Abstract
The ErbB4 receptor tyrosine kinase is expressed at high levels in human and mouse colitis, and inhibits colon epithelial cell apoptosis in the presence of pro-inflammatory cytokines. In this study, we investigated the molecular mechanisms responsible for ErbB4-induced cell survival. In cultured mouse colon epithelial cells, ErbB4 overexpression resulted in increased levels of cyclooxygenase-2 (COX-2) mRNA and protein; in contrast, ErbB4 knockdown with siRNA blocked COX-2 accumulation in response to tumor necrosis factor. While ErbB4 is expressed as up to four different isoforms in epithelial tissues, its ability to promote COX-2 expression was isoform-independent. ErbB4-stimulated COX-2 induction was associated with an increase in mRNA half-life and was blocked by inhibition of Src, phosphatidylinositol 3-kinase, or epidermal growth factor (EGF) receptor (R). Furthermore, ErbB4 expression promoted EGFR phosphorylation in the presence of heregulin, implicating ErbB4-EGFR heterodimerization in these responses. With regard to the cellular responses to ErbB4 activation, increased survival of ErbB4-expressing cells in the presence of pro-inflammatory cytokines was sensitive to the COX-2 inhibitor celecoxib. Furthermore, ErbB4-overexpressing cells acquired the ability to form colonies in soft agar, indicative of cellular transformation, also in a celecoxib-sensitive manner. Together our data indicate that ErbB4 is a key regulator of COX-2 expression and cellular survival in colon epithelial cells, acting in concert with EGFR through a Src and phosphatidylinositol 3-kinase dependent mechanism. These results suggest that chronic overexpression of ErbB4 in the context of inflammation could contribute to colitis-associated tumorigenesis by inhibiting colonocyte apoptosis.
Keywords: Cell survival, colon epithelial cells, cyclooxygenase-2, ErbB4, inflammatory bowel diseases, receptor tyrosine kinases
The ErbB4 receptor tyrosine kinase is a member of the epidermal growth factor receptor (EGFR)-related ErbB family, along with EGFR/ErbB1, HER2/ErbB2, and HER3/ErbB3. It is broadly expressed in fetal and adult mammalian tissues (1), and following ligand binding becomes active either as homodimers, heterodimers with other ErbBs, or as a constitutively active intracellular domain proteolytic cleavage product (2). ErbB4 expression and activity have been linked to a variety of cellular processes including differentiation (3, 4), cell survival (5), migration (6), proliferation (3, 7), growth arrest (8), and tumorigenesis (9, 10) in different tissues. However, only limited data are available on the intracellular signaling pathways involved in these responses. ErbB4 has several unusual biochemical properties—the ability to bind both heregulin/neuregulin growth factors and a subset of EGFR ligands (11), the expression and metalloproteinase processing of up to four alternatively spliced isoforms (6, 8), and association with a more restricted suite of SH2-containing targets than ErbB1-3 (12)—which make it unique both in terms of its signaling and its possible role in disease. Further characterization of the molecular pathways downstream of ErbB4 activation will be key to advancing understanding of the receptor’s biology.
ErbB4 is expressed at elevated levels in the inflamed colonic mucosa of Crohn’s disease patients, is induced in cultured cells by the potent inflammatory cytokine tumor necrosis factor (TNF), and promotes survival of cultured colon epithelial cells in a phosphatidylinositol (PI) 3-kinase dependent manner (13). Furthermore, ErbB4 deletion from colorectal cancer cells promotes apoptosis (14). Together, these observations suggest that ErbB4 is a key regulator of colonocyte survival in inflammation and tumorigenesis, though the underlying signaling pathways remain undetermined.
Cyclooxygenase-2 (COX-2), the inducible form of the mammalian prostaglandin synthase, catalyzes the production of prostaglandins from arachidonic acid, simultaneously modulating inflammatory responses (15) and promoting intestinal epithelial cell survival (16, 17). COX-2 is present at high levels during inflammation and the response to injury in a number of tissues, including the digestive tract (18). It has also been identified as a key participant in colorectal carcinogenesis (19, 20). A better understanding of the cellular mechanisms regulating COX-2 expression and activity is likely to open new therapeutic avenues for both inflammatory diseases and cancer.
In this study, we tested the hypothesis that COX-2 is a target of ErbB4 signaling. We report that ErbB4 overexpression in mouse colon epithelial cells resulted in elevated COX-2 expression. This effect was associated with increased COX-2 mRNA half-life and was blocked by EGFR, Src, or PI 3-kinase inhibition. Additionally, ErbB4 expression in these cells promoted cell survival and anchorage-independent growth which were sensitive to the COX-2 inhibitor celecoxib, and ErbB4 knockdown from colon cancer cells resulted in decreased COX-2 expression and increased sensitivity to TNF-induced cell death. Together these results suggest that ErbB4 promotes colonocyte survival through COX-2 expression, and that this pathway may represent a useful therapeutic target for inflammatory disorders and cancer.
Materials and Methods
Cell culture
The non-transformed, conditionally immortalized young adult mouse colon (YAMC) epithelial cell line was provided by Dr. Robert Whitehead and the Vanderbilt Digestive Diseases Research Center Novel Cell Line Core (21). These cells express a temperature-sensitive SV40 Large T antigen which confers conditional immortalization, but not transformation, under permissive conditions [33 °C in RPMI 1640 with 5% FBS, 5 units/ml mouse interferon-γ (Intergen, Norcross, GA), 100 U/ml penicillin and streptomycin, 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenous acid (BD Biosciences, San Jose, CA)]. YAMC cells express low but detectable levels of ErbB4 in the resting state (13). Parental YAMC and ErbB4-infected YAMC cell lines were maintained under permissive conditions and transferred to nonpermissive conditions (RPMI 1640 containing 0.5% FBS, streptomycin and penicillin without IFN-γ, insulin, transferrin, or selenous acid, at 37 °C) for 24h before signaling experiments. LIM 2405 human colon cancer cells were also provided by Dr. Whitehead (22) and were grown in RPMI 1640 plus 5% FBS, 100 U/ml penicillin and streptomycin, 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenous acid.
Transfections and constructs
pCDNA3.1-ErbB4 (JM-a/CYT-1, JM-a/CYT-2, JM-b/CYT-1, and JM-b/CYT-2 isoforms) expression vectors were kindly provided by Graham Carpenter (Vanderbilt University). Inserts from these constructs were PCR amplified (primers: 5′-ATGGCGATCGCATGAAGCCGGCGACAGGACTTTG-3′, Sgf I site; 5′-TTGGGCCGGACCGGCCTTACACCACAGTATTCCGGTG-3′, Sfi I site) then cut with Sfi I and Sgf I and ligated into linearized bicistronic LZRS-GFP vector (Albert Reynolds, Vanderbilt). Recombinant plasmids were screened by Sfi I/Sgf I digestion. Phoenix packaging cells (Steve Hanks, Vanderbilt) were transiently transfected with LZRS-GFP or LZRS-ErbB4-GFP and YAMC cells were subjected to 5 rounds of infection with filtered supernatant supplemented with 4 μg/ml polybrene. Infected populations were expanded and GFP-positive cells were sorted at the Vanderbilt Medical Center Flow Cytometry Shared Resource using a Becton-Dickinson FACSAria; top 20% GFP-expressing cells were maintained as pools.
Non-targeting control and ErbB4-specific siRNA pools were purchased from Dharmacon (Lafayette, CO) and transfected into YAMC cells (100 nM siRNA) with Lipofectamine RNAiMax (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol. Control and ErbB4-targeting lentiviral shRNA particles were purchased from Santa Cruz and introduced to LIM 2405 cells according to the manufacturer’s instructions.
Soft agar colony formation
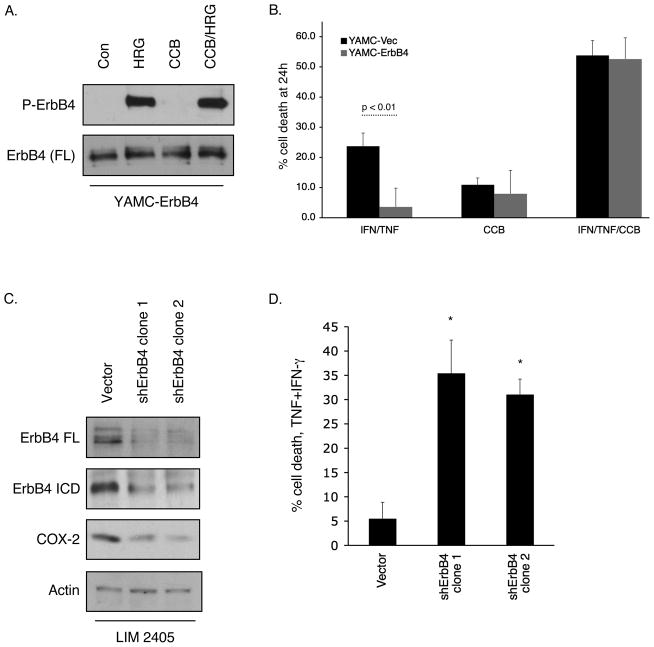
YAMC cell pools expressing LZRS-GFP (YAMC-Vec) and cells expressing LZRS-ErbB4 [JM-b/CYT-2]-GFP (YAMC-ErbB4) were embedded in a 0.35% Noble agar (Sigma)/growth medium gel overlaid on a 0.5% agar/growth medium support. The ErbB4 ligand heregulin-1β (HRG; 100 ng/ml) or the COX-2 inhibitor celecoxib (5 μM, Ray DuBois, Vanderbilt University) were added to both the overlay and supporting gels in some wells. Cultures were maintained under permissive conditions, to model cooperation of ectopic ErbB4 with the SV40 Large T antigen expressed in these cells, for three weeks. After 3 weeks in culture at permissive temperature, colonies were stained with MTT and counted by an investigator blinded to experimental conditions.
Antibodies, cytokines, and growth factors
Antibodies were purchased from: polyclonal anti-ErbB4 (c-18), monoclonal anti-HuR, monoclonal anti-CUGBP-2, and goat polyclonal anti-COX-2, Santa Cruz Biotechnology (Santa Cruz, CA); monoclonal anti-RBM3, Novus Biologicals (Littleton, CO); monoclonal anti-actin, Sigma Corp. (St. Louis, MO); anti-phospho-Y1284 ErbB4, phospho-Y1068 EGFR, phospho-S536 p65 NF-κB and HRP-conjugated secondary antibodies, Cell Signaling (Danvers, MA). Recombinant HRG was purchased from R&D Systems (Minneapolis, MN). Murine TNF was purchased from Peprotech (Rocky Hill, NJ). All HRG and TNF cell treatments of were at 100 ng/ml.
Cell lysates, immunoprecipitation, and Western blot analysis
Cellular proteins were extracted in 50 mM Tris, pH 7.4 containing 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 0.2% sodium deoxycholate, 0.1% SDS, and 0.1% protease and phosphatase inhibitor cocktails (Sigma Corp.) and cleared by centrifugation. Whole cell lysates were immediately subjected to protein assay and boiled in Laemmli loading buffer. For immunoprecipitation, 1 mg lysate was precleared then incubated with 2 μg antibody for 1h at 4 °C followed by 1h at 4 °C with protein A/G-agarose beads (Santa Cruz). Immunocomplexes were washed 4x in lysis buffer and eluted by boiling in Laemmli buffer. Samples were separated on SDS-polyacrylamide gels (6–10% as appropriate) and blotted on nitrocellulose membranes using the manufacturer’s instructions for each antibody. Loading was monitored by Western blot for actin and at least one additional uninvolved protein for whole cell lysates.
RNA isolation and analysis
Total RNA was purified with RNeasy columns (Qiagen, Valencia, CA) including on-column DNase treatment. COX-2 mRNA expression was determined by real-time quantitative PCR (RT-qPCR) using an independent cDNA synthesis step with iScript (Bio-Rad, Hercules, CA), SYBR Green reaction mix (Sigma), and iCycler with IQ5 software (Bio-Rad). Relative mRNA levels were determined using the 2−ΔΔCT method with 18S RNA as the reference. Primers used were: 5′-CGTCTGCCCTATCAACTTTCG (18s Fwd); 5′-CCTTCCTTGGATGTGGTAGCC (18s Rev); 5′-CTCCCTGAAGCCGTACACAT (COX-2 Fwd); ATGGTGCTCCAAGCTCTACC (COX-2 Rev).
Cytotoxicity assays
Cells were plated in 96-well dishes (10,000 per well), grown for 24h, shifted to nonpermissive conditions in the presence of 150 U/ml γ-IFN for 24h, then exposed to 100 ng/ml TNF for 24h. Cells were counted using an MTS-based colorimetric proliferation assay kit (Promega Corp.) and % cell kill calculated relative to untreated control wells. Reported values reflect averages of at least 4 replicate wells.
Statistics and replicates
All data are representative of at least three independent experiments. Statistical analyses and mRNA half-life calculations were performed using Prism software (GraphPad Inc., La Jolla, CA). Statistical significance of differences from controls was assessed by ANOVA analysis with Tukey post-test. Error bars indicate standard error of means.
Results
Full-length ErbB4 increases COX-2 levels in colon epithelial cells
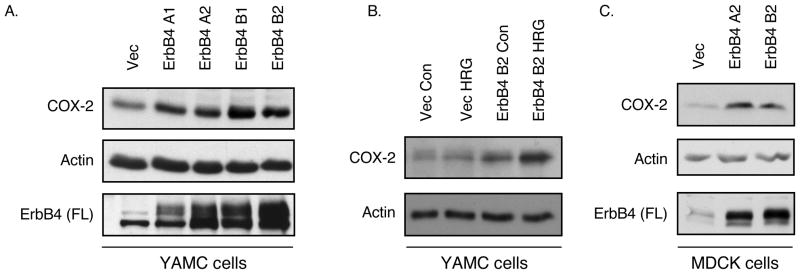
In colonocytes, ErbB4 is induced by, and promotes cell survival in the presence of, inflammatory cytokines such as TNF (13). To investigate the mechanisms of this cellular response, we screened ErbB4-overexpressing cells for changes in expression of molecules associated with both cell survival and inflammation. An intriguing target identified was COX-2, which is involved in inflammation, cell survival, and colon carcinogenesis (23, 24). Western blot analysis of YAMC mouse colon epithelial cells infected with empty vector or retroviral expression constructs for each of the four ErbB4 isoforms (JM-a/CYT-1, JM-a/CYT-2, JM-b/CYT-1, JM-b/CYT-2) showed increased steady-state levels of COX-2 protein (Figure 1A). Interestingly, this effect did not depend on a particular ErbB4 isoform, but seemed to parallel levels of full-length receptor expressed. Subsequent experiments using ErbB4 overexpression compared cells expressing vector (termed YAMC-Vec below) with cells expressing the ‘minimal’ isoform JM-b/CYT-2 ErbB4 (termed YAMC-ErbB4 below). COX-2 expression was further enhanced by addition of the ErbB4 ligand HRG for 3h (Figure 1B). Elevated COX-2 was also detected in MDCK cocker spaniel kidney epithelial cells overexpressing ErbB4 (Figure 1C), showing that this effect is not specific to the YAMC cell line or the colon. COX-2 activity is generally controlled through changes in message and protein expression, but regulatory phosphorylation on the enzyme has been reported (25). Thus we immunoprecipitated COX-2 from YAMC and YAMC-ErbB4 cells with or without HRG treatment, and blotted with antibodies to phospho-tyrosine, -threonine, and -serine. However, no ErbB4- or HRG-induced phosphorylation on COX-2 was detected (data not shown).
Figure 1. ErbB4 stimulates COX-2 expression in colon epithelial cells.
(A) YAMC cells were infected with retroviral vectors expressing each ErbB4 isoform (A1, JM-a/CYT-1; A2, JM-a/CYT-2; B1, JM-b/CYT-1; B2, JM-b/CYT-2). Whole cell lysates were prepared and subjected to Western blot analysis for full-length (FL) ErbB4 and COX-2 expression. Actin blot included as loading control. (B) JM-b/CYT-2 expressing cells (YAMC-ErbB4) were exposed to HRG (100 ng/ml) for 3h; COX-2 protein levels were determined by immunoblot analysis. (C) MDCK cells were infected with Vector or ErbB4-expressing constructs and COX-2 levels were determined by Western blot analysis.
ErbB4 promotes TNF-stimulated COX-2 accumulation
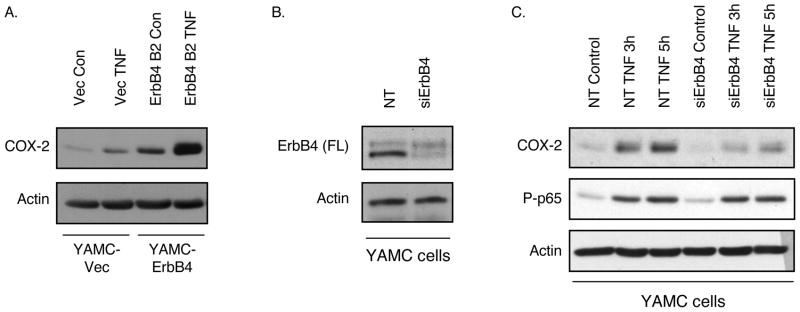
Both ErbB4 (13) and COX-2 (23) are induced in colon epithelial cells by the pro-inflammatory cytokine TNF. To test whether these pathways are linked, we treated YAMC-Vec and YAMC-ErbB4 cells with TNF (100 ng/ml) and determined COX-2 expression by Western blot analysis. TNF induced COX-2 accumulation by 3h in YAMC-Vec cells; this response was significantly enhanced by ErbB4 overexpression (Figure 2A). As noted above, these results use the YAMC-ErbB4 cells expressing the JM-b/CYT-2 isoform; similar outcomes were observed with lines expressing other ErbB4 isoforms (not shown), further supporting the conclusion that ErbB4-mediated COX-2 induction does not require specific JM or CYT sequences.
Figure 2. ErbB4 enhances TNF-induced COX-2 expression.
(A) YAMC-ErbB4 cells were exposed to TNF (100 ng/ml) for 3h; COX-2 protein levels were determined by immunoblot analysis. (B) YAMC cells were transfected with non-targeting or ErbB4-specific siRNA pools for 72h. (C) siRNA-transfected cells were stimulated with TNF; whole cell lysates were prepared and subjected to immunoblot analysis for COX-2 induction as well as phosphorylation of the p65 NF-κB subunit as control for active TNF signaling.
Unchallenged YAMC cells express low but detectable basal levels of ErbB4, which increase following cell exposure to TNF. We have previously used siRNA knockdown to show that this endogenous ErbB4 expression regulates acute TNF activation of PI 3-kinase/Akt signaling (13). To test the requirement for endogenous ErbB4 in COX-2 regulation by TNF, YAMC cells were transfected with non-targeting or ErbB4-specific siRNA pools. As in our prior studies, we obtained >85% knockdown of endogenous ErbB4 with siRNA transfection (Figure 2B). Following TNF treatment, whole cell protein lysates were prepared and COX-2 induction was assessed by immunoblot analysis. TNF-induced COX-2 expression was significantly attenuated by ErbB4 knockdown (Figure 2C). Other TNF-induced signaling targets, including phosphorylation of MAP kinases (13) and the p65 subunit of NF-κB, were not sensitive to ErbB4 deletion.
ErbB4 enhancement of COX-2 levels in YAMC cells is Src and PI 3-kinase-dependent
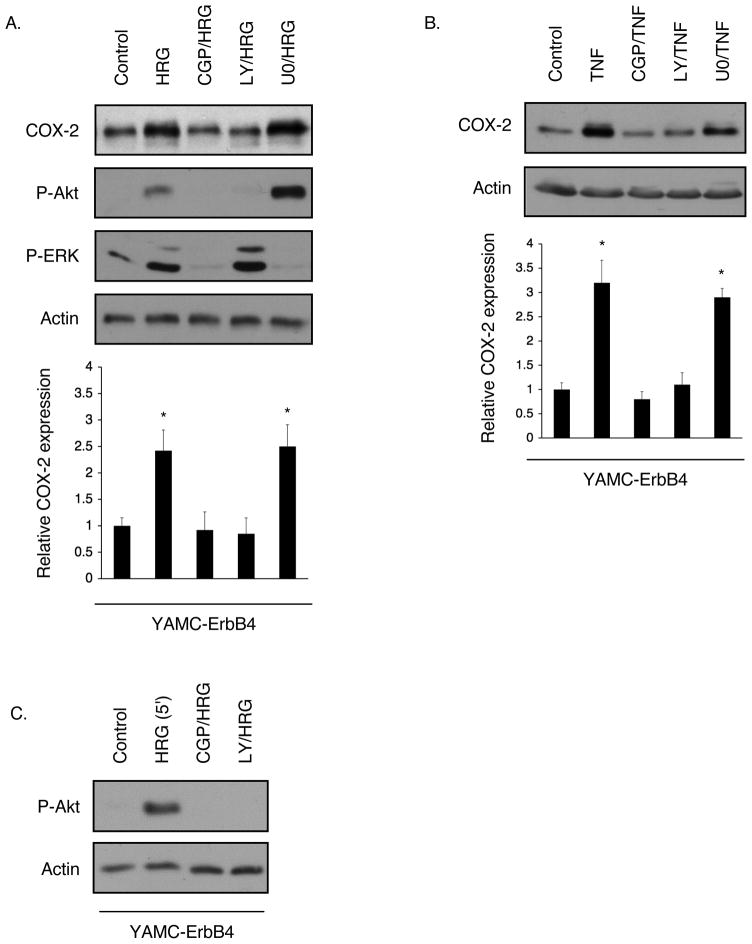
To investigate the molecular pathways involved in ErbB4-stimulated COX-2 expression, YAMC-ErbB4 cells were exposed to HRG or TNF for 3h in the presence of Src (CGP77675, 1 μM), PI 3-kinase (LY294002, 5 μM), and MEK (U0126, 10 μM) inhibitors. Blockade of Src or PI 3-kinase, but not MEK, signaling abrogated COX-2 induction in these cells (Figure 3A, B). Results were confirmed with the use of additional Src and PI 3-kinase inhibitors (PP2 and wortmannin respectively, data not shown). Interestingly, Src inhibition also blocked Akt phosphorylation in response to 3h HRG exposure (Figure 3A), suggesting that Src is upstream of PI 3-kinase/Akt in ErbB4-induced signaling. To further explore this possible relationship we tested Akt phosphorylation after acute (5 min) HRG treatment. Src inhibition abrogated HRG-stimulated Akt phosphorylation at this early time point (Figure 3C), supporting the notion that Src is required for activation of the PI 3-kinase pathway by ErbB4 in colon epithelial cells.
Figure 3. Src and PI 3-kinase activity are required for COX-2 induction in YAMC-ErbB4 cells.
YAMC-ErbB4 cells were exposed to CGP 77675 (Src inhibitor, 1 μM), LY294002 (PI 3-kinase inhibitor, 5 μM), or U0126 (MEK inhibitor, 10 μM) for 30 min before incubation with either (A) TNF or (B) HRG for 3h. COX-2 levels were determined by immunoblot analysis. Graphs show densitometry for 4 experiments. *, p <0.01 vs. control. (C) YAMC-ErbB4 cells were exposed to HRG for 5 min with or without CGP 77675 or LY294002 pretreatment. Akt phosphorylation was determined by Western blot analysis.
ErbB4 enhancement of COX-2 levels requires EGFR
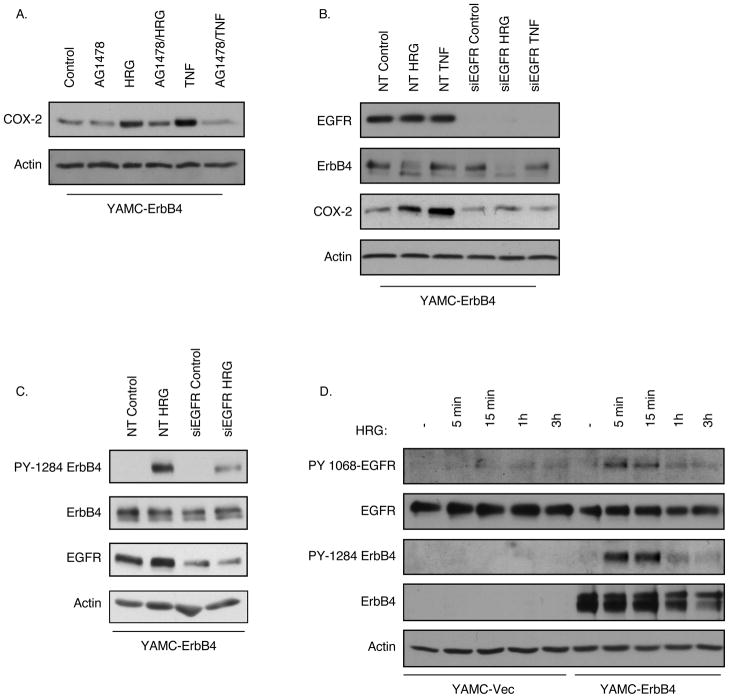
Treatment with EGF also promotes COX-2 expression levels in YAMC cells (Hobbs and Polk, unpublished observations), raising the possibility that ErbB4 enhances COX-2 by heterodimerization with, or transactivation of, EGFR. Therefore we exposed YAMC-ErbB4 cells to the EGFR inhibitor AG1478 (150 nm, 30 min pretreatment) before TNF or HRG treatment. Whole cell lysates were prepared and COX-2 levels were assessed by Western blot analysis. EGFR inhibition completely blocked both TNF-and HRG-stimulated COX-2 induction in YAMC-ErbB4 cells (Figure 4A). Similarly, transfection with EGFR-specific siRNA abrogated COX-2 induction in the context of ErbB4 overexpression (Figure 4B). Furthermore, siRNA knockdown of EGFR expression attenuated ErbB4 phosphorylation in response to HRG (Figure 4C), suggesting a role for EGFR in ligand-induced ErbB4 activation. Using antibodies specific for EGFR phosphorylated on Y1068, we also observe EGFR phosphorylation by 5 min in ErbB4-overexpressing cells (Figure 4D), paralleling the onset of ErbB4 phosphorylation [note that phosphorylation of the low levels of endogenous ErbB4 were also detectable in YAMC-Vec cells after HRG exposure, albeit only at longer blot exposure times (not shown)]. In contrast, in vector-expressing YAMC cells HRG had minimal effect on EGFR phosphorylation/activation at any time point studied. Thus, while EGFR does not bind HRG directly (26), it can be activated by this ligand in the presence of ErbB4 and is required for maximal ligand-driven ErbB4 phosphorylation and COX-2 induction.
Figure 4. EGFR regulates ErbB4 activation and COX-2 expression.
(A) YAMC-ErbB4 cells were incubated with the EGFR inhibitor AG1478 (150 nM) for 30 min before 3h treatment with TNF or HRG. COX-2 levels were determined by Western blot analysis. (B) YAMC-ErbB4 cells were transfected with non-targeting or EGFR-specific siRNA pools for 72h, then stimulated with TNF or HRG. EGFR, ErbB4, and COX-2 levels were determined by immunoblot analysis. (C) YAMC-ErbB4 cells were transfected with non-targeting or EGFR-specific siRNA pools, then stimulated with HRG for 15 min. ErbB4 phosphorylation was determined by Western blot analysis. (D) YAMC-Vec and YAMC-ErbB4 cells were exposed to HRG for indicated times; EGFR and ErbB4 phosphorylation were determined by Western blot analysis.
COX-2 mRNA levels are elevated and message half-life extended in ErbB4-expressing cells
COX-2 expression in the mammalian cell is controlled at multiple levels. To investigate the point at which ErbB4 signaling is involved in this regulation, we treated cells with inhibitors of protein translation and RNA synthesis. 5h exposure of YAMC-Vec and YAMC-ErbB4 cells to the translation inhibitor cycloheximide had no appreciable effect on basal COX-2 levels (data not shown). Given that ErbB4 expression enhances COX-2 accumulation in as little as 3h following exposure to a stimulus (see Figure 1B, E) regulation of protein stability/turnover is therefore unlikely to account for observed differences.
In contrast, preincubation with 10 μg/ml Actinomycin D to stop RNA synthesis completely blocked HRG-and TNF-stimulated COX-2 protein expression in YAMC-ErbB4 cells (Figure 5A), suggesting regulation of mRNA, either at the level of transcription or message stability. RT-qPCR analysis of isolated RNA confirmed an effect on RNA levels; YAMC-ErbB4 cells showed elevated basal COX-2 steady-state mRNA vs. YAMC-Vec (Figure 5B), and an enhanced mRNA accumulation in response to either HRG or TNF. Furthermore, siRNA knockdown of ErbB4 from YAMC cells blunted COX-2 mRNA accumulation in response to TNF (Figure 5C), as it did with protein accumulation (Figure 2B). To test whether these responses might reflect a change in COX-2 message half-life, YAMC-Vec and YAMC-ErbB4 cell cultures were subjected to timed incubations with Actinomycin D followed by RT-qPCR of isolated RNA and regression analysis. This analysis showed that the half-life of COX-2 mRNA is extended from 0.87h in YAMC-Vec cells to 2.79h in YAMC-ErbB4 cells (Figure 5D). Additionally, Western blot analysis for CUGBP-2, HuR, and RBM3, proteins which regulate COX-2 message stability (27–29), revealed a modest increase in RBM3, but no change in CUGBP-2 or HuR, in YAMC-ErbB4 cells versus YAMC-Vec (Figure 5E). No apparent change in sub-cellular localization of these regulatory proteins with ErbB4 expression was observed by immunofluorescence localization analysis (data not shown); in both YAMC-Vec and YAMC-ErbB4 cells CUGBP-2, HuR, and RBM3 are all expressed primarily in the nucleus.
Figure 5. ErbB4 regulates COX-2 mRNA expression.
(A) Cells were exposed to HRG or TNF in the presence of Actinomycin D (ActD; 10 μg/ml) and COX-2 protein levels were determined by Western blot analysis. (B) JM-b/CYT-2 expressing cells (YAMC-ErbB4) were exposed to tumor necrosis factor (TNF, 100 ng/ml) or HRG (100 ng/ml) for 3h; COX-2 mRNA levels were determined by RT-qPCR. (C) YAMC cells were transfected with non-targeting or ErbB4-specific siRNA pools for 72h. Cells were stimulated with TNF and COX-2 levels determined by RT-qPCR. (D) Cells were exposed to Actinomycin D for indicated times and COX-2 mRNA levels were determined by RT-qPCR. (E) Expression of RBM3, HuR, and CUGBP-2 protein in YAMC-Vec and YAMC-ErbB4 cells was determined by Western blot analysis.
ErbB4-induced cell survival requires COX-2 activity
We have previously reported that ErbB4 expression protects cells from cytokine-induced apoptosis (13). We used the COX-2 inhibitor celecoxib to ask whether ErbB4 induces cell survival through COX-2 dependent mechanisms. After confirming that celecoxib does not nonspecifically interfere with ErbB4 phosphorylation in response to HRG (Figure 6A), we exposed YAMC-Vec and YAMC-ErbB4 cells to a cytotoxic cytokine cocktail (interferon-γ, 150 units/ml; TNF, 100 ng/ml) to stimulate apoptosis (30) in the presence or absence of celecoxib (5 μM). Cell loss was determined using an MTS-based cell count assay. ErbB4 protection from TNF-induced cell death was reversed by the presence of celecoxib (Figure 6B), indicating that COX-2 activity is involved in ErbB4-mediated cell survival. Consistent with these results, shRNA-mediated knockdown of ErbB4 in LIM 2405 human colorectal carcinoma cells, which express high levels of endogenous ErbB4, decreased both steady-state COX-2 protein expression and cell survival in the presence of TNF plus interferon-γ (Figure 6C, D).
Figure 6. ErbB4-induced cell survival correlates with COX-2 expression.
(A) YAMC-ErbB4 cells were exposed to HRG for 15 min with or without the COX-2 inhibitor celecoxib (CCB; 5 μM, 30 min pretreatment). Cell lysates were prepared and ErbB4 phosphorylation determined by Western blot. (B) YAMC-Vec and YAMC-ErbB4 cells were primed with interferon-γ (150 units/ml) overnight, then exposed to TNF+interferon-γ for 24h with or without CCB. Cell loss was determined by an MTS-based cell count assay. (C, D) LIM 2405 cells were infected with lentiviral particles to express either control or ErbB4 targeting shRNA constructs and selected with 5 μg/ml Puromycin. (C) ErbB4 and COX-2 protein levels were determined by Western blot analysis. (D) Cells were exposed to a TNF+IFN-γ cocktail for 24h and cell loss determined by MTS cell count. *, p <0.01 vs. control.
ErbB4 confers anchorage-independent growth through COX-2
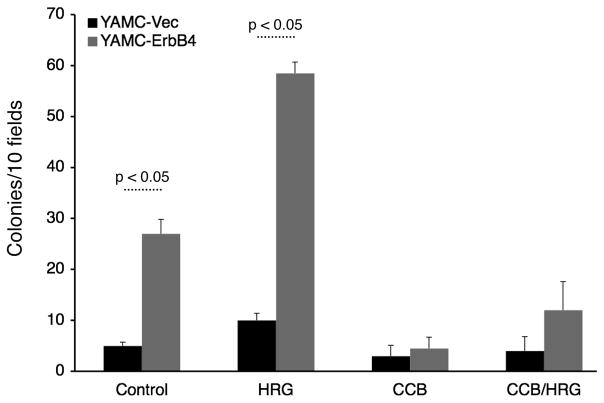
As a loss of appropriate cell death can contribute to cellular transformation and tumorigenesis, we asked whether ErbB4 promotes soft agar colony formation of colon epithelial cells. YAMC-Vec and YAMC-ErbB4 cells were embedded in 0.35% agar and maintained at permissive conditions for three weeks. Three-dimensional cell clusters and colonies were then counted as a measure of transformation. Vector-expressing cells formed only a very few small cell clusters, while in contrast ErbB4-expressing cells formed numerous detectable colonies (Figure 7). The ErbB4 ligand HRG enhanced this response, while celecoxib blocked ErbB4-stimulated anchorage-independent growth/colony formation either with or without HRG, suggesting that COX-2 activity is required for ErbB4-induced colon epithelial cell transformation.
Figure 7. ErbB4 promotes mouse colon epithelial cell transformation.
YAMC-Vec and YAMC-ErbB4 were subjected to a soft agar colony-formation assay in the presence or absence of HRG and celecoxib. After 3 weeks colonies (>30 cells) were photographed and counted.
Discussion
In this study we show that ErbB4 promotes colon epithelial cell survival in a COX-2 dependent manner. COX-2 induction by ErbB4 was associated with increased mRNA half-life, and required EGFR, Src, and PI 3-kinase activity. These data suggest that elevated ErbB4 expression in inflammatory bowel disease (13) could contribute to inappropriate cell survival in the inflammatory milieu, thus promoting colitis-associated development of colorectal tumors. Decreased survival of colorectal cancer cells transfected with ErbB4 shRNA constructs [(14) and Figure 6] and the ability of ErbB4-expressing cells to form colonies in soft agar in a celecoxib-dependent manner (Figure 7) are consistent with this possibility.
In contrast to the fairly clear association of other ErbB family members with tumorigenesis, data in the literature on ErbB4 in cancer are complex. Overexpression has been reported in several cancers including endometrial (31) and non-small cell lung (10, 32, 33), but this correlation is not seen in all tumor types (34–36), and studies of breast cancer have yielded contradictory results (37, 38). In the GI tract the limited available data support a role for ErbB4 in carcinogenesis, with reports noting somatic mutation of the ErbB4 gene in colorectal cancer (33) or ErbB2/ErbB4 coexpression in late-stage tumors (39). Leung and colleagues recently reported high ErbB4 protein expression in a substantial subset of colorectal tumors examined by immunohistochemical staining (40). Taken together with these observations, our results linking ErbB4 to expression of COX-2, cell survival, and anchorage-independent growth indicate that further investigation into the possible role of ErbB4 in colorectal carcinogenesis is warranted. As it recognizes a uniquely broad subset of EGF-related ligands (heregulin/neuregulin growth factors, betacellulin, HB-EGF, and epiregulin) while at the same time binding a more restricted suite of SH2 and PTB domain-containing downstream signaling partners than other ErbBs (12), ErbB4 may provide a unique therapeutic target amongst this receptor family.
The result that COX-2 induced by ErbB4 is dependent on PI 3-kinase signaling (Figure 3) is consistent with our previous data showing that cell survival conferred by ErbB4 overexpression requires this pathway (13). Interestingly, this was the case even with expression of CYT-2 isoforms of the molecule, which lack the YTPM motif required for PI 3-kinase binding in NIH 3T3 cells (6). The sensitivity of HRG-stimulated Akt activation to Src inhibitors (Figure 3) suggests ErbB4 can stimulate PI 3-kinase indirectly. Furthermore, heterodimerization with EGFR or ErbB3 could result in PI 3-kinase activation and cell survival signaling without the need for YXXM motifs on ErbB4 itself (41). For example, a recent report from the Threadgill laboratory suggests that ErbB3/ErbB4 heterodimers are important for survival of HCT116 colon cancer cells (14); in a context in which other ErbBs are activated, the CYT-1 YTPM motif on ErbB4 would likely be dispensable for PI 3-kinase activation.
We find that EGFR is rapidly phosphorylated by HRG in YAMC-ErbB4 cells, and conversely EGFR is required for maximal ErbB4 phosphorylation in response to HRG (Figure 4). The most likely interpretation of these data is formation of ErbB4/EGFR heterodimers. However, it is also possible that ErbB4 expression is promoting EGFR transactivation through other mechanisms. EGFR can be activated as a result of stimulated ligand release through metalloproteinase activation such as in the T84 colon epithelial cell response to carbachol (42) or corneal epithelial wound healing accelerated by lysophosphatidic acid (43). Ligand-independent intracellular EGFR transactivation pathways have also been demonstrated (44). Interestingly, both the ligand-release and intracellular signaling transactivation mechanisms appear to require Src, which directly binds ErbB4-derived phosphopeptides in vitro (12). As ErbB4 expression and activation are TNF-responsive in colon epithelial cells (13), and ErbB4-stimulated COX-2 induction requires Src activity (Figure 4), it is possible that either of these mechanisms are involved in EGFR activation by HRG in YAMC-ErbB4 cells.
ErbB4 expression is typically low in unchallenged colon epithelial cells, but is induced by injury and inflammation (13), and promotes cellular survival and transformation. Our data connecting ErbB4 to COX-2 mRNA half-life and thus both basal and stimulated expression levels are novel and consistent with the hypothesis that elevated ErbB signaling during injury and inflammation could promote a tissue environment with elevated EGFR activity, PI 3-kinase/Akt signaling (13), and COX-2 expression. This environment would present a favorable niche for the expansion of cells which acquire mutations and subsequent cancer development. Thus, ErbB4 may represent a novel target for intervention in colorectal cancers, particularly those associated with chronic inflammatory diseases. Modulation of ErbB4-mediated signaling, possibly through use of blocking or inactivating antibodies (45, 46) may provide an avenue to block excessive activation of the PI 3-kinase and COX-2 signaling axes.
Acknowledgments
The authors wish to thank Jessica Bernard and Lindsay Kuhnhein for expert technical support. This work was supported by NIH grant K01DK077956 (MRF), American Cancer Society Institutional Research Grant #IRG-58-009-50 (MRF), a Senior Scientist Award from the Crohn’s and Colitis Foundation of America (DBP), NIH Award R01DK056008 (DBP), and the Vanderbilt Digestive Diseases Research Center (VDDRC; NIH Award P30DK058404), including the VDDRC Novel Cell Line Development and Histology core laboratories. The VMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the VDDRC (DK058404).
Grant support: NIH grant K01DK077956 (MRF), American Cancer Society Institutional Research Grant #IRG-58-009-50 (MRF), a Senior Scientist Award from the Crohn’s and Colitis Foundation of America (DBP), NIH Award R01DK056008 (DBP), and the Vanderbilt Digestive Diseases Research Center (NIH Award P30DK058404).
Abbreviations used
- COX-2
cyclooxygenase-2
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- HRG
heregulin-1β
- PI
phosphatidylinositol
- RT-qPCR
real-time quantitative PCR
- TNF
tumor necrosis factor
- YAMC
young adult mouse colon
Footnotes
The authors of this study have no conflicts of interest to disclose.
References
- 1.Srinivasan R, Poulsom R, Hurst HC, Gullick WJ. Expression of the c-erbB-4/HER4 protein and mRNA in normal human fetal and adult tissues and in a survey of nine solid tumour types. J Pathol. 1998;185(3):236–245. doi: 10.1002/(SICI)1096-9896(199807)185:3<236::AID-PATH118>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Linggi B, Cheng QC, Rao AR, Carpenter G. The ErbB-4 s80 intracellular domain is a constitutively active tyrosine kinase. Oncogene. 2006;25(1):160–163. doi: 10.1038/sj.onc.1209003. [DOI] [PubMed] [Google Scholar]
- 3.Long W, Wagner K-U, Lloyd KCK, Binart N, Shillingford JM, Hennighausen L, et al. Impaired differentiation and lactational failure of Erbb4-deficient mammary glands identify ERBB4 as an obligate mediator of STAT5. Development. 2003;130(21):5257–5268. doi: 10.1242/dev.00715. [DOI] [PubMed] [Google Scholar]
- 4.Vaskovsky A, Lupowitz Z, Erlich S, Pinkas-Kramarski R. ErbB-4 activation promotes neurite outgrowth in PC12 cells. J Neurochem. 2000;74(3):979–987. doi: 10.1046/j.1471-4159.2000.0740979.x. [DOI] [PubMed] [Google Scholar]
- 5.Erlich S, Goldshmit Y, Lupowitz Z, Pinkas-Kramarski R. ErbB-4 activation inhibits apoptosis in PC12 cells. Neuroscience. 2001;107(2):353–362. doi: 10.1016/s0306-4522(01)00350-5. [DOI] [PubMed] [Google Scholar]
- 6.Kainulainen V, Sundvall M, Maatta JA, Santiestevan E, Klagsbrun M, Elenius K. A natural ErbB4 isoform that does not activate phosphoinositide 3-kinase mediates proliferation but not survival or chemotaxis. J Biol Chem. 2000;275(12):8641–8649. doi: 10.1074/jbc.275.12.8641. [DOI] [PubMed] [Google Scholar]
- 7.Tang CK, Goldstein DJ, Payne J, Czubayko F, Alimandi M, Wang LM, et al. ErbB-4 ribozymes abolish neuregulin-induced mitogenesis. Cancer Res. 1998;58(15):3415–3422. [PubMed] [Google Scholar]
- 8.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294(5549):2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan R, Gillett CE, Barnes DM, Gullick WJ. Nuclear expression of the c-erbB-4/HER-4 growth factor receptor in invasive breast cancers. Cancer Res. 2000;60(6):1483–1487. [PubMed] [Google Scholar]
- 10.Starr A, Greif J, Vexler A, Ashkenazy-Voghera M, Gladesh V, Rubin C, et al. ErbB4 increases the proliferation potential of human lung cancer cells and its blockage can be used as a target for anti-cancer therapy. Int J Cancer. 2006;119(2):269–274. doi: 10.1002/ijc.21818. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter G. ErbB-4: mechanism of action and biology. Exp Cell Res. 2003;284(1):66–77. doi: 10.1016/s0014-4827(02)00100-3. [DOI] [PubMed] [Google Scholar]
- 12.Kaushansky A, Gordus A, Budnik BA, Lane WS, Rush J, MacBeath G. System-wide investigation of ErbB4 reveals 19 sites of Tyr phosphorylation that are unusually selective in their recruitment properties. Chem Biol. 2008;15(8):808–817. doi: 10.1016/j.chembiol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frey MR, Edelblum KL, Mullane MT, Liang D, Polk DB. The ErbB4 growth factor receptor is required for colon epithelial cell survival in the presence of TNF. Gastroenterology. 2009;136(1):217–226. doi: 10.1053/j.gastro.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee D, Yu M, Lee E, Kim H, Yang Y, Kim K, et al. Tumor-specific apoptosis caused by deletion of the ERBB3 pseudo-kinase in mouse intestinal epithelium. J Clin Invest. 2009;119(9):2702–2713. doi: 10.1172/JCI36435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5(6):698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 16.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83(3):493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Tang XM, Half E, Kuo MT, Sinicrope FA. Cyclooxygenase-2 overexpression reduces apoptotic susceptibility by inhibiting the cytochrome c-dependent apoptotic pathway in human colon cancer cells. Cancer Res. 2002;62(21):6323–6328. [PubMed] [Google Scholar]
- 18.Singer, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115(2):297–306. doi: 10.1016/s0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- 19.Buchanan FG, Holla V, Katkuri S, Matta P, DuBois RN. Targeting cyclooxygenase-2 and the epidermal growth factor receptor for the prevention and treatment of intestinal cancer. Cancer Res. 2007;67(19):9380–9388. doi: 10.1158/0008-5472.CAN-07-0710. [DOI] [PubMed] [Google Scholar]
- 20.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87(5):803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 21.Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci U S A. 1993;90(2):587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitehead RH, Zhang HH, Hayward IP. Retention of tissue-specific phenotype in a panel of colon carcinoma cell lines: relationship to clinical correlates. Immunol Cell Biol. 1992;70 (Pt 4):227–236. doi: 10.1038/icb.1992.30. [DOI] [PubMed] [Google Scholar]
- 23.Tong X, Yin L, Joshi S, Rosenberg DW, Giardina C. Cyclooxygenase-2 regulation in colon cancer cells: modulation of RNA polymerase II elongation by histone deacetylase inhibitors. J Biol Chem. 2005;280(16):15503–15509. doi: 10.1074/jbc.M411978200. [DOI] [PubMed] [Google Scholar]
- 24.Tang X, Sun YJ, Half E, Kuo MT, Sinicrope F. Cyclooxygenase-2 overexpression inhibits death receptor 5 expression and confers resistance to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human colon cancer cells. Cancer Res. 2002;62(17):4903–4908. [PubMed] [Google Scholar]
- 25.Parfenova H, Balabanova L, Leffler CW. Posttranslational regulation of cyclooxygenase by tyrosine phosphorylation in cerebral endothelial cells. Am J Physiol. 1998;274(1 Pt 1):C72–81. doi: 10.1152/ajpcell.1998.274.1.C72. [DOI] [PubMed] [Google Scholar]
- 26.Pinkas-Kramarski R, Shelly M, Guarino BC, Wang LM, Lyass L, Alroy I, et al. ErbB tyrosine kinases and the two neuregulin families constitute a ligand-receptor network. Mol Cell Biol. 1998;18(10):6090–6101. doi: 10.1128/mcb.18.10.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhopadhyay D, Houchen CW, Kennedy S, Dieckgraefe BK, Anant S. Coupled mRNA stabilization and translational silencing of cyclooxygenase-2 by a novel RNA binding protein, CUGBP2. Mol Cell. 2003;11(1):113–126. doi: 10.1016/s1097-2765(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 28.Sengupta S, Jang BC, Wu MT, Paik JH, Furneaux H, Hla T. The RNA-binding protein HuR regulates the expression of cyclooxygenase-2. J Biol Chem. 2003;278(27):25227–25233. doi: 10.1074/jbc.M301813200. [DOI] [PubMed] [Google Scholar]
- 29.Sureban SM, Ramalingam S, Natarajan G, May R, Subramaniam D, Bishnupuri KS, et al. Translation regulatory factor RBM3 is a proto-oncogene that prevents mitotic catastrophe. Oncogene. 2008;27(33):4544–4556. doi: 10.1038/onc.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright K, Kolios G, Westwick J, Ward SG. Cytokine-induced apoptosis in epithelial HT-29 cells is independent of nitric oxide formation. Evidence for an interleukin-13-driven phosphatidylinositol 3-kinase-dependent survival mechanism. J Biol Chem. 1999;274(24):17193–17201. doi: 10.1074/jbc.274.24.17193. [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan R, Benton E, McCormick F, Thomas H, Gullick WJ. Expression of the c-erbB-3/HER-3 and c-erbB-4/HER-4 growth factor receptors and their ligands, neuregulin-1 alpha, neuregulin-1 beta, and betacellulin, in normal endometrium and endometrial cancer. Clin Cancer Res. 1999;5(10):2877–2883. [PubMed] [Google Scholar]
- 32.al Moustafa AE, Alaoui-Jamali M, Paterson J, O’Connor-McCourt M. Expression of P185erbB-2, P160erbB-3, P180erbB-4, and heregulin alpha in human normal bronchial epithelial and lung cancer cell lines. Anticancer Res. 1999;19(1A):481–486. [PubMed] [Google Scholar]
- 33.Soung YH, Lee JW, Kim SY, Wang YP, Jo KH, Moon SW, et al. Somatic mutations of the ERBB4 kinase domain in human cancers. Int J Cancer. 2006;118(6):1426–1429. doi: 10.1002/ijc.21507. [DOI] [PubMed] [Google Scholar]
- 34.Memon AA, Sorensen BS, Melgard P, Fokdal L, Thykjaer T, Nexo E. Expression of HER3, HER4 and their ligand heregulin-4 is associated with better survival in bladder cancer patients. Br J Cancer. 2004;91(12):2034–2041. doi: 10.1038/sj.bjc.6602251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rotterud R, Nesland JM, Berner A, Fossa SD. Expression of the epidermal growth factor receptor family in normal and malignant urothelium. BJU Int. 2005;95(9):1344–1350. doi: 10.1111/j.1464-410X.2005.05497.x. [DOI] [PubMed] [Google Scholar]
- 36.Edwards J, Traynor P, Munro AF, Pirret CF, Dunne B, Bartlett JM. The role of HER1-HER4 and EGFRvIII in hormone-refractory prostate cancer. Clin Cancer Res. 2006;12(1):123–130. doi: 10.1158/1078-0432.CCR-05-1445. [DOI] [PubMed] [Google Scholar]
- 37.Junttila TT, Sundvall M, Lundin M, Lundin J, Tanner M, Harkonen P, et al. Cleavable ErbB4 isoform in estrogen receptor-regulated growth of breast cancer cells. Cancer Res. 2005;65(4):1384–1393. doi: 10.1158/0008-5472.CAN-04-3150. [DOI] [PubMed] [Google Scholar]
- 38.Tovey SM, Witton CJ, Bartlett JM, Stanton PD, Reeves JR, Cooke TG. Outcome and human epidermal growth factor receptor (HER) 1–4 status in invasive breast carcinomas with proliferation indices evaluated by bromodeoxyuridine labelling. Breast Cancer Res. 2004;6(3):R246–251. doi: 10.1186/bcr783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JC, Wang ST, Chow NH, Yang HB. Investigation of the prognostic value of coexpressed erbB family members for the survival of colorectal cancer patients after curative surgery. Eur J Cancer. 2002;38(8):1065–1071. doi: 10.1016/s0959-8049(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 40.Leung SP, Griffith OL, Masoudi H, Gown A, Jones S, Phang T, et al. Clinical utility of type 1 growth factor receptor expression in colon cancer. Am J Surg. 2008;195(5):604–610. doi: 10.1016/j.amjsurg.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 41.Chuu CP, Chen RY, Barkinge JL, Ciaccio MF, Jones RB. Systems-level analysis of ErbB4 signaling in breast cancer: a laboratory to clinical perspective. Mol Cancer Res. 2008;6(6):885–891. doi: 10.1158/1541-7786.MCR-07-0369. [DOI] [PubMed] [Google Scholar]
- 42.McCole DF, Keely SJ, Coffey RJ, Barrett KE. Transactivation of the epidermal growth factor receptor in colonic epithelial cells by carbachol requires extracellular release of transforming growth factor-alpha. J Biol Chem. 2002;277(45):42603–42612. doi: 10.1074/jbc.M206487200. [DOI] [PubMed] [Google Scholar]
- 43.Xu KP, Yin J, Yu FS. Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor. Invest Ophthalmol Vis Sci. 2007;48(2):636–643. doi: 10.1167/iovs.06-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caruso R, Pallone F, Fina D, Gioia V, Peluso I, Caprioli F, et al. Protease-activated receptor-2 activation in gastric cancer cells promotes epidermal growth factor receptor trans-activation and proliferation. Am J Pathol. 2006;169(1):268–278. doi: 10.2353/ajpath.2006.050841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X, Levkowitz G, Tzahar E, Karunagaran D, Lavi S, Ben-Baruch N, et al. An immunological approach reveals biological differences between the two NDF/heregulin receptors, ErbB-3 and ErbB-4. J Biol Chem. 1996;271(13):7620–7629. [PubMed] [Google Scholar]
- 46.Hollmen M, Maatta JA, Bald L, Sliwkowski MX, Elenius K. Suppression of breast cancer cell growth by a monoclonal antibody targeting cleavable ErbB4 isoforms. Oncogene. 2009;28(10):1309–1319. doi: 10.1038/onc.2008.481. [DOI] [PubMed] [Google Scholar]