Abstract
Mouse models are increasingly contributing to our understanding of the neural genetics of sensory processing and memory. For example, strain differences have helped elucidate basic mechanisms of age-related hearing loss and auditory fear conditioning. Assessing sensory differences arising in acoustic communication contexts is also important for understanding natural audition. While this topic has not been well studied, it is currently being addressed through auditory neuroethological studies in the CBA/CaJ strain, where insights will help lay a foundation for future neural genetic studies. Here, we focus on the responses of adult females to ultrasonic vocalizations of males. We tested a group of female mice in a place-preference paradigm before and after auditory and olfactory experience with a male. A control group was housed with other female cagemates between trials. All females showed an initial preference for male calls that rapidly decayed over the course of a trial. However, only females that had been pair-housed with a male during the inter-trial interval displayed a reinstated interest in male vocalizations, suggesting possible group differences in the assessment of the calls’ behavioral relevance. These findings provide a timeframe during which auditory processing of male ultrasounds might be expected to show a difference depending on behavioral relevance, and also suggest an importance of social interactions in maintaining call recognition.
Introduction
Mouse models have been invaluable in revealing the genetic bases of auditory phenomena such as age-related hearing loss and fear conditioning. For example, studies incorporating strain comparisons were critical for identifying ahl as a gene involved in presbycusis (Erway et al., 1993; Johnson et al., 1997), and loci on chromosomes 1 and 10 for auditory fear conditioning (Wehner et al., 1997). Given such successes, there is hope that mouse models may be helpful in investigating more complex auditory tasks, such as the processing of behaviorally relevant communication sounds. This has previously been studied in several neuroethological animal models (Wang, 2000; Wilczynski et al., 2001; Sisneros and Bass, 2003; Clayton et al., 2009), and is more recently beginning to be explored in the mouse (Moles et al., 2004; Shu et al., 2005; Kimchi et al., 2007; Pierman et al., 2008; Scattoni et al., 2008b; Wang et al., 2008; Portfors et al., 2009), where one paper has even explicitly studied the impact of genetics on acoustic communication behavior (Panksepp et al., 2007). Further developing a vocal communication model in the mouse will not only increase our understanding of complex social behavior, but perhaps point toward therapeutic targets for genetic disorders involving communication deficits (Andres, 2002; Scattoni et al., 2008a).
Previous work on mouse communication has demonstrated that the auditory cortical processing of at least one class of ultrasonic vocalizations undergoes neural plasticity. Specifically, mothers and pup-naïve virgin females represent isolation-induced pup calls differently in a way that may enhance the calls’ detection and discrimination for the former (Liu and Schreiner, 2007; Galindo-Leon et al., 2009). However, since large hormonal changes accompany motherhood, dissecting the mechanisms underlying this plasticity is complicated (Miranda and Liu, 2009). Hence, an alternative context that does not conflate experience and hormonal changes may provide a better platform for investigating the neural processes responsible for recognition of species-specific communication sounds.
Here, we begin laying the foundation for one such model: adult male-female ultrasonic communication. While male mice are known to produce calls before and during mating with a female (Sales, 1972; Nyby, 1983; White et al., 1998), the purpose of these calls has not been well elucidated. Researchers have speculated that they serve a “courtship” role because the vocalizations have acoustic structure analogous to bird song (Holy and Guo, 2005). However, relatively little is known about the female’s response to the vocalizations – a prerequisite to investigating the neural basis of this acoustic communication. Earlier work has shown that adult females have an innate interest in intact vocalizing males (Pomerantz et al., 1983), but a recent study indicates females habituate rapidly to the pure playback of male calls in the absence of real males (Hammerschmidt et al., 2009). In order to validate this potential model of experience-dependent changes in the processing of social stimuli, we designed a behavioral apparatus to look at the female’s response to courtship calls before and after they gain behavioral relevance.
Methods
Animals
The Emory University Institutional Animal Care and Use Committee approved all procedures. Experiments were carried out on 31 female CBA/CaJ mice between 12 and 22 weeks old. The mice were weaned at approximately 21 days and housed by sex in groups of 2 to 4. Importantly, after weaning, females had no contact with male mice. The animals were housed in cages padded with Alpha Dri bedding with ad libitum access to standard rodent chow and water. The cages were housed in a temperature-controlled colony room on a reverse light cycle (14 hr. light/10 hr. dark). All experiments took place between 9:00 and 13:00 during the dark phase of the light cycle, but testing occurred in red light and care was taken to keep the animals in darkness immediately before and after testing.
Each animal was tested in our behavioral apparatus twice, with approximately six days between trials. Efforts were taken to perform both tests around the same time of day. The animals were divided into two experimental groups: male-exposed and litter housed. Litter housed animals were returned to their litter after the first trial and lived as usual during the interval between trials. Male-exposed animals were housed with their litters for 3 days immediately following their first behavioral test. Then, they were removed from their litters and housed in a divided cage with a male mouse for 72 hours. We limited the pair-housing period to 72 hours because we wanted to test the effect of experience without introducing hormonal changes caused by long-term exposure to male pheromones that may bias the results (Whitten, 1956). Their second trial took place the day after the pair-housing phase ended. Immediately before and after pair-housing with a male, these females were housed with their littermates. Divided cages used for pair-housing were obtained from Tecniplast (Exton, PA, USA). The cages measured 14″L × 6″W × 5.5″D and were split in half lengthwise by a translucent red divider that appears opaque to murine species. Each animal had its own water supply and access to a shared food compartment. Two arrays of holes (each with 3mm diameter) were punched into the divider to allow the transmission of auditory, olfactory, and very restricted visual stimuli. Only males that vocalized regularly when presented with fresh bedding from a female cage were chosen as partners for pair-housing. All males were between 14 and 22 weeks old. Previous research has shown that males emit courtship vocalizations in response to direct contact with females or olfactory stimulation from female urine (Dizinno et al., 1978; Holy and Guo, 2005).
Behavioral Testing
Animals were tested in an anechoic chamber, in an arena made of clear polycarbonate, measuring 18″L × 10″W × 8″H. The arena was bisected width-wise with a plastic divider to delineate two distinct chambers. A doorway in the divider allowed for animals to travel freely between the chambers. Two 2″×2″ arrays of 3mm holes were drilled into a long side of the arena such that each chamber would have one array centered between the divider and the far wall. Two speakers were placed on the outside of the cage, aligned with the arrays of holes. Prior to testing, a layer of clean Alpha-Dri bedding was added to the arena. Between trials, the bedding was changed and the arena cleaned with a 5% bleach solution.
The day before testing, animals were removed from their litters and allowed 10 minutes to freely explore the testing apparatus. No sound playback occurred during this habituation phase. Both the habituation period and the behavioral test were monitored from the outside via video recording equipment. On the day of testing, the animal was removed from her home cage and given 5 minutes to explore the arena before the trial began. At the 5-minute mark, the trial began when video tracking software (TopScan by Cleversys, Reston, VA, USA) was prompted to begin recording the animal’s position. Data on the animal’s position was fed into a playback system (Tucker-Davis Technologies, Alachua, FL, USA) whose output was dependent on which chamber the animal was in (Figure 1a). At any time, the speaker projecting into the same chamber as the female was active, while the one in the opposite chamber was inactive. In a given trial, one speaker was programmed to broadcast courtship vocalizations while the other was to remain silent even while “active”. The females could therefore choose to listen to courtship calls or silence. A bat detector placed in the anechoic chamber confirmed playback of the test stimuli. The playback speaker was counterbalanced across females, and analysis of habituation videos confirmed the lack of an innate preference for a particular chamber. Ten minutes after the initiation of the test, the experiment was terminated and the female was returned to her home cage.
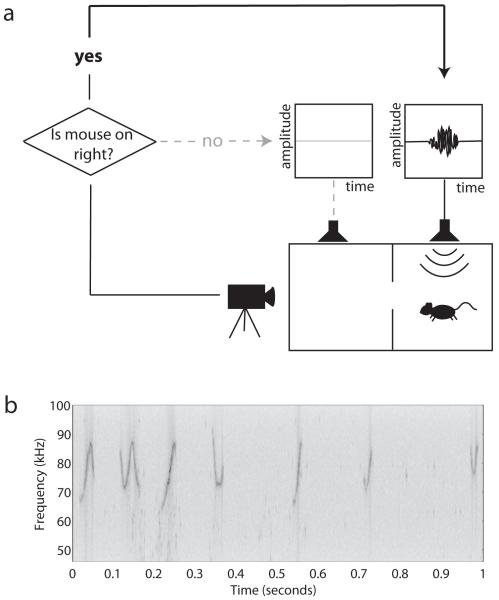
Figure 1.
Behavioral apparatus and clip of playback sound
a During the trial, the mouse moves freely throughout an arena with two distinct chambers. A video camera feeds footage of the trial into a desktop computer equipped with video tracking software. The tracking program discriminates which chamber the mouse is in and sends this information to a TDT playback system, which activates the speaker that projects into the chamber where the mouse is. In a given trial, the sound file associated with one speaker contains only silence while the other contains recorded male vocalizations. b A one-second long sample from the minute-long recording used as stimulus. The recording was made from an adult male CBA/CaJ mouse in response to female olfactory stimuli. The sample shown here is a one-second long clip taken from the middle of the recording (approximately 2 seconds in).
To obtain the recordings of male calls we played back to the females, CBA/CaJ males were screened for their inclination to vocalize when presented with soiled bedding from a female cage. Of the recordings gathered, one (elicited from a 15-week-old male) was selected for its fairly high density of call bouts. A one-minute clip of this recording was extracted, filtered in MATLAB (The MathWorks, Natick, MA, USA) between 46 and 100 kHz to remove lower frequency background noise, and programmed in RPVdsEx (Tucker-Davis Technologies, Alachua, FL, USA) to play through a TDT playback system (223214.625 samples/second). The calls were played back so that the loudest calls had a maximum sound pressure level of 76-79 dBSPL, though the amplitudes of other calls were variable because they were recorded naturally. Figure 1b shows a one-second sample of the minute-long clip we used during experimentation. Although the audio file was looped to play continuously, the speakers tuned in and out of the playback file depending on which chamber the animal was in. The same clip was played to each female during both of her experimental sessions.
TopScan animal tracking software was used to generate a record of the animal’s position throughout the trial. In MATLAB, the data file was parsed into a series of minute-long blocks to allow for behavioral analysis on a relatively fine timescale. With the tracking data, we were also extract behavioral information from each trial. Amount of time spent near the speaker was calculated for each chamber (playback and silent) by defining a rectangular zone around the speaker and counting the number of frames the animal could be found within its boundaries. Distance travelled was quantified by integrating the path length of the animal over the course of a pre-defined block of time (usually one minute).
Vaginal Cytology
To determine the mouse’s state of estrous, vaginal cytology was examined using methods similar to those previously described (Goldman et al., 2007). Briefly, the vagina was flushed with 0.9% sodium chloride, then the fluid was drawn back into the pipette and dropped onto pre-cleaned glass microscope slides. The slides were evaluated fresh and unstained on a light microscope. A smear containing only cornified epithelial cells was characteristic of estrus. Metestrus was characterized by a high density of cornified epithelial cells and the presence of small numbers of leukocytes. Heavily leukocytic swabs indicated diestrus. Proestrus could be identified by a low density of nucleated epithelial cells and the absence of leukocytes. Females who did not cycle regularly were excluded from the study.
Statistical Analysis
Statistical tests were carried out in MATLAB and Excel. Prior to comparing means, a two-tailed Lilliefors test was used to determine goodness-of-fit to a normal distribution. If either population in a desired comparison was determined to be statistically different from a normal distribution, a Mann-Whitney U test was used to determine whether the medians of the two data sets were the same. If both data sets were found to be normally distributed, a two-tailed t test was used to compare means. If they were not normally distributed, a Wilcoxon rank sum test was used to compare the samples. Paired t-tests were used to examine the effects of repeated testing within the different housing groups.
Results
During the first trial, in which all animals were naïve to courtship vocalizations, a significant preference for the chamber broadcasting vocalizations was observable across all 31 animals during the first 60 s (>30 s, t(30) = 3.76, p < 0.005). After the first minute, the proportion of time spent in the vocalization chamber dropped to a level no different than chance (Figure 2). In trial two, after half the animals had been pair-housed with adult males for approximately 3 days, a preference for the chamber broadcasting vocalizations was apparent only among these male-exposed females in the first 60 s (>30 s, t(15) = 3.77, p < 0.005). Females that had been litter-housed during the inter-trial interval displayed a lasting habituation to the sounds, showing no preference for the vocalization chamber (32.2 s, t(14) = 0.66, p = 0.51).
Figure 2.
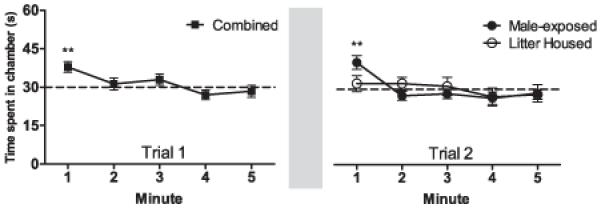
Innate and experience-dependent preference for courtship calls
At left: Amount of time during trial 1 spent in the chamber broadcasting vocalizations, broken down into minute-long blocks. Data are pooled across groups, as there were no significant differences between male-exposed and litter housed groups at these time points (n = 31). In the minute after the animals first heard vocalizations, a significant preference was shown for the chamber where the calls were being played, but this effect quickly diminished over time. The gray bar shown represents the delay (~6 days) between trials 1 and 2.
At right: Amount of time during trial 2 spent in the chamber broadcasting vocalizations. Litter housed animals (n = 15) showed no preference for the chamber where calls were being played back. Females that had been pair-housed (n = 16) initially showed a significant preference for the vocalizations that diminished during subsequent minutes. **p < 0.005. Data are represented as means +/− SEM.
Taking a more detailed look at changes within animals, it is apparent that all 31 animals lost interest in the male calls over the course of the first trial, as they showed a significant decrease (−9.5 s, t(30) = 3.80, p < 0.001) in preference from beginning to end of trial 1 (Figure 3). Comparing each animal’s change in preference from the beginning of trial 1 to the beginning of trial 2 within groups shows that while the litter housed females incurred a decrease in preference for the vocalizations (−7.9 s, paired t(14) = 2.21, p < 0.05), male-exposed females did not display a significant change in their preference (+4.9 s, paired t(15) = 1.12, p = 0.28). Moreover, comparing performance from the end of trial 1 to the beginning of trial 2, litter housed females did not show a change in interest in male calls (+2.3 s, paired t(14) = 0.44, p = 0.67), while animals that had been pair-housed with a male between trials showed a significant increase in preference for the vocalizations (+13.7 s, paired t(15) = 2.81, p < 0.05).
Figure 3.
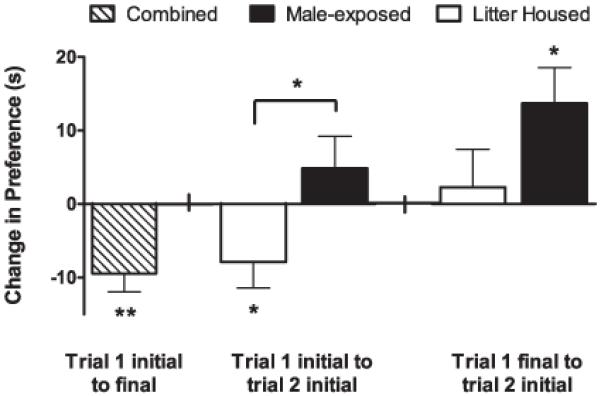
Within-animal changes in preference for calls from trial to trial
At far left: From the beginning (minute 1) of the first trial to the end (minute 5) of the first trial, all animals (n = 31) showed a significant decrease in preference for the male calls, representing a habituation to the sounds. Middle: Litter housed animals (n = 15) showed a significant decrease in preference for male calls from the beginning (minute 1) of the first trial to the beginning (minute 1) of the second trial. In contrast, male-exposed females (n = 16) did not exhibit a significant change in their preference during this time.
At far right: The preference of litter housed animals (n = 15) for male calls did not change between the end of trial 1 (minute 5) and the beginning of trial 2 (minute 1). Meanwhile, the females that had been pair-housed (n = 16) during the inter-trial interval showed a significant increase in their preference calls from the end of trial 1 (minute 5) to the beginning of trial 2 (minute 1). *p < 0.05, **p < 0.005. Data are represented as means +/− SEM.
We looked to the recordings taken by our video tracking system to characterize the locomotor behavior of each animal, but we did not observe any significant trends. During their first trial, the animals did not spend significantly more time next to the speaker in the playback chamber than they did near the speaker in the silent chamber (+17.3 s, t(30) = 1.72, p = 0.10). No new biases arose upon testing the animals a second time (male-exposed: +30.3 s, t(45) = 0.11, p = 0.48; litter-housed: +35.7 s, t(44) = 0.16, p = 0.31). We attempted to quantify the animals’ activity level by measuring how far they traveled over the course of their trial, but means for the male-exposed and litter-housed groups in their second trial were no different when compared to the group mean for the first trial (for minute one, male-exposed: +11.8 cm., t(45) = 0.08, p = 0.57; litter-housed: −16.9 cm., t(44) = 0.12, p = 0.43; no significant differences observed at later timepoints, as well). We counted the number of crossings between chambers as another assay of activity level, but again, mean number of crossings for the male-exposed and litter-housed animals were no different when compared to the group mean for the first trial (for minute one, male-exposed: +1.1 crossings, t(45) = 0.21, p = 0.17; litter-housed: + 1.5 crossings, t(44) = 0.24, p = 0.11; no significant differences observed at later timepoints, as well). Thus, although the animals exhibited preferences early in each trial as measured by time, their interest was not reflected in average measures of their locomotor behavior.
Estrous status was taken into consideration (Figure 4), but estrous females did not differ in their preference for the vocalizations compared to nonestrous females during the first minute of their first (1.4 s, t(14) = 0.05, p = 0.84) or second trials (1.2 s, t(14) = 0.05, p = 0.84). No significant differences were observed in subsequent minutes either. Therefore, estrous and nonestrous animals were combined for analysis.
Figure 4.
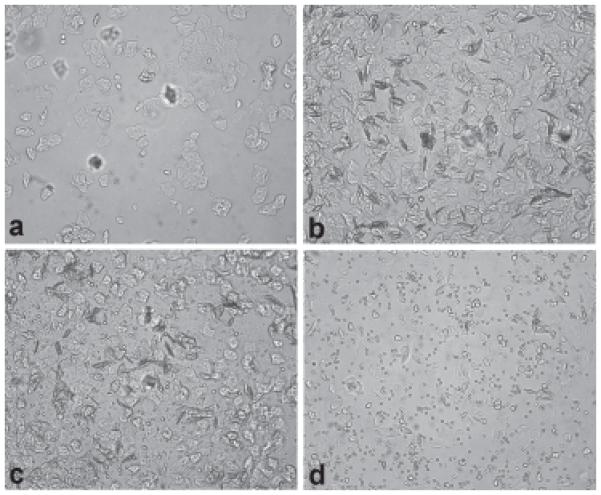
Vaginal cytology
a Proestrus is characterized by a low cell density and the presence of nucleated and some cornified epithelial cells. Leukocytes should be absent. b Estrus is indicated by a swab dominated by cornified epithelial cells. Again, leukocytes are largely absent. c During metestrus, the sample will contain a dense mixture of cornified epithelial cells and leukocytes. Some nucleated epithelial cells may be apparent. d Diestrus swabs are heavily leukocytic, with light expression of epithelial cells.
Discussion
Our study yielded three main findings. First, virgin female mice had an innate interest in adult male ultrasonic vocalizations, but expression of this preference decayed rapidly in an artificial setting without a real male mouse. Second, male-exposed females showed a reinstated preference for these calls upon retest, while litter-housed females remained habituated to the vocalizations. Finally, preference for the calls was not modulated by the female’s estrous state. These results suggest that an initial behavioral relevance of male calls requires reinforcement to retain significance, and this can be provided through a meaningful social interaction with males.
The precise influences that establish the behavioral relevance of the calls are yet to be identified, although our experiments provide some clues. In the divided cage where pair-housing took place, females were exposed to olfactory and acoustic cues produced by the male. It may be that acoustic exposure to courtship calls alone was sufficient to rekindle the female’s interest in the sounds. However, this seems unlikely because litter-housed females should have then demonstrated some reinstatement of preference during their second trial as a result of simply hearing the calls during their first trial. Alternatively, the female may have acquired important information about the significance of the calls through the natural pairing of acoustic and olfactory stimuli from the male. Females are innately attracted to male pheromones (Moncho-Bogani et al., 2002; Ramm et al., 2008; Martinez-Garcia et al., 2009), to the extent that pheromones can be used to induce a conditioned place preference (Martinez-Ricos et al., 2007). If male pheromones are so attractive they can be used as positive reinforcers in behavioral tests, it is possible that during pair-housing with a male, the females we tested began associating male ultrasonic vocalizations with innately rewarding olfactory cues. In our experiment then, male calls may have served as a conditioned stimulus that exerted its own attractive power over females when they were tested after pair-housing with a male.
From their responses, we argue that the animals likely formed different memories of the male calls’ behavioral relevance. Male-exposed females probably formed an associative memory during the paired-housing period that lent the calls initial attractiveness when they were played back later. In contrast, litter-housed females had heard the same calls before, but these sounds were not associated with anything relevant, and so they came to carry no particular meaning. The different behavioral responses may simply reflect different motivational states of the two groups. Additionally though, this behavioral disparity may also correlate with differences in auditory cortical coding. Indeed, previous work in a different behavioral context has shown that the neural representation of mouse pup vocalizations changes when a virgin female mouse becomes a mother (Fichtel and Ehret, 1999; Liu et al., 2006; Liu and Schreiner, 2007; Galindo-Leon et al., 2009). Specifically, pup call-evoked neural activity in mothers compared to virgins tends to improve the formers’ ability to detect and/or discriminate those calls. Our current study may provide a new behavioral context in which to explore experience-dependent plasticity for communication sounds within the auditory system. A male-female communication model may even provide additional advantages, since the hormonal changes associated with pregnancy and lactation in the maternal model may well interact with experience to produce auditory plasticity (Miranda and Liu, 2009). Since estrus state did not modulate the preference of the virgin female in the current study, the role of experience may be less confounded here by hormonal factors.
Overall, the results of our study were consistent with the conclusions of a recent study by Hammerschmidt et al. (2009), which showed that sexually inexperienced C57/6NCrl females expressed an innate preference for male calls, irrespective of estrous state. They additionally found that neither control tones nor ultrasonic pup calls elicited preferences from virgins. Hence, the two studies provide compelling evidence that the ultrasonic calls of male mice are intrinsically interesting to females. As in our case though, the preference reported by Hammerschmidt et al. (2009) was attenuated upon retest. Our results suggest a reason: the initial interest in the calls quickly dissipated without reinforcement by the natural source of the calls – the male.
Despite the overall similarity in our conclusions, the Hammerschmidt et al. (2009) study differed from ours in important details. Female preferences in our case were smaller in both magnitude and duration of expression. During the first trial, our females spent approximately 63% of their time in the chamber where they heard vocalizations, and this preference was only observable during the first minute of the session. In contrast, the females studied in Hammerschmidt et al. (2009) spent upwards of 90% of their time in the chamber where they had heard vocalizations and demonstrated this preference for three minutes. This disparity could be due to differences in mouse strains. Previous studies have shown that CBA/CaJ and C57 mice react differently in open field and elevated plus mazes. For example, two groups found that CBA mice show less activity in the open field than C57s (Southwick, 1968; Avgustinovich et al., 2000). Others found that CBAs exhibit low emotional reactivity in the plus maze compared to C57s, as indicated by their willingness to enter different arms of the maze (Griebel et al., 2000). Open field and plus maze apparatuses are often used to assess anxiety, and though these results are conflicting in terms of what they tell us about anxiety, they may help us explain the strain differences in preference expression we see here. If performance in the plus maze reflects emotional reactivity as Griebel et al. (2000) suggest, the robust expression of preference shown by C57 females may be due to greater reactivity to the courtship calls.
An alternative explanation for the quantitative preference differences in the two studies may lie with the differing behavioral measures employed. Our study was designed to assess the valence of male vocalizations: communication sounds were played as long as the female was in the chamber associated with the calls. Therefore, longer times meant that the calls actively kept the females there longer. It may have become self-evident early on that no male was actually producing calls, prompting a rapid extinction in preference. On the other hand, the results of Hammerschmidt et al. (2009) would have been more sensitive to how well the memory of calls could elicit female approach and investigation. There, calls were played from one side of the behavioral apparatus for one minute (irrespective of the female’s position in the box) and the female’s preference was only quantified following cessation of playback. Hence, the absence of a male would have been congruent with the absence of calls during that period, so that interest in the call chamber would not have been actively extinguished.
The development of animal models to explore the mechanisms underlying naturally occurring auditory plasticity is important. Studies of the neural representation of meaningful sounds have so far mostly used artificial contexts – behavioral conditioning or training – to make sounds behaviorally relevant (Weinberger, 2004). For instance, studies of primary auditory cortical plasticity induced by auditory fear conditioning have found that receptive fields display shifts toward the frequency of the conditioned stimulus immediately following training (Bakin and Weinberger, 1990). Similarly, training an animal to discriminate tones within a frequency band can result in an expanded representation of that frequency band in A1 over the course of many training sessions (Recanzone et al., 1993). However, whether the same forms of sensory plasticity occur in natural contexts when communication sounds acquire significance is less clear. Unlike the artificial sounds used in conditioning experiments, animal vocalizations are made meaningful through social processes, probably activating brain areas involved in arousal and reward differently than in fear or operant conditioning paradigms. Furthermore, to successfully interpret communication sounds, animals must simultaneously detect, discriminate, and categorize acoustic stimuli before making an appropriate behavioral response, whereas artificial paradigms usually require their subjects to perform only one of these tasks. For instance, a mouse dam will need to detect her pups’ calls in varying levels of background noise, discriminate these calls from similar sounds, and categorize all her pups’ calls as such despite individual differences in call structure.
By pursuing an ethological model of auditory processing in the mouse, we hope to eventually identify specific genetic loci that play a role in the recognition of species-specific vocalizations. We have taken a first step towards this by characterizing a behavioral response to a class of communication calls that is modifiable with experience and reproducible in different mouse strains. Although ethological models of acoustic communication are being studied, few take advantage of genomic information, which is readily available in the mouse. By further developing this line of study, we will gain a better understanding of the genetic control of neural plasticity in natural communication contexts, as well as potentially identify genes implicated in disorders that involve social communication deficits (Scattoni et al., 2008a).
Acknowledgements
The authors thank Tamara Ivanova for mouse husbandry; the National Institutes of Health (R01 DC008343 to RCL and T32 GM008605 to KS) and the National Science Foundation’s Center for Behavioral Neuroscience (IBN-9876754) for funding.
References
- Andres C. Molecular genetics and animal models in autistic disorder. Brain Research Bulletin. 2002;57:109–119. doi: 10.1016/s0361-9230(01)00642-6. [DOI] [PubMed] [Google Scholar]
- Avgustinovich DF, Lipina TV, Bondar NP, Alekseyenko OV, Kudryavtseva NN. Features of the genetically defined anxiety in mice. Behav Genet. 2000;30:101–109. doi: 10.1023/a:1001999020138. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Clayton DF, Balakrishnan CN, London SE. Integrating genomes, brain and behavior in the study of songbirds. Current Biology. 2009;19:R865–R873. doi: 10.1016/j.cub.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizinno G, Whitney G, Nyby J. Ultrasonic vocalizations by male mice (Mus-Musculus) to female sex-pheromone - Experiential determinants. Behavioral Biology. 1978;22:104–113. [Google Scholar]
- Erway LC, Willott JF, Archer JR, Harrison DE. Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hear Res. 1993;65:125–132. doi: 10.1016/0378-5955(93)90207-h. [DOI] [PubMed] [Google Scholar]
- Fichtel I, Ehret G. Perception and recognition discriminated in the mouse auditory cortex by c-Fos labeling. Neuroreport. 1999;10:2341–2345. doi: 10.1097/00001756-199908020-00022. [DOI] [PubMed] [Google Scholar]
- Galindo-Leon EE, Lin FG, Liu RC. Inhibitory plasticity in a lateral band improves cortical detection of natural vocalizations. Neuron. 2009;62:705–716. doi: 10.1016/j.neuron.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. Female mice respond to male ultrasonic ‘songs’ with approach behaviour. Biol Lett. 2009;5:589–592. doi: 10.1098/rsbl.2009.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Liu RC, Schreiner CE. Auditory cortical detection and discrimination correlates with communicative significance. PLoS Biol. 2007;5:e173. doi: 10.1371/journal.pbio.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci. 2006;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia F, Martinez-Ricos J, Agustin-Pavon C, Martinez-Hernandez J, Novejarque A, Lanuza E. Refining the dual olfactory hypothesis: pheromone reward and odour experience. Behav Brain Res. 2009;200:277–286. doi: 10.1016/j.bbr.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Martinez-Ricos J, Agustin-Pavon C, Lanuza E, Martinez-Garcia F. Intraspecific communication through chemical signals in female mice: reinforcing properties of involatile male sexual pheromones. Chem Senses. 2007;32:139–148. doi: 10.1093/chemse/bjl039. [DOI] [PubMed] [Google Scholar]
- Miranda JA, Liu RC. Dissecting natural sensory plasticity: hormones and experience in a maternal context. Hear Res. 2009;252:21–28. doi: 10.1016/j.heares.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D’Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Moncho-Bogani J, Lanuza E, Hernandez A, Novejarque A, Martinez-Garcia F. Attractive properties of sexual pheromones in mice: innate or learned? Physiol Behav. 2002;77:167–176. doi: 10.1016/s0031-9384(02)00842-9. [DOI] [PubMed] [Google Scholar]
- Nyby J. Ultrasonic vocalizations during sex behavior of male house mice (Mus musculus): a description. Behav Neural Biol. 1983;39:128–134. doi: 10.1016/s0163-1047(83)90722-7. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierman S, Sica M, Allieri F, Viglietti-Panzica C, Panzica GC, Bakker J. Activational effects of estradiol and dihydrotestosterone on social recognition and the arginine-vasopressin immunoreactive system in male mice lacking a functional aromatase gene. Horm Behav. 2008;54:98–106. doi: 10.1016/j.yhbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz SM, Nunez AA, Bean NJ. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiol Behav. 1983;31:91–96. doi: 10.1016/0031-9384(83)90101-4. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Roberts PD, Jonson K. Over-representation of species-specific vocalizations in the awake mouse inferior colliculus. Neuroscience. 2009;162:486–500. doi: 10.1016/j.neuroscience.2009.04.056. [DOI] [PubMed] [Google Scholar]
- Ramm SA, Cheetham SA, Hurst JL. Encoding choosiness: female attraction requires prior physical contact with individual male scents in mice. Proc Biol Sci. 2008;275:1727–1735. doi: 10.1098/rspb.2008.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales GD. Ultrasound and mating behavior in rodents with some observations on other behavioral situations. Journal of Zoology. 1972;68:149. &. [Google Scholar]
- Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: A tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2008a doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L, Crawley JN. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav Brain Res. 2008b;187:371–378. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W, Cho JY, Jiang Y, Zhang M, Weisz D, Elder GA, Schmeidler J, De Gasperi R, Sosa MA, Rabidou D, Santucci AC, Perl D, Morrisey E, Buxbaum JD. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci U S A. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisneros JA, Bass AH. Seasonal plasticity of peripheral auditory frequency sensitivity. Journal of Neuroscience. 2003;23:1049–1058. doi: 10.1523/JNEUROSCI.23-03-01049.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick CHC LH. Interstrain differences in aggressive behavior and exploratory activity of inbred mice. Communications in Behavioral Biology A. 1968:49–59. [Google Scholar]
- Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One. 2008;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. On cortical coding of vocal communication sounds in primates. Proc Natl Acad Sci U S A. 2000;97:11843–11849. doi: 10.1073/pnas.97.22.11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner JM, Radcliffe RA, Rosmann ST, Christensen SC, Rasmussen DL, Fulker DW, Wiles M. Quantitative trait locus analysis of contextual fear conditioning in mice. Nat Genet. 1997;17:331–334. doi: 10.1038/ng1197-331. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NR, Prasad M, Barfield RJ, Nyby JG. 40- and 70-kHz vocalizations of mice (Mus musculus) during copulation. Physiol Behav. 1998;63:467–473. doi: 10.1016/s0031-9384(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Modification of the oestrous cycle of the mouse by external stimuli associated with the male. J Endocrinol. 1956;13:399–404. doi: 10.1677/joe.0.0130399. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Rand AS, Ryan MJ. Evolution of calls and auditory tuning in the Physalaemus pustulosus species group. Brain Behavior and Evolution. 2001;58:137–151. doi: 10.1159/000047268. [DOI] [PubMed] [Google Scholar]