Abstract
The molecular events associated with the development of pathological hypertrophy have been shown to be stimulated through G-protein coupled receptors (GPCRs) that activate Gq signaling pathways in neonatal cardiomyocytes and in transgenic (TG) and knockout (KO) mice. We demonstrated that CaMKII, a multifunctional Ca2+ regulated protein kinase, was activated through GPCR and InsP3 mediated Ca2+ release and suggested that CaMKII was a downstream mediator of Gq -coupled hypertrophic signaling. This was supported by the demonstration of CaMKII activation by pressure overload (TAC) and induction of hypertrophy by transgenic (TG) CaMKII expression. CaMKII also phosphorylates Ca2+ handling proteins including the ryanodine receptor (RyR2), phosphorylation of which markedly increases sarcoplasmic reticulum (SR) Ca2+ leak. Increased RyR2 phosphorylation is associated with heart failure development in CaMKII TG mice, and mice genetically deleted for CaMKII (KO) have attenuated RyR2 phosphorylation, SR Ca2+ leak and heart failure development following long term TAC. Genetic ablation of CaMKII also decreases development of heart failure in Gq TG mice, and decreases infarct size, while improving functional recovery in mice subject to ischemia/reperfusion and preventing adverse remodeling following coronary artery occlusion. The underlying mechanisms are currently under study.
Keywords: CaMKII, Hypertrophy, Heart Failure, GPCR, ischemia/reperfusion
Involvement of CaMKII in cardiac hypertrophy
Cardiac hypertrophy is a mechanism by which the heart responds to extrinsic signals or injury in an effort to minimize cardiac wall stress. Physiologic hypertrophy is exemplified by the heart’s adaptive response to athletic conditioning, while pathologic hypertrophy occurs in response to disease states such as hypertension or myocardial infarction (1). Pathologic cardiac hypertrophy is characterized by increased cell size, changes in protein synthesis, cardiac remodeling, myofilament reorganization and increased expression of fetal genes (2). In the neonatal rat ventricular myocyte (NRVM) model of hypertrophy, GPCR agonists such as norepinephrine (NE), endothelin-1 (ET-1), phenylephrine (PE) or prostaglandin F2 alpha (PGF2α) mimic these responses, inducing increases in cell size, reorganization of sarcomeric proteins and re-expression of fetal genes such as ANF, BNP, β-MHC and α skeletal actin (3-10). Signaling through these GPCRs is initiated by coupling to the heterotrimeric G protein Gq (11;12), and expression of the α subunit of Gq (Gαq) in NRVMs in the absence of agonist can also induce hypertrophy as evidenced by marked increases in cell size, hypertrophic gene expression and myofilament organization (13-15). In addition, hypertrophy can be elicited in vivo by cardiac transgenic Gαq overexpression (16). There is also evidence that Gq activation mediates hypertrophy in vivo in response to pressure overload (TAC). This was first shown in mice with cardiac specific overexpression of a specific Gαq inhibitor peptide, in which there was a reduced hypertrophic response to transverse aortic constriction (TAC) compared to control animals (17). In addition, mice with cardiac-specific deletion of Gαq family proteins show no ventricular hypertrophy in response to pressure overload (18).
The downstream target of Gαq is phospholipase C (11;12) and early work from our laboratory demonstrated that hypertrophic agonists induce phosphoinositide (PI) hydrolysis in NRVMs (7;19-21). PI hydrolysis results in the generation of diacylglycerol and activation of protein kinase C (PKC) as well as formation of inositol trisphosphate (InsP3) which releases Ca2+ . Numerous laboratories including our own have examined and suggested involvement of PKC as well as various MAP kinase cascades in hypertrophic signaling (1;22;23). We also suggested that Ca2+/CaM dependent protein kinase II (CaMKII) serves as a downstream mediator of GPCR induced hypertrophy based on the finding that inhibition of CaMKII with KN-93 blocked phenylephrine-induced hypertrophy in NRVMs (24). CaMKII is activated in response to elevated Ca2+ and its interaction with Ca2+/Calmodulin. How GPCR agonist induced PI hydrolysis would activate CaMKII in the heart is not clear. One possibility is via effects of PKC on Ca2+ channels, resulting in enhanced Ca2+ entry and release. Another could be via InsP3 mediated Ca2+ release. In either case, these GPCR mediated changes in Ca2+ release would be superimposed upon the large and highly controlled changes in intracellular Ca2+ cycling that occur as part of excitation-contraction coupling. We recently addressed the question of whether localized, rather than global increases in Ca2+ induced by the Gq receptor coupled hypertrophic agonist ET-1 might lead to activation of CaMKII and elicit hypertrophic responses. This possibility was suggested by the observation that in cardiomyocytes, type 2 InsP3 (InsP3R2) receptors are concentrated in the nucleus (25) and that a splice variant of the predominant cardiac CaMKII isoform, CaMKII δB , contains a nuclear localization sequence which targets it to the nucleus (26;27). We determined in this study that CaMKII can be activated through an InsP3 receptor dependent mechanism by demonstrating that either ET-1 or the InsP3 receptor agonist adenophostin increased CaMKII autophosphorylation and that this response was attenuated by inhibition of InsP3 receptor signaling using 2-APB (28). We also used myocytes from InsP3R2 knock out mice (29) to show that CaMKII was not activated by ET-1 in the absence of InsP3R2 (28). We further demonstrated that CaMKII activation by localized InsP3 dependent increases in Ca2+ led to HDAC export from the nucleus whereas a more global increase in Ca2+ did not (26). Class II HDACs including HDAC regulate activity of the transcription factor MEF2 thereby affecting hypertrophic gene expression (30;31). Thus the concept of excitation-transcription coupling, whereby agonist-induced increases in nuclear Ca2+ activate CaMKII to mediate HDAC dependent gene transcription, was proposed (Figure 1).
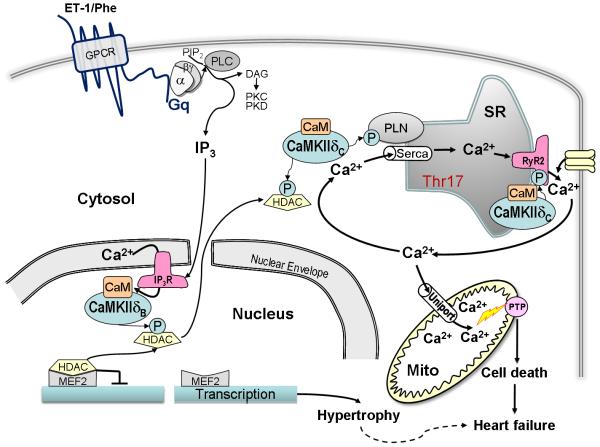
Figure 1.
Activation and targets of CaMKII δ in hypertrophy and heart failure. Abbreviations: ET-1, Endothelin-1; Phe, Phenylephrine; GPCR, G-Protein Coupled Receptor; α, α subunit of the Gq-protein; β◻, β◻ subunit of the Gq-protein; PIP2, Phosphatidylinositol 4,5-Bisphosphate; PLC, Phospholipase C; DAG, Diacylglycerol; PKC, Protein Kinase C; PKD, Protein Kinase D; CaM, Calmodulin; PLN, Phospholamban; SR, Sarcoplasmic Reticulum; SERCA, SR Ca2+ ATPase; CaMKII δ, Calcium Calmodulin Dependent Protein Kinase II; RyR2, Ryanodine Receptor 2; IP3, Inositol (1,4,5)-Trisphosphate; HDAC, Histone Deacetylase; IP3R, IP3 Receptor; Mito; Mitochondria; PTP, Permeability Transition Pore; MEF2, Myocyte Enhancer Factor-2.
Role of CaMKII in in vivo hypertrophy
To examine the possibility that CaMKII might be a mediator of cardiac hypertrophy development following pressure overload, CaMKII activation was measured in mice subject to TAC. We observed increases in autophosphorylated CaMKII (indicative of kinase activation) at 1 to 7 days post TAC (32-34). To determine whether increased CaMKII activity was a sufficient stimulus to induce hypertrophy, we generated cardiac specific CaMKII δ transgenic (TG) mice using the α-myosin heavy chain (αMHC) promoter. Both CaMKII δB and δC TG mice were generated. The CaMKII δB splice variant differs from δC by the inclusion of a nuclear localization sequence (26). Immunohistochemical analysis indicated that CaMKII δB was largely limited to the nucleus, while CaMKII δC was primarily cytosolic (27;33;35). There was cardiac enlargement at eight weeks in both CaMKII δB and δC TG mice compared to WT mice and both mice showed increases in expression of hypertrophic genes such as ANF, BNP, β-MHC and α skeletal actin (33;35;36). Thus both nuclear and cytosolic CaMKII δ overexpression induce hypertrophic responses.
Our earlier in vitro studies suggested that CaMKII, activated by localized nuclear InsP3 signaling, phosphorylated HDAC within the nucleus, promoting its export to the cytosol to enhance gene expression (36-39). The in vivo data suggested, however, that CaMKII δ can also phosphorylate HDAC when expressed in the cytosol, preventing it from returning to the nucleus and repressing gene expression (36). Consistent with this hypothesis, we saw increases in cytosolic HDAC and transcriptional activation of MEF2 dependent gene expression in both CaMKII δB and δC TG mice (36, Figure 1).
Role of CaMKII in heart failure
While both CaMKII δB and CaMKII δC TG mice exhibited hypertrophy which ultimately became maladaptive, the CaMKII δC mice showed a remarkably rapid progression to heart failure, with all of its commonly associated phenotypic changes (27;33;35). CaMKII δC TG mice showed premature death compared to wild type mice with only 50% survival at 15 weeks of age, and displayed progressive cardiac enlargement and ventricular dilation at 6-13 weeks. This was coupled with a marked loss of ventricular function measured by fractional shortening (33;40). In addition, specific changes in Ca2+ handling proteins were seen in the CaMKII δC TG mice that were not recapitulated in the CaMKII δB line (33;36;40). One notable finding in the CaMKII δC TG mice was increased association of CaMKII with the ryanodine receptor RyR2 (33;40). There was also increased phosphorylation of RyR2 at Ser2815, a known CaMKII phosphorylation site (33;40). Ca2+ sparks in cardiomyocytes isolated from CaMKII δC TG mice were significantly increased in number, frequency and width, leading to a 4.3 fold increase in diastolic SR “calcium leak” compared to wild type cardiomyocytes. Indeed, the “leak” was so substantial that SR Ca2+ content, measured by the caffeine-induced Ca2+ transient, was decreased by more than 60% in the CaMKII δC TG mice relative to wild type (40).
To determine whether the decreased SR Ca2+ content that resulted from the SR Ca2+ leak was a major contributor to contractile dysfunction and heart failure development in the CaMKII δC TG mice, these mice were crossed with phospholamban (PLN) knockout mice generated by the Kranias lab (41). PLN deletion restored SR Ca2+ loading and Ca2+ transients in the CaMKII δC TG as anticipated. Remarkably however, PLN KO/TG mice did not show improvement, but rather an exaggerated heart failure phenotype compared to the δC TG mice i.e. they showed greater mortality, ventricular dilation, and myocyte death (42). Characterization of cardiomyocytes from the PLN KO/TG mice revealed that recovery of the SR Ca2+ load (facilitated by PLN deletion) coupled with the increased P-RyR (due to CaMKII δC overexpression) conspired to markedly increase Ca2+ sparks. Of significant interest, this increase in diastolic SR Ca2+ leak was shown to result in mitochondrial Ca2+ loading, which did not occur when the SR Ca2+ leak was minimized (42). Experiments using ryanodine to minimize the leak and Ru360 to reduce mitochondrial Ca2+ overload showed that survival of cardiomyocytes from the PLN KO/TG mice was markedly enhanced (42). Current studies are aimed at determining the importance of the mitochondrial PT pore and other mechanisms by which heart failure progression occurs in the CaMKII δC TG and double mutant mice.
Is CaMKII required for hypertrophy and transition to heart failure?
To further explore the role of CaMKII δ in hypertrophy and heart failure we generated CaMKII δ knockout mice (43). The CaMKII δ knockout mice (KO) exhibited no gross baseline changes in ventricular structure and function. When cardiac hypertrophy was induced by 2-week isoproterenol stimulation, comparable hypertrophy developed in WT and KO mice (unpublished observation). In addition, CaMKII δ deletion had no effect on hypertrophy induced by 2-week transverse aortic constriction (TAC), as evidenced by gravimetric analysis, echocardiographic parameters and cell size measurement (43). Consistent with this we found equivalent upregulation of hypertrophic gene expression in WT and KO mice subject to TAC, and there was no difference in the phosphorylation of the CaMKII target, HDAC5. The observation that CaMKII δ was not required for the development of hypertrophy in response to pressure overload was unexpected and led us to examine involvement of other kinase signaling pathways that could compensate for CaMKII δ deletion. The gamma (γ) isoform of CaMKII is normally present at low amounts in the heart, and its expression is not affected by CaMKII δ deletion (43). However, confirming a previous report (32), we found that expression of CaMKII ◻ was increased by pressure overload and this occurred to a similar extent in WT and KO mice (43). We are currently testing the hypothesis that maintenance of hypertrophy in the CaMKII δ KO mice is explained by the concomitant upregulation in expression and activation of CaMKII γ. We also find that protein kinase D (PKD) activation is increased by pressure overload and to similar extents in WT and KO mice (43). Thus, an alternative hypothesis is that PKD, which has been implicated in the development of TAC induced hypertrophy (44), and also functions as an efficacious kinase for class II HDACs including HDAC5 (38;39), provides signals that mediate cardiac hypertrophy in the absence of CaMKII δ.
Remarkably, while hypertrophy is intact in the CaMKII δ KO mice, the transition from hypertrophy to heart failure is not (43). Specifically, WT mice 6 weeks post TAC show a range of phenotypic changes (e.g. chamber dilation, ventricular dysfunction, lung edema, cardiac fibrosis, apoptosis) associated with heart failure development. All of these responses are significantly attenuated in CaMKII δ KO mice, and survival after TAC is dramatically improved (43). These salutary effects of CaMKII δ deletion on the response to long-term pressure overload are observed in the absence of any diminution in the initial development of hypertrophy, as indicated above. Thus we suggest that CaMKII δ deletion does not prevent decompensation simply because it prevents the development of pathological hypertrophy, but rather that it plays a more fundamental role in the transition to heart failure.
Role of CaMKII in Ca2+ handling and heart failure development
It is well accepted that disturbed Ca2+ handling contributes to the development of heart failure. Heart failure is associated with significant quantitative changes in the level of expression of intracellular Ca2+ regulatory proteins including the sarcoplasmic or endoplasmic reticulum calcium ATPase2 (SERCA2), the predominant cardiac IP3 receptor (IP3R2) and the ryanodine receptor (RyR2) (40;45). Expected changes in expression of these proteins were observed in WT mice subject to long term TAC but were attenuated in CaMKII δ KO mice. Of particular interest, WT mice showed increases in IP3R2 and decreases in RyR2 expression, modest at 2 weeks and more significant at 6 weeks after TAC (43). The increase in IP3R2 and decrease in RyR2 was attenuated in CaMKII δ KO mice. Thus the elevated IP3R2 to RyR2 ratio associated with TAC induced heart failure is markedly diminished in the KO mice (43). Taken together, these results suggest that CaMKII δ contributes to the altered expression of Ca2+ regulatory proteins during the development of pressure overload-induced heart failure.
Of further interest, we demonstrated that the fraction of RyR2 phosphorylated at the CaMKII site was increased in response to TAC both prior to and during development of heart failure but that this did not occur in CaMKII δ KO mice (43). We suggest that increases in CaMKII-phosphorylated RyR2 contribute to the decompensation of pressure overload induced heart failure in WT mice. As mentioned earlier, RyR2 phosphorylation in CaMKII δC TG mice enhanced diastolic Ca2+ leak and was suggested to be causally related to the development of heart failure (40). To assess SR Ca2+ leak as a functional correlate of the RyR2 phosphorylation induced by TAC, we measured Ca2+ sparks in cardiomyocytes isolated from WT and KO mice. There was no difference in the Ca2+ spark frequency in WT versus KO mice at baseline. A highly significant difference in spark frequency was observed, however, in mice subjected to 6-week TAC, with SR Ca2+ leak increasing in cardiomyocytes from WT but not KO mice (43). We suggest that the ability of CaMKII δ deletion to prevent TAC-induced SR Ca2+ leak underlies its salutary effect in this mouse model of pressure overload induced heart failure.
In the knockout model described above, CaMKII activity is absent beginning in early development and deleted in tissues other than the heart. We have used cardiac specific CaMKII δ KO mice to reproduce our observation that heart failure development is blunted, thus the effect we see is due to loss of CaMKII in cardiomyocytes (unpublished). Whether inhibiting CaMKII after initiation of hypertrophy can prevent heart failure development or improve its course is a critical question that is currently under study. We are also attempting to determine if CaMKII mediated SR Ca2+ leak can be blocked pharmacologically and if this has beneficial effects on heart failure development. Of further interest is the possibility that the β-adrenergic receptor signals through CaMKII, at least under more chronic conditions, and that this may contribute to the efficacy of β-adrenergic blockade in heart failure. The evidence for a role of CaMKII in β-adrenergic induced heart failure was recently reviewed (46). Extrapolating from mouse models to the therapy of human heart failure is clearly premature, but we are encouraged by the phenotypic similarities between heart failure induced through and dependent upon CaMKII δ and that seen clinically and in other genetic heart failure models.
Role of CaMKII in Gq induced hypertrophy and heart failure
Our previous studies demonstrated that Gαq expression in NRVMs stimulates cardiac hypertrophy (13-15), consistent with the hypertrophic effect of Gq-coupled receptor agonists (3-10;47). Pioneering experiments from Dorn’s laboratory established that this occurs in vivo, i.e. overexpression of Gαq induces a hypertrophic phenotype similar to that elicited by pressure overload (16;48). While Gαq expression in vivo induces hypertrophy, high levels of Gαq expression or accentuated stress signaling elicited by aortic banding or pregnancy in Gαq TG mice is associated with hypertrophic decompensation (14;16;48). Progression of Gαq-mediated hypertrophy results in part from induction of cardiac apoptosis. Gαq TG mice that develop peripartum cardiomyopathy were shown to exhibit massively increased apoptosis (14;49). A mitochondrial death pathway involving the Bcl2 family protein Nix, was implicated in Gq induced apoptosis (13-15;50;51). We have also shown that enhanced Gαq signaling induced by expression of a constitutively activated form of Gαq causes cardiomyocyte apoptosis (13-15). Recent observations suggest that CaMKII expression is increased in Gαq TG mice and that CaMKII is activated by Gαq expression in NRVMs, consistent with the possibility that CaMKII signals downstream of Gq activation (manuscript in preparation, Ling et al). Accordingly we asked whether genetic deletion of CaMKII protected against development of heart failure induced by Gαq overexpression. Gαq TG mice were crossed with CaMKII δ KO mice. Echocardiography demonstrated that CaMKII δ deletion attenuated the left ventricular chamber dilation and dysfunction seen in Gq mice. Hemodynamic measurements also confirmed that there was improved heart function in the double mutant mice compared to the Gαq TG mice heart functions. Other hallmarks of heart failure such as arrhythmias, cardiac fibrosis and apoptosis were also significantly prevented by CaMKII δ deletion in mice overexpressing Gαq (manuscript in preparation, Ling et al).
CaMKII in I/R injury and in response to MI
Reperfusion is essential to salvage ischemic tissue, but it also contributes to myocardial damage referred to as reperfusion injury. CaMKII inhibition with KN-93 or through cardiac expression of the CaMKII inhibitor AC3-I has been shown to protect against ischemic damage resulting from myocardial infarction (MI) or ex vivo ischemia/reperfusion (I/R) injury (52;53). Using our recently generated CaMKII δ KO mice, the role of CaMKII in ischemic injury was examined further. WT and KO mice were subjected to ex vivo global I/R (22min /1hour), left anterior descending coronary artery (LAD) occlusion to induce ischemia for 1-hr followed by reperfusion for 24 hours (in vivo I/R), or permanent LAD occlusion for 6 weeks (MI). CaMKII δ deletion significantly reduced infarct size following either ex vivo or in vivo I/R and there was improved recovery of cardiac function in the absence of CaMKII. In response to MI, CaMKII δ ablation was found to inhibit the development of left ventricular dilation as assessed by echocardiography. Preliminary results indicate that adverse remodeling was diminished and cardiac function improved in KO compared to WT mice (manuscript in preparation, Ling et al). These findings provide further evidence that CaMKII δ plays a deleterious role in the development of myocardial injury and heart failure in MI and I/R as well as pressure overload models.
Is there a differential role for CaMKII δB and δC in heart failure?
In an effort to better understand the mechanism by which CaMKII δC TG mice developed heart failure, we crossed the CaMKII δC mice with several other genetic mouse models that had potential to rescue the heart failure phenotype. As described above, replenishing the SR Ca2+ load via PLN ablation actually exacerbated the phenotype (42). Interestingly two other rescue strategies, inhibition of CaMKII δC at the SR with SR targeted AIP (54) and preventing mitochondrial permeability transition pore formation by cyclophilin D ablation (55) also failed to rescue the heart failure seen in CaMKII δC TG mice (Huke S et al. in revision; Elrod J et al. submitted). Increased dilation or apoptosis also appear to accompany the increased lethality in these models. These results are consistent with the suggestion that CaMKII δC is involved in pro-apoptotic signaling and sensitizes towards activation of mitochondrial death pathways (56). Several recent studies have also demonstrated that whereas CaMKII δC is proapoptotic, CaMKII δB has protective effects (57;58). In this regard it is notable that CaMKII δB TG mice do not rapidly transition from hypertrophy to heart failure, although secondary changes in protein phosphatase activity ultimately affect SR Ca2+ uptake and predispose to ventricular dilation (35). Future studies using CaMKII δB versus δC subtype specific KO and TG mice will allow us to gain insight into the function of the individual CaMKII δ splice variants and their contribution to Ca2+ handling, transcriptional regulation and cell viability, and how this impacts the development of heart failure. It will also be informative to examine changes in CaMKII expression and activity in human heart failure patients in light of the potential for opposing effects of the CaMKII δB and δC subtypes, as well as for compensatory effects of increased CaMKII ◻
Acknowledgments
Funding was supported by NIH grants 5P01 HL080101-05 and 2T32GM007752-31
Footnotes
CaMKII in hypertrophy and heart failure
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heineke J, Molkentin JD. Nat. Rev. Mol. Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 2.Frey N, Olson EN. Annu. Rev. Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 3.Adams JW, Sah VP, Henderson SA, Brown JH. Circ. Res. 1998;83:167–178. doi: 10.1161/01.res.83.2.167. [DOI] [PubMed] [Google Scholar]
- 4.Adams JW, Migita DS, Yu MK, Young R, Hellickson MS, Castro-Vargas FE, Domingo JD, Lee PH, Bui JS, Henderson SA. J. Biol. Chem. 1996;271:1179–1186. doi: 10.1074/jbc.271.2.1179. [DOI] [PubMed] [Google Scholar]
- 5.Simpson P, McGrath A, Savion S. Circ. Res. 1982;51:787–801. doi: 10.1161/01.res.51.6.787. [DOI] [PubMed] [Google Scholar]
- 6.Simpson P. J. Clin. Invest. 1983;72:732–738. doi: 10.1172/JCI111023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shubeita HE, McDonough PM, Harris AN, Knowlton KU, Glembotski CC, Brown JH, Chien KR. J. Biol. Chem. 1990;265:20555–20562. [PubMed] [Google Scholar]
- 8.Ito H, Hirata Y, Adachi S, Tanaka M, Tsujino M, Koike A, Nogami A, Murumo F, Hiroe M. J. Clin. Invest. 1993;92:398–403. doi: 10.1172/JCI116579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadoshima J-I, Xu Y, Slayter HS, Izumo S. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 10.Knowlton KU, Michel MC, Itani M, Shubeita HE, Ishihara K, Brown JH, Chien KR. J. Biol. Chem. 1993;268:15374–15380. [PubMed] [Google Scholar]
- 11.Smrcka AV, Hepler JR, Brown KO, Sternweis PC. Science. 1991;251:804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- 12.Taylor SJ, Chae HZ, Rhee SG, Exton JH. Nature. 1991;350:516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- 13.Dorn GW, Brown JH. Trends Cardiovasc. Med. 1999;9:26–34. doi: 10.1016/s1050-1738(99)00004-3. [DOI] [PubMed] [Google Scholar]
- 14.Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH, Dorn GW. Proc. Natl. Acad. Sci. USA. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams JW, Pagel AL, Means CK, Oksenberg D, Armstrong RC, Brown JH. Circ. Res. 2000;87:1180–1187. doi: 10.1161/01.res.87.12.1180. [DOI] [PubMed] [Google Scholar]
- 16.D’Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW. Proc. Natl. Acad. Sci. USA. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 18.Wettschureck N, Rutten H, Zywietz A, Gehring D, Wilkie TM, Chen J, Chien KR, Offermanns S. Nat. Med. 2001;7:1236–1240. doi: 10.1038/nm1101-1236. [DOI] [PubMed] [Google Scholar]
- 19.Brown JH, Buxton IL, Brunton LL. Circ. Res. 1985;57:532–537. doi: 10.1161/01.res.57.4.532. [DOI] [PubMed] [Google Scholar]
- 20.Hilal-Dandan R, Ramirez MT, Villegas S, Gonzalez A, Endo-Mochizuki Y, Brown JH, Brunton LL. Am. J. Physiol. 1997;272:H130–H137. doi: 10.1152/ajpheart.1997.272.1.H130. [DOI] [PubMed] [Google Scholar]
- 21.Brown JH, Masters SB. Trends Pharmacol. Sci. 1984;5:417–419. [Google Scholar]
- 22.Dorn GW, Force T. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clerk A, Sugden PH. Circ. Res. 2006;99:455–458. doi: 10.1161/01.RES.0000241053.89089.c3. [DOI] [PubMed] [Google Scholar]
- 24.Sei CA, Irons CE, Sprenkle AB, McDonough PM, Brown JH, Glembotski CC. J. Biol. Chem. 1991;266:15910–15916. [PubMed] [Google Scholar]
- 25.Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. J Biol Chem. 2005;280:15912–15920. doi: 10.1074/jbc.M414212200. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan M, Edman CF, Schulman H. J. Cell Biol. 1994;126:839–852. doi: 10.1083/jcb.126.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez MT, Zhao X, Schulman H, Brown JH. J. Biol. Chem. 1997;272:31203–31208. doi: 10.1074/jbc.272.49.31203. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. J. Clin. Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Circ. Res. 2005;96:1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c. [DOI] [PubMed] [Google Scholar]
- 30.McKinsey TA, Zhang CL, Olson EN. Proc. Natl. Acad. Sci. USA. 2000;97:14400–14405. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson EN, Schneider MD. Genes Dev. 2003;17:1937–1956. doi: 10.1101/gad.1110103. [DOI] [PubMed] [Google Scholar]
- 32.Colomer JM, Mao L, Rockman HA, Means AR. Mol Endocrinol. 2003;17:183–192. doi: 10.1210/me.2002-0350. [DOI] [PubMed] [Google Scholar]
- 33.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr., Bers DM, Brown JH. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 34.Saito T, Fukuzawa J, Osaki J, Sakuragi H, Yao N, Haneda T, Fujino T, Wakamiya N, Kikuchi K, Hasebe N. J Mol Cell Cardiol. 2003;35:1153–1160. doi: 10.1016/s0022-2828(03)00234-7. [DOI] [PubMed] [Google Scholar]
- 35.Zhang T, Johnson EN, Gu Y, Morissette MR, Sah VP, Gigena MS, Belke DD, Dillmann WH, Rogers TB, Schulman H, Ross J, Jr., Brown JH. J Biol Chem. 2002;277:1261–1267. doi: 10.1074/jbc.M108525200. [DOI] [PubMed] [Google Scholar]
- 36.Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS, Olson EN, Brown JH. J. Biol. Chem. 2007;282:35078–35087. doi: 10.1074/jbc.M707083200. [DOI] [PubMed] [Google Scholar]
- 37.Lu J, McKinsey TA, Nicol RL, Olson EN. Proc. Natl. Acad. Sci. USA. 2000;97:4070–4075. doi: 10.1073/pnas.080064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Mol. Cell Biol. 2004;24:8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006 doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 41.Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Circ. Res. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- 42.Zhang T, Guo T, Mishra S, Dalton ND, Kranias EG, Peterson KL, Bers DM, Brown JH. Circ. Res. 2009;106:354–362. doi: 10.1161/CIRCRESAHA.109.207423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Heller BJ. J. Clin. Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fielitz J, Kim MS, Shelton JM, Qi X, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3059–3063. doi: 10.1073/pnas.0712265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Circ. Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 46.Grimm M, Brown JH. J. Mol. Cell Cardiol. 2009;48:322–330. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown JH, Martinson EA. Trends Cardiovasc. Med. 1992;2:209–214. doi: 10.1016/1050-1738(92)90026-O. [DOI] [PubMed] [Google Scholar]
- 48.Sakata Y, Hoit BD, Liggett SB, Walsh RA, Dorn GW. Circ. 1998;97:1488–1495. doi: 10.1161/01.cir.97.15.1488. [DOI] [PubMed] [Google Scholar]
- 49.Hayakawa Y, Chandra M, Miao W, Shirani J, Brown JH, Dorn GW, II, Armstrong RC, Kitsis RN. Circ. 2003;108:3036–3041. doi: 10.1161/01.CIR.0000101920.72665.58. [DOI] [PubMed] [Google Scholar]
- 50.Yussman MG, Toyokawa T, Odley A, Lynch RA, Wu G, Colbert MC, Aronow BJ, Lorenz JN, Dorn GW. Nat. Med. 2002;8:725–730. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- 51.Diwan A, Matkovich SJ, Yuan Q, Zhao W, Yatani A, Brown JH, Molkentin JD, Kranias EG, Dorn GW. J. Clin. Invest. 2009;119:203–212. doi: 10.1172/JCI36445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 53.Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, Hajjar RJ, Kranias EG, Mundina-Weilenmann C, Mattiazzi A. Cardiovasc. Res. 2007;73:689–698. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Ji Y, Li B, Reed TD, Lorenz JN, Kaetzel MA, Dedman JR. J Biol Chem. 2003;278:25063–25071. doi: 10.1074/jbc.M302193200. [DOI] [PubMed] [Google Scholar]
- 55.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 56.Zhu W, Woo AY, Yang D, Cheng H, Crow MT, Xiao RP. J. Biol. Chem. 2007;282:10833–10839. doi: 10.1074/jbc.M611507200. [DOI] [PubMed] [Google Scholar]
- 57.Peng W, Zhang Y, Zheng M, Cheng H, Zhu W, Cao CM, Xiao RP. Circ. Res. 2009 doi: 10.1161/CIRCRESAHA.109.210914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Little GH, Saw A, Bai Y, Dow J, Marjoram P, Simkhovich B, Leeka J, Kedes L, Kloner RA, Poizat C. J. Biol. Chem. 2009;284:24857–24868. doi: 10.1074/jbc.M109.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]