Abstract
Transcriptional cascades are required for specification of 5-HT neurons and 5-HT modulated behaviors. Expression of several cascade factors extends across lifespan suggesting their control of behavior may not be temporally restricted to programming normal numbers of 5-HT neurons. We applied new mouse conditional targeting approaches to investigate ongoing requirements for Pet-1, a cascade factor required for the initiation of 5-HT synthesis but whose expression persists into adulthood. We found that Pet-1 was required after 5-HT neuron generation, for multiple steps in 5-HT neuron maturation including axonal innervation to the somatosensory cortex, firing properties, and 5-HT1A and 5-HT1B autoreceptor expression. Targeting Pet-1 in adult 5-HT neurons showed that it was still needed to preserve normal anxiety-related behaviors through direct autoregulated control of serotonergic gene expression. These findings show that Pet-1 is required across lifespan and therefore behavioral pathogenesis can result from both developmental and adult-onset alterations in serotonergic transcription.
Introduction
The brain serotonin (5-HT) transmitter system is a critical homeostatic modulator of neural circuits that shape emotional behaviors in response to stressors in the environment 1. A widely discussed theory supported by a rich literature emphasizes the importance of 5-HT function for the maturation of neural circuits and the development of normal adult emotional behaviors 2. Altered serotonergic signaling and gene expression during embryonic development disrupts cortical dendritic arborization 3, differentiation, and patterning of forebrain afferents 4,5. Other studies show that postnatal perturbation of the serotonergic system can cause emotional disorders in adult animals 6–8. These findings together with correlative studies of serotonergic indices and gene variants in monkeys and humans support the idea that alterations in serotonergic function are involved in establishing vulnerability for several mood and neurological disorders 1,9.
The likelihood that altered serotonergic function during development contributes to behavioral pathogenesis has stimulated interest in the genetic mechanisms that direct the formation of the 5-HT system 10. A cascade (Supplementary Fig. 1) of transcriptional regulators has been identified that progressively restricts multi-potent neuronal progenitors to a 5-HT neuron fate in the embryonic ventral hindbrain 11. Gene targeting of factors in the cascade causes alterations in adult 5-HT-modulated emotional responses 12,13, thus providing a link between transcriptional regulation of 5-HT neuron birth and adult behavior. Nevertheless, the mechanisms through which transcription factors in the cascade regulate behavior are poorly understood and may not be, simply, the result of programming normal 5-HT neuron numbers and 5-HT levels. For example, although all of the factors known to compose the cascade have been shown to be necessary for the initiation of 5-HT synthesis at the cell fate specification stage, transcriptional control of subsequent steps in 5-HT system maturation may also be crucial for programming normal 5-HT-modulated behaviors. However, whether or not factors in the cascade are responsible for additional transcriptional events in the maturation of the system has not been investigated. Furthermore, it is not known whether the critical period for transcription directed by these developmental determinants extends into adulthood to regulate maintenance of 5-HT signaling and preserve behavioral integrity. The concept of a transcriptional maintenance mechanism is potentially of critical importance in understanding the regulation of behavioral and psychiatric pathogenesis as drug, toxin, and dietary perturbation studies in adults including humans demonstrate the importance of ongoing presynaptic serotonergic function in emotional and behavioral processing 9.
Expression of the rodent Pet-1 ETS domain transcription factor (human orthologue, Fev) is restricted in the CNS to 5-HT neurons and is induced in postmitotic precursors just prior to the initiation of 5-HT synthesis in the ventral hindbrain 14. Pet-1 plays a pivotal role in the cascade through its coordinate induction of the enzymatic pathway responsible for 5-HT synthesis in immature postmitotic precursors 12. Interestingly, Pet-1 expression is never extinguished and appears to continue undiminished in all adult 5-HT neurons 14. This persistent expression suggests that Pet-1 may be required for events in 5-HT neuron maturation that occur subsequent to their specification and possibly in adulthood for transcriptional maintenance of the 5-HT system. Here, we applied new 5-HT neuron-specific and temporally-restricted conditional targeting approaches to investigate requirements for continued Pet-1-dependent transcription in the 5-HT system.
Results
Conditional deletion of Pet-1 after 5-HT neuron generation
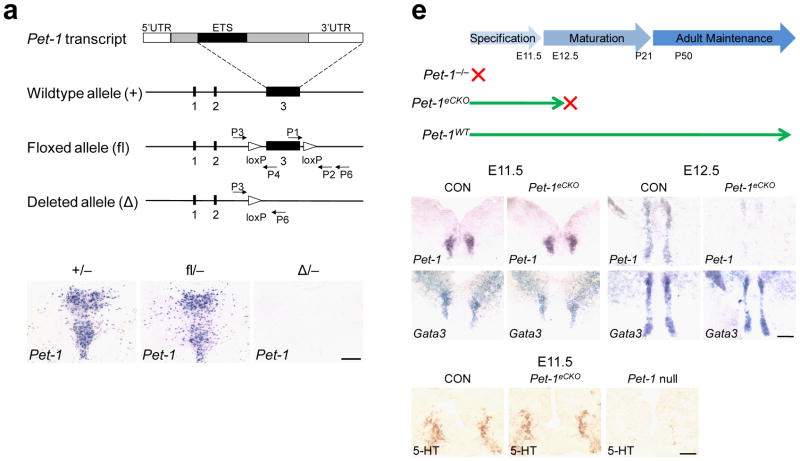
To investigate Pet-1 function after its initial role in 5-HT neuron generation, we inserted two loxP sites in introns on each side of exon 3, which encodes most of the Pet-1 protein coding sequences including the ETS DNA binding domain (Fig. 1a). In situ hybridization (Fig. 1b, c) and quantitative reverse transcriptase PCR (RT-qPCR) (Supplementary Fig. 2) indicated that Pet-1 expression was indistinguishable in mice carrying either one copy of the wildtype (+) or floxed (fl) Pet-1 allele together with a constitutive null allele. Excision of exon 3 generated a deleted Pet-1 allele (Δ) in which all protein coding sequences except those encoding 42 amino acids at the N terminus were eliminated (Fig. 1a). In situ hybridization showed that Pet-1 expression was absent in mice heterozygous for the deleted and null Pet-1 alleles (Fig. 1d). Furthermore, normal numbers of TPH+ neurons were present in Pet-1fl/−mice but not in Pet-1 Δ−/−mice (Supplementary Fig. 2). Thus, the unrecombined floxed allele is functionally equivalent to the wildtype allele and the conditionally deleted Pet-1 allele is functionally equivalent to the Pet-1 constitutive null allele.
Figure 1.
Conditional deletion of Pet-1 after 5-HT neuron fate specification. (a) Targeting strategy. From the top, schematic of the Pet-1 mRNA; wildtype Pet-1 allele (+); floxed Pet-1 allele (fl); and the conditionally deleted Pet-1 allele (Δ, bottom). (b–d) In situ hybridization to detect Pet-1 transcripts in the dorsal raphe (DRN) of mice heterozygous for the Pet-1 null allele and either the wild-type (b), floxed (c) or conditionally deleted Pet-1 alleles (d). (e) Time frame of Pet-1 expression in Pet-1 /− , Pet-1eCKO (Pet-1fl/−, ePet::Cre), and wildtype mice. (f–i) In situ hybridization to detect Pet-1 and Gata3 mRNAs in control (Pet-1fl/+, ePet::Cre) and Pet-1eCKO mice. (j–l) 5-HT immunostaining in control, Pet-1eCKO, and Pet-1−/−mice at E11.5. Scale bars are 100 μm in (i, l) and 200 μm in (d).
We crossed floxed Pet-1 mice with ePet::Cre transgenic mice 15, which express Cre recombinase only in postmitotic 5-HT neurons, to generate Pet-1 early conditional knockout mice (Pet-1fl/−, ePet::Cre, designated Pet-1eCKO). Nearly all 5-HT neurons derived from progenitors in rhombomere 1 and 2 are born by embryonic days 10 and 11 respectively 16,17. However, we showed previously 18 that reduced serotonergic gene expression is not evident with this ePet::Cre transgene until about E12.5. Thus, Pet-1 expression should be maintained in Pet-1eCKO mice for about 2 days after fulfilling its early role in 5-HT neuron generation. Based on this reasoning, we predicted that normal numbers of 5-HT neurons would be generated in Pet-1eCKO mice.
Consistent with our expectation, we found that expression of Pet-1 and other 5-HT neuron markers including tryptophan hydroxylase 2 (Tph2), serotonin transporter (Sert or Slc6a4) and another transcription factor Gata3 was indistinguishable between Pet-1eCKO and the wild-type controls in the anterior hindbrain at E11.5 (Fig. 1f, h, and Supplementary Fig. 3). Furthermore, immunohistochemistry, using anti-sera against 5-HT showed that normal numbers of 5-HT+ neurons were generated in Pet-1eCKO mice as compared with the controls (Fig. 1j, k). In comparison, very few 5-HT+ cells could be detected in Pet-1−/−mice at this stage (Fig. 1l). Reduced Pet-1 transcripts were not observed until E12.5 (Fig. 1g), while the expression of Gata3 was not altered (Fig. 1i). Concomitant with the conditional deletion of Pet-1 at E12.5 was diminished expression of Tph2, Sert and 5-HT in Pet-1eCKO mice (Supplementary Fig. 3). Importantly, Pet-1 is not required for 5-HT neuron survival 19, and all Pet-1 deficient cells were present in the Pet-1eCKO brain through adulthood (Supplementary Fig. 4). These findings indicate that Pet-1eCKO mice provide a means to investigate Pet-1 function in 5-HT system maturation after it has fulfilled its initial role in 5-HT neuron generation.
Continued Pet-1 function controls serotonergic innervation
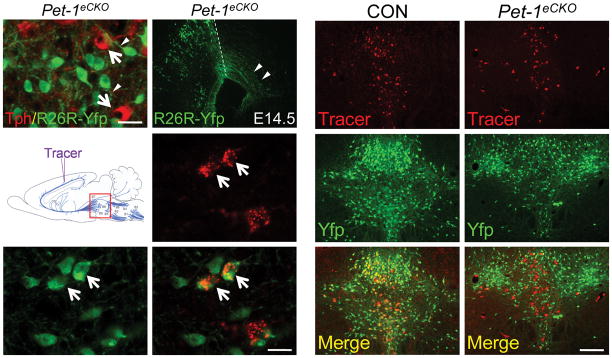
Immediately following the birth of 5-HT neurons, maturation of the 5-HT system depends on proper routes of cell body migration, axon pathfinding, and innervation in terminal fields 20. To facilitate an investigation of Pet-1 function in these maturation events, we used Cre-mediated activation of the R26RYfp (ref 21) reporter allele to permanently mark Pet-1 deficient 5-HT neurons in Pet-1eCKO mice. Pet-1 deletion was spared in a small subset (~15%) of 5-HT neurons in Pet-1eCKO mice (Supplementary Fig. 4) and therefore Pet-1 deficient Tph – cells were situated side by side with untargeted Tph+ 5-HT neurons (Fig. 2a, arrows). Examination of R26R-Yfp+ cells in Pet-1eCKO mice showed these Pet-1 deficient 5-HT neurons extended axons from their cell bodies, similar to intermingled wildtype Tph+ cells, and therefore Pet-1 was not essential for proximal axonal outgrowth (Fig. 2a, arrowheads). Indeed, Yfp immunostaining in Pet-1eCKO mice revealed that axon bundles from Pet-1 deficient 5-HT neurons crossed the midbrain-hindbrain boundary and entered the midbrain at E14.5 (Fig. 2b).
Figure 2.
Continued Pet-1 function is required for maturation of serotonergic axonal innervation patterns. (a) Co-immunostaining of Yfp and Tph in adult Pet-1eCKO mice; white arrows indicate untargeted 5-HT neurons; arrowheads indicate proximal axons extending from cell bodies of Pet-1 deficient 5-HT neurons. (b) Yfp immunostaining of Pet-1 deficient 5-HT neuron axon bundles at E14.5 in Pet-1eCKO mice. Arrowheads mark axons that have crossed the MHB and entered the midbrain (dashed lines, midbrain-hindbrain boundary, MHB). (c) Schematic of the retrograde tracing experiment. Tracer injected into the somatosensory cortex labeled (d) Yfp+ Pet-1 deficient 5-HT neurons (e) in the dorsal raphe. (f), merge of (d) and (e). (g–l) Significantly fewer retrogradely labeled cells were found in the DRN of the Pet-1eCKO brain (g, h; 49.9±3.1%, mean ± s.e.m, relative to control, n=6 for each genotype). Overlay of tracer signal with Yfp immunostaining (i, j) showed that 83.0±1.8%, mean ± s.e.m, of the retrogradely labeled DRN cells in control mice were ePet::Yfp+ 5-HT neurons (k), whereas only 19.3±1.7% Yfp+ Pet-1 deficient 5-HT neurons (l) were labeled by the same tracer injection in Pet-1eCKO brain. *** p<0.001, two tailed t test. Scale bars are 20 μm in (a, f) and 200 μm in (l).
To determine whether these axons could properly reach their forebrain targets, we performed a retrograde tracing experiment by injecting tracer into the somatosensory cortex, which receives serotonergic afferents mainly from 5-HT neurons located in the dorsal raphe nucleus (DRN) (Fig. 2c) 22. Stereotaxic injection into 3 week-old wildtype and Pet-1eCKO mice resulted in the retrograde labeling of both 5-HT and non-5-HT neurons in the DRN (Fig. 2d, e, f). Tracer intensity observed in Pet-1 deficient 5-HT neurons was comparable to that in other retrogradely labeled cells, suggesting Pet-1-deficient cells were able to retrogradely transport tracer (Fig. 2f). Interestingly, we found a significant decrease in the total number of tracer labeled cells across the entire DRN in Pet-1eCKO mice (Fig. 2g, h). In wildtype mice, the majority of retrogradely labeled cells were 5-HT neurons (Fig. 2i, k). In contrast, far fewer Pet-1 deficient Yfp+ 5-HT neurons were labeled with similarly performed tracer injections (Fig. 2j, l), indicating significantly disrupted serotonergic innervation of the somatosensory cortex from DRN 5-HT neurons in the Pet-1eCKO brain.
Continued Pet-1 function controls autoreceptor pathways
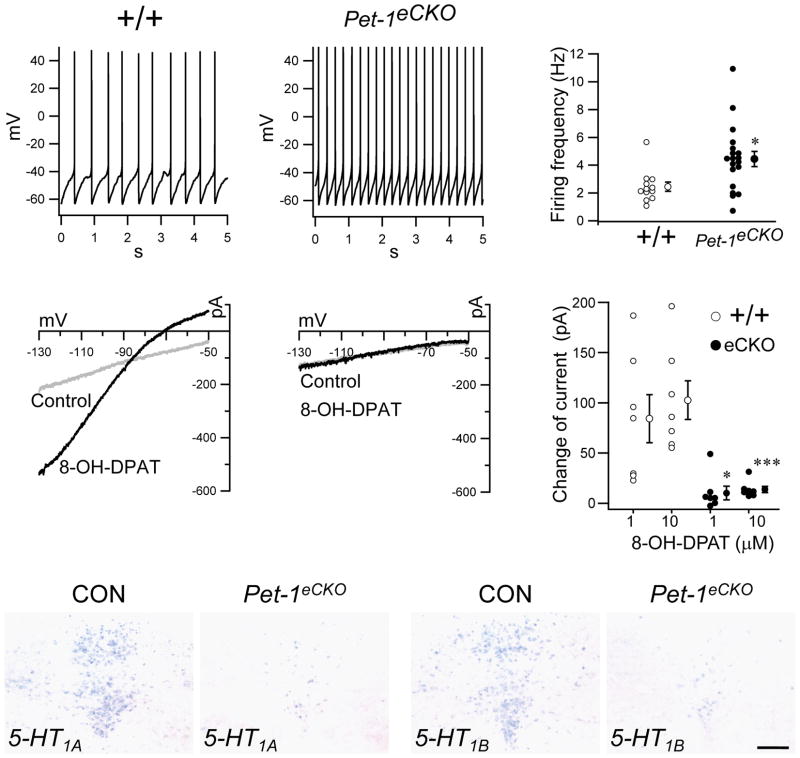
Another critical event in the maturation of brain 5-HT neuron function is the acquisition of 5-HT neuron specific firing properties 23. To study whether continued Pet-1 function is required for normal firing of 5-HT neurons, we analyzed Pet-1 deficient R26R-Yfp+ cells in postnatal brain slices from Pet-1eCKO mice with whole cell recordings under current clamp conditions. Compared with the aged-matched controls, many Pet-1 deficient cells demonstrated increased spontaneous firing of action potentials (Fig. 3a, b, c). The increased excitability could be due to alterations in 5-HT1A autoreceptor signaling that normally inhibits the firing of 5-HT neurons through negative feedback inhibition 24. As previously described 25, activation of 5-HT1A receptor with its specific agonist 8-OH-DPAT elicited strong inwardly rectifying potassium currents in control Yfp+ 5-HT neurons under voltage clamp (Fig. 3d, f). In striking contrast, 8-OH-DPAT at either low (1 μM) or high (10 μM) concentrations did not elicit a change in baseline currents in Pet-1 deficient Yfp+ 5-HT neurons (Fig. 3e, f). To investigate the mechanism that accounts for the loss of 8-OH-DPAT responses, we used in situ hybridization to examine the expression of the 5-HT1A receptor and found greatly reduced levels of 5- HT1A receptor mRNA in the Pet-1eCKO DRN (Fig. 3g, h).
Figure 3.
Continued Pet-1 function is required for 5-HT neuron firing properties and inhibitory autoreceptor function. (a, b) Whole cell current clamp recordings measuring spontaneous firing of Yfp+ neurons with indicated genotypes (c) Quantification of firing frequencies in (a, b), +/+, n=12; Pet-1eCKO, n=19; * p<0.05, two tailed t-test. (d, e) Whole cell voltage clamp recordings measuring current changes of Yfp+ neurons induced with either 1 or 10 μM of 5-HT1A agonist 8-OH-DPAT. A ramp voltage was applied at 200 mV/s. The intersection voltage, -87 mV, of the control and 8-OH-DPAT trace in (d) was close to the estimated K+ equilibrium potential (-99 mV), considering that recordings were not corrected for the liquid junction potential of around 10 mV. (f) Quantification of current changes at -110 mV in (d, e); * p<0.05, *** P<0.001; two tailed t-test (g–j) In situ hybridization of 5-HT1A (g, h) and 5-HT1B (i, j) in control and Pet-1eCKO mice. Scale bar is 200 μm. Error bar is mean ± s.e.m.
A second prominent serotonergic autoreceptor that functions in serotonergic presynaptic terminals for regulation of 5-HT release is the 5-HT1B receptor 26. In situ hybridization in Pet-1eCKO mice revealed that Pet-1 was also required for expression of the 5-HT1B gene (Fig. 3i, j). The residual expression of 5-HT1A and 5-HT1B receptors mRNAs is most likely from the remaining untargeted 5-HT neurons in Pet-1eCKO mice since the expression of the two autoreceptors in the DRN was almost completely abolished in the Pet-1−/−mice (Supplementary Fig. 5). Our in situ hybridization studies (data not shown) indicated that the onset of 5-HT1A and 5-HT1B autoreceptor gene expression occurs after embryonic day 14 in nearly all 5-HT neurons, which is consistent with their onset in the forebrain at E14.5 27. Thus, our findings show that ongoing Pet-1 expression is required, well after it has completed its role in the initiation of 5-HT synthesis in immature precursors, for maturation of essential serotonergic autoreceptor characteristics that controls firing patterns and transmitter release.
Gata3 is not required for 5-HT1A autoreceptor responses
We investigated whether the establishment of normal 5-HT neuron firing properties requires parallel ongoing activity of other serotonergic developmental control genes or Pet-1 plays a special role in this event. Germ line targeting of the zinc finger transcription factors, Gata2 and Gata3, has demonstrated requirements for both factors in 5-HT neuron differentiation 28. We found that Gata2 protein expression began to dramatically decline in differentiated 5-HT neurons at E12.5 and was not detectable at E14.5. In contrast, Gata3 expression persisted in all 5-HT neurons through adulthood (Supplementary Fig. 6).
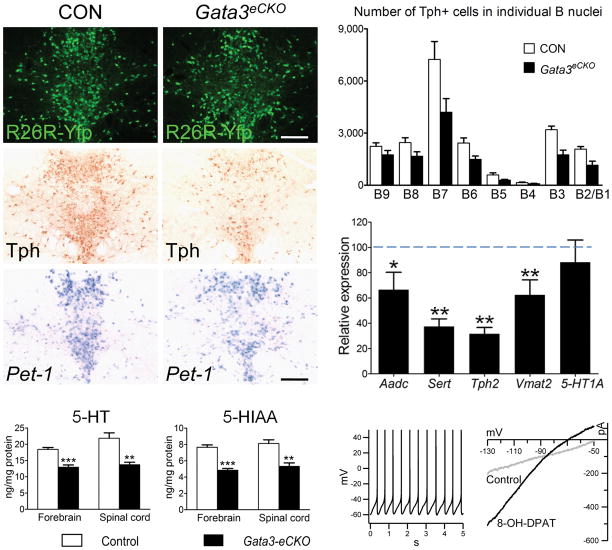
To compare the role of Gata3 in differentiated 5-HT neurons with that of Pet-1, we crossed floxed Gata3 mice 29 with ePet::Cre mice to generate Gata3 conditional knockout mice (Gata3fl/fl, ePet::Cre, designated as Gata3eCKO). Like Pet-1, Gata3 was not required for cell survival as normal numbers of Gata3 deficient 5-HT neurons survived in the adult brain (Fig. 4a). However, we did observe a substantial reduction in the number of Tph immunoreactive cells (Fig. 4b, d), 5-HT levels (Fig. 4f, g) and expression of several other 5-HT genes (Fig. 4e) in the Gata3eCKO DRN. Persistent Gata3 and Pet-1 expression appears to be maintained by independent regulatory pathways, as neither of them was required for each other’s expression (Fig. 4c and Fig.1i). These findings suggest that, Gata3 and Pet-1 function in parallel pathways to coordinate the expression of normal levels of serotonergic gene expression and 5-HT in the brain.
Figure 4.
Continued Gata3 expression is needed to maintain 5-HT gene expression but not autoreceptor function. (a) Yfp Immunostaining in adult DRN. (b) Tph immunostaining. (c) In situ hybridization of Pet-1 mRNA. (d)Counts of Tph+ cell bodies in control versus Gata3eCKO mice in individual adult B nuclei, n=3 for each genotype. (e) RT-qPCR of eCKO Aadc, Sert, Tph2, Vmat2, and 5-HT1A mRNAs in control versus Gata3 mice, control n=7, normalized to 100%; Gata3eCKO n=11, * p<0.05, ** p<0.01, two tailed t test. (f, g) HPLC analysis of 5-HT (f) and 5-HIAA (g) levels in forebrain and spinal cord of control (n=7) and Gata3eCKO (n=5) mice. ** p<0.01, *** p<0.001, two tailed t test. (h) Whole cell current clamp recordings of spontaneous firing of R26R-Yfp+ Gata3 deficient cells. (i) Whole cell voltage clamp recordings of current changes in response to 5-HT1A agonist, 8-OH-DPAT, in R26R-Yfp+ Gata3 deficient cells. Scale bars are 200 μm. Error bars represent s.e.m except for s.d in (d).
Although Gata3 and Pet-1 share several common transcriptional targets, Gata3 deficient 5-HT neurons demonstrated normal 5-HT1A expression (Fig. 4e) indicating distinct requirements for Pet-1 and Gata3 in the regulation of gene expression in 5-HT neurons. These findings further suggest that Gata3 may not be required for serotonergic firing characteristics. Indeed, whole cell recordings of slices from Gata3eCKO mice revealed firing properties and 5-HT1A agonist responses typical of wildtype 5-HT neurons (Fig. 4h, i).
Targeting of Pet-1 in the adult ascending 5-HT system
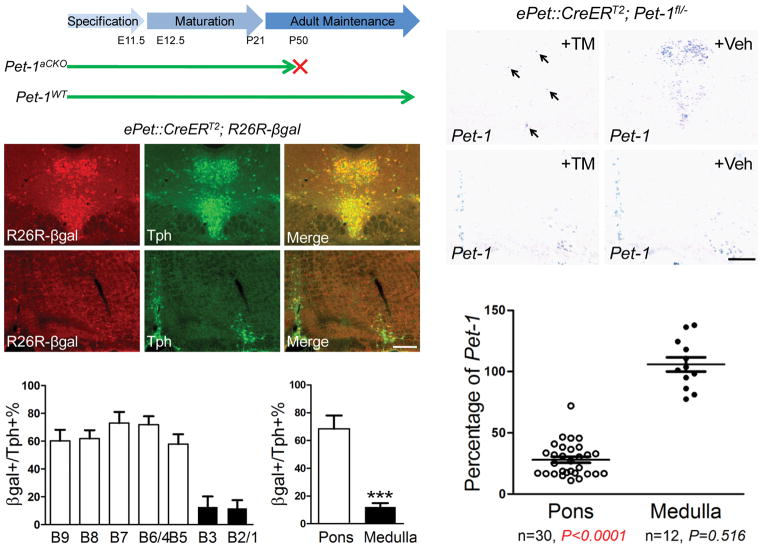
Having demonstrated that ongoing Pet-1 function is needed for multiple steps in 5-HT system maturation, we sought to determine whether a Pet-1-dependent transcriptional program still operates in adulthood to support serotonergic function and 5-HT modulated behaviors. Thus, we used Pet-1 regulatory elements to generate a transgene that directed tamoxifen (TM) inducible CreERT2 (ref 30) expression specifically in brain 5-HT neurons. We identified several different founder lines with inducible Cre activity by injecting pregnant females with a single dose of TM (150 μg/g, intraperitoneal, i.p.) at E11.5 and scoring at E16.5 for Cre-activated βgal expression from the R26Rβgal allele (Supplementary Fig. 7). Eight week-old Cre reporter mice (R26Rβgal+, ePet::CreERT2) were then given a single daily dose of TM or vehicle for five consecutive days. Expression of βgal, 5 or 30 days after the last TM treatment, was detected in the majority of 5-HT neurons in the DRN and median raphe nucleus (MRN). Recombination was strictly dependent upon TM treatment as no Cre activity could be detected in the absence of TM. Importantly, Cre activity was not detected in other regions of the CNS after TM injections (Supplementary Fig. 7). In one of the transgenic lines, designated ePet::CreERT2ascend, we found differential targeting efficacies following adult TM treatments between 5-HT neuron raphe nuclei that give rise to ascending and descending 5-HT systems (Fig. 5b, c). Double-labeling to detect β-galactosidase and Tph revealed TM-activated Cre activity in up to 80% of 5-HT neurons in the DRN (B6, B7), MRN (B5, B8), and B9 nucleus. In contrast, far fewer 5-HT neurons were β-galactosidase+ in the medullary nuclei (B1-B3) in response to TM treatments (Fig. 5d, e).
Figure 5.
Stage-specific disruption of Pet-1 in the adult ascending 5-HT system. (a) Adult stage specific deletion of Pet-1 in Pet-1aCKO mice. (b, c) Co-immunostaining of βgal and Tph in adult dorsal (b) and medullary (c) raphe. (d, e) Percentage of TPH+ cells expressing CreER activated βgal in individual adult B nuclei (d) and in 5-HT neurons of ascending versus descending pathways (e, 68.5±9.5% in the pons (B4-B9) and 12.1±6.8% in medullary nuclei (B1-B3). n=7, mean ± s.d, *** p<0.001, two tailed t test). (f–i) In situ hybridization of Pet-1 mRNA in coronal sections of adult Pet-1aCKO mice treated witheither TM or vehicle (Veh). (j) RT-qPCR of Pet-1 mRNA in TM-treated adult Pet-1aCKO mice (pons, n=30, 28.0±2.5% relative to control, n=35; medulla, n=12, 105.9±5.8% relative to the control, n=15). Each dot represents a sample from the indicated group, mean ± s.e.m, two tailed t test. Scale bars are 200 μm.
To determine the efficacy of the ePet::CreERT2ascend line for excision of Pet-1 in Pet-1fl/−mice, we crossed ePet::CreERT2ascend, Pet-1−/−and Pet-1fl/fl mice to generate Pet-1fl/−; ePet::CreERT2ascend mice, designated as Pet-1aCKO. Six to eight week-old Pet-1aCKO mice were given single daily TM treatments for 5 days and sacrificed either 5 or 30 days after TM treatments for evaluation of Pet-1 expression in the adult brain. Using in situ hybridization, we observed that TM treatment abolished the majority of Pet-1 expression in adult DRN (B6, B7 nuclei), MRN (B5, B8 nuclei) and the B9 group of 5-HT neurons (Fig. 5f, g). In contrast, Pet-1 mRNA was not decreased in the B1-B3 groups of 5-HT neurons in the ventral medulla (Fig. 5h, i). The reduction of Pet-1 in Pet-1aCKO(+TM) mice was further quantified with RT-qPCR, which revealed a more than 70% loss of Pet-1 mRNA in pontine tissue containing B5-B9 serotonergic nuclei, but no significant change in tissue containing the medullary B1-B3 nuclei (Fig. 5j). The loss of Pet-1 mRNA in B5-B9 nuclei demonstrated that ePet::CreERT2ascend could be used for highly reproducible and stage-specific disruption of Pet-1 expression in adult ascending 5-HT neurons.
Adult Pet-1 is required for normal anxiety-like behaviors
Germ line targeting of Pet-1 results in increased anxiety-like behaviors in the adult. It remains unknown, however, whether Pet-1 dependent transcription is only needed during development or is also required in adulthood to modulate normal anxiety responses. To address this question, we treated 6–8 week old Pet-1aCKO mice with TM to delete Pet-1 in the ascending 5-HT system. The impact of adult Pet-1 deletion on anxiety-related behaviors was then investigated 4 weeks after the last TM treatment.
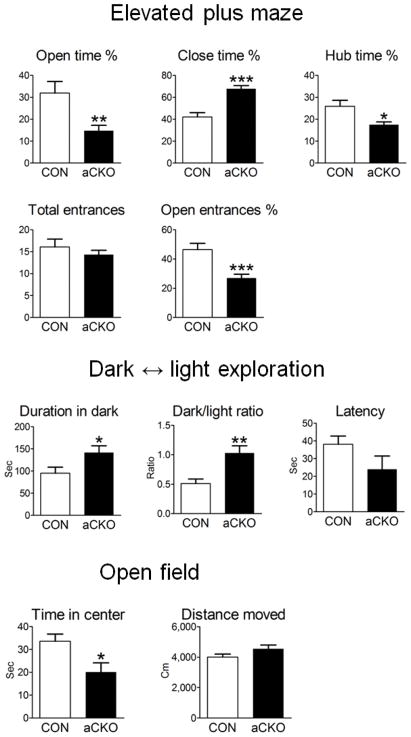
Using similar dosages, it was recently demonstrated in mice that multiple tamoxifen treatments have no effect in several anxiety-related behavioral tests 31. We verified this finding on a separate cohort of wild type mice treated with either vehicle or TM (Supplementary Fig. 8). We then examined TM-treated control and Pet-1aCKO mice with the elevated plus maze test and found that Pet-1aCKO(+TM) mice spent significantly less time and initiated fewer entries into the open unprotected arms of the maze (Fig. 6a, e). In addition, Pet-1aCKO mice spent less time in the hub area but significantly more time in the closed arm (Fig. 6b, c). No differences between genotypes were found in overall explorative activities determined as number of total open/closed arm entrances (Fig. 6d). The increased avoidance of the aversive properties of height and openness suggests an augmented anxiety-like behavior in Pet-1aCKO(+TM) mice. To further study this behavior, we tested the same mice in the light ↔ dark exploration paradigm, which presents the mice with a similar conflict between the curiosity to explore a novel environment versus the aversive features of a brightly illuminated open field. As compared to TM-treated littermate controls, Pet-1aCKO(+TM) mice spent significantly more time in the dark chamber (Fig. 6f, g) with a trend of reduced latency to enter the dark area from the beginning of the test (Fig. 6h). The increased time in the dark area and thus avoidance of the bright open areas further supported an increased anxiety-like behavior in Pet-1aCKO(+TM) mice. Finally, in further support of increased anxiety-like behavior, Pet-1aCKO(+TM) mice spent significantly less time (Fig. 6i) than controls in the center of an open field. Importantly, a second independent cohort of control and Pet-1aCKO mice showed similar significant increases in all three tests of anxiety-like behaviors in Pet-1aCKO(+TM) mice (Supplementary Fig. 9). Furthermore, the increased anxiety-like behavior seen in all three tests depended on reduced Pet-1 levels as no differences were observed in a separate cohort of Pet-1aCKO mice treated with vehicle (Supplementary Fig 10). Overall growth measured as body weight was similar in control and Pet-1aCKO mice following TM treatments (data not shown).
Figure 6.
Disruption of Pet-1-dependent transcription in the adult ascending 5-HT system causes elevated anxiety-like behavior. Six to eight-week-old male Pet-1aCKO mice (n=12) and their littermate controls (Pet-1fl/−, n=12) were treated with TM for 5 consecutive days and then acclimated for another 4 weeks before behavioral testing. (a–e) Elevated plus maze, * p<0.05, ** p<0.01, *** p<0.001; two tailed t test. (f–h) Dark ↔ light exploration, * p<0.05, ** p<0.01; two tailed t test. (i, j) Open field, * p<0.05; two tailed t test. Error bars represent s.e.m.
Adult Pet-1 is required for serotonergic gene expression
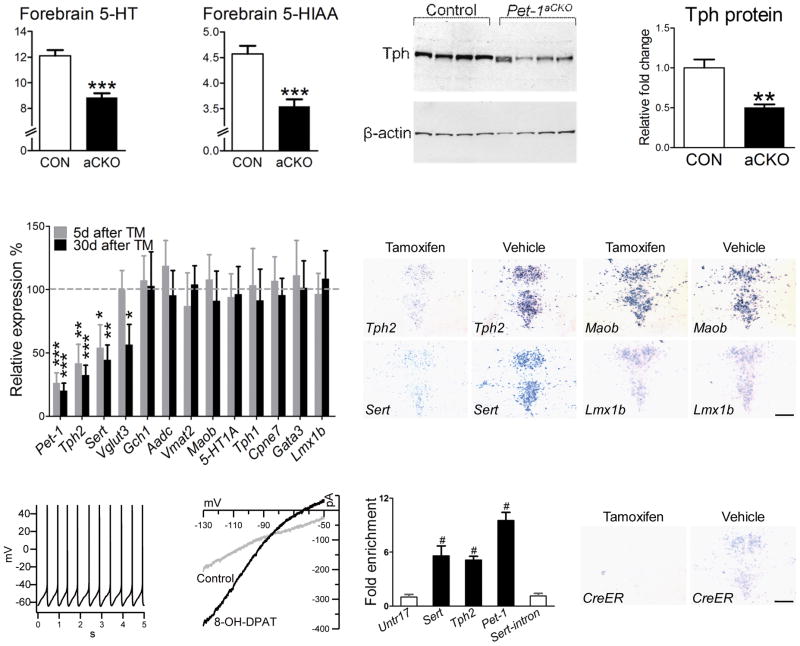
The altered anxiety-like behaviors in Pet-1aCKO(+TM) mice demonstrate that Pet-1 is required in adulthood to maintain serotonergic function. To explore the mechanisms underlying the alterations in serotonergic function in Pet-1aCKO(+TM) mice, we first measured levels of brain 5-HT and 5-HIAA in Pet-1aCKO and control mice sacrificed 5 days after TM treatments. HPLC analysis demonstrated that both 5-HT and 5-HIAA were significantly reduced in the forebrain of Pet-1aCKO(+TM) mice (Fig. 7a, b), but as predicted, 5-HT levels were not altered in the spinal cord (data not shown), which is innervated by the descending 5-HT system. Western blotting using a monoclonal antibody against both Tph1 and Tph2 indicated that Tph protein levels were reduced about 50% in Pet-1aCKO(+TM) mice relative to controls (Fig. 7c, d). Consistent with these findings, we found a comparable decrease in Tph2 mRNA levels in Pet-1aCKO(+TM) mice as early as five days after the last TM treatment (Fig. 7e, f). Further, Tph2 mRNA was still decreased 30 days after the last treatment and we did not find a compensatory increase in Tph1 expression with the loss of Tph2 (Fig. 7e). Together these findings indicate that Pet-1 is required in adult 5-HT neurons to regulate 5-HT synthesis through maintenance of Tph2 expression.
Figure 7.
5-HT synthesis and Sert expression is maintained in the adult ascending 5-HT system through positively autoregulated direct Pet-1 transactivation. (a, b) HPLC analysis of 5-HT and 5-HIAA levels in the forebrain of TM treated control (n=7) and Pet-1aCKO (n=7) mice, *** p<0.001, two tailed t test. (c, d) Western blotting analysis of Tph protein in DRN of TM treated control (n=8) and Pet-1aCKO mice (n=7, 50.3±4.0% relative to the control), ** p<0.01, two tailed test. (e) RT-qPCR analysis of serotonergic gene expression in Pet-1aCKO or control mice either 5 days (control, n=11; Pet-1aCKO, n=11) or 30 days (control n=14; Pet-1aCKO, n=16) after TM or vehicle treatments, * p<0.05, ** p<0.01, *** p<0.001. (f–i) In situ hybridization to detect Tph2, Sert, Maob, and Lmx1b mRNAs in coronal sections of Pet-1aCKO mice treated with either TM or vehicle. (j) Whole cell current clamp recordings of spontaneous firing of Yfp+ Pet-1 deficient cells in TM treated Pet-1aCKO mice. (k) Whole cell voltage clamp recordings of current changes in response to 5-HT1A agonist, 8-OH-DPAT, in Yfp+ Pet-1 deficient cells. (l) RT-qPCR analysis of chromatin immunoprecipitations. Values represent fold enrichment in binding to the indicated regions as compared to negative control region (Untr17). Untr, untranscribed genomic region; #, p<0.0001 for Tph2, Sert, Pet-1 versus Untr17 or Sert-intron, one-way ANOVA with Bonferroni's Multiple Comparison Test. (m, n) In situ hybridization to detect CreERT2 mRNA in adult Pet-1aCKO mice treated with either TM or vehicle. Scale bars are 200 μm. Error bars represent s.e.m except for s.d in (l).
To determine whether disruption of the 5-HT1A autoreceptor pathway might have contributed to the abnormal anxiety-like behavior, we performed whole cell recordings in Pet-1aCKO(+TM) slices but found that Pet-1 was no longer required in the adult brain for spontaneous firing, autoreceptor agonist responses (Fig. 7j, k), or 5-HT1A gene expression (Fig. 7e). To explore other potential deficits beyond the loss of 5-HT, we analyzed the set of additional genes that are known to depend on Pet-1 in the embryonic hindbrain as well as other genes that are important for 5-HT synthesis and metabolism (Fig. 7e, f–i). Sert and the vesicular glutamate transporter 3 (Vglut3 or Slc17a8) were also significantly reduced (Fig. 7e, h), but for Vglut3, decreased expression was not observed until 30 days after TM treatments (Fig. 7e). In contrast to the dramatic decreases in Tph2, Sert, and Vglut3 gene expression, Pet-1 was no longer required in adulthood to maintain mRNA levels for Aadc and Vmat2 whose embryonic expression is dependent on Pet-1 function (Fig. 7e).
Direct autoregulation of serotonergic gene expression
The studies presented so far do not distinguish direct vs. indirect transcriptional control of target genes by Pet-1. We previously identified a consensus Pet-1 binding sequence GGAART upstream of Sert and showed that Pet-1 protein interacted with these sites in vitro 14. Further analyses identified conserved putative Pet-1 ETS binding sites in highly conserved upstream regulatory regions of Tph2 and Sert genes (Supplementary Fig. 11). To probe the mechanism through which Pet-1 regulates Tph2 and Sert in 5-HT neurons, we investigated the possibility that Pet-1 directly regulates their transcription through interactions with conserved upstream regulatory elements.
Because we have been unsuccessful in preparing a suitable Pet-1 antibody for chromatin immunoprecipitation (ChIP), we generated a new transgenic mouse line that expresses a mycepitope tagged Pet-1 protein in the Pet-1−/−brain with Pet-1 promoter/enhancer sequences 15. We found that expression of the ePet::mycPet-1 transgene recapitulated endogenous Pet-1 expression in both developing and adult hindbrain resulting in the rescue of normal numbers of 5-HT neurons in the Pet-1−/− brain (Supplementary Fig. 12). We then used this rescue line for ChIP to determine whether Pet-1 directly interacted with Tph2 and Sert promoter sequences, in vivo. Chromatin was harvested from E12.5 mouse hindbrain before extensive 5-HT neuron dispersion began to scatter these cells, although the dissected tissue was still largely composed of non-serotonergic cells. Sheared chromatin was immunoprecipitated with an anti-myc antibody and analyzed by RT-qPCR for anti-myc enrichment of genomic fragments that included predicted Pet-1 binding sites upstream of Tph2 and Sert as well as in the intron of Sert (+11390). Compared to the control, no enrichment was detected near the Sert intron sequence. In contrast, several fold enrichment of upstream Tph2 and Sert sequences was observed compared to both negative control region untr17 and to the Sert intron sequence in two independent immunoprecipitation assays (Fig. 7l and data not shown).
Finally, we used the ePet::CreERT2ascend transgene as a reporter for Pet-1-dependent regulation of its own enhancer in Pet-1aCKO mice. We found CreERT2 expression in the adult DRN was significantly reduced in Pet-1aCKO mice treated with TM, but not in Pet-1aCKO mice treated with vehicle, thus demonstrating that adult expression of Pet-1 depends on positive autoregulation (Fig. 7m, n). Inspection of the upstream Pet-1 promoter/enhancer sequences 32 revealed conserved Pet-1 consensus binding sites at -465 and -621 relative to predicted transcription start site (Supplementary Fig.11). ChIP for genomic fragments with these binding sites revealed a ten-fold enrichment relative control immunoprecipitations (Fig. 7l and data not shown). These findings suggest that transcriptional regulation of 5-HT synthesis and serotonergic gene expression in adulthood depends on direct positive autoregulatory maintenance of Pet-1 expression.
Discussion
In this study, we tested the idea that Pet-1, a critical component of an embryonic transcriptional cascade that generates 5-HT neurons in the ventral hindbrain, continues to regulate subsequent milestones in 5-HT system maturation and 5-HT function in adulthood. We find that Pet-1 function is not restricted to the induction of serotonergic characteristics in embryonic 5-HT neuron precursors. Instead, our findings demonstrate that ongoing Pet-1 directed transcription is required across life span for multiple regulatory events that shape and maintain the serotonergic neurotransmitter system. A further conclusion supported by our findings is that the etiology of behavioral pathogenesis is not limited to dysfunction of the serotonergic system during development but may also result from adult onset alterations in serotonergic transcription.
The present findings together with our previously published studies 12 define three general but distinct stages of Pet-1 function. The initial stage occurs during serotonergic neurogenesis in which Pet-1 regulates a late phase of 5-HT neuron generation by coordinating the induction of key serotonergic genes required for transmitter synthesis, reuptake and vesicular transport in immature postmitotic precursors 12. Here, we uncovered a second stage of Pet-1 function using a conditional targeting approach that did not interfere with Pet-1 expression until about 2 days after the completion of serotonergic neurogenesis in the anterior hindbrain. This transcriptional stage coincides with the prolonged period of 5-HT neuron maturation when these cells must negotiate complex axonal growth and pathfinding decisions and are acquiring their characteristic firing properties. We identified multiple requirements for Pet-1 at this second stage, which showed that Pet-1 is essential for proper 5-HT system maturation. For example, retrograde tracing of R26R-Yfp-marked Pet-1 deficient 5-HT neurons revealed a substantial deficit in the number of serotonergic projections to the somatosensory cortex. However, initial serotonergic axon-like outgrowth did not appear compromised in Pet-1eCKO mice suggesting that Pet-1 dependent transcription regulates subsequent pathfinding decisions that help to build the ascending serotonergic system. The innervation defects in Pet-1eCKO mice were not likely contributed by the reduction of brain 5-HT. Although pharmacological disruption of embryonic 5-HT signaling alters neuronal organizations in the presubicular cortex 33 and 5-HT regulates thalamocortical axon pathfinding by modulating axonal responsiveness to guidance cues 5, recent studies of Tph2 targeted mice, which are devoid of brain 5-HT synthesis, do not detect defects in serotonergic innervation patterns 34.
We also identified a special role for continued Pet-1 directed transcription, relative to Gata3, in regulating the maturation of 5-HT neuron firing frequency through the control of 5-HT autoreceptor mediated inhibitory responses. Similar to the innervation defects, the defects in firing frequencies and autoreceptor mediated inhibitory responses were not likely caused simply by reduced 5-HT as 5-HT is also reduced in the Gata3eCKO brain. Instead, our findings showed that Pet-1 transcriptionally controls spontaneous firing frequency and inhibitory responses through regulation of 5- HT1A autoreceptor gene expression. We further found that Pet-1 was required for expression of the presynaptic 5-HT1B autoreceptor. Because expression of 5-HT1A and 5-HT1B do not normally occur until several days after 5-HT neuron generation, our findings strongly support the hypothesis that persistent Pet-1 directed transcription is essential for maturation steps during which 5-HT neurons acquire key functional characteristics.
We identified a third stage of Pet-1 function using a tamoxifen-inducible targeting approach that resulted in a severe and selective reduction of Pet-1 in the adult ascending 5-HT system. Significantly, the targeting of Pet-1 in adult 5-HT neurons revealed that this late stage of Pet-1 function is required in the adult ascending 5-HT system to maintain emotional behaviors. Our conclusion of altered emotional behavior in Pet-1aCKO(+TM) mice was supported with three different tests of rodent anxiety-related behavior performed on two independent cohorts of mice that were tested several months apart from one another. The simplest interpretation of our findings is that the accompanying reduction of 5-HT levels in the ascending serotonergic system accounted for the elevated anxiety in Pet-1aCKO(+TM) mice. However, the literature regarding the effect of adult neurotoxin-mediated depletion of 5-HT on anxiety in rats is conflicting with both anxiogenic and anxiolytic effects reported depending on experimental design 35–37. Furthermore, as Pet-1 is likely to control a complex network of downstream transcriptional targets, the increased anxiety-like behavior in Pet-1aCKO(+TM) mice may have resulted from multiple alterations in adult serotonergic function. Indeed, we found a reduction in Sert and Vglut3 gene expression, which suggests complex changes in the 5-HT neuron genetic network. Interestingly, a recent study demonstrated that at least part of the ascending 5-HT system engages in dual serotonergic/glutamatergic fast synaptic transmission 38. Reduced Vglut3 expression in Pet-1aCKO(+TM) mice was not observed until 30 days after the TM treatments and thus, adult loss of Pet-1 expression may have elicited gradual plasticity changes in glutamatergic transmission that contributed to the behavioral phenotype of Pet-1aCKO(+TM) mice.
The normal expression of 5-HT1A autoreceptor and 5-HT1A mediated inhibitory responses in Pet-1aCKO(+TM) mice indicates that the observed increased anxiety-like behaviors following adult deletion of Pet-1 were not due to deficiencies in 5-HT1A signaling pathway. This result is consistent with the findings that although germ line targeting of 5-HT1A leads to increased anxiety-related behaviors 39,40 reduced 5-HT1A autoreceptor signaling in adulthood does not 41. These findings, therefore suggest further that anxiety-like behavior in Pet-1aCKO(+TM) may be caused by a different process than that responsible for increased anxiety in Pet-1−/− mice. Nevertheless, our findings provide the first direct evidence in support of the concept that adult 5-HT-modulated behaviors are not hardwired during development but are transcriptionally regulated in the adult brain. Moreover, they highlight the potential importance of perturbations in serotonergic transcription at any stage of life in emotional pathogenesis.
Several characteristics of Pet-1 expression and function suggest it is a terminal selector gene analogous to the C. elegans ETS terminal selector gene, ast-1 that coordinates induction and maintenance of dopamine synthesis and transport in postmitotic neurons through a common conserved terminal selector motif 42. Consistent with the fundamental properties of a terminal selector gene, Pet-1 is expressed throughout the life of postmitotic 5-HT neurons and is required not only to determine serotonergic-type identity but also to maintain it. However, like a terminal selector gene, it is not required for generic neuronal identity. Further key features of Pet-1 function that fits with the terminal selector gene classification is that it directly regulates and maintains expression of terminal differentiation genes that define serotonergic-type identity and positively autoregulates its own expression all through conserved ETS binding motifs 43. It remains to be determined whether Pet-1 induces other transcription factors that then function cooperatively in a feedforward loop for control serotonergic-type identity.
Our findings raise the question of why is Pet-1 still needed in adult 5HT neurons for regulation of a subset of its known embryonic targets but not for others such as Aadc, Vmat2 and 5-HT1A genes. Adult CNS expression of Tph2 and Sert are restricted to Pet-1 expressing 5-HT neurons and are rate-limiting for the essential serotonergic functions of 5-HT synthesis and reuptake. Interestingly, expression of Tph2 and Sert in the adult DRN are regulated by external stimuli such as selective serotonin reuptake inhibitors and different stress paradigms 44,45. In addition, we showed earlier that the levels of Tph2 and Sert expression, in vivo, were sensitive to the levels of Pet-1 expression 46. We hypothesize (Supplementary Fig. 1) that environmentally induced alterations in Tph2 and Sert expression may be mediated through direct transcriptional activation by Pet-1, which itself is subject to extrinsic regulation 47, thus providing an efficient homeostatic transcriptional mechanism acting across life span to alter serotonergic function in response to environmental challenges.
Methods
Mice
Animal procedures used in this study were approved by the CWRU School of Medicine Institutional Animal Care in compliance with the National Institutes of Health guide for the care and use of laboratory animals.
Floxed Pet-1 mice
An eleven kb genomic fragment that included Pet-1 was subcloned into a targeting construct designed to insert loxP sites around exon 3. Several rounds of electroporation and G418 selection were performed on genetically modified R1 ES cells containing a protamine Cre transgene 48. A total of 176 colonies were isolated and screened by Southern blot analysis using an NcoI restriction digest and a 5′ external probe. Nine positive clones were identified and rescreened using a KpnI digestion and 3′ external probe. The 5′ probe hybridized to an 8.4 kb fragment in wildtype DNA and an 11.2 kb fragment in targeted DNA. The 3′ probe hybridized to an 8.0 kb fragment in wildtype DNA and a 10.6 kb fragment in targeted DNA. Two clones, i5h and i7c, were chosen for blastocyst injection. All resulting chimeras displayed germline transmission and were bred to mice of mixed 129Sv and C57BL/6 backgrounds. The F1 pups from male chimeras were screened for mice carrying either a floxed Pet-1 allele or a conditionally deleted Pet-1 allele using PCR genotyping with following primers p1: 5’-ACTCTGGCTTCCCTTTCTCC-3’; p2: 5’-ACTTGGAGGCCTTTTGCTCT-3’; p3: 5’-TAGGAGGGTCTGGTGTCTGG-3’; p4: 5’-GCGTCCTTGTGTGTAGCAGA-3’; p6: 5’-ATGCAAGAAGTTTCGGATGG-3’ as shown in Supplementary Figure 2.
ePet::CreERT2
DNA sequences encoding a fusion protein of Cre recombinase with a mutated estrogen receptor (CreERT2, a gift from Dr. Pierre Chambon via Dr. Susan Dymecki) were first subcloned into the pSG5 vector (Stratagene, Cedar Creek, TX) between the β-globin intron and the simian virus 40 polyadenylation sequences. The β-globin-intron/CreERT2 /poly (A) cassette was then released from the pSG5 vector and subcloned downstream of the β-globin minimal promoter in a modified BGZA vector in which LacZ was removed. The β-globin promoter/β-globin-intron/CreERT2 /poly (A) region was subcloned downstream of the 40 kb ePet genomic fragment present in the modified pBACe3.6 vector 15. The transgene was released from vector with an AscI digest and purified for pronuclear injections into hybrid c57B6/129 zygotes. Founders were identified by PCR with 5’-AAAATTTGCCTGCATTACCG-3’ and 5’-ATTCTCCCACCGTCAGTACG-3’ primers.
ePet::mycPet-1
DNA sequences encoding a myc tagged Pet-1 protein (gift from Dr. Qiufu Ma) as well as a simian virus 40 polyadenylation region were first subcloned downstream of the β-globin minimal promoter in a modified BGZA vector. The β-globin/Pet-1/poly (A) cassette was then released and subcloned downstream of ePet enhancer sequence present in pBACe3.6. The transgene was released from vector with an AscI digest and purified for pronuclear injections into Pet-1−/−fertilized eggs in a mixed C57BL/6 and 129 background. Founders were identified by PCR with 5’-GGGCCTATCCAAACTCAACTT-3’ and 5’-GGGAGGTGTGGGAGGTTTT-3’primers.
Histology
Fluorescent and Diaminobenzidine (DAB) immunohistochemistry were performed as described 46. The following primary antibodies were used: rabbit anit-5-HT (1:10,000, ImmunoStar, Hudson, WI), mouse anti-TPH (1:200, Sigma, St. Louis, MO), rabbit anti-GFP (1:1,000, Invitrogen, Carlsbad, CA), rabbit anti-Vmat2 (1:200, Millipore, Billerica, MA), goat anti-CHAT (1:200, Millipore, Billerica, MA), chicken anti-TH (1:100, Aves, Tigard, Oregon), mouse anti-NeuN (1:500, Millipore, Billerica, MA), mouse anti-GFAP (1:200, Imgenex, San Diego, CA), rabbit anti-β-galactosidase (1:5,000, MP Biomedicals, Solon, OH), rabbit anti-Cre (1:500, Covance, Princeton, NJ), Mouse anti-myc (9E10, Sigma).Secondary antibodies including FITC, TexRed, and Cyanine3 (1:200) were from Jackson ImmunoResearch (West Grove, PA). Fluorescent and bright field images were collected using a SPOT RT color digital camera (Diagnostic Instruments, Sterling Heights, MI) attached to an Olympus Optical BX51 microscope (Center Valley, PA). Confocal images were taken on a Zeiss LSM 510 confocal laser microscope.
Retrograde Tracing
Six wildtype (ePet::Yfp+) and six Pet-1eCKO mice (~P22) were deeply anesthetized by 1.5% isoflurane in the air flow. Mice were placed into a stereotaxic frame and a small opening was made in the skull directly over the injection site (−0.5mm, 3mm, 0.5mm from bregma). Coordinates for stereotaxic injections were obtained from the Paxinos mouse brain atlas. About 1 μl of Texas Red conjugated dextran (5% diluted in 0.5XPBS, 3000 Mw, Invitrogen, Carlsbad, CA) was pressure injected into the somatosensory barrel cortex using a Hamilton syringe. After injection, animals were allowed to survive for another 3 days before being sacrificed for histology.
In Situ Hybridization
Gene specific DNA oligonucleotide primers (Supplementary Table 1) were designed to amplify ~600bp fragments using cDNA synthesized from adult raphe mRNA. Forward and reverse primers contained bacteriophage T7 or T3 promoter sequences at 5’ ends so that PCR products could be directly used as templates to synthesize digoxigenin (Roche, Burlington, NC) labeled sense and antisense riboprobes. In situ hybridization was performed using previously published lab protocols 14.
Western Blot Analysis
Mice were sacrificed thirty days after the last TM treatment. DRN tissue was dissected and homogenized in RIPA buffer containing 1x proteinase inhibitor (Sigma, St. Louis, MO). Proteins were quantified using the BCA Protein Assay Kit (Pierce, Rockford, IL). Seven micrograms of each protein extract was separated by 10% SDS-PAGE (BioRad, Hercules, CA) and then transferred to a 0.45 μm nitrocellulose membrane (BioRad, Hercules, CA). Antibodies used were a monoclonal anti-TPH antibody (1:2000, Sigma), an HRP conjugated anti-mouse secondary antibody (1:2000, Cell Signaling Technology, Beverly, MA), and an anti-beta actin (1:3000, Millipore, Billerica, MA). The film was developed and then scanned on a HP Scanjet 8200. The mean band density was measured using ImageJ (http://rsb.info.nih.gov/ij).
Tamoxifen Preparation and Treatment
Tamoxifen (Sigma, St. Louis, MO) was dissolved in corn oil at 20 mg/ml according to Joyner Lab’s protocol http://www.mskcc.org/mskcc/html/77387.cfm. For TM treatment in the embryo, one single dose of TM (150 μg/g body weight) either by i.p. injection or oral gavage was given to the mother at E11.5. For treatment in adulthood, 5 single daily doses of TM (150 μg/g body weight) were given to adult mice by i.p. injections.
Electrophysiology
Coronal slices including dorsal raphe (250 μm thick) were cut from brainstem of Pet-1eCKO , Pet-1aCKO(+TM), and control mice aged 3–5 weeks postnatally. Mice were anesthetized with isoflurane and decapitated. The brainstem was cooled and sliced in ice cold solution containing (in mM) 87, NaCl; 75, sucrose; 2.5, KCl; 0.5, CaCl2; 7, MgCl2; 1.25, NaH2PO4; 25, NaHCO3; and 20, glucose bubbled with 95% O2 and 5% CO2 using a vibratome (VT1000S, Leica). Slices were stored for at least 1 hour at room temperature in recording artificial cerebrospinal fluid containing (in mM) 124, NaCl; 3, KCl; 2.5, CaCl2; 1.2, MgSO4; 1.23, NaH2PO4; 26, NaHCO3, and 10, glucose bubbled with 95% O2 and 5% CO2. YFP+ cells were visually identified under an upright microscope (DMLFSA, Leica) equipped with a monochromator system (Polychrome IV, TILL Photonics). Whole-cell recordings were made from the cells in the dorsomedial subregion of the B7 DRN. During recordings, slices were continuously perfused with the external solution containing 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX), 20 μM D-(-)-2-Amino-5-phosphonopentanoic acid (D-AP5) and 20 μM picrotoxin at room temperature. Patch pipettes (2–4 MΩ) were filled with an internal solution with the following composition (in mM) 140, K-methylsulfate; 4, NaCl; 10, HEPES; 0.2, EGTA; 4, Mg-ATP; 0.3, Na-GTP and 10, Tris-phosphocreatine (pH 7.3, adjusted with KOH). Membrane currents or voltages were recorded with an EPC10/2 amplifier (HEKA). The signals were filtered at 3 kHz and digitized at 50 kHz. The PatchMaster software (HEKA) was used for control of voltage and data acquisition. Offline analysis was performed with Igor Pro software (Wavemetrics). 5-HT1A agonist, (±)-8-Hydroxy-2-dipropylaminotetralin hydrobromide (8-OH-DPAT) was purchased from Tocris and bath-applied to slices.
Quantitative Real-time PCR
Mice were anesthetized with Avertin (0.5 g tribromoethanol/39.5 ml H20, 0.02 ml/g body weight) and sacrificed by rapid decapitation. Brains were dissected and placed in RNase-free tissue culture plates. A sterile razor blade was used to cut a transverse section at Bregma area -2.92 mm and then again at Bregma area -5.46 mm to isolate the area containing the DRN and MRN. The tissue was placed immediately in Trizol (Invitrogen, Carlsbad, CA) and RNA was extracted according to the manufacturer’s manual. Genomic DNA was removed by DNase I treatment (Roche, Burlington, NC) and 1 μg RNA was used for first-strand cDNA synthesis (Invitrogen, Carlsbad, CA). For real-time RT-qPCR, a SYBR green detection system (Molecular Probes, Eugene, OR), fluorescein calibration dye (Bio-Rad, Hercules, CA), Platinum Taq (Invitrogen, Carlsbad, CA), specific primers (Supplementary Table 2), and 2 μl of undiluted cDNA were used in 20 μl PCR reactions. Each reaction was performed in triplicate. All real time RT-PCR reactions were performed in 40 cycles on the iCycler (Bio-Rad, Hercules, CA). Relative gene expression and statistics analysis were determined using Relative Expression Software Tool (http://www.gene-quantification.de/rest-paper.html).
HPLC Analysis
Tissues were collected as described 46. HPLC analysis was performed by the Neurochemistry Core Lab at Vanderbilt University, Center for Molecular Neuroscience, Nashville, TN.
Sequence Analysis
Three kilobases upstream of predicted the human and mouse Tph2, Sert, and Pet-1 transcription start sites were compared using ECR browser tool (http://ecrbrowser.dcode.org/) as previously described 19. The minimum criterion for significant sequence conservation was 70% identity over 100 bp. Gene annotation information was derived from NCBI (Pet-1, GeneID, 260298; Tph2, GeneID, 216343; Sert (Slc6a4), GeneID, 15567). Predicted conserved Pet-1 consensus binding sites (GGAAR(T)) were identified using rVista 2.0 (http://rvista.dcode.org/).
Chromatin Immunoprecipitation (ChIP) Assays
Hindbrain tissue from the mesencephalic flexure to the cervical flexure was removed from 56 E12.5 ePet::mycPet-1 transgenic embryos and quickly frozen on dry ice. MycPet-1 occupancy of genomic regions was tested by GenPathway, Inc. (San Diego), using goat anti-Myc antibody (Abcam ab9132) and quantitative PCR (qPCR) according to their protocols. Binding was tested in triplicate for the negative control region (untranscribed genomic region Untr17) and regions in or near predicted Pet-1 binding sites. Data are expressed as fold enrichment for each sample relative to binding at Untr17. Differences in binding among regions were calculated using one-way ANOVA with Bonferroni's Multiple Comparison Test (Prism 5.0, GraphPad Software, La Jolla, CA). Replication of the entire assay gave similar results. Sequences of primers used for qPCR and their positions relative to the predicted conserved Pet-1 binding sites for each of the test genes are shown below. Primers sequences are underlined. Pet-1 binding sites are shown in bold and italic characters.
Tph2
TTTCCTGTGGCTTTCTAAAGTTGGAAAAGTACAAATATAATCTTGTCTATGCCTGTCAAATTGCTGGGTCTGATCAGGTCATAGATGGAGAGCAATAAAATTGTATCAGAAGAGTATCAAAGGAATGATGGGCCTATGGGCATTTCATTTCC
Sert
CCCCTTCTTTCCGCTCTATCTTGATTAGCTAGGTCAGCCTCAGGTGGTTGCTGGGGAGATTCCAGGCCTACTGTGGTGGACATCCGAAACAAGAGATTCCCTGAGAGGGAGGGGTGTGGTAGCCATTTCCTGGGCCTAAGAAGAAGCCCACAAGGAAGGGAGAGCTTCCTCTTCTGTCACGGTGTAAACAGAACACAGGCAGACAGACAGATGGCACCGAGAGCTTCC
Sert-intron
CATCCTCAGTCCAGAAGAGAGAGCCCAGCTCCTTCCCTGTGCCCCGTCCCGGCAGTGAAATGAAGGTACAGCCT
Pet-1
GGAAACCAGGAAATCGAGGAGGGGATGGGTCTCTAGGGACCTAAAGAGAGTAGGAAAAAAGGAGGGAGAAGGCACGGGGGTGGGCAAAGATAAAGGGAGCCACGGCAGCGCGGTAGCGCGGCTGGGAGCGCAGCGACAGGCGAGAGGGAGGGAAGCGGAA AT
Behavioral Tests
All tests were carried out in the Case Western Reserve University Rodent Behavior Core. Six to eight week-old Pet-1aCKO (ePet::CreERT2; Pet-1fl/−) and littermate controls (Pet-1fl/−) were treated with TM for 5 consecutive days. After the last TM injection, mice were rested for another 4 weeks prior to testing with access to food and water ad libitum. All tests were performed during the light cycle between 10:30 am and 6:00 pm. Equipment was cleaned thoroughly with 70% ethanol between each test to remove odor cues. The elevated plus maze test was conducted first due to its sensitivity to prior experience. Individual tests were performed at least 48 hours apart from one another. The tester was blind to group identification. Cohort 1 was tested in autumn and Cohort 2 in spring.
Elevated Plus Maze
The elevated plus maze, equipped with infrared grid and video tracking system (Med Associates Inc, St. Albans, VA), was ~1 m high and consisted of two open and two closed arms forming the shape of a cross. Mice were placed in the center of the maze facing the open arm and their activity was recorded for five minutes. Total time spent in the open arm, closed arm, hub, and number of entries into each arm were measured. We did not observe differences in frequency of defecation, urination and head dips between control and Pet-1aCKO(+TM) mice.
Light ↔ Dark exploration
The light/dark box consists of two square dark gray chambers. The lit open chamber (20X20 cm) was illuminated with a 100 W light 40 cm above the chamber floor and the “dark” chamber (15X15 cm) was entirely enclosed with a solid black plastic top. Mice were placed in the open chamber, facing away from the dark side, and their exploration pattern was tracked for 5 minutes. Latency to cross over into the dark chamber and total duration in light was scored. We did not detect differences in the number of re-entries into the illuminated chamber between control and Pet-1aCKO(+TM) mice.
Open field
The open field consisted of a 40 cm x 40 cm box located in a dimly lit room. Using EthoVision XT 5.0 (Noldus, Leesburg, VA), the area was digitally subdivided into a 20 cm x 20 cm center area and a peripheral area. The peripheral area was also divided into middle (inner 10 cm) and an outer area (outer 10 cm) to determine thigmotaxic behavior. Animals were placed in the open field and allowed to explore the enclosure freely for 15 minutes. During this period locomotor parameters such as total distance moved, velocity, angular velocity, and heading degrees were measured to determine basic locomotor activity and presence of stereotypies. Frequency and duration in the center, periphery and outer quadrants were collected to determine anxiety-like behavior. Additionally, data was nested into 5 minutes bins and distance moved during each of these 3 periods was recorded to evaluate habituation differences across groups.
Statistics
All statistical measures on normally distributed data were done using either a two tailed t test between the control and mutant mice or one – way ANOVA with Bonferroni’s multiple comparison test to compare means between all combinations of groups. Statistical analysis in RT-qPCR experiment was carried out by using Pair Wise Fixed Reallocation Randomization Test (http://www.gene-quantification.de/rest-paper.html).
Supplementary Material
Acknowledgments
We thank Dr. Lynn Landmesser for helpful suggestions on retrograde tracing; Drs. Susan Dymecki and Pierre Chambon for CreERT2 plasmids; Dr. Qiufu Ma for the mycPet-1 vector; Dr. Jingfang Zhu for the floxed Gata3 mice; Katherine Lobur for outstanding assistance with genotyping of mice and Jennifer Reeves for behavioral testing in the Case Rodent Behavior Core. We thank Drs. Landmesser, Maricich, and Silver for helpful comments on the manuscript. This work was supported by MH062723 and MH078028 to E.S.D.
Footnotes
Author Contributions
E.S.D conceived the project. C.L. made the transgenic and targeting constructs, characterized all new mouse lines, generated all histological, RT-PCR, and retrograde tracing data and images. C.L. and S.C.W. performed western blot analyses. C.L. and G.C. performed behavioral analyses. T. M. and S.H. generated the electrophysiology data. C.L., S.H., T.M., G.C., S. C. W., and E.S.D. analyzed the data. E.S.D and C.L. designed the experiments and wrote the manuscript.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008 doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders. Current opinion in pharmacology. 2007;7:8–17. doi: 10.1016/j.coph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Vitalis T, Cases O, Passemard S, Callebert J, Parnavelas JG. Embryonic depletion of serotonin affects cortical development. Eur J Neurosci. 2007;26 :331–344. doi: 10.1111/j.1460-9568.2007.05661.x. [DOI] [PubMed] [Google Scholar]
- 4.Salichon N, et al. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- 6.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 7.Maciag D, et al. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross C, et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- 9.Jans LA, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12:522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- 10.Scott MM, Deneris ES. Making and breaking serotonin neurons and autism. Int J Dev Neurosci. 2005;23:277–285. doi: 10.1016/j.ijdevneu.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Cordes SP. Molecular genetics of the early development of hindbrain serotonergic neurons. Clinical genetics. 2005;68:487–494. doi: 10.1111/j.1399-0004.2005.00534.x. [DOI] [PubMed] [Google Scholar]
- 12.Hendricks TJ, et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- 13.Dai JX, et al. Enhanced contextual fear memory in central serotonin-deficient mice. Proc Natl Acad Sci U S A. 2008;105:11981–11986. doi: 10.1073/pnas.0801329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendricks T, Francis N, Fyodorov D, Deneris E. The ETS Domain Factor Pet-1 is an Early and Precise Marker of Central 5-HT Neurons and Interacts with a Conserved Element in Serotonergic Genes. J Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott MM, et al. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci, USA. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob J, et al. Transcriptional repression coordinates the temporal switch from motor to serotonergic neurogenesis. Nat Neurosci. 2007;10:1433–1439. doi: 10.1038/nn1985. [DOI] [PubMed] [Google Scholar]
- 17.Pattyn A, et al. Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev. 2003;17:729–737. doi: 10.1101/gad.255803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao ZQ, et al. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krueger KC, Deneris ES. Serotonergic transcription of human FEV reveals direct GATA factor interactions and fate of Pet-1-deficient serotonin neuron precursors. J Neurosci. 2008;28:12748–12758. doi: 10.1523/JNEUROSCI.4349-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lidov HG, Molliver ME. An immunohistochemical study of serotonin neuron development in the rat: ascending pathways and terminal fields. Brain Res Bull. 1982;8:389–430. doi: 10.1016/0361-9230(82)90077-6. [DOI] [PubMed] [Google Scholar]
- 21.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- 23.Le Francois B, Czesak M, Steubl D, Albert PR. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008;55 :977–985. doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 24.Fornal CA, et al. Single-unit responses of serotonergic dorsal raphe neurons to 5-HT1A agonist and antagonist drug administration in behaving cats. J Pharmacol Exp Ther. 1994;270:1345–1358. [PubMed] [Google Scholar]
- 25.Bayliss DA, Li YW, Talley EM. Effects of serotonin on caudal raphe neurons: activation of an inwardly rectifying potassium conductance. J Neurophysiol. 1997;77:1349–1361. doi: 10.1152/jn.1997.77.3.1349. [DOI] [PubMed] [Google Scholar]
- 26.Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Bonnin A, Peng W, Hewlett W, Levitt P. Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience. 2006;141:781–794. doi: 10.1016/j.neuroscience.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 28.Craven SE, et al. Gata2 specifies serotonergic neurons downstream of sonic hedgehog. Development. 2004;131:1165–1173. doi: 10.1242/dev.01024. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 30.Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 31.Vogt MA, et al. Suitability of tamoxifen-induced mutagenesis for behavioral phenotyping. Exp Neurol. 2008;211:25–33. doi: 10.1016/j.expneurol.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Scott MM, Krueger KC, Deneris ES. A differentially autoregulated Pet-1 enhancer region is a critical target of the transcriptional cascade that governs serotonin neuron development. J Neurosci. 2005;25:2628–2636. doi: 10.1523/JNEUROSCI.4979-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janusonis S, Gluncic V, Rakic P. Early serotonergic projections to Cajal-Retzius cells: relevance for cortical development. J Neurosci. 2004;24:1652–1659. doi: 10.1523/JNEUROSCI.4651-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutknecht L, et al. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J Neural Transm. 2008;115:1127–1132. doi: 10.1007/s00702-008-0096-6. [DOI] [PubMed] [Google Scholar]
- 35.Ludwig V, Schwarting RK. Neurochemical and behavioral consequences of striatal injection of 5,7-dihydroxytryptamine. J Neurosci Methods. 2007;162:108–118. doi: 10.1016/j.jneumeth.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Pum ME, Huston JP, Muller CP. The role of cortical serotonin in anxiety and locomotor activity in Wistar rats. Behav Neurosci. 2009;123:449–454. doi: 10.1037/a0014478. [DOI] [PubMed] [Google Scholar]
- 37.Sommer W, et al. Local 5,7-dihydroxytryptamine lesions of rat amygdala: release of punished drinking, unaffected plus-maze behavior and ethanol consumption. Neuropsychopharmacology. 2001;24:430–440. doi: 10.1016/S0893-133X(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 38.Varga V, et al. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–453. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- 39.Heisler LK, et al. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramboz S, et al. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson-Jones JW, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flames N, Hobert O. Gene regulatory logic of dopamine neuron differentiation. Nature. 2009;458:885–889. doi: 10.1038/nature07929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hobert O. Regulatory logic of neuronal diversity: terminal selector genes and selector motifs. Proc Natl Acad Sci U S A. 2008;105:20067–20071. doi: 10.1073/pnas.0806070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardner KL, Hale MW, Lightman SL, Plotsky PM, Lowry CA. Adverse early life experience and social stress during adulthood interact to increase serotonin transporter mRNA expression. Brain Res. 2009;1305:47–63. doi: 10.1016/j.brainres.2009.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shishkina GT, Kalinina TS, Dygalo NN. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience. 2007;150:404–412. doi: 10.1016/j.neuroscience.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci. 2008;11:1001–1003. doi: 10.1038/nn.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rivera HM, Oberbeck DR, Kwon B, Houpt TA, Eckel LA. Estradiol increases Pet-1 and serotonin transporter mRNA in the midbrain raphe nuclei of ovariectomized rats. Brain Res. 2009;1259:51–58. doi: 10.1016/j.brainres.2008.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci U S A. 1997;94:14602–14607. doi: 10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.