et al., 2006) and human (Deakin et al., 2008; Jogia et al., 2008) studies showing selective regional reductions in task-related BOLD signal activation following lamotrigine treatment. BOLD signal activation appears to be related, at least in part, to glutamate release (Baslow et al., 2005; Gsell et al., 2006). Therefore, the decrease in activation in the posterior right hippocampus in the lamotrigine group is consistent with a reduction in glutamate release.
Results suggest that in addition to memory improvement, lamotrigine therapy was associated with changes in hippocampal activation consistent with a reduction in glutamate release. The findings also suggest that fMRI may be more sensitive to lamotrigine effects on the hippocampus than is structural MRI. A strength of the study is the randomized, double-blind, placebo-controlled design. Limitations are the modest sample size and an assessment period of only 24 weeks.
Acknowledgments
Role of funding sources: Supported by NIH grant MH01725 and an investigator-initiated grant from GlaxoSmithKline. The sponsors had no further role in study design, collection, analysis and interpretation of data, writing of the report; or the decision to submit the paper for publication.
Acknowledgements: None.
Footnotes
Contributors: Dr. Brown designed the study and wrote much of the manuscript. Dr. Zaidel performed the data analysis and contributed substantially to the writing of the manuscript. Dr. McColl contributed to the data acquisition and analysis and reviewed and made contributions to the manuscript. Dr. Allen supervised the data analysis and made contributions to the manuscript. Dr. Vazquez assisted with participant enrollment and reviewed and contributed to the manuscript. Dr. Ringe contributed to administration of the activation task and reviewed the manuscript.
Conflicts of interest: Dr. Brown has research funding from NIMH, NIDA, NIAAA, the Stanley Medical Research Institute, and Forest, and receives study drug from Astra Zeneca and GlaxoSmithKline. Dr. Vazquez has research support from NIDDK. Dr. Allen has funding from NIMH. Drs. Ringe, Zaidel and McColl report no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baslow MH, Dyakin VV, Nowak KL, Hungund BL, Guilfoyle DN. 2-PMPA, a NAAG peptidase inhibitor, attenuates magnetic resonance BOLD signals in brain of anesthetized mice: evidence of a link between neuron NAAG release and hyperemia. J Mol Neurosci. 2005;26:1–15. doi: 10.1385/JMN:26:1:001. [DOI] [PubMed] [Google Scholar]
- Brown ES, Vazquez M, Nakamura A. Randomized, placebo-controlled, crossover trial of memantine for cognitive changes with corticosteroid therapy. Biol Psychiatry. 2008a;64:727–729. doi: 10.1016/j.biopsych.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Brown ES, Wolfshohl J, Shad MU, Vazquez M, Osuji IJ. Attenuation of the effects of corticosteroids on declarative memory with lamotrigine. Neuropsychopharmacology. 2008b;33:2376–2383. doi: 10.1038/sj.npp.1301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES, Woolston JD, Frol A, Bobadilla L, Khan DA, Hanczyc M, Rush AJ, Fleckenstein J, Babcock E, Cullum CM. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol Psychiatry. 2004;55:538–545. doi: 10.1016/j.biopsych.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Coluccia D, Wolf OT, Kollias S, Roozendaal B, Forster A, de Quervain DJ. Glucocorticoid therapy-induced memory deficits: acute versus chronic effects. J Neurosci. 2008;28:3474–3478. doi: 10.1523/JNEUROSCI.4893-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Lipton PA. Towards a functional organization of the medial temporal lobe memory system: role of the parahippocampal and medial entorhinal cortical areas. Hippocampus. 2008;18:1314–1324. doi: 10.1002/hipo.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gsell W, Burke M, Wiedermann D, Bonvento G, Silva AC, Dauphin F, Buhrle C, Hoehn M, Schwindt W. Differential effects of NMDA and AMPA glutamate receptors on functional magnetic resonance imaging signals and evoked neuronal activity during forepaw stimulation of the rat. J Neurosci. 2006;26:8409–8416. doi: 10.1523/JNEUROSCI.4615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 2001;14:413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- Jogia J, Haldane M, Cobb A, Kumari V, Frangou S. Pilot investigation of the changes in cortical activation during facial affect recognition with lamotrigine monotherapy in bipolar disorder. Br J Psychiatry. 2008;192:197–201. doi: 10.1192/bjp.bp.107.037960. [DOI] [PubMed] [Google Scholar]
- Kida I, Smith AJ, Blumenfeld H, Behar KL, Hyder F. Lamotrigine suppresses neurophysiological responses to somatosensory stimulation in the rodent. Neuroimage. 2006;29:216–224. doi: 10.1016/j.neuroimage.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Li SJ, Li Z, Wu G, Zhang MJ, Franczak M, Antuono PG. Alzheimer Disease: evaluation of a functional MR imaging index as a marker. Radiology. 2002;225:253–259. doi: 10.1148/radiol.2251011301. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri M, Sinforiani E, Bono G, Vignati F, Berselli ME, Attanasio R, Nappi G. Memory impairment in Cushing’s disease. Acta Neurol Scand. 1993;87:52–55. doi: 10.1111/j.1600-0404.1993.tb04075.x. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AL. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry. 1999;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- Peters J, Suchan B, Koster O, Daum I. Domain-specific retrieval of source information in the medial temporal lobe. Eur J Neurosci. 2007;26:1333–1343. doi: 10.1111/j.1460-9568.2007.05752.x. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s disease. Biol Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Schteingart DE. Improvement in learning associated with increase in hippocampal formation volume. Biol Psychiatry. 2003;53:233–238. doi: 10.1016/s0006-3223(02)01750-x. [DOI] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol. 1992;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]