Abstract
The cytokine melanoma differentiation associated gene 7 (mda-7) was identified by subtractive hybridization as a protein whose expression increased during the induction of terminal differentiation, and that was either not expressed or was present at low levels in tumor cells compared to non-transformed cells. Based on conserved structure, chromosomal location and cytokine-like properties, MDA-7, was classified as a member of the interleukin (IL)-10 gene family and designated as MDA-7/IL-24. Multiple studies have demonstrated that expression of MDA-7/IL-24 in a wide variety of tumor cell types, but not in corresponding equivalent non-transformed cells, causes their growth arrest and rapid cell death. In addition, MDA-7/IL-24 has been noted to radiosensitize tumor cells which in part is due to the generation of reactive oxygen species (ROS) and ceramide that cause endoplasmic reticulum stress and suppress protein translation. Phase I clinical trial data has shown that a recombinant adenovirus expressing MDA-7/IL-24 (Ad.mda-7 (INGN-241)) was safe and had measurable tumoricidal effects in over 40% of patients, strongly arguing that MDA-7/IL-24 could have significant therapeutic value. This review describes what is presently known about the impact of MDA-7/IL-24 on tumor cell biology and its potential therapeutic applications.
Keywords: MDA-7, IL-24, Apoptosis, Autophagy, Ceramide, ROS, Ca2+, Clinical trial, Signal transduction, PERK, ER stress, MCL-1
1. Background to MDA-7/IL-24
MDA-7/IL-24 was discovered using a subtraction hybridization approach by exposing melanoma cells to the terminal differentiation-inducing agents interferon beta and mezerein (Jiang & Fisher, 1993; Jiang et al., 1993, 1995). Based on a conserved amino acid signature sequence, chromosomal location and cytokine-like properties, mda-7, has been classified as a member of the expanding interleukin (IL)-10 gene family, which includes IL-10, IL-19, IL-20, IL-22 and IL-26, and has been designated as mda-7/IL-24 (Jiang et al., 1995; Ekmekcioglu et al., 2001; Huang et al., 2001; Ellerhorst et al., 2002; Wolk et al., 2002; Pestka et al., 2004). MDA-7/IL-24 protein expression is decreased in advanced melanomas, with nearly undetectable levels in metastatic disease, in general agreement with this gene product being classified as a tumor suppressor (Jiang et al., 1995; Jiang et al., 1996; Huang et al., 2001; Wolk et al., 2002). Other published studies over the last 15 years have demonstrated that enforced expression of MDA-7/IL- 24, either by transfection of a plasmid containing the cDNA for mda-7/IL-24 or by use of a recombinant adenovirus to deliver the gene, Ad.mda-7, rapidly inhibits the growth of a broad-spectrum of cancer cells, resulting in tumor cell death within 24–48 h (Jiang et al., 1993; Jiang & Fisher, 1993; Jiang et al., 1995; Su et al., 1998; Huang et al., 2001; Ekmekcioglu et al., 2001; Wolk et al., 2002; Ellerhorst et al., 2002; Kotenko, 2002; Caudell et al., 2002; Pestka et al., 2003, 2004; Fisher, 2005; Lebedeva et al., 2005, 2007b). When expressed, MDA-7/IL-24 is secreted from cells, as would be expected for a cytokine. Of considerable note, when MDA-7/IL-24 was over-expressed in non-transformed cells little change was observed in either cell growth or cell viability (e.g., Jiang et al., 1996).
Initial studies using mammalian cell-synthesized MDA-7/IL-24 protein; a protein that is a dimer and glycosylated, demonstrated that purified MDA-7/IL-24 interacted with two type II cytokine hetero-dimeric receptor complexes: IL-20R1/IL-20R2 (type 1 IL-20R) and IL-22R1/IL-20R2 (type 2 IL-20R) (Parrish-Novak et al., 2002). In one of the first of these studies, non-transformed BHK cells stably transfected with IL-20 and IL-22 receptors were treated with MDA-7/IL-24; at low pM concentrations of MDA-7/IL-24 (<100 pM) growth was promoted whereas at higher concentrations (>100 pM) it inhibited cell proliferation. In transfected cells, MDA-7/IL-24 activated multiple STAT transcription factors. However, in ovarian carcinoma cells, which express endogenous IL-20 receptor complexes, it was noted that MDA-7/IL-24 at low nM concentrations promoted growth inhibition without altering STAT transcription factor phosphorylation/function (Parrish-Novak et al., 2002; Chada et al., 2004a,b). Other studies have demonstrated using tumor cells, which lack STAT1 or STAT3 function or with blocked Janus kinase function that STAT pathway signaling is not required for MDA-7/IL-24-induced growth arrest or tumor cell killing (Sauane et al., 2003).
More recently, experiments indicate a difference in the cell signaling and cell killing properties of bacterial synthesized unglycosylated and monomeric GST-MDA-7/IL-24 and mammalian cell-synthesized glycosylated dimeric MDA-7/IL-24 with FLAG or (His)6 tags to assist in isolation and purification. In multiple studies using a wide variety of transformed cell lines, GST-MDA-7/IL-24 has been noted to promote cell growth arrest and apoptosis in a transformed cell-specific fashion and has been noted to cause these effects independently of expression of IL-20 receptors, in a similar manner to Ad.mda-7 (Sauane et al., 2004a; Lebedeva et al., 2007b; Emdad et al., 2009; and references therein). This would suggest that cancer cells take up GST-MDA-7/IL-24 in an interleukin receptor-independent fashion (Sauane et al., 2004a). In contrast to GST-MDA-7/IL-24 and Ad.mda-7, purified MDA-7/IL-24, synthesized in mammalian cells, does not appear to have any biologic effect on cells lacking expression of IL-20 receptor complexes (Sauane et al., 2008). Of note however, and in a similar manner to GST-MDA-7/IL-24 and Ad.mda-7, in cells where IL-20 receptor complexes were expressed, mammalian synthesized MDA-7/IL-24-induced cell killing was independent of STAT transcription factor activation. For example, in A549 human lung carcinoma cells, which lack expression of the IL-20 receptor complexes, extracellular treatment with mammalian cell-synthesized MDA-7/IL-24 results in no biologic effect on cell growth/viability, whereas treatment with GST-MDA-7/IL-24, or viral infection with Ad.mda-7 or Ad.mda-7 signal peptide null (SP-), which expressed a non-secreted form of MDA-7/IL-24 or transfection of a plasmid to express MDA-7/IL-24 all results in tumor cell growth arrest and cell death (Nishikawa et al., 2004; Sauane et al., 2004a,b; Pataer et al., 2007). Furthermore, although it has been noted that MDA-7/IL-24, IL-20, and IL-19 all activated STAT transcription factors in IL-20 receptor expressing cancer cells, only MDA-7/IL-24 has the ability to cause cell death (Chada et al., 2006). Collectively, this data argues that the direct tumoricidal effects of MDA-7/IL-24 when expressed intracellularly are independent of IL-20 receptor complex signaling and instead are dependent on an additional biological property of MDA-7/IL-24.
2. MDA-7/IL-24 and apoptosis
The pathways by which Ad.mda-7 (or: transfection with a cDNA to express MDA-7/IL-24; treatment with bacterial synthesized GST-MDA-7/IL-24 or eukaryotic cell generated His6-MDA-7/IL-24) enhances apoptosis in tumor cells are still not completely understood, however, over the last 7 years a large amount of evidence from multiple studies has demonstrated the involvement of proteins important in the regulation of endoplasmic reticulum (ER) stress and mitochondrial integrity (Lebedeva et al., 2003a,b; Gupta et al., 2006a; Lebedeva et al., 2007a; Yacoub et al., 2008a; Park et al., 2009; Yacoub et al., 2010a; Hamed et al., 2010; Yacoub et al., 2010b) (Fig. 1). Some studies have argued that MDA-7/IL-24 promoted activation of the double stranded RNA-activated kinase, Protein Kinase R (PKR), which was correlated with enhanced eIF2 alpha phosphorylation and MDA-7/IL-24-stimulated cell death. In this study PKR null fibroblasts were resistant to IL-24-induced apoptosis, although subsequent studies from the same group have argued that PKR does not always play a role in the lethal effects of MDA-7/IL-24 (Pataer et al., 2002, 2005).
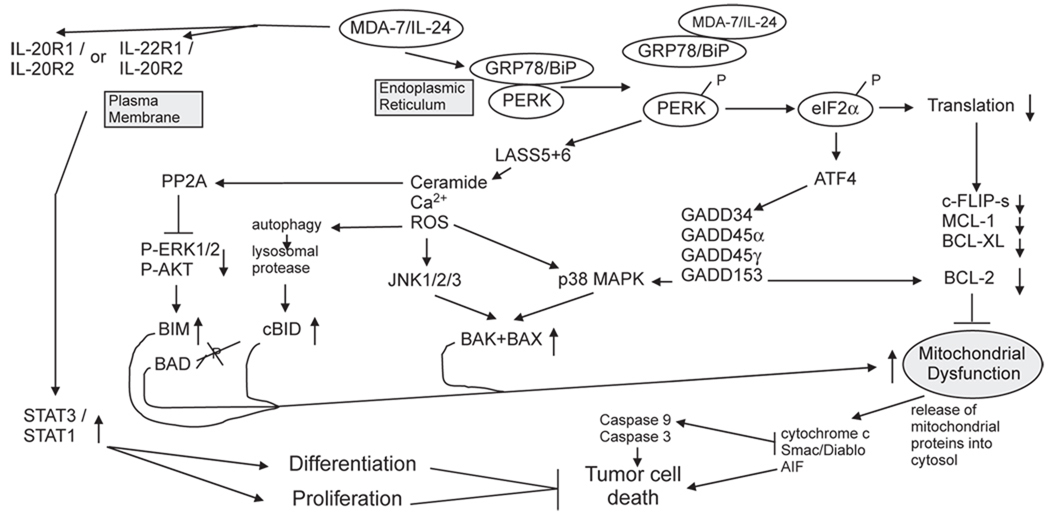
Fig. 1.
Molecular pathways by which MDA-7/IL-24 regulates cell viability and cell growth. MDA/IL-24 has two major targets in cells: the IL-20/IL-22 receptor complexes and the HSP70 family chaperone GRP78/BiP. MDA-7/IL-24 binding to its cognate receptors activates STAT family transcription factors and activation of these factors can promote differentiation and proliferation in a cell type dependent manner. STAT transcription factors play no role in MDA-7/IL-24 toxicity. MDA-7/IL-24 binds to GRP78/BiP; it is possible that entry of bacterial synthesized GST-MDA-7 into tumor cells is mediated by binding to cell surface GRP78/BiP. The majority of GRP78/BiP is present in the endoplasmic reticulum and is bound to PKR-like endoplasmic reticulum kinase (PERK); the chaperone inhibits PERK kinase activity. MDA-7/IL-24 disrupts the association of GRP78/BiP with PERK permitting PERK to phosphorylate eIF2α; phospho-eIF2α suppresses the translation of the majority of cellular proteins resulting in the rapid loss of protective proteins that have short half lives such as MCL-1 and BCL-XL, and via ATF4 promotes the transcription of a specific subset of genes e.g. GADD34 that promote apoptosis. PERK signaling promotes increased LASS6 (ceramide synthase 6) levels that promote increased Ca2+ mobilization leading to elevated ROS levels. Increased ceramide/Ca2+/ROS activate JNK and p38 signaling that promotes activation of the toxic BH3 domain proteins BAX and BAK.
In studies from our laboratories we noted that MDA-7/IL-24 binds to the HSP70 family chaperone BiP/GRP78. Binding of MDA-7/IL-24 to BiP/GRP78 inactivates the chaperone function of the protein promoting its dissociation from PKR-like endoplasmic reticulum kinase (PERK) (Gupta et al., 2006b). Over-expression of BiP/GRP78 suppresses MDA-7/IL-24-induced toxicity (Yacoub et al., 2008a, 2010a). Dissociation of BiP/GRP78 from PERK promotes PERK trans-phosphorylation and activation, and subsequently the phosphorylation and activation of eIF2 alpha. The phosphorylation of eIF2 alpha in turn leads to the global suppression of protein translation which, with respect to its tumor cell killing properties, results in reduced expression of anti-apoptotic proteins that have short half lives such as MCL-1, BCL-XL and c-FLIP-s (Fels & Koumenis, 2006; Fritsch et al., 2007; Raven & Koromilas, 2008). Indeed, some of the earliest correlative observations regarding MDA-7/IL-24 toxicity were that the cytokine decreased expression of BCL-XL and enhanced expression of toxic BH3 domain proteins such as BAX and BAK (Su et al., 2006; Fritsch et al., 2007; and references therein) (Fig. 1). Very recent data from our laboratories has also demonstrated a central role for the PERK-dependent generation of the lipid second messenger species ceramide and dihydro-ceramide in response to MDA-7/IL-24 (Bhutia et al., 2010; Sauane et al., 2010; Yacoub et al., 2010a). One mechanism by which MDA-7/IL-24 likely increases dihydro-ceramide levels in a PERK-dependent fashion is via increasing ceramide synthase 6 protein stability. Elevated ceramide levels facilitate calcium ion-dependent generation of reactive oxygen species that in combination all play a central role in modulation of signaling pathway function and mitochondrial integrity.
What is perhaps more unusual with respect to the cancer therapeutic properties of MDA-7/IL-24 compared to multiple other FDA approved anti-cancer agents, and in a manner consistent with the phrase “water always wins” is that in all tumor and transformed cells tested to date, intracellular delivery of this cytokine protein causes cell death; however, the precise mode of cytokine lethality exhibits subtle differences between tumor cells of different tissue origins (Gupta et al., 2006a; Lebedeva et al., 2007b; Sarkar et al., 2007a; Emdad et al., 2009). One difference between cell types is the degree to which different toxic BH3 domain proteins play as upstream agonists promoting mitochondrial dysfunction. For example, the ability of Ad.mda-7 to induce apoptosis in the prostate cancer cell line, DU145, which does not produce BAX, indicates that MDA-7/IL-24 can mediate apoptosis in tumor cells by a BAX-independent pathway (Lebedeva et al., 2003a,b, 2007a). In multiple primary human glioblastoma cells we noted downstream of PERK activation and lysosomal dysfunction that cathepsin B-dependent cleavage of BID played a central role in cytokine-induced mitochondrial dysfunction and lethality (Yacoub et al., 2008b; Hamed et al., 2010; Yacoub et al., 2010b). In a cell type-dependent fashion MDA-7/IL-24 inactivates the ERK1/2 and activates the JNK signaling pathways leading to: dephosphorylation of BAD S112 and BIM, which promotes BAD activation and BIM protein stabilization and activation of BAX and BAK, respectively (Fig. 1). In melanoma cell lines, but not in normal melanocytes, infected by Ad.mda-7 it was noted that a significant decrease in both BCL-2 and BCL-XL levels occurred, with a more modest up-regulation of BAX and BAK expression (Lebedeva et al., 2002). This data supports a hypothesis that Ad.mda-7 enhances the ratio of pro-apoptotic to anti-apoptotic proteins in cancer cells, thereby facilitating induction of apoptosis (Su et al., 1998).
Recently we reported that in prostate carcinoma cells, the MDA-7/IL-24-induced ER stress response causes apoptosis by translational inhibition of the anti-apoptotic protein myeloid cell leukemia-1 (MCL-1) (Dash et al., 2010b). Forced expression of MCL-1 blocked MDA-7/IL-24 lethality, whereas RNA interference or gene knockout of MCL-1 markedly sensitized transformed cells to MDA-7/IL-24. MCL-1 down-regulation by MDA-7/IL-24 relieved its association with the pro-apoptotic protein BAK, causing oligomerization of BAK and leading to cell death. These observations show the profound role of the BCL-2 protein family member MCL-1 in regulating cancer-specific apoptosis induced by this cytokine. Earlier, we reported the importance of BCL-XL in protecting against MDA-7/IL-24 lethality (Lebedeva et al., 2003a). Both BCL-XL and MCL-1 can sequester BAK; however, it is important to note that ectopic expression of MDA-7/IL-24 can moderately downregulate BCL-XL while, at the same time point, MCL-1 expression in prostate cancer cells disappeared completely. The functional redundancy of these two anti-apoptotic proteins depends on the fact that only one of them is tightly regulated by the therapeutic treatment of MDA-7/IL-24. Overall, our studies highlight the possibility that MDA-7/IL-24 may cooperate synergistically with MCL-1 small-molecule inhibitors to induce cancer cell death, which is an area for future investigation.
3. MDA-7/IL-24, endoplasmic reticulum stress and autophagy
Increased mitochondrial dysfunction caused by MDA-7/IL-24 has been linked to cytokine-induced ER stress; PERK signaling both suppresses the expression of MCL-1 and BCL-XL but also, via PERK-dependent increases in ROS/ceramide levels that cause activation of the JNK pathway which in turn promotes BAX and BAK activation; and this then promotes mitochondrial dysfunction (Lebedeva et al., 2003b; Yacoub et al., 2008a; Sauane et al., 2010). In some cell types, notably ovarian and renal carcinoma cells, MDA-7/IL-24 has been shown to cause activation of the extrinsic apoptosis pathway, in particular the death receptor CD95 (Park et al., 2009; Yacoub et al., 2010b). In ovarian cancer cells, CD95 activation was ligand-independent and required MDA-7/IL-24-induced ceramide generation (Yacoub et al., 2010b). Downstream of the CD95 receptor, cleavage of BID again played a central role in mediating cytokine toxicity, though in ovarian and renal carcinoma cells BID cleavage (and its activation) is caspase 8-dependent, rather than cathepsin-dependent as was noted in glioblastoma cells. These observations highlight the fact that MDA-7/IL-24 can promote dissimilar changes in diverse cancer cells that culminate in lethality in a cancer-specific manner.
One particular response of tumor cells to MDA-7/IL-24 exposure is the induction of autophagy. Autophagy is a ubiquitous process that occurs in all eukaryotes (rev'd in Bursch, 2001; Dice, 2007; Levine & Kroemer, 2008). It ismorphologically distinct fromapoptosis, a different complement of proteins is activated, and, unlike apoptosis, may occur under both normal and stressed growth conditions. Autophagy is a nonselective process in which cytoplasm and organelles are (apparently) randomly assorted into the autophagosome, where they are degraded. The autophagic process is activated by both extracellular stimuli (e.g. starvation, hormone treatment, chemotherapy) and intracellular stimuli (e.g. accumulation of unfolded proteins in the ER) (Yang et al., 2005; Ogata et al., 2006; Yorimitsu et al., 2006).
There are three well-recognized types of autophagy: micro-autophagy, macro-autophagy, and chaperone-mediated autophagy (CMA). CMA is the only form of autophagy in which apparently no vesicular traffic is involved (Bursch, 2001; Dice, 2007; Tasdemir et al., 2007; Levine & Kroemer, 2008). During this process, specific proteins (containing the lysosome-tag sequence KFERQ) are tagged by the CMA substrate chaperone complex, and are then routed to lysosomal/endosomal compartments for degradation. Micro-autophagy differs from macro-autophagy in that the lysosome invaginates, degrading cytosolic proteins directly. Macro-autophagy involves the sequestration of organelles and long-lived proteins in a double membrane bound vesicle called the autophagosome. This vesicle then fuses with the endosomal/lysosomal compartment, and its contents are degraded by lysosomal acidic hydrolases, e.g., cathepsin and calpain family proteases. The term “mitophagy” was developed to describe the removal of mitochondria by autophagy, but the precise nature of the process is still controversial (George et al., 2000; Yu et al., 2004; Kim et al., 2007). There is evidence that the process of mito-autophagy may be both selective and non-selective. In yeast mitochondrial removal occurs more by micro-autophagy (the intracellular pinocytosis by the vacuolar membrane) than by macro-autophagy (double membrane autophagosomes). In mammalian cells, macro-autophagy appears to be the main mechanism of mitochondrial removal.
Macro-autophagy is mediated by two ubiquitin-like conjugation systems, ATG12-ATG5 and ATG8 (microtubule-associated protein 1 light-chain 3, LC3)-phosphatidylethanolamine (PE) (George et al., 2000; Yu et al., 2004; Kim et al., 2007). ATG12 and ATG5 conjugate almost immediately after synthesis, and their bond is irreversible. After activation by the additional conjugation of ATG16, the ATG12-ATG5-ATG16 complex can associate with a small, crescent-shaped membranous structure, the immature autphagosome (the pre-autophagosomal structure (PAS)). The complex is not associated with the mature autophagosome. LC3, the mammalian orthologue of the yeast protein ATG8, is lipid modified and recruited by the ATG12-ATG5-ATG16 complex to the PAS and autophagosome, where it remains after the dissociation of ATG5-ATG12-ATG16 conjugate. Expression of a GFP-conjugated form of LC3 (ATG8) has thus provided a useful tool for researchers to study the earliest stages of autophagy induction. After formation of the autophagosome, this doublemembrane structure fuses with an acidic endosome. The proteins and/or organelles in the lumen of this compartment are then degraded and recycled by the cell.
Apoptosis pathways have been linked with the regulation of autophagy, e.g., knock down of caspase 8 expression can induce autophagic cell death (Yu et al., 2004). Beclin1, an essential protein in the activation of the class III PI3K vps34 whose function is obligatory for PAS and autophagosome formation, contains a BH3 domain that binds to the mitochondrial protective proteins BCL-2/BCL-XL/MCL-1 and release of Beclin1 from these proteins permits its binding to vps34, with concomitant increased PI3K activity, and to the induction of autophagy (Maiuri et al., 2007). Vps34 is believed to be the main target of 3-methyl adenine (3MA), a small-molecule inhibitor frequently used to inhibit autophagy. The serine–threonine kinase mTOR is one other well-recognized gatekeeper in the autophagy process, exerting an inhibitory effect; mTOR acts both in a signal transduction cascade that activates anti-autophagic transcription and translation and by inhibiting the ATG proteins directly by phosphorylation. The mTOR inhibitor rapamycin is one tool used in both the laboratory and in the clinic to promote autophagy. Hence, by implication, the class I PI3K/AKT pathway also is involved in down-regulation of autophagy, by its ability to activate mTOR.
The role of autophagy as a process in tumor cell survival or tumor cell death remains controversial and data from a wide number of laboratories indicates that autophagy in a cell type- and stimulus/drug-dependent fashion may maintain cell viability or may act to cause cell death; with death either being due to autophagy-dependent induction of apoptosis or due to a direct toxic effect of the process (Bursch, 2001; Ogata et al., 2006; Yorimitsu et al., 2006). For example, in hepatoma cells, treatment with the drug combination of sorafenib and vorinostat enhances autophagy in a CD95-dependent fashion that is a protective event against drug toxicity (Park et al., 2008), i.e., activation of CD95 simultaneously produces a “toxic” signal, caspase 8 activation, and a “protective” signal, autophagy. However, in the same tumor cell types the celecoxib derivative OSU-03012 has been shown to cause cell killing that is dependent on a toxic form of autophagy (Gao et al., 2008). The BCL-2/BCL-XL/MCL-1 inhibitor Obatoclax (GX15-070) as a single agent causes release of Beclin1 from the protective BCL-2 proteins that results in autophagy and tumor cell killing whereas inhibitors of growth factor receptors or of mTOR can promote autophagy that has been shown to have protective effects; inhibition of growth factor receptors further increases Obatoclax-induced autophagy and tumor cell killing (Martin et al., 2009). Thus, autophagy paradoxically directs cell survival towards viability or death in a cell type- and stimulus-dependent fashion.
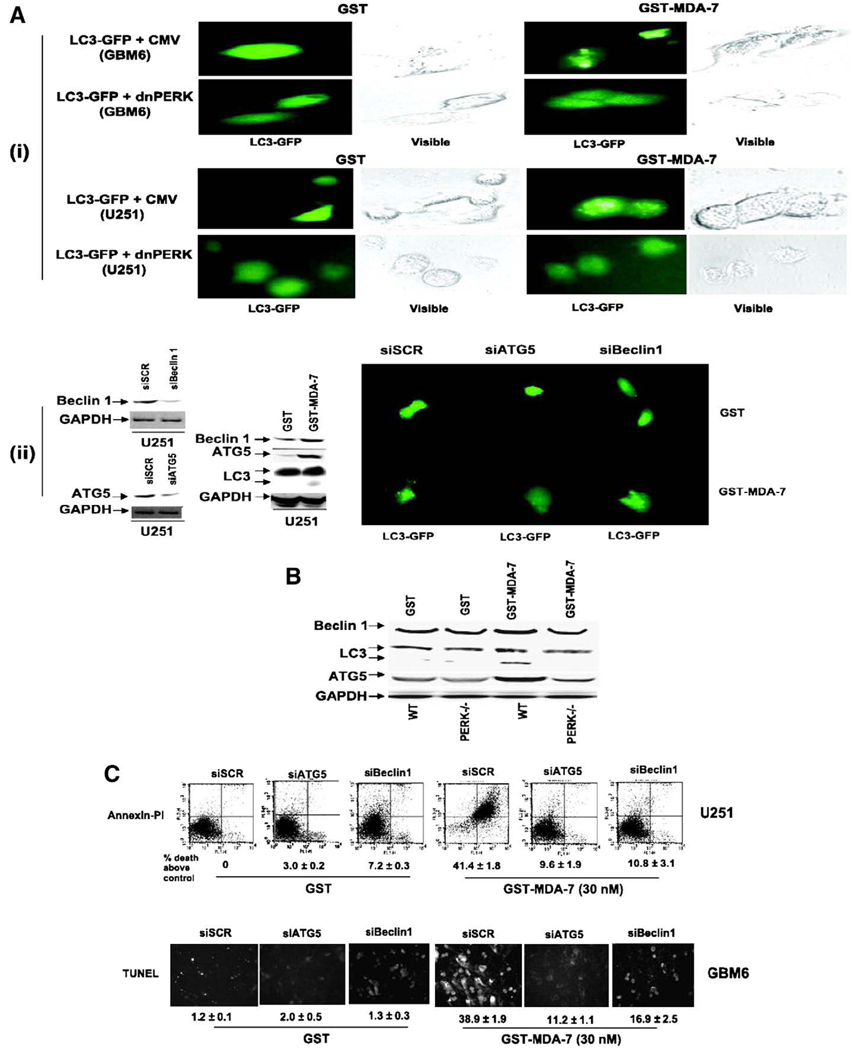
With respect to MDA-7/IL-24, the role of autophagy in cytokine-induced toxicity has been recently defined in GBM, prostate and ovarian tumor cells. In GBM cells MDA-7/IL-24 binding to PERK results in elevated levels of ceramide, intracellular Ca2+ and ROS. These signaling molecules in turn promote autophagy as judged by increased numbers of punctate intracellular vesicles labeled with a GFP-tagged form of ATG8; with the processing of endogenous ATG8 and increased expression of ATG5 and Beclin1; and with increased staining for lysosomal acidic vesicles (Yacoub et al., 2008b) (Fig. 2). In Fig. 2 MDA-7/IL-24-induced autophagy is blocked by expression of dominant negative PERK or by knock down of ATG5 or Beclin1. Over-expression of the MDA-7/IL-24 binding protein BiP/GRP78, HSP70 or knock down of ATG5 or Beclin1 suppressed MDA-7/IL-24 toxicity in GBM cells (Fig. 2, data not shown).
Fig. 2.
GST-MDA-7 causes LC3-GFP vesicularization in transformed cells in a PERK-dependent manner. A, (i), GBM6 and U251 cells were plated in four-well chamber glass slides in triplicate and 12 h after plating transfected with a plasmid to express LC3-GFP and in parallel co-transfected with either a vector control plasmid (CMV) or with a plasmid to express dnPERK. Twelve hours after transfection, cells were treated with GST or GST-MDA-7 (100 nmol/L). Twenty-four hours after GST-MDA-7 exposure, the GBM6 and U251 cells were examined under visual light (visible) or under fluorescent light (LC3-GFP). Representative images from the triplicate plating (n = 2). (ii), U251 cells were plated in four-well chamber glass slides in triplicate and 12 h after plating transfected with a plasmid to express LC3-GFP and in parallel co-transfected with either a vector control plasmid to express a nonspecific scrambled siRNA (siSCR) or plasmids to knockdown expression of Beclin-1 (siBeclin-1) or ATG5 (siATG5). Parallel studies also transfected cells with plasmids to express scrambled siRNA and untagged GFP. Twelve hours after transfection, cells were treated with GST or GST-MDA-7 (100 nmol/L). Twenty-four hours after GST-MDA-7 exposure, the U251 cells were examined under fluorescent light (LC3-GFP and GFP). Representative images from the triplicate plating (n = 3). Immunoblotting, cells transfected with siRNA constructs to modulate the expression of ATG5 and Beclin-1 were immunoblotted to determine the expression of Beclin-1 and ATG5 48 h after transfection. Cells treated with GST-MDA-7 and GST were immunoblotted 48 h after treatment to determine the expression of Beclin-1, ATG5, the cleavage status of LC3 and GAPDH (n = 2). B, Transformed MEFs (WT; deleted for PERK, PERK−/−) 24 h after plating were treated with GST or GST-MDA-7 (100 nmol/L). Twenty-four hours after GST-MDA-7 treatment, cells were isolated and subjected to SDS-PAGE to determine the expression of Beclin-1, ATG5, the cleavage status of LC3 and GAPDH (n = 2). C, GBM6 and U251 cells were plated in four-well chamber glass slides in triplicate and 12 h after plating transfected with either a vector control plasmid to express a nonspecific scrambled siRNA or plasmids to knockdown expression of Beclin-1 or of ATG5. Twelve hours after transfection, cells were treated with GST or GST-MDA-7 (30 nmol/L). Forty-eight hours after GST-MDA-7 exposure, the viability of the GBM6 and U251 cells was determined by: terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay or; by Annexin V-PI flow cytometry on isolated cells (±SE; n = 3). Data shown are for GBM6 (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) and U251 cells was determined by Annexin V/PI flow cytometry (±SE; n = 2). Data reproduced with permission from Yacoub et al. Caspase-, cathepsin and PERK-dependent regulation of MDA-7/IL-24-induced cell killing in primary human glioma cells. Mol Cancer Ther 7 (2008), 297–313.
GBM cells as part of their highly invasive biology express elevated levels of the lysosomal protease cathepsin B (Colin et al., 2009). Thus, one consequence of MDA-7/IL-24 promoting high levels of lysosomal protease activation within a GBM cell, naturally over-expressing such proteases, is the release of activated cathepsin B into the cytosol. Cytosolic active cathepsin B causes cleavage and activation of the proapoptotic BH3 domain protein BID. Active BID in turn promotes activation of BAX and BAK leading to mitochondrial dysfunction, pore formation and recruitment of the intrinsic apoptosis pathway, resulting ultimately in GBM cell death. In agreement with these findings, inhibiting the abilities of protective BCL-2 family proteins to sequester BAX and BAK using the drugs HA14-1 or Obatoclax, or knock down of MCL-1, enhances MDA-7/IL-24 toxicity in GBM cells (unpublished data). This effect correlated with increased levels of autophagy (unpublished data).
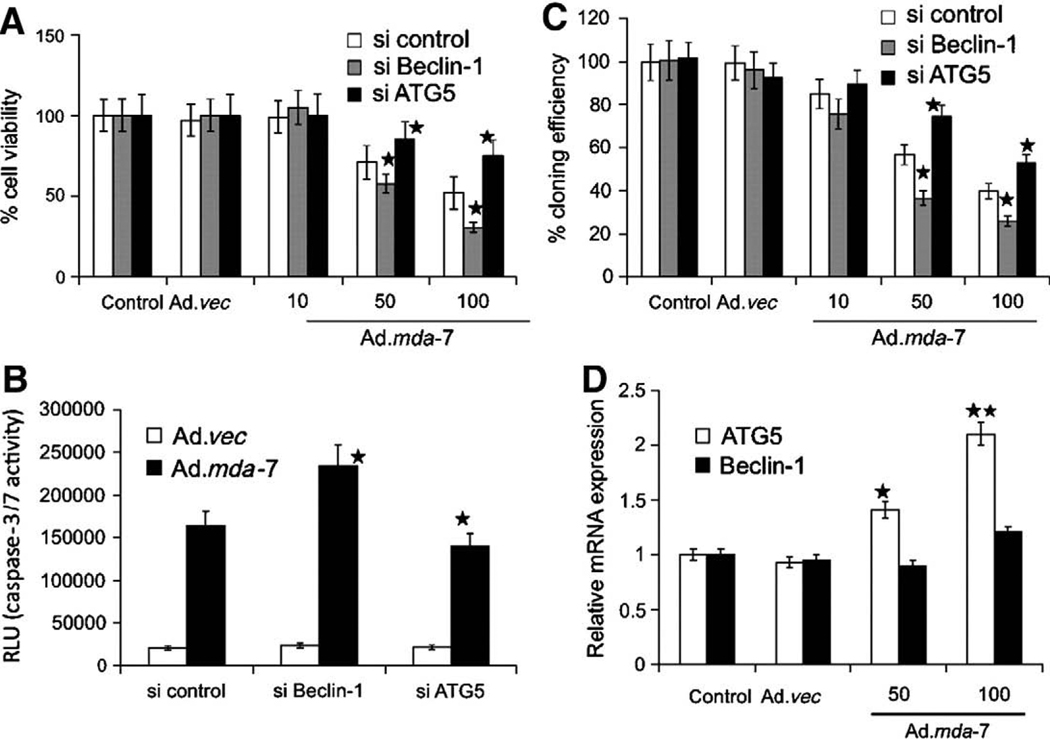
In prostate cancer cells, however, the role of autophagy in MDA-7/IL-24 cytokine lethality was noted to be significantly different to that in GBM cells. As was discovered in GBM, MDA-7/IL-24 also induces a PERK-dependent form of autophagy in prostate cancer cells (Bhutia et al., 2010) (Fig. 3). However, in prostate cancer cells suppression of autophagy using either 3MA or knock down of Beclin1 enhances MDA-7/IL-24 lethality (Fig. 3, data not shown). In contrast, knock down of ATG5 expression suppresses both MDA-7/IL-24-induced autophagy and apoptosis in these cells (Fig. 3). This apparent discrepancy between the effects of Beclin1 and ATG5 knock down and the suppression of autophagy and altered cell survival was understood when it was noted that in prostate cancer cells ATG5 was cleaved in an autophagy- and calpain-dependent fashion into a ~25 kDa proapoptotic fragment that has been previously shown to cause mitochondrial dysfunction via activation of BAX and BAK, resulting in enhanced apoptosis (Xia et al., 2010) (Fig. 3). Thus, in prostate cancer cells MDA-7/IL-24-induced autophagy was primarily a protective event but that in a secondary fashion, without autophagy and calpain activation, ATG5 could not be processed into its active proapoptotic form to facilitate prostate cancer cell death.
Fig. 3.
Role of Beclin-1 and ATG5 in Ad.mda-7-induced apoptosis. DU-145 cells were transfected with the indicated siRNAs, and cell viability was determined by MTT assay (A), Caspase-Glo 3/7 assay for caspase-3 expression (B), and colony formation assays (C) 48 hours after infection with Ad.mda-7 (10, 50, or 100 pfu/cell) or Ad.vec (100 pfu/cell). C, colony formation assays in monolayer culture. Colonies were fixed, stained, and counted (>50 cells) 2 weeks after plating. D, Expression profile of Beclin-1 and ATG5 at the mRNA level 48 h after Ad.mda-7 infection (50 or 100 pfu/cell) of DU-145 cells. ★, P < 0.05; ★★, P < 0.001, compared with control. Data reproduced with permission from Bhutia et al. Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation associated gene 7/interleukin-24. Cancer Res. 70 (2010), 3667–3676.
In renal and ovarian cancer cells the role of autophagy in survival and how autophagy was induced by MDA-7/IL-24 was again subtly different compared to the mechanisms previously described in GBM and prostate cancer cells (Park et al., 2009, 2010; Yacoub et al., 2010b). MDA-7/IL-24-induced activation of PERK again played an important role in the induction of autophagy in ovarian and renal cancer cells. In both cell types, MDA-7/IL-24-induced apoptosis was dependent on activation of CD95; CD95 activation was also in these systems largely responsible for MDA-7/IL-24-induced autophagy. Prior studies in liver, renal, pancreatic and melanoma cells had shown that drug-induced activation of CD95 led to induction of a protective form of autophagy that acted to negate CD95/caspase 8-induced apoptosis (Park et al., 2008). In renal and ovarian cancer cells, however, despite the fact that the majority of autophagy was CD95 dependent, it was noted that knock down of ATG5 or Beclin1 reduced MDA-7/IL-24-induced apoptosis. Thus, elevated autophagy is a widely observed response of diverse tumor cell types to increased MDA-7/IL-24 expression but that the precise role of autophagy in the cellular response to MDA-7/IL-24 is tumor cell type-dependent.
4. MDA-7/IL-24 as a therapeutic tool
As noted previously,MDA-7/IL-24 is a secreted protein, and secreted MDA-7/IL-24 has been shown in several studies to have a “toxic bystander” effect on distant tumor cells in vitro. From the standpoint of MDA-7/IL-24 as a gene therapeutic tool, based on simple mass action effects, it is not possible to infect every tumor cell within a tumor using an adenovirus even with intra-tumoral injection, and this has been one possible reason why so many gene therapy approaches have failed in the clinic. In addition, systemic IV administration of any recombinant adenovirus will not result in productive infection of all disseminated tumors due to rapid first pass sequestration of virus by the liver coupled to neutralization by any pre-existing anti-adenovirus antibodies. Based on its selective and potent anti-cancer activity in vitro and in animal models, a Phase I clinical trial was performed in advanced carcinomas and melanomas using a replication incompetent serotype 5 adenovirus to express MDA-7/IL-24; Ad.mda-7 (INGN-241) (Fisher et al., 2003; Cunningham et al., 2005; Tong et al., 2005; Fisher et al., 2007; Lebedeva et al., 2007b; Eager et al., 2008). These studies employed repeated intra-tumoral injection of Ad.mda-7 into the tumors of patients with advanced disease and indicated that repeated administration of Ad.mda-7 into the tumor and tissue surrounding the tumor was safe and this gene could induce apoptosis in a large percentage of tumor volume with a measurable clinical response rate of ~44%.
It was evident in this first clinical trial that infection of a small proportion of tumor cells with non-replicating Ad.mda-7 resulted in detectable MDA-7/IL-24 protein levels and increased tumor cell apoptosis many centimeters from the site of any virally infected neoplastic cell in the tumor, indicating, as was observed in animals, and now in patients, that secreted MDA-7/IL-24 was having a “toxic bystander” effect on uninfected tumor cells (Sauane et al., 2004b; Su et al., 2005; Sauane et al., 2006; Sauane et al., 2008). In addition, we have noted in prostate, renal and glioblastoma xenograft tumors that Ad.mda-7 infection of an established tumor growing on one flank of an animal results in growth arrest and apoptosis in an uninfected tumor growing on the opposite flank of the animal (Sarkar et al., 2005a,b, 2007b, 2008; Park et al., 2010; unpublished results). Clearly, however, at sites more distant to viral administration where MDA-7/IL-24 concentrations may be only growth inhibitory and not cytotoxic, the combination of MDA-7/IL-24 therapy with established therapeutic agents to enhance the toxicity of MDA-7/IL-24 would be of considerable utility. The toxic effects of MDA-7/IL-24 therapy could be combined with other treatment modalities to achieve an improved profound clinical response.
5. MDA-7/IL-24 radiosensitizes tumor cells
MDA-7/IL-24 causes the generation of ROS in tumor cells, but not in non-transformed cells, and quenching of ROS production suppresses MDA-7/IL-24 toxicity (Lebedeva et al., 2003b; Yacoub et al., 2003a,b,c, 2004; Lebedeva et al., 2006, 2007a, 2008a,b; Yacoub et al., 2010a). Several long-established therapeutic modalities also generate ROS in tumor cells as part of their toxic biology (Pelicano et al., 2004). For example, ionizing radiation causes ionizing events in water, generating hydroxyl radicals that can impact on the function of mitochondria in cells, which in turn amplify the initial free radical signaling, generating large amounts of reactive oxygen and nitrogen species (Valerie et al., 2007). In addition, radiation can cause DNA damage and activate poly ADP ribosyl polymerase (PARP) leading to an altered cellular redox status due to NADPH depletion, which can also be sensed by mitochondria (Hagan et al., 2007). Radiation exposure also increases ceramide levels in tumor cells (Stancevic & Kolesnick, 2010).
Radiotherapy is used as a primary modality for the treatment of many malignancies including those of the breast, brain, prostate, and lung. Based on the tumoricidal effects of both radiation and MDA-7/IL-24, it has been a logical step for investigators to determine whether MDA-7/IL-24 had radiosensitizing potential. Several laboratories have demonstrated that Ad.mda-7, GST-MDA-7/IL-24 and MDA-7/IL-24 can radiosensitize a wide variety of tumor cell lines in vitro and in vivo (Kawabe et al., 2002; Su et al., 2003; Yacoub et al., 2003b,c; Emdad et al., 2006, 2007). In studies using human glioma and prostate carcinoma cells, the ability of both ionizing radiation and MDA-7/IL-24 to generate reactive oxygen species (ROS) was directly linked to the radiosensitizing properties of MDA-7/IL-24. MDA-7/IL-24 activates ceramide synthase 6 as part of its toxic effects and ceramide synthase 6 was also linked to MDA-7/IL-24 toxicity (Yacoub et al., 2010a); others have shown that radiotherapy utilizes ceramide synthase 6 to kill tumor cells (Mesicek et al., 2010). Other therapeutic agents have also been shown to act, in part, by generating ROS, including arsenic trioxide, 4-hydroxyphenyl-retinamide (4-HPR), vitamin E and perillyl alcohol (Yacoub et al., 2003a; Lebedeva, 2007a, 2008a,b). In general agreement with ROS enhancing the lethal actions of MDA-7/IL-24, combined treatment of renal, brain, lung, breast, pancreatic and prostate carcinoma cells with MDA-7/IL-24 and radiotherapy or arsenic trioxide or 4-HPR resulted in a highly synergistic potentiation of tumor cell killing that was not manifested in non-transformed epithelial cell counterparts. Collectively, these findings argue that established and novel therapeutic modalities, which generate ROS can promote MDA-7/IL-24 lethality in cancer cells thereby enhancing the therapeutic index.
6. MDA-7/IL-24 inhibits cyto-protective signaling pathways and activates cytotoxic signaling pathways in tumor cells that control the apoptotic threshold
The regulation of signal transduction pathway functions by Ad.mda-7 and GST-MDA-7/IL-24 protein, particularly when combined with therapeutic modalities that generate ROS and ceramide, is as apparently complicated as the number of mechanisms by which MDA-7/IL-24 has been reported to induce cell death. As noted in a prior section, activation of STAT transcription factors does not appear to significantly modulate MDA-7/IL-24-lethality, despite MDA-7/IL-24 activating STAT transcription factors through IL-20 receptor complexes (Sauane et al., 2003; Chada et al., 2004a,b). Data in several tumor cell types has argued that either Ad.mda-7 or MDA-7/IL-24 protein promote activation of the p38 mitogen activated protein kinase (MAPK) pathway, which via GADD34 (CHOP) promotes growth arrest and cell death (Sarkar et al., 2002; Su et al., 2003) (Fig. 1). In part, this may be explained by data suggesting MDA-7/IL-24 causes PKR/PERK activation in some tumor cell types, which is a known upstream activator of both p38 MAPK and GADD34. However, in some tumor cell types MDA-7/IL-24-induced p38 MAPK signaling clearly also plays a “switch-hitter” role with respect to growth and survival, wherein low concentrations of MDA-7/IL-24 induce a level of p38 MAPK signaling that facilitates growth arrest and cell survival with higher MDA-7/IL-24 concentrations causing an intense sustained pathway activation that leads to tumor cell death (Yacoub et al., 2008a,b). Several studies have linked the c-Jun NH2-terminal kinase (JNK) pathway as a mediator of MDA-7/IL-24 toxicity; as MDA-7/IL-24 increases ROS and ceramide levels and as these messengers have been widely shown by many groups to strongly activate JNK pathway signaling, this finding is perhaps not too surprising (e.g., Wagner & Nebreda, 2009). Other studies have demonstrated that MDA-7/IL-24 inhibits PI3K/AKT and ERK1/2 pathway function, which in the case of ERK1/2 signaling is mediated by MDA-7/IL-24-induced activation of PERK; this reduction in ERK1/2 activity further promotes the MDA-7/IL-24-induced reduction in MCL-1 levels and facilitates JNK pathway activation (Yacoub et al., 2008a).
As a single agent ionizing radiation-induced cell killing in a variety of cancer cells has been linked to the activation of the JNK pathway (Dent et al., 2003a,b). When combined with ionizing radiation, MDA-7/IL-24 has been suggested to promote radiation toxicity by modulating JNK1/2/3 pathway signaling (Yacoub et al., 2003c,b, 2004). For example, lung cancer cells were radiosensitized by Ad.mda-7 via JNK1/2 signaling, without radiation further enhancing MDA-7/IL-24-induced JNK1/2 activation (Kawabe et al., 2002; Nishikawa et al., 2004). Use of established rodent and human glioma cell lines, as well as primary human glioma cell isolates, demonstrated that Ad.mda-7 caused radiosensitization in vitro and in vivo, and that in vitro sensitization was dependent on JNK1/2/3 activation and in vivo sensitization correlated with increased JNK1/2/3 phosphorylation (Yacoub et al., 2003b,c, 2004). Many groups have argued that prolonged intense JNK1/2/3 pathway signaling is involved in cell death processes.
7. Conclusions
MDA-7/IL-24 is a multi-facetted killer of cancer cells that has shown significant clinical benefit in patients as a single agent. Future expanded clinical studies will be required to determine whether MDA-7/IL-24 represents a viable therapeutic in glioblastoma, and other cancers, and whether MDA-7/IL-24 can be rationally combined with other established cancer treatments to improve tumor killing properties in patients. Based on the remarkable efficacy shown by Ad.mda-7 and paracrine induction of MDA-7/IL-24 using direct tumor injection in patients with advanced cancers, we are very optimistic that this molecule will display profound activity in patients with diverse cancers, especially when combined with therapeutic agents that promote ER stress responses (Fisher, 2005; Lebedeva et al., 2007a,b; Emdad et al., 2009). We are actively pursuing these combinatorial studies as well as investigating improved and unique ways of effectively delivering mda-7/IL-24 in vivo.
One mechanism to increase the total amount of MDA-7/IL-24 being delivered to the site of the tumor is by use of a conditionally replicative adenovirus (CRAd) also termed cancer terminator viruses (CTV) (Sarkar et al., 2005a,b, 2007b, 2008). A virus that only replicates in tumor cells will result in viral replication-dependent tumor cell killing as well as the synthesis and release of MDA-7/IL-24 that will kill and suppress the growth of uninfected tumor cells, as well as promoting further expression of MDA-7/IL-24. Due to a lack of expression of the coxsackie and adenovirus receptor (CAR) many tumor cells cannot be infected by serotype 5 adenovirus and the development of viruses with chimeric knob proteins to deliver gene therapeutics is also being explored: a type 5/type 3 recombinant adenovirus to deliver MDA-7/IL-24 was recently shown by us to be a more effective therapeutic for prostate and GBM tumors in vivo than a “standard” type 5 virus (Dash et al., 2010a; Hamed et al., 2010). Finally, it is possible that lethal though highly immunogenic forms of MDA-7/IL-24, e.g., GST-MDA-7, could be delivered to tumors via their encapsulation in micro-bubbles, that plus ultra-sound, will target delivery of this cytokine to cancers (Greco et al., 2010). Additionally, it was predicted and has recently been shown recently that stem cells can also be used to deliver mda-7/IL-24, therapeutically for brain cancers (Fisher, 2005; Germano et al., 2010). Thus, the possible approaches to deliver MDA-7/IL-24 are diverse in nature and all of the noted approaches will, we hope, be translated into the clinic for evaluation over the up-coming 5 years.
Acknowledgments
Support was provided by P01-CA104177, R01-CA108325, R01-DK52825; R01-CA063753, R01-CA077141, R01-CA097318; R01-CA127641; R01-CA098712; P01-NS031492, the Samuel Waxman Cancer Research Foundation (SWCRF) and the National Foundation for Cancer Research (NFCR). P.D. is The Universal Professor in Signal Transduction and P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research and is a SWCRF Investigator.
Abbreviations
- ERK
extracellular regulated kinase
- MEK
mitogen activated extracellular regulated kinase
- JNK
c-Jun NH2-terminal kinase
- PI3K
phosphatidyl inositol 3 kinase
- MDA-7
melanoma differentiation associated gene 7
- IL-24
interleukin-24
- PERK
protein kinase R-like endoplasmic reticulum kinase
- MAPK
mitogen activated protein kinase
- ca
constitutively active
- dn
dominant negative
- EGFR
epidermal growth factor receptor
- PTEN
phosphatase and tensin homologue on chromosome ten
References
- Bhutia SK, Dash R, Das SK, Azab B, Su ZZ, Lee SG, et al. Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Res. 2010;70:3667–3676. doi: 10.1158/0008-5472.CAN-09-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch W. The autophagosomal ± lysosomal compartment in programmed cell death. Cell Death and Differ. 2001;8:569–581. doi: 10.1038/sj.cdd.4400852. [DOI] [PubMed] [Google Scholar]
- Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, et al. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. 2002;168:6041–6046. doi: 10.4049/jimmunol.168.12.6041. [DOI] [PubMed] [Google Scholar]
- Chada S, Mhashilkar AM, Ramesh R, Mumm JB, Sutton RB, Bocangel D, et al. Bystander activity of Ad-mda7: human MDA-7 protein kills melanoma cells via an IL-20 receptor-dependent but STAT3-independent mechanism. Mol Ther. 2004a;10:1085–1095. doi: 10.1016/j.ymthe.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Chada S, Sutton RB, Ekmekcioglu S, Ellerhorst J, Mumm JB, Leitner WW, et al. MDA-7/IL-24 is a unique cytokine-tumor suppressor in the IL-10 family. Int Immunopharmacol. 2004b;4:649–667. doi: 10.1016/j.intimp.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Chada S, Mhashilkar AM, Liu Y, Nishikawa T, Bocangel D, Zheng M, et al. mda-7 gene transfer sensitizes breast carcinoma cells to chemotherapy, biologic therapies and radiotherapy: correlation with expression of bcl-2 family members. Cancer Gene Ther. 2006;13:490–502. doi: 10.1038/sj.cgt.7700915. (PMID: 16282987). [DOI] [PubMed] [Google Scholar]
- Colin C, Voutsinos-Porche B, Nanni I, Fina F, Metellus P, Intagliata D, et al. High expression of cathepsin B and plasminogen activator inhibitor type-1 are strong predictors of survival in glioblastomas. Acta Neuropathol. 2009;118:745–754. doi: 10.1007/s00401-009-0592-2. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, et al. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: A phase I study. Mol Ther. 2005;11:149–159. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Dash R, Dmitriev I, Su ZZ, Bhutia SK, Azab B, Vozhilla N, et al. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) improves therapeutic efficacy in low CAR prostate cancer cells. Cancer Gene Ther. 2010a;17(7):447–456. doi: 10.1038/cgt.2009.91. (PMID: 20150932). [DOI] [PubMed] [Google Scholar]
- Dash R, Richards JE, Su ZZ, Bhutia SK, Azab B, Rahmani M, et al. Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10 related cytokine. Cancer Res. 2010b;70:5034–5045. doi: 10.1158/0008-5472.CAN-10-0563. (PMID: 20501829). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003a;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003b;22:5885–5896. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- Dice JF. Chaperone-mediated autophagy. Autophagy. 2007;3:295–299. doi: 10.4161/auto.4144. [DOI] [PubMed] [Google Scholar]
- Eager R, Harle L, Nemunaitis J. Ad-MDA-7; INGN 241: A review of preclinical and clinical experience. Expert Opin Biol Ther. 2008;8:1633–1643. doi: 10.1517/14712598.8.10.1633. [DOI] [PubMed] [Google Scholar]
- Ekmekcioglu S, Ellerhorst J, Mhashilkar AM, Sahin AA, Read CM, Prieto VG, et al. Downregulated melanoma differentiation associated gene (mda-7) expression in human melanomas. Int J Cancer. 2001;94:54–59. doi: 10.1002/ijc.1437. [DOI] [PubMed] [Google Scholar]
- Ellerhorst JA, Prieto VG, Ekmekcioglu S, Broemeling L, Yekell S, Chada S, et al. Loss of MDA-7 expression with progression of melanoma. J Clin Oncol. 2002;20:1069–1074. doi: 10.1200/JCO.2002.20.4.1069. [DOI] [PubMed] [Google Scholar]
- Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sarkar D, Settleman J, Fisher PB. Combinatorial treatment of non-small-cell lung cancers with gefitinib and Ad.mda-7 enhances apoptosis induction and reverses resistance to a single therapy. J Cell Physiol. 2007;210:549–559. doi: 10.1002/jcp.20906. [DOI] [PubMed] [Google Scholar]
- Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sauane M, Dash R, et al. Historical perspective and recent insights into our understanding of the molecular and biochemical basis of the antitumor properties of mda-7/IL-24. Cancer Biol Ther. 2009;8:391–400. doi: 10.4161/cbt.8.5.7581. [DOI] [PubMed] [Google Scholar]
- Emdad L, Sarkar D, Lebedeva IV, Su ZZ, Gupta P, Mahasreshti PJ, et al. Ionizing radiation enhances adenoviral vector expressing mda-7/IL-24-mediated apoptosis in human ovarian cancer. J Cell Physiol. 2006;208:298–306. doi: 10.1002/jcp.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fels DR, Koumenis C. The PERK/eIF2α/ATF4 Module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5:723–728. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- Fisher PB. Is mda-7/IL-24 a "magic bullet" for cancer? Cancer Res. 2005;65:10128–10138. doi: 10.1158/0008-5472.CAN-05-3127. (Review. PMID: 16287994). [DOI] [PubMed] [Google Scholar]
- Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, et al. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther. 2003;2:S23–S37. [PubMed] [Google Scholar]
- Fisher PB, Sarkar D, Lebedeva IV, Emdad L, Gupta P, Sauane M, et al. Melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24): Novel gene therapeutic for metastatic melanoma. Toxicol Appl Pharmacol. 2007;224:300–307. doi: 10.1016/j.taap.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch RM, Schneider G, Saur D, Scheibel M, Schmid RM. Translational repression of MCL-1 couples stress-induced eIF2 alpha phosphorylation to mitochondrial apoptosis initiation. J Biol Chem. 2007;282:22551–22562. doi: 10.1074/jbc.M702673200. [DOI] [PubMed] [Google Scholar]
- Gao M, Yeh PY, Lu YS, Hsu CH, Chen KF, Lee WC, et al. OSU-03012, a novel celecoxib derivative, induces reactive oxygen species-related autophagy in hepatocellular carcinoma. Cancer Res. 2008;68:9348–9357. doi: 10.1158/0008-5472.CAN-08-1642. [DOI] [PubMed] [Google Scholar]
- George MD, Baba M, Scott SV, Mizushima N, Garrison BS, Ohsumi Y, Klionsky DJ. Apg5p functions in the sequestration step in the cytoplasm-to-vacuole targeting and macroautophagy pathways. Mol Biol Cell. 2000;11:969–982. doi: 10.1091/mbc.11.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germano IM, Emdad L, Qadeer ZA, Binello E, Uzzaman M. Embryonic stem cell (ESC)-mediated transgene delivery induces growth suppression, apoptosis and radiosensitization, and overcomes temozolomide resistance in malignant gliomas. Cancer Gene Ther. 2010;17:664–674. doi: 10.1038/cgt.2010.31. (PMID:20523363). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco A, Di Benedetto A, Howard CM, Kelly S, Dementieva Y, Miranda M, et al. Eradication of therapy-resistant human prostate tumors using an ultrasound guided site-specific cancer terminator virus delivery approach. Mol Ther. 2010;18:295–306. doi: 10.1038/mt.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Su ZZ, Lebedeva IV, Sarkar D, Sauane M, Emdad L, et al. mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther. 2006a;111:596–628. doi: 10.1016/j.pharmthera.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Walter MR, Su ZZ, Lebedeva IV, Emdad L, Randolph A, et al. BiP/GRP78 is an intracellular target for MDA-7/IL-24 induction of cancer-specific apoptosis. Cancer Res. 2006b;66:8182–8191. doi: 10.1158/0008-5472.CAN-06-0577. [DOI] [PubMed] [Google Scholar]
- Hagan MP, Yacoub A, Dent P. Radiation-induced PARP activation is enhanced through EGFR-ERK signaling. J Cell Biochem. 2007;101:1384–1393. doi: 10.1002/jcb.21253. [DOI] [PubMed] [Google Scholar]
- Hamed HA, Yacoub A, Park MA, Eulitt P, Sarkar D, Dimitriev IP, et al. OSU-03012 enhances Ad.mda-7-induced GBM cell killing via ER stress and autophagy and by decreasing expression of mitochondrial protective proteins. Cancer Biol Ther. 2010;9 doi: 10.4161/cbt.9.7.11116. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed HA, Yacoub A, Park MA, Eulitt PJ, Dash R, Sarkar D, et al. Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Mol Ther. 2010;18:1130–1142. doi: 10.1038/mt.2010.29. (PMID: 20179672). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Huang EY, Madireddi MT, Gopalkrishnan RV, Leszczyniecka M, Su ZZ, Lebedeva IV, et al. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene. 2001;20:7051–7063. doi: 10.1038/sj.onc.1204897. [DOI] [PubMed] [Google Scholar]
- Jiang H, Fisher PB. Use of a sensitive and efficient subtraction hybridization protocol for the identification of genes differentially regulated during the induction of differentiation in human melanoma cells. Mol Cell Differ. 1993;1:285–299. [Google Scholar]
- Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–2486. [PubMed] [Google Scholar]
- Jiang H, Su ZZ, Boyd J, Fisher PB. Gene expression changes associated with reversible growth suppression and the induction of terminal differentiation in human melanoma cells. Mol Cell Differ. 1993;1:41–66. [Google Scholar]
- Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe S, Nishikawa T, Munshi A, Roth JA, Chada S, Meyn RE. Adenovirus-mediated mda-7 gene expression radiosensitizes non-small cell lung cancer cells via TP53-independent mechanisms. Mol Ther. 2002;6:637–644. [PubMed] [Google Scholar]
- Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotenko SV. The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev. 2002;13:223–240. doi: 10.1016/s1359-6101(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, et al. mda-7/IL-24, novel anticancer cytokine: Focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience (Review) Int J Oncol. 2007b;31:985–1007. [PubMed] [Google Scholar]
- Lebedeva IV, Sarkar D, Su ZZ, Gopalkrishnan RV, Athar M, Randolph A, et al. Molecular target-based therapy of pancreatic cancer. Cancer Res. 2006;66:2403–2413. doi: 10.1158/0008-5472.CAN-05-3510. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Sarkar D, Su ZZ, Kitada S, Dent P, Stein CA, et al. Bcl-2 and Bcl-xL differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24. Oncogene. 2003a;22:8758–8773. doi: 10.1038/sj.onc.1206891. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Sauane M, Gopalkrishnan RV, Sarkar D, Su Z-z, Gupta P, et al. mda-7/IL-24: Exploiting cancer's Achilles' heel. Mol Ther. 2005;11:4–18. doi: 10.1016/j.ymthe.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, Fisher PB. The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene. 2002;21:708–718. doi: 10.1038/sj.onc.1205116. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Sarkar D, Kitada S, Dent P, Waxman S, et al. Melanoma differentiation associated gene-7, mda-7/interleukin-24, induces apoptosis in prostate cancer cells by promoting mitochondrial dysfunction and inducing reactive oxygen species. Cancer Res. 2003b;63:8138–8144. [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Vozhilla N, Chatman L, Sarkar D, Dent P, et al. Mechanism of in vitro pancreatic cancer cell growth inhibition by melanoma differentiation-associated gene-7/ interleukin-24 and perillyl alcohol. Cancer Res. 2008a;68:7439–7447. doi: 10.1158/0008-5472.CAN-08-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Vozhilla N, Chatman L, Sarkar D, Dent P, et al. Chemoprevention by perillyl alcohol coupled with viral gene therapy reduces pancreatic cancer pathogenesis. Mol Cancer Ther. 2008b;7:2042–2050. doi: 10.1158/1535-7163.MCT-08-0245. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Washington I, Sarkar D, Clark JA, Fine RL, Dent P, et al. Strategy for reversing resistance to a single anticancer agent in human prostate and pancreatic carcinomas. Proc Natl Acad Sci USA. 2007a;104:3484–3489. doi: 10.1073/pnas.0700042104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, et al. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007b;3:374–376. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- Martin AP, Mitchell C, Rahmani M, Nephew KP, Grant S, Dent P. Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol Ther. 2009;8:2084–2096. doi: 10.4161/cbt.8.21.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesicek J, Lee H, Feldman T, Jiang X, Skobeleva A, Berdyshev EV, et al. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 2010 Apr 18; doi: 10.1016/j.cellsig.2010.04.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Ramesh R, Munshi A, Chada S, Meyn RE. Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation. Mol Ther. 2004;9:818–928. doi: 10.1016/j.ymthe.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Ogata M, Hino SI, Saito A, Morikawa K, Kondo S, Kanemoto S, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Zhang G, Martin AP, Hamed H, Mitchell C, Hylemon PB, et al. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther. 2008;7:1648–1662. doi: 10.4161/cbt.7.10.6623. (PMID:18787411). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Walker T, Martin AP, Allegood J, Vozhilla N, Emdad L, et al. MDA-7/IL-24-induced cell killing in malignant renal carcinoma cells occurs by a ceramide/CD95/PERK-dependent mechanism. Mol Cancer Ther. 2009;8:1280–1291. doi: 10.1158/1535-7163.MCT-09-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Mitchell C, Hamed HA, Allegood J, Dmitriev IP, Ogretmen B, et al. A serotype 5/3 adenovirus expressing MDA-7/IL-24 infects renal carcinoma cells and promotes paracrine-induced MDA-7/IL-24 and apoptosis in uninfected cells. Mol. Cancer Ther. 2010 submitted. [Google Scholar]
- Parrish-Novak J, Xu W, Brender T, Yao L, Jones C, West J, et al. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J Biol Chem. 2002;277:47517–47523. doi: 10.1074/jbc.M205114200. [DOI] [PubMed] [Google Scholar]
- Pataer A, Bocangel D, Chada S, Roth JA, Hunt KK, Swisher SG. Enhancement of adenoviral MDA-7-mediated cell killing in human lung cancer cells by geldanamycin and its 17-allyl-amino-17-demethoxy analogue. Cancer Gene Ther. 2007;14:12–18. doi: 10.1038/sj.cgt.7700989. [DOI] [PubMed] [Google Scholar]
- Pataer A, Vorburger SA, Barber GN, Chada S, Mhashilkar AM, Zou-Yang H, et al. Adenoviral transfer of the melanoma differentiation-associated gene 7 (mda7) induces apoptosis of lung cancer cells via upregulation of the double-stranded RNA-dependent protein kinase (PKR) Cancer Res. 2002;62:2239–2243. [PubMed] [Google Scholar]
- Pataer A, Vorburgerm SA, Chada S, Balachandran S, Barber GN, Roth JA, et al. Melanoma differentiation-associated gene-7 protein physically associates with the double-stranded RNA activated protein kinase PKR. Mol Ther. 2005;11:717–723. doi: 10.1016/j.ymthe.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Pestka S, Kotenko SV, Fisher PB. IL-24. In: Henry HL, Norman AW, editors. Encyclopedia of Hormones. San Diego, CA: Academic Press; 2003. pp. 507–513. [Google Scholar]
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- Raven JF, Koromilas AE. PERK and PKR: old kinases learn new tricks. Cell Cycle. 2008;7:1146–1150. doi: 10.4161/cc.7.9.5811. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Lebedeva IV, Gupta P, Emdad L, Sauane M, Dent P, et al. Melanoma differentiation associated gene-7 (mda-7)/IL-24: a ‘magic bullet’ for cancer therapy? Expert Opin Biol Ther. 2007a;7:577–586. doi: 10.1517/14712598.7.5.577. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Lebedeva IV, Su Z-z, Park E-S, Chatman L, Vozhilla N, et al. Eradication of therapy resistant human prostate tumors using a cancer terminator virus. Cancer Res. 2007b;67:5434–5442. doi: 10.1158/0008-5472.CAN-07-0195. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, et al. mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci U S A. 2002;99:10054–10059. doi: 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Su Z-z, Park E-S, Vozhilla N, Dent P, Curiel DT, et al. A cancer terminator virus (CTV) eradicates both treated primary and untreated distant human melanomas. Cancer Gene Ther. 2008;15:293–302. doi: 10.1038/cgt.2008.14. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su Z-z, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci USA. 2005a;102:14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Su Z-z, Vozhilla N, Park ES, Randolph A, Valerie K, et al. Targeted virus replication plus immunotherapy eradicates primary and distant pancreatic tumors in nude mice. Cancer Res. 2005b;65:9056–9063. doi: 10.1158/0008-5472.CAN-05-1261. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Choo HT, Gupta P, Lebedeva IV, Yacoub A, et al. Mechanistic aspects of mda-7/IL-24 cancer cell selectivity analyzed via a bacterial fusion protein. Oncogene. 2004a;23:7679–7690. doi: 10.1038/sj.onc.1207958. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Lebedeva I, Mei MX, Sarkar D, Su ZZ, et al. Mda-7/IL-24 induces apoptosis of diverse cancer cell lines through JAK/STAT-independent pathways. J Cell Physiol. 2003;196:334–345. doi: 10.1002/jcp.10309. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gupta P, Lebedeva IV, Su ZZ, Sarkar D, Randolph A, et al. N-glycosylation of MDA-7/IL-24 is dispensable for tumor cell-specific apoptosis and “bystander” antitumor activity. Cancer Res. 2006;66:11869–11877. doi: 10.1158/0008-5472.CAN-06-1887. [DOI] [PubMed] [Google Scholar]
- Sauane M, Lebedeva IV, Su ZZ, Choo HT, Randolph A, Valerie K, et al. Melanoma differentiation associated gene-7/interleukin-24 promotes tumor cell-specific apoptosis through both secretory and nonsecretory pathways. Cancer Res. 2004b;64:2988–2993. doi: 10.1158/0008-5472.can-04-0200. [DOI] [PubMed] [Google Scholar]
- Sauane M, Su ZZ, Dash R, Liu X, Norris JS, Sarkar D, et al. Ceramide plays a prominent role in MDA-7/IL-24-induced cancer-specific apoptosis. J Cell Physiol. 2010;222:546–555. doi: 10.1002/jcp.21969. [DOI] [PubMed] [Google Scholar]
- Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, Fisher PB. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc Natl Acad Sci USA. 2008;105:9763–9768. doi: 10.1073/pnas.0804089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancevic B, Kolesnick R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 2010;584:1728–1740. doi: 10.1016/j.febslet.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, et al. Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 by normal cells. Oncogene. 2005;24:7552–7566. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Lebedeva IV, Sarkar D, Emdad L, Gupta P, Kitada S, et al. Ionizing radiation enhances therapeutic activity of mda-7/IL-24: Overcoming radiation- and mda-7/IL-24-resistance in prostate cancer cells overexpressing the antiapoptotic proteins bcl-xL or bcl-2. Oncogene. 2006;25:2339–2348. doi: 10.1038/sj.onc.1209271. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Lebedeva IV, Sarkar D, Gopalkrishnan RV, Sauane M, Sigmon C, et al. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene. 2003;22:1164–1180. doi: 10.1038/sj.onc.1206062. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Madireddi MT, Lin JJ, Young CSH, Kitada S, Reed JC, et al. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci USA. 1998;95:14400–14405. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasdemir E, Maiuri MC, Tajeddine N, Vitale I, Criollo A, Vicencio JM, et al. Cell cycle-dependent induction of autophagy, mitophagy and reticulophagy. Cell Cycle. 2007;6:2263–2267. doi: 10.4161/cc.6.18.4681. [DOI] [PubMed] [Google Scholar]
- Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, Dent P. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- Xia HG, Zhang L, Chen G, Zhang T, Liu J, Jin M, et al. Control of basal autophagy by calpain1 mediated cleavage of ATG5. Autophagy. 2010;6:61–66. doi: 10.4161/auto.6.1.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub A, Gupta P, Park MA, Rhamani M, Hamed H, Hanna D, et al. Regulation of GST-MDA-7 toxicity in human glioblastoma cells by ERBB1, ERK1/2, PI3K, and JNK1-3 pathway signaling. Mol Cancer Ther. 2008a;7:314–329. doi: 10.1158/1535-7163.MCT-07-2150. [DOI] [PubMed] [Google Scholar]
- Yacoub A, Hamed HA, Allegood J, Mitchell C, Spiegel S, Lesniak MS, et al. PERK-dependent regulation of ceramide synthase 6 and thioredoxin play a key role in mda-7/IL-24-induced killing of primary human glioblastoma multiforme cells. Cancer Res. 2010a;70:1120–1129. doi: 10.1158/0008-5472.CAN-09-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub A, Liu R, Park MA, Hamed HA, Dash R, Schramm DN, et al. Cisplatin enhances protein kinase R-like endoplasmic reticulum kinase- and CD95-dependent melanoma differentiation-associated gene-7/interleukin-24-induced killing in ovarian carcinoma cells. Mol Pharmacol. 2010b;77:298–310. doi: 10.1124/mol.109.061820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub A, Mitchell C, Brannon J, Rosenberg E, Qiao L, McKinstry R, et al. MDA-7 (interleukin-24) inhibits the proliferation of renal carcinoma cells and interacts with free radicals to promote cell death and loss of reproductive capacity. Mol Cancer Ther. 2003a;2:623–632. [PubMed] [Google Scholar]
- Yacoub A, Mitchell C, Hong Y, Gopalkrishnan RV, Su ZZ, Gupta P, et al. MDA-7 regulates cell growth and radiosensitivity in vitro of primary (non-established) human glioma cells. Cancer Biol Ther. 2004;3:739–751. doi: 10.4161/cbt.3.8.968. [DOI] [PubMed] [Google Scholar]
- Yacoub A, Mitchell C, Lebedeva IV, Sarkar D, Su ZZ, McKinstry R, et al. mda-7 (IL-24) Inhibits growth and enhances radiosensitivity of glioma cells in vitro via JNK signaling. Cancer Biol Ther. 2003c;2:347–353. doi: 10.4161/cbt.2.4.422. [DOI] [PubMed] [Google Scholar]
- Yacoub A, Mitchell C, Lister A, Lebedeva IV, Sarkar D, Su ZZ, et al. Melanoma differentiation-associated 7 (interleukin 24) inhibits growth and enhances radiosensitivity of glioma cells in vitro and in vivo. Clin Cancer Res. 2003b;9:3272–3281. [PubMed] [Google Scholar]
- Yacoub A, Park MA, Gupta P, Rahmani M, Zhang G, Hamed H, et al. Caspase-, cathepsin and PERK-dependent regulation of MDA-7/IL-24-induced cell killing in primary human glioma cells. Mol Cancer Ther. 2008b;7:297–313. doi: 10.1158/1535-7163.MCT-07-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YP, Liang ZQ, Gu ZL, Qin ZH. Molecular Mechanism and regulation of autophagy. Acta Pharm Sinica. 2005;26:1421–1434. doi: 10.1111/j.1745-7254.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]