Abstract
Type 2 diabetes mellitus is associated with dyslipidemia and with an increased risk of coronary heart disease (CHD). Our objective was to compare the effects of hormone replacement therapy (HRT) on plasma lipoproteins and coronary disease progression in postmenopausal women with and without diabetes. Study subjects were participants in the Estrogen Replacement and Atherosclerosis trial, a placebo-controlled, randomized trial of HRT (conjugated equine estrogen 0.625 mg/day with or without medroxyprogesterone acetate 2.5 mg/day) in postmenopausal women with established CHD (men age 65±7 y). Plasma remnant lipoprotein levels and HDL subpopulation levels were measured at baseline and year 1. Quantitative coronary angiography was assessed at baseline and at follow-up. At baseline, remnant lipoprotein levels were significantly higher and HDL-C levels significantly lower in diabetic women than in women without diabetes. HRT lowered remnant lipoproteins and increased HDL-C and large HDL particle levels in both groups. However, during HRT, levels of these parameters were still significantly worse in diabetic women than in non-diabetic women. A significant interaction between HRT and diabetes status, with greater increases in plasma atheroprotective HDL α1 particles in non-diabetic women than in diabetic women during HRT, was observed. CHD progressed significantly more in women with diabetes than in women without diabetes. Our findings indicate that diabetes attenuates the HRT-related increase in atheroprotective HDL α1 particles. Faster progression of coronary atherosclerosis in women with diabetes could be mediated in part by a worse lipoprotein profile in these women than in women without diabetes, both before and during HRT.
Keywords: Hormone replacement therapy, diabetes mellitus, lipoproteins, cholesterol, triglycerides, coronary heart disease
INTRODUCTION
Coronary heart disease (CHD) is the main cause of death in postmenopausal women (1). Type 2 diabetes mellitus increases the risk of CHD (2;3) and there is some evidence that the risk conferred by diabetes is greater in women than in men (4). The increased risk of CHD with diabetes is in part mediated by the associated dyslipidemia, characterized by elevated plasma triglycerides (TG) and reduced HDL cholesterol (HDL-C) levels, in addition to elevated small dense LDL levels (5). Other important mediators of increased CHD risk in diabetes are obesity, hypertension, and increased inflammation. The typical dyslipidemia observed in diabetes is due to the insulin resistance state, which causes increased hepatic synthesis of TG-rich lipoproteins and a faster clearance of HDL (6–8).
In randomized clinical trials conducted mostly in older women, hormone replacement therapy (HRT) has been shown to either offer no protection against CHD (9;10) or to increase the risk of CHD (11;12). Use of HRT in younger postmenopausal women may reduce the risk of CHD (13;14). While HRT is no longer prescribed for the prevention of CHD, postmenopausal women are still prescribed HRT for the relief of menopausal symptoms. By lowering plasma LDL-C and increasing HDL-C levels, HRT has the potential of improving the dyslipidemia associated with diabetes, with the exception of potentially further increasing plasma TG levels. In a randomized study of HRT in postmenopausal women with CHD, it was shown that coronary artery disease progression was faster in women with glucose intolerance randomized to HRT than in normoglycemic women randomized to HRT (15).
The aims of this study were to compare the lipoprotein subpopulation response to HRT in non diabetic and diabetic women with established CHD.
METHODS
Study design
Subjects were postmenopausal women participating in the Estrogen Replacement and Atherosclerosis (ERA) trial (9;16). Women with established CHD, defined as ≥30% stenosis of at least one epicardial coronary artery by quantitative coronary angiography, were enrolled into the study. Exclusion criteria were: history of uncontrolled diabetes or hypertension, deep-vein thrombosis or pulmonary embolism, kidney disease, symptomatic gallstones, or TG levels >400 mg/dL. The study had a randomized, placebo-controlled, double-blind design and consisted of three parallel phases: a) oral conjugated equine estrogen (CEE 0.625 mg/day, as Premarin®, Wyeth-Ayerst), b) oral CEE and medroxyprogesterone acetate (CEE 0.625 mg/day and MPA 2.5 mg/day, as Prempro®, Wyeth-Ayerst), and c) placebo.
Participants were followed for a mean of 3.2 years. Of the 309 enrolled subjects, paired plasma samples at baseline and at year 1 for the assessment of plasma lipoprotein subpopulations were available in 250 women. Paired baseline and follow-up coronary angiography assessments were available in 217 women. Diabetes was defined as use of hypoglycemic medications or a fasting glucose level ≥126 mg/dl.
Measurement of plasma lipids, remnant lipoproteins and HDL subpopulations
Fasting blood samples were obtained from study participants at the baseline and the year-1 visits. Blood was drawn in tubes containing 0.1% EDTA and plasma was separated by centrifugation at 1000 x g for 30 min at 4 °C. Plasma total cholesterol (TC) and TG concentrations were measured by automated assays (17). HDL-C levels were measured after precipitation of apo B-containing lipoproteins with heparin-manganese (18). LDL-C levels were calculated with the Friedewald formula (19). Plasma apolipoprotein (apo) A-I and apo C-III levels were measured with immunoturbidimetric assays (Wako Diagnostics, Richmond, VA) (20).
Plasma concentrations of remnant-like lipoprotein cholesterol (RLP-C) were measured using an immunoseparation technique (Polymedco, Cortlandt Manor, NY) (21).
Apo A-I-containing HDL subpopulations were measured by 2-dimensional gel electrophoresis, as previously described (22). HDL were separated into 8 subpopulations (pre-β1, pre-β2, α1, α2, α3, pre-α1, pre-α2, and pre-α3) according to charge, size, and composition. The concentration of each HDL subpopulation was calculated multiplying its percentage by the total plasma apo A-I concentration and was expressed as mg/dl of apo A-I.
Plasma CRP levels were measured with an enzyme-linked immunosorbent assay (American Laboratory Products Co., Windham, NH) (23).
Coronary angiography
Quantitative coronary angiography was performed at baseline and after approximately 3.2 y of follow-up, as previously described (9;16). Analysis of angiograms was performed in pairs with a previously validated system of cine-projection (SME 3500, Sony, Park Ridge, NJ). The mean intra-operator difference between blinded duplicate measurements of minimal diameter was 0.02 mm. The reference, minimal (the point of greatest narrowing), and average luminal diameters were obtained for 10 proximal epicardial coronary artery segments, as previously described (9;16).
Statistical analysis
All analyses were based on intent-to-treat. Skewed variables were log-transformed before analysis. Changes in plasma lipid and lipoprotein variables were calculated as: Value year 1 – Value baseline. The change in mean minimum coronary artery diameter (MMD) was calculated as: MMD follow-up − MMD baseline. We have previously reported a similar effect of CEE and CEE+MPA on plasma lipoproteins (24). Therefore women randomized to either one of these two treatments were combined in one group and compared to placebo-treated women. The effects of treatment on plasma concentrations of lipids, apolipoproteins, and lipoprotein subspecies were tested by multiple regression analysis. The model was adjusted for age, race, smoking, and use of lipid-lowering medications. Diabetes and the interaction between diabetes and treatment were also included in the model. Analysis of covariance was used to test the hypothesis of an association between changes in MMD and changes in lipoprotein subspecies. The model was adjusted for baseline minimal coronary lumen diameter, age, BMI, race, smoking, use of lipid-lowering medications, A P value <0.05 was set as statistically significant. The magnitude of treatment effect, or effect size, was assessed with the formula by Cohen: d = M1 - M2/SDpooled, where M1 is the mean change during placebo, M2 is the mean change during HRT, and SDpooled is = √[(SD 1+ SD 2)/2] (25). According to Cohen (25), an absolute d value of less than 0.3 is defined as a small effect size, while a d greater than 0.8 is considered a large effect size.
RESULTS
At baseline, 182 of the ERA subjects participating in this study were free of diabetes and 68 had type 2 diabetes mellitus (Table 1). Women with diabetes were significantly younger and had a higher BMI than women without diabetes. The percentage of African-Americans was higher in the diabetes group than in the group without diabetes. Baseline fasting plasma glucose levels were higher in diabetic than non-diabetic women, but blood pressure and coronary artery mean minimum lumen diameter were similar between the two groups of women.
Table 1.
Baseline characteristics of CHD women without and with diabetes in the ERA trial.
| No Diabetes (N=182) |
Diabetes (N=68) |
P * | |
|---|---|---|---|
| Age, y | 66 (7) | 64 (7) | 0.01 |
| BMI, kg/m2 | 28.22 (7.7) | 33.8 (6.7) | 0.0001 |
| Race, % | 0.001 | ||
| White | 88 | 69 | |
| Black | 8 | 26 | |
| Other | 4 | 5 | |
| Smoking, % | 24 | 16 | 0.24 |
| Lipid lowering medications, % | 33 | 34 | 0.88 |
| Plasma glucose, mg/dl | 99 (16) | 170 (51) | 0.0001 |
| Systolic blood pressure, mm Hg | 134 (18) | 138 (16) | 0.13 |
| Diastolic blood pressure, mm Hg | 74 (8) | 75 (9) | 0.52 |
| (N=158) | (N=59) | ||
| Min lumen diameter, mm | 1.93 (0.33) | 1.90 (0.40) | 0.49 |
Data are mean (SD);
P value, Mann-Whitney test for continuous variables and Fisher exact test for categorical variables
Plasma TG, remnant lipoprotein cholesterol (RLP-C), and apo C-III levels, but not plasma TC or LDL-C levels, were significantly higher in diabetic than in non-diabetic women at baseline (Table 2). Plasma HDL-C levels were significantly lower in diabetic women. However, no differences were observed in plasma apo A-I levels or in the apo A-I-containing HDL subpopulation profile between the two groups of women, with the exception of a slight elevation in levels of small pre-α3 particles in diabetic women (Table 2). Plasma CRP levels were significantly higher in women with diabetes than in non-diabetic women (Table 2). At year 1, after adjustment for treatment effects, the differences in plasma TG, remnant lipoproteins, and HDL-C between subjects without and with diabetes were even more marked. In addition, at year 1, the mean plasma concentration of HDL α2 particles was significantly lower in diabetic than in non diabetic women (Table 2). However, the difference in plasma CRP levels was no longer present.
Table 2.
Baseline and year-1 plasma lipid, lipoprotein, and CRP levels in CHD women without and with diabetes in the ERA trial.
| Baseline |
Year 1 |
|||||
|---|---|---|---|---|---|---|
| No Diabetes (N=182) | Diabetes (N=68) | P* | No Diabetes (N=182) | Diabetes (N=68) | P** | |
| mg/dl | mg/dl | mg/dl | mg/dl | |||
| TC | 217 (40) | 218 (46) | 0.50 | 209 (30) | 211 (42) | 0.63 |
| LDL-C | 137 (35) | 133 (43) | 0.09 | 122 (34) | 123 (39) | 0.45 |
| TG | 184 (104) | 225 (120) | 0.004 | 188 (116) | 238 (140) | 0.0001 |
| RLP-C | 11.8 (9.9) | 15.5 (12.3) | 0.007 | 10.8 (11) | 13.7 (10.7) | 0.003 |
| Apo C-III | 13.7 (4.7) | 15.8 (6.1) | 0.02 | 13.6 (4.6) | 15.9 (5.9) | 0.004 |
| HDL-C | 45 (12) | 42 (13) | 0.04 | 51 (15) | 45 (14) | 0.004 |
| Apo A-I | 126 (19) | 126 (19) | 0.80 | 140 (24) | 135 (24) | 0.14 |
| HDL subpopulations | ||||||
| pre-β1 | 23.5 (9.1) | 22.8 (8.1) | 0.59 | 24.1 (8.7) | 23.6 (9.2) | 0.56 |
| pre-β2 | 2.0 (1.2) | 2.3 (1.4) | 0.46 | 2.1 (1.3) | 2.6 (1.7) | 0.18 |
| α1 | 12.9 (8.1) | 12.2 (8.8) | 0.42 | 17.4 (10.4) | 15.4 (10.7) | 0.11 |
| α2 | 38.3 (10.6) | 37.0 (9.7) | 0.25 | 43.2 (12.2) | 38.9 (10.2) | 0.02 |
| α3 | 38.0 (8.8) | 39.3 (8.8) | 0.19 | 39.8 (9.9) | 40.6 (7.3) | 0.15 |
| pre-α1 | 2.6 (2.2) | 2.5 (2.0) | 0.96 | 3.4 (2.6) | 3.1 (2.4) | 0.98 |
| pre-α2 | 4.3 (1.9) | 4.5 (2.2) | 0.82 | 5.1 (2.3) | 4.8 (2.2) | 0.35 |
| pre-α3 | 4.7 (1.8) | 5.3 (2.1) | 0.02 | 4.8 (2.0) | 5.3 (2.3) | 0.09 |
| CRP | 0.52 (0.68) | 0.79 (0.74) | 0.03 | 0.77 (1.20) | 0.76 (0.59) | 0.09 |
Mean (SD);
P value, model covariates: age, race, smoking, and use of lipid-lowering medications;
model includes all covariates in * and treatment. Skewed variables were log-transformed before analysis.
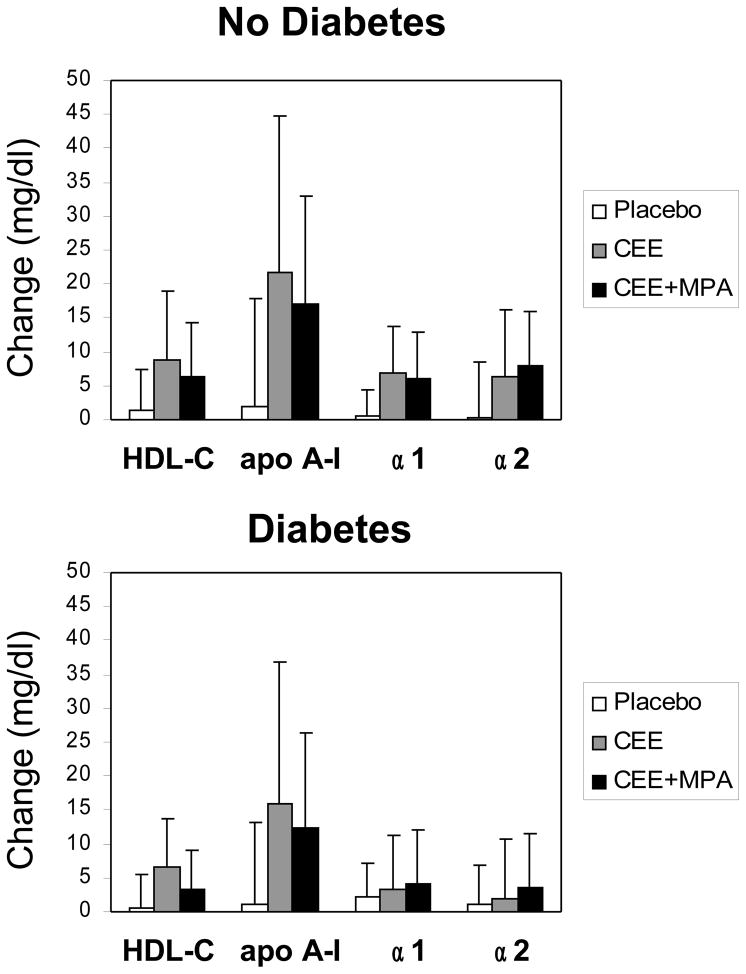
In a multiple regression analysis adjusted for age, race, use of lipid-lowering medications, and smoking, the on-trial changes in TC and LDL-C levels were significantly associated with treatment (Table 3). HRT did not significantly affect plasma TG or apo C-III levels. Nevertheless, plasma RLP-C levels were significantly reduced by HRT. Plasma HDL-C and apo A-I levels and the HDL subpopulations pre-β1, α1, α2, α3 and pre-α1 were significantly increased by HRT. Overall, HRT caused similar and significant changes in plasma lipoproteins in both diabetic and non-diabetic women, but the changes in HDL-C and preα2 particle levels were significantly lower in women with diabetes than those in healthy women. There was a statistically significant interaction (p<0.02) between HRT and diabetes status for the change in HDL α1 particle levels, with lower increases in α1 levels in diabetic women on HRT, relative to placebo, than in women without diabetes (Table 3). A trend for a similar interaction between HRT and diabetes was also observed for α2 particles. This is also indicated by the large effect size for α1 and α2 in non-diabetic but small effect size in diabetic women. The observed interaction was not affected by the type of HRT, as similar changes in HDL, apo A-I, α1 and α2 particle levels were observed in the CEE and CEE+MPA arms of the study, when analyzed by diabetes status (Figure 1).
Table 3.
Changes in plasma lipid, lipoprotein, and CRP levels, and coronary atherosclerosis progression in the placebo and the HRT arms of the ERA study by diabetes status.
| No diabetes |
Diabetes |
P*(treatment) | P*(diabetes) | P***(interaction) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (N=59) | HRT (N=123) | effect size d | Placebo (N=27) | HRT (N=41) | effect size d | ||||
| TC, mg/dl | 3.2 (37) | −11.6 (40) | 0.38 | −1.9 (30 | −7.5 (37) | 0.16 | 0.02 | 0.69 | 0.45 |
| LDL-C, mg/dl | −0.2 (31) | −18.5 (39) | 0.52 | 7 (21) | −14.5 (42) | 0.64 | 0.0001 | 0.13 | 0.86 |
| TG, mg/dl | 6.7 (89) | 1.4 (69) | 0.07 | 7.0 (128) | 20.5 (124) | −0.11 | 0.86 | 0.12 | 0.43 |
| RLP-C, mg/dl | 1.7 (9) | −2.3 (6.2) | 0.53 | −0.6 (8) | −2.6 (10.1) | 0.22 | 0.001 | 0.64 | 0.42 |
| Apo C-III, mg/dl | 0.3 (3.4) | −0.2 (3.3) | 0.15 | −0.2 (3.3) | 0.6 (4.3) | −0.21 | 0.96 | 0.76 | 0.15 |
| HDL-C, mg/dl | 1.8 (7) | 7.7 (8.9) | −0.74 | 0.4 (6) | 4.6 (7.2) | −0.63 | 0.0001 | 0.02 | 0.38 |
| Apo A-I, mg/dl | 1.8 (17) | 19.5 (20) | −0.95 | 1.2 (12) | 13.9 (18) | −0.83 | 0.0001 | 0.14 | 0.21 |
| HDL subpopulations, mg/dl | |||||||||
| pre-β1 | −1.1 (6.8) | 1.5 (6.4) | −0.39 | −2.0 (7.2) | 2.7 (7.5) | −0.64 | 0.0001 | 0.63 | 0.39 |
| pre-β2 | −0.1 (0.7) | 0.2 (0.8) | −0.40 | 0.2 (1.0) | 0.3 (1.1) | −0.10 | 0.06 | 0.33 | 0.38 |
| α1 | 0.5 (4.5) | 6.5 (7.2) | −0.99 | 2.3 (5.4) | 3.9 (8.3) | −0.23 | 0.0001 | 0.38 | 0.02 |
| α2 | 0.4 (8.4) | 7.0 (9.1) | −1.07 | 0.8 (5.9) | 2.7 (8.7) | −0.26 | 0.0001 | 0.07 | 0.06 |
| α3 | 0.4 (7.9) | 2.4 (7.5) | −0.26 | −0.9 (7.2) | 2.9 (7.8) | −0.50 | 0.02 | 0.70 | 0.63 |
| pre-α1 | 0.2 (1.4) | 1.1 (1.6) | −0.60 | 0.5 (1.5) | 0.8 (1.8) | −0.18 | 0.0001 | 0.65 | 0.09 |
| pre-α2 | 0.6 (1.5) | 0.9 (1.4) | −0.21 | 0.2 (1.7) | 0.4 (1.7) | −0.12 | 0.14 | 0.03 | 0.86 |
| pre-α3 | 0.5 (1.9) | −0.1 (1.5) | 0.35 | 0.1 (2.0) | 0.0 (1.9) | 0.05 | 0.04 | 0.53 | 0.37 |
| CRP, mg/dl | −0.05 (1.43) | 0.38 (1.10) | −0.34 | −0.18 (0.54) | 0.06 (0.58) | −0.48 | 0.01 | 0.08 | 0.52 |
| (N=49) | (N=108) | (N=22) | (N=37) | ||||||
| mean min lumen diameter, mm |
−0.09 (0.19) | −0.08 (0.18) | −0.05 | −0.19 (0.18) | −0.14 (0.26) | −0.22 | 0.44 | 0.04 | 0.46 |
Mean(SD)
Effect size: Cohen’s d
P value for treatment effect, model adjusted for age, race, smoking, and use of lipid-lowering medications
P value for diabetes effect, model as above
P value for the interaction between treatment and diabetes, model as above
Figure 1.
Changes in HDL-C, apo A-I, and α1 and α2 particles according to treatment phase in postmenopausal women without diabetes (upper panel) and with diabetes (lower panel). Data shown as mean changes and SD. CEE, conjugated equine estrogen arm, CEE+MPA, conjugated equine estrogen plus medroxyprogesterone arm.
Plasma CRP levels were significantly increased by HRT, relative to placebo, but there was no interaction between treatment and diabetes status (Table 3).
Coronary artery atherosclerosis progressed significantly more in diabetic women than in healthy women, but HRT treatment did not affect the degree of progression in either group.
We have previously reported in this population a significant association of on trial changes in remnant lipoprotein cholesterol and HDL pre-β1 levels with progression of coronary atherosclerosis (24). As shown in Table 4, even though these associations were no longer significant when analyses were carried out separately in subjects without or with diabetes, the magnitude of the effect was similar in the two groups.
Table 4.
Association between progression in coronary atherosclerosis and change in plasma lipoproteins in women in the HRT arms of the ERA study.
| All |
No Diabetes |
Diabetes |
||||
|---|---|---|---|---|---|---|
| B | P | B | P | B | P | |
| RLP-C | 0.005 | 0.008 | 0.005 | 0.05 | 0.005 | 0.11 |
| Apo A-I | 0.001 | 0.20 | 0.000 | 0.68 | 0.003 | 0.17 |
| HDL-C | −0.001 | 0.77 | −0.001 | 0.53 | 0.000 | 0.96 |
| HDL subpopulations | ||||||
| Pre-β1 | 0.005 | 0.02 | 0.005 | 0.06 | 0.007 | 0.11 |
| α1 | 0.002 | 0.40 | 0.001 | 0.81 | 0.004 | 0.37 |
| α2 | 0.000 | 0.85 | −0.002 | 0.34 | 0.006 | 0.18 |
B: unstandardized coefficient; this provides a measure of the change in minimum lumen diameter (mm) per each 1 mg/dl change in dependent variable
P: P value
DISCUSSION
Type 2 diabetes mellitus significantly increases the risk of CHD(2). In this study, women with diabetes had a higher BMI, and were more likely to be African-Americans. These findings are in agreement with the current literature (2;26). Mean minimum coronary artery diameter was similar in diabetic and non-diabetic women, but diabetic women were significantly younger, indicating earlier disease with diabetes.
Subjects with diabetes characteristically display higher plasma TG and lower HDL-C levels than normal subjects. The increase in plasma TG levels is caused in part by an increased synthesis of TG-rich very low-density lipoproteins (VLDL) by the liver (7). In diabetes, insulin resistance promotes the release of free fatty acids from adipose tissue, resulting in greater hepatic availability of substrate for VLDL synthesis (27). In addition, a reduction in TG-rich lipoprotein clearance has been observed in insulin-resistance states, due to an increase in plasma levels of apo C-III, an inhibitor of lipoprotein lipase (LPL) (28). The combination of increased synthesis and delayed clearance results in higher concentrations of circulating TG-rich and remnant lipoproteins and an increased atherogenic potential. TG-rich lipoproteins also act as substrate for the cholesteryl ester transfer protein (CETP): in hypertriglyceridemic states, the increased CETP activity, coupled with an increase in the expression of hepatic lipase, leads to increased catabolism of the large HDL particles resulting in decreased overall HDL size and HDL-C levels (8). Likewise, in our study, women with diabetes had significantly higher plasma levels of TG and remnant lipoproteins and lower plasma levels of HDL-C than non-diabetic women. These observations are consistent with findings from the Framingham Offspring study (29). In addition, we found significantly higher plasma levels of apo C-III in women with diabetes, in agreement with previous findings (30) (31). Apo C-III gene expression is down-regulated by insulin (32).
Women with diabetes had a faster progression of coronary disease, relative to women without diabetes, but HRT did not affect disease progression in either group. Similarly to our findings, Howard et al (15) had found faster atherosclerosis progression in diabetic women than in non-diabetic women participating in the Women’s Angiographic Vitamin and Estrogen (WAVE) Study. However, when the effect of HRT on coronary disease progression was examined in the latter study, it was found that HRT was associated with significantly faster atherosclerosis progression in coronary segments initially free of disease in diabetic women, but not in non-diabetic women (15). These effects were accompanied by a worsening of CRP levels with HRT in women with diabetes but not in women without diabetes (15). No effect of HRT was observed on the progression in diseased segments (15). These results are somewhat in disagreement with our data, which indicate no interaction between HRT and diabetes status on CRP or atherosclerosis progression. However, in our analysis, we did not distinguish between diseased and non-diseased coronary artery segments. In a cross-sectional study of 212 diabetic and 411 non-diabetic postmenopausal women, Dubuisson et al (33) found greater carotid artery intima-media thickness in women with diabetes, relative to women without diabetes. However, current and former HRT use was associated with lower intima-media thickness in the internal but not the common carotid artery, relative to non-users, both in diabetic and non-diabetic women (33). The observational nature of the study may explain the protective effect of HRT on atherosclerosis, as most observational studies have suggested a reduction in CHD risk with HRT (34).
HRT has been shown to increase plasma TG levels by increasing the production of TG-rich VLDL particles (35). Thus, HRT would be expected to worsen hypertriglycridemia in diabetes. We found that women with diabetes had a non-significant increase in TG levels during HRT, but this was not statistically different from the effect of HRT on TG levels in women without diabetes. On the other hand, both groups of women experienced a significant, and similar in magnitude, reduction in remnant lipoproteins. A significant reduction in the postprandial elevation in TG levels in diabetic women treated with HRT, relative to placebo, has been reported (36), and a reduction of intestinal TG-rich lipoproteins during treatment with estrogen has been reported in non-diabetic women (37). The reduction in postprandial TG and in remnant lipoprotein levels with HRT could be mediated by a faster clearance of TG particles, either via enhanced receptor-mediated clearance or by increased lipolysis. Estrogen has been shown to increase the expression of hepatic LDL receptors in rats (38) and the expression of LPL in heart muscle in mice (39). In our study, apo C-III was not significantly modified by HRT, making it unlikely that apo C-III contributed to the reduction in remnant lipoproteins with HRT. HRT has been shown to improve insulin-resistance in postmenopausal women (13), but it is not known if this plays a role in the lipoprotein changes. HRT is also known to reduce plasma LDL-C and increase plasma HDL-C levels. We found that HRT was effective in lowering LDL-C both in women with and without diabetes. In addition, HRT resulted in significant increases in plasma HDL-C and apo A-I levels.
During HRT, a significant increase in the level of the major HDL subpopulations was observed. We observed an interaction between treatment and presence of diabetes for the change in HDL particles, with a significantly smaller increase in large α1 particle levels and a trend for smaller increases in α2 particle levels by HRT in diabetic compared to non-diabetic women. Moreover, α2 particle levels were significantly lower and α1 particle levels tended to be lower in women with diabetes than in women without diabetes at year 1, after adjustment for treatment effects, suggesting that diabetes is associated with both lower levels of the large HDL particles and with a reduced response to HRT. The reduced increase in α1 and α2 particles following HRT treatment in diabetic women, compared to non-diabetic women, may be due to the fact that diabetic women had significantly higher TG levels than non-diabetic women and therefore an impaired HDL metabolism. It is well documented that high plasma levels of α1 and α2 particles are significantly associated with less atherosclerosis (40–42). Therefore, it may be hypothesized that, in diabetic women, lower levels of large HDL particles lead to a less efficient reverse cholesterol transport and contribute to a faster atherosclerosis progression. The faster coronary atherosclerosis progression in diabetic women than in non-diabetic women is consistent with the increased risk of CHD conferred by the presence of diabetes and indicates that “beneficial” changes in plasma lipoproteins with HRT do not significantly retard the progression of atherosclerosis in diabetic women. Moreover, since post-treatment levels of TG-rich lipoprotein and HDL were still worse in diabetic than in non-diabetic women, these differences may still have contributed to the faster progression of CHD in diabetic women.
A limitation of this study is that our data do not provide definitive information but rather suggest provocative patterns that need to be more fully explored in larger data sets. Since HRT is still used by postmenopausal women for the relief of vasomotor symptoms, if diabetes does indeed attenuate the favorable effects of HRT on HDL particles, our results would shed additional light on the results of HRT clinical trials and provide additional motivation for glucose regulation in women contemplating HRT.
In conclusion, HRT-related changes in markers of CHD risk are significantly affected by the presence of abnormal glucose metabolism. More research is warranted to determine if normalization of glucose metabolism will lead to more favorable effects of HRT on lipoprotein profiles.
Acknowledgments
We are indebted to Georgia Saylor for database construction and analyses. This work was supported by the National Institutes of Health/National Heart Lung and Blood Institute grant R01 HL70081 to S.L.-F., and by the U.S. Department of Agriculture, under agreement No. 58-1950-7-707. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture.
Footnotes
Conflict of interest: The authors have no relevant conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov registry number: NCT00000549
References
- 1.American Heart Association. Heart disease and stroke statistics - 2008 update. American Heart Association; 2008. pp. 1–40. [Google Scholar]
- 2.The Expert Panel. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Krolewski AS, Rosner B, et al. A prospective study of maturity-onset diabetes and risk of coronary heart disease and stroke in women. Arch Int Med. 1991;151:1141–47. [PubMed] [Google Scholar]
- 4.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective studies. BMJ. 2006;332:73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RD, Cupples A, Schaefer EJ, Wilson WPF. Lipoproteins, apolipoproteins, and low-density lipoprotein size among diabetics in the Framingham offspring study. Metabolism. 1996;45:1267–72. doi: 10.1016/s0026-0495(96)90246-2. [DOI] [PubMed] [Google Scholar]
- 6.Olefsky JM, Farguhar JW, Reaven GM. Reappraisal of the role of insulin in hypertriglyceridemia. Am J Med. 1974;57:551–60. doi: 10.1016/0002-9343(74)90006-0. [DOI] [PubMed] [Google Scholar]
- 7.Cummings MH, Watts GF, Umpleby AM, Hennessy TR, Naoumova R, Slavin BM, et al. Increased hepatic secretion of very-low-density lipoprotein apolipoprotein B-100 in NIDDM. Diabetologia. 1995;38:959–67. doi: 10.1007/BF00400586. [DOI] [PubMed] [Google Scholar]
- 8.Frenais R, Ouguerram K, Maugeais C, Mahot P, Maugere P, Krempf M, et al. High density lipoprotein apolipoprotein AI kinetics in NIDDM: a stable isotope study. Diabetologia. 1997;40:578–83. doi: 10.1007/s001250050718. [DOI] [PubMed] [Google Scholar]
- 9.Herrington DM, Reboussin DM, Brosnihan B, Sharp PC, Shumaker SA, Snyder TE, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522–29. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- 10.The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. The Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 11.Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 12.Hulley SB, Grady D, Bush T, Furberg CD, Herrington DM, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 13.Salpeter SR, Walsh JME, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacemernt therapy on components of the metabolic syndrome in postmenopausal women. Diab Obes Metab. 2006;8:538–54. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 14.Rossouw J, Prentice R, Manson JA, Wu L, Barad D, Barnabei V, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 15.Howard BV, Hsia J, Ouyang P, Van Voorhees L, Lindsay J, Silverman A, et al. Postmenopausal hormone therapy is associated with atherosclerosis progression in women with abnormal glucose tolerance. Circulation. 2004;110:201–6. doi: 10.1161/01.CIR.0000134955.93951.D5. [DOI] [PubMed] [Google Scholar]
- 16.Herrington DM, Reboussin DM, Klein K, Sharp PC, Shumaker SA, Snyder TE, et al. Estrogen Replacement and Atherosclerosis (ERA) study: study design and baseline characteristics of the cohort. Control Clin Trials. 2000;21:257–85. doi: 10.1016/s0197-2456(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 17.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma lipoprotein fractions. Clin Chim Acta. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 18.Burstein M, Samaille J. Sur un dosage rapide du cholesterol lie aux alpha- et aux beta-lipoproteines du serum. Clin Chim Acta. 1960;5:609. doi: 10.1016/0009-8981(58)90020-2. [DOI] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.Contois J, McNamara J, Lammi-Keefe C, Wilson P, Massov T, Schaefer E. Reference intervals for plasma apolipoprotein A-I determined with a standardized commercial immunoturbidimetric assay: results from the Framingham Offspring Study. Clin Chem. 1996;42:507–14. [PubMed] [Google Scholar]
- 21.McNamara JR, Shah PK, Nakajima K, Cupples LA, Wilson WPF, Ordovas JM, et al. Remnant lipoprotein cholesterol and triglyceride reference ranges from the Framingham Heart Study. Clin Chem. 1998;44:1224–32. [PubMed] [Google Scholar]
- 22.Asztalos BF, Sloop CH, Wong L, Roheim PS. Two-dimensional electrophoresis of plasma lipoproteins: recognition of new apo A-I-containing subpopulations. Biochim Biophys Acta. 1993;1169:291–300. doi: 10.1016/0005-2760(93)90253-6. [DOI] [PubMed] [Google Scholar]
- 23.Lakoski SG, Brosnihan B, Herrington DM. Hormone therapy, C-reactive protein, and progression of atherosclerosis: data from the Estrogen Replacement on progression of coronary artery Atherosclerosis (ERA) trial. Am Heart J. 2005;150:907–11. doi: 10.1016/j.ahj.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 24.Lamon-Fava S, Herrington DM, Reboussin BA, Sherman M, Horvath KV, Schaefer EJ, et al. Changes in remnant and high-density lipoproteins associated with hormone therapy and progression of coronary artery disease in postmenopausal women. Atherosclerosis. 2009;205:325–30. doi: 10.1016/j.atherosclerosis.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 26.Bowman SA. Socioeconomic characteristics, dietary and lifestyle patterns, and health and weight status of older adults in NHANES, 1999–2002: a comparison of Caucasians and African Americans. J Nutr Elderly. 2009;28:30–46. doi: 10.1080/01639360802633938. [DOI] [PubMed] [Google Scholar]
- 27.Lewis G. Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol. 1997;8:146–53. doi: 10.1097/00041433-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Chan DC, Watts GF, Nguyen MN, Barrett P. Apolipoproteins C-III and A-V as predictors of very-low-density lipoprotein triglyceride and apolipoprotein B-100 kinetics. Arterioscler Thromb Vasc Biol. 2006;26:590–596. doi: 10.1161/01.ATV.0000203519.25116.54. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer EJ, McNamara JR, Shah PK, Nakajima K, Cupples LA, Ordovas JM, et al. Elevated remnant-like particle cholesterol and triglyceride levels in diabetic men and women in the Framingham Offspring Study. Diabetes Care. 2002;25:989–94. doi: 10.2337/diacare.25.6.989. [DOI] [PubMed] [Google Scholar]
- 30.Olivieri O, Bassi A, Stranieri C, Trabetti E, Martinelli N, Pizzolo F, et al. Apolipoprotein C-III, metabolic syndrome, and risk of coronary artery disease. J Lipid Res. 2003;44:2374–81. doi: 10.1194/jlr.M300253-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Cohn J, Patterson BW, Uffelman KD, Davignon J, Steiner G. Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C-III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:3949–55. doi: 10.1210/jc.2003-032056. [DOI] [PubMed] [Google Scholar]
- 32.Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, et al. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest. 2004;114:1493–503. doi: 10.1172/JCI19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubuisson JT, Wagenknecht LE, D’Agostino RB, Haffner S, Rewers M, Saad MF, et al. Association of hormone replacement therapy and carotid wall thickness in women with and without diabetes. Diabetes Care. 1998;21:1790–1796. doi: 10.2337/diacare.21.11.1790. [DOI] [PubMed] [Google Scholar]
- 34.Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of epidemiologic evidence. Prev Med. 1991;20:47–63. doi: 10.1016/0091-7435(91)90006-p. [DOI] [PubMed] [Google Scholar]
- 35.Walsh BW, Schiff I, Rosner B, Greenberg LJ, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325:1196–204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- 36.Friday KE, Dong C, Fontenot RU. Conjugated equine estrogen improves glycemic control and blood lipoproteins in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2001;86:48–52. doi: 10.1210/jcem.86.1.7094. [DOI] [PubMed] [Google Scholar]
- 37.Westerveld H, Kock LAW, Rijn HJM, Erkelens D, Bruin TWA. 17 beta-estradiol improves postprandial lipid metabolism in postmenopausal women. J Clin Endocrinol Metab. 1995;80:249–53. doi: 10.1210/jcem.80.1.7829621. [DOI] [PubMed] [Google Scholar]
- 38.Windler EET, Kovanen PT, Chao YS, Brown MS, Havel RJ, Goldstein JL. The estradiol-stimulated lipoprotein receptor in the rat liver. J Biol Chem. 1980;255:10464–71. [PubMed] [Google Scholar]
- 39.Liu D, Deschamps A, Korach KS, Murphy E. Estrogen-enhanced gene expression of lipoprotein lipase in heart is antagonized by progesterone. Endocrinology. 2008;149:711–16. doi: 10.1210/en.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asztalos BF, Cupples LA, Demissie S, Horvath KV, Cox CE, Batista M, et al. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 2004;24:2181–87. doi: 10.1161/01.ATV.0000146325.93749.a8. [DOI] [PubMed] [Google Scholar]
- 41.Asztalos BF, Collins D, Cupples LA, Demissie S, Horvath KV, Bloomfield HE, et al. Value of high-density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the Veterans Affairs HDL Intervention Trial. Arterioscler Thromb Vasc Biol. 2005;25:2185–91. doi: 10.1161/01.ATV.0000183727.90611.4f. [DOI] [PubMed] [Google Scholar]
- 42.Asztalos BF, Batista M, Horvath KV, Cox CE, Dallal G, Morse JS, et al. Change in alpha1 HDL concentration predicts progression in coronary artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23:847–52. doi: 10.1161/01.ATV.0000066133.32063.BB. [DOI] [PubMed] [Google Scholar]