Abstract
Objective
Our objective was to determine signaling molecules and apoptosis rate in the term placenta of a baboon model of maternal nutrient reduction (MNR).
Study Design
Female baboons were fed ad libitum for controls (CTR; n=7) or 70% of CTR diet (MNR; n=6) from 30 to 165 days of gestation (dG) with necropsy at 165 dG. Placental tissues were collected, fixed for immunohistochemistry or snap frozen to measure ERK, AKT, JNK, XIAP and caspase 3. Placental villous apoptosis was determined by TUNEL and cytokeratin 18 cleavage.
Results
Compared to CTR, MNR placentas demonstrated: reduced placental weight (p<0.02), decreased p-ERK (p<0.04), increase placental villous apoptosis (p<0.001), increased villous cytokeratin 18 cleavage, increased XIAP protein (p<0.007) and increased active caspase 3 (p<0.02).
Conclusion
We conclude that placental apoptosis is increased in this baboon model of MNR at term and that the increase in XIAP may be a protective mechanism against this apoptosis.
Keywords: Apoptosis, XIAP, Caspase 3, placenta, MNR
Introduction
Placental development is important for fetal growth and development. Abnormal placentation is associated with the development of complicated pregnancies in humans. Poor maternal nutrition is a common problem in the developing world, which can have many adverse effects on the fetus including intrauterine growth restriction (IUGR). 1 A higher degree of syncytiotrophoblast apoptosis is found in placentas from pregnancies complicated by fetal growth restriction and pre-eclampsia. 2–5 Apoptosis, an active process of cellular destruction, serves an essential remodeling function in multi-cellular organisms and is a component of normal development and differentiation in most tissues including the placenta. 6, 7 Abnormal regulation of apoptosis in different tissues has been implicated in the onset and progression of a broad range of diseases. 2
The X-linked inhibitor of apoptosis protein (XIAP) is the most potent member of a group of inhibitor of apoptosis proteins (IAPs) that regulate cell death. 4 IAP posses a defining structural motif, called Baculovirus Inhibitor of Apoptosis Repeat (BIR), wich is responsible for the inhibition of caspases 3, 7 and 9. 8 Caspases are a family of cysteine proteases that plays a central role in initiating and executing the apoptosis cascade. 6, 9 In trophoblast cells, caspases are known to cleave the cytokeratin 18 proteinwhich produces a neo-peptide that is used as a marker of apoptosis. 10 Controlling the activity of caspases is essential for the appropriate execution of cell death and the regulation of cell survival. XIAP is present in trophoblast throughout placental development, but expression is significantly decreased near delivery when apoptosis is maximal. 11 A decrease in XIAP protein is associated with the presence of increased in apoptosis during IUGR in the pregnant sheep. 12 This suggests a role for XIAP in the regulation of trophoblast apoptosis in normal and abnormal processes in pregnancy.
Signal transduction cascades serve to elicit a cellular response initiated at the cell surface. These cascades contain multiple components involving series of posphorylation steps. The extracellular signal-regulated kinase 1/2 (ERK1/2) and the protein kinase B (AKT) pathways are found in many cells types and play various roles. These proteins mediate biological responses like cellular growth, proliferation and cell survival. 13 The c-Jun Amino Terminal Kinase (JNK) is another signaling molecule that is activated to diverse stimuli including DNA damage, UV radiation, heat shock and others. 14 There is a reduction of these signaling proteins in other models of IUGR. 15
While there are many studies on the effects of controlled reductions in maternal nutrient intake on apoptosis in rodents and sheep, data are lacking in non-human primates. 16–19 In the present study, we evaluated placental apoptosis and changes in key signaling molecules in the placentas of control pregnant baboons fed ad libitum and mothers exposed to nutrient reduction (MNR) fed 70 % of the feed consumed by the controls. Previous studies on the placentas showed that MNR decreased placental size near-term when villous volume and surface area, capillary surface area, and the villous isomorphic coefficient were all decreased and intervillous space hydraulic diameter was increased. 1, 20
The pregnant baboon presents several advantages for the study of effects of maternal nutrition on placental and fetal growth compared with other non-human primates including fetal size, and similarities in placentation and placental structure with those of human pregnancy. 1, 16 We hypothesized that maternal nutrient reduction would increase placental villous apoptosis and decrease abundance of XIAP at the end of gestation. Since this model is associated with a decrease in placental size, we further hypothesized an increase in cytokeratin 18 cleavage and active caspase 3 in placentas from MNR mothers. We also sought to determine changes in cell signaling proteins associated with cell proliferation (ERK), cell survival (AKT) and cell stress (JNK) in the placenta of controls and MNR animals.
Materials and Methods
Animal care
All procedures were approved by the Southwest Foundation for Biomedical Research (SFBR) Institutional Animal Care and Use Committee and conducted in AAALAC-approved facilities. Animal care was done as described by Schlabritz-Loutsevitch et al. (2007). 1, 16 Briefly, the subjects of this study were 13 female baboons aged 8–15 years maintained in group housing and bred as previously described in detail. 1, 16 Baboons were observed twice a day for wellbeing and three times a week for turgescence (sex skin swelling) and signs of vaginal bleeding to enable timing of pregnancy. 1 Pregnancy was dated initially by observing the changes in the swelling of the sex skin and confirmed at 30 days of gestation by ultrasonography. Animals (Papio sp.) were fed Purina Monkey Diet 5038 (Purina, St. Louis, MO). Diet was closely monitored in seven animals fed ad libitum (control group) and their food intake calculated weekly on a per kilogram basis. From confirmation of pregnancy, the six pregnant baboons in the MNR group received 70% of the average daily amount of feed eaten (on a weight-adjusted basis) by control (CTR) animals at the same gestational age. Cesarean sections were performed at 165.9±1.1 days of gestation (dG) in CTR and 162.1±4.6 dG in MNR animals. Placental tissues were obtained from a central area of a cotyledon and fixed in formalin (10% buffered formalin), and embedded in paraffin for immunohistochemistry or snap frozen in liquid nitrogen for protein studies.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)
Placental sections were used for these experiments. TUNEL protocol was followed as suggested by the manufacturer (Chemicon, Inc.). Briefly, slides were dewaxed and post-fixed using a solution of ethanol:acetic Acid (2:1) for 5 min. The equilibration buffer was added to the slide for 10 sec followed by incubation with the TdT enzyme for 1hr at 37°C. Following this, the anti-digoxigenin conjugate was incubated on the slide for 30 min and 4’,6-diamidino-2-phenylindole,dihydrochloride (DAPI) was used for nuclear staining. Slides were viewed using fluorescein excitation and emission filters. Analysis was performed in 2 placental slides per animal (3 controls; 3 MNR, selected randomly) and 20–30 fields were counted per slide with a mean count generated per slide for analysis purposes. The percent apoptosis was calculated in the placental slides (12 slides total) as the number of TUNEL positive cells divided by the total number of cells in 20 to 30 fields × 100.
Western blot analysis
Western blot was performed as previously described by Arroyo et al., (2008). 12 Briefly, frozen placental tissues were homogenized and protein tissue lysates (50µg) were separated on 4–12% Bis-Tris gel SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were incubated with an antibody against mouse XIAP (at a dilution of 1:200; Transduction Laboratories, Lexington, KY) or antibodies against rabbit active (cleaved) caspase 3, caspase 3, phospho-AKT, total AKT, phospho-ERK or total ERK, and phospho-JNK or total JNK (all at 1:500; Cell Signaling Technology, Dancers, MA). A secondary anti-mouse or anti-rabbit Ig-horseradish peroxidase (HRP) antibody (dilution 1:10,000) (Upstate Cell Signaling solutions, Lake Placid, NY) was incubated for 1 hr at room temperature. The membranes were incubated with ECL substrate (Amersham, Princeton, NJ) for 5 min and the emission of light was detected using x-ray film. To determine loading consistencies, each membrane was stripped of antibodies and reprobed utilizing an antibody against mouse beta-actin at a dilution of 1:4,000 (MP Biomedicals, Aurora Ohio). Presence of these proteins was confirmed and quantified.
M30 Cytodeath Immunohistochemistry (IHC)
IHC was performed on paraffin-embedded placental sections. Slides were de-waxed with 100% xylene. Slides were washed in PBS and sections were blocked for 1 hr using 10% normal goat serum / PBS. Slides were then incubated for 1 hr with a mouse monoclonal primary antibody against M30 Cytodeath (the neo-peptide produced from cytokeratin 18 cleavage; dilution 1:200) (Roche, Indianapolis, IN) or a mouse IgG1 (dilution of 1:500) for negative control. Sections were washed in 1X PBS. Sections were then incubated for 45 min with a biotin-labeled anti-Mouse secondary antibody. Slides were washed in 1X PBS, incubated in streptavidin-biotin-horseradish peroxidase solution, and developed with diaminobenzidine (DAB) Vectastain ABC DAB kit (Vector Laboratories, Inc., Burlingame, CA). DAB (brown) was used to stain for the M30 positive cells in serial placental sections. Hematoxylin was used for nuclear counterstaining. Slides were mounted using Permount mounting media.
Statistical analysis
Comparisons were made between control and MNR groups using a rank sum test (Mann-Whitney U test) for the following: fetal and placental weights, and XIAP, caspase 3, ERK and AKT western blot analysis. For TUNEL assay, comparison of mean number of apoptotic cell counts per slide between both groups (n=5) slides per group were check for normality followed by students t-test. Data are shown as mean ± SE and a P value of <0.05 was considered significant for the statistical comparisons.
Results
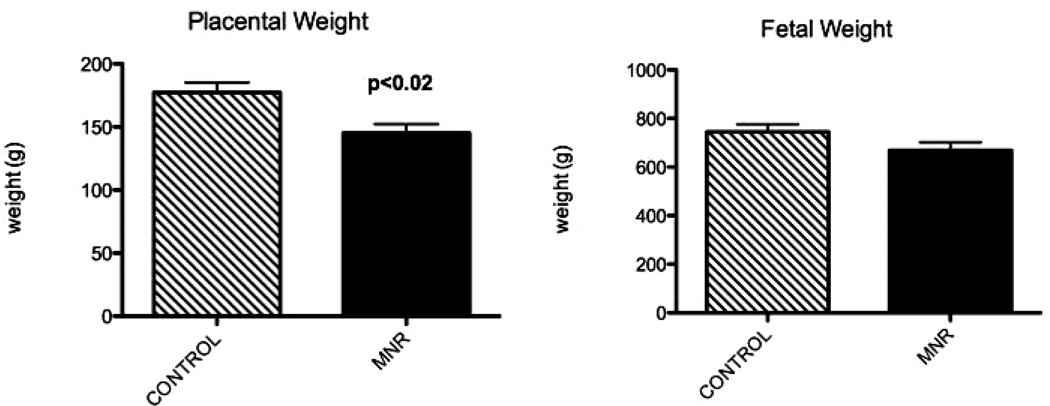
MNR baboons showed a significant decrease in placental weight (177g±8 vs. 145g±7; p<0.02) and mean fetal weight decreased by 7% (744g±32 vs. 669g±33; p=0.1) at 165 dG (Figure 1). In our study, there were no pregnancy miscarriages or fetal demises, fetal growth restriction or fetal abnormalities.
Figure 1.
Fetal and placental weights in fetuses of control and maternal nutrient reduction (MNR) groups at 165 days of gestation
Placental apoptosis
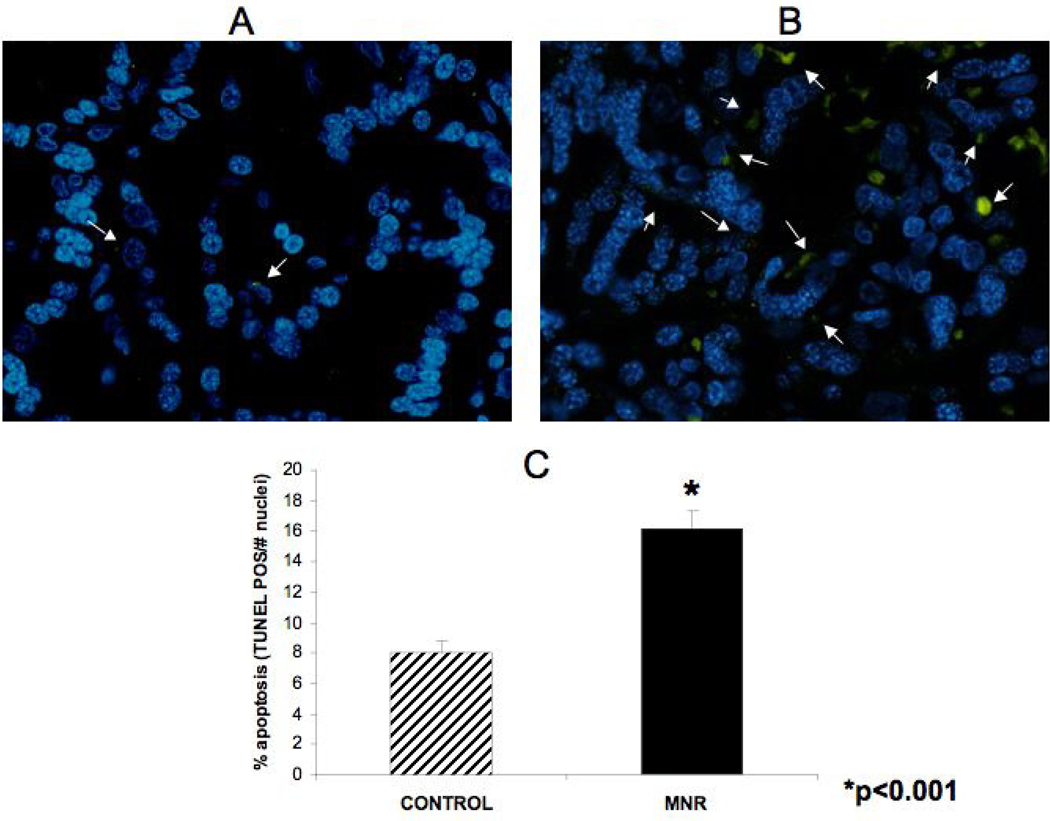
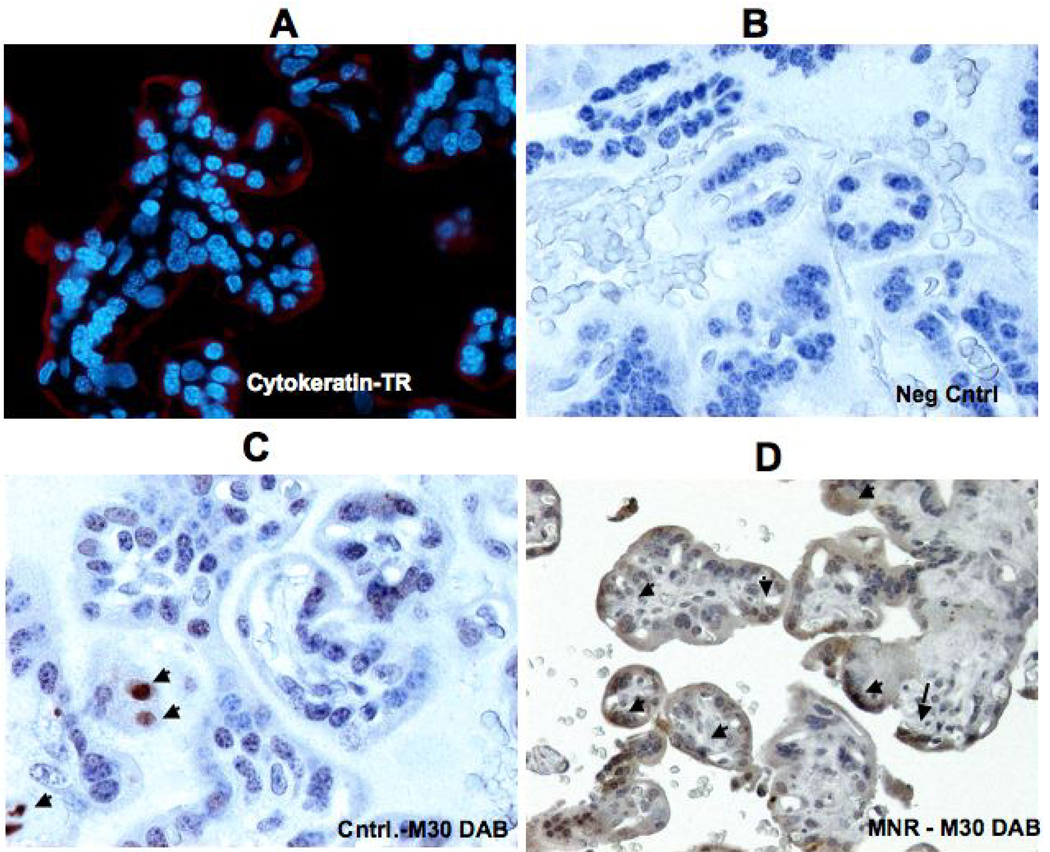
For the TUNEL studies, approximately 265 microscopic fields were available for analysis (MNR group = 120 fields; control group = 145 fields). Figure 2 demonstrates the difference in apoptosis achieved by TUNEL staining in A) placenta of control ad libitum fed mother and B) placenta of mother in the nutrient reduction group. It can be seen (Fig. 2C) that the percentage of apoptosis increased by approximately 100% in the presence of maternal nutrient reduction. Immunoflourescence was performed to identify trophoblast cells depicted in red in Figure 3 A. Figure 3 shows the presence of M30 in negative control (Panel B), treated (Panel D) and control animals (Panel C). Panel A depicts immufluorescence staining for pan-cytokeratin to identify the trophoblast cells. Immunohistochemistry for M30 showed an increased cytokeratin 18 cleavage in the trophoblast of placentas from mothers in the MNR group (3D) compared to controls (Figure 3C).
Figure 2.
Apoptosis demonstrated by Tunnel staining in A) placenta of a control ad libitum fed mother and B) placenta of a nutrient restricted mother. Quantification of percent apoptotic cells between groups (C).
Figure 3.
Cytokeratin 18 cleavage (M30) in placenta of control and MNR animals. Cytokeratin 7 immunofluorescence (red) in the baboon placenta (A). Placental sections for neg control (B), control ad libitum fed mother (C), and maternal nutrient reduction group (D).
Placental XIAP and caspase 3 proteins
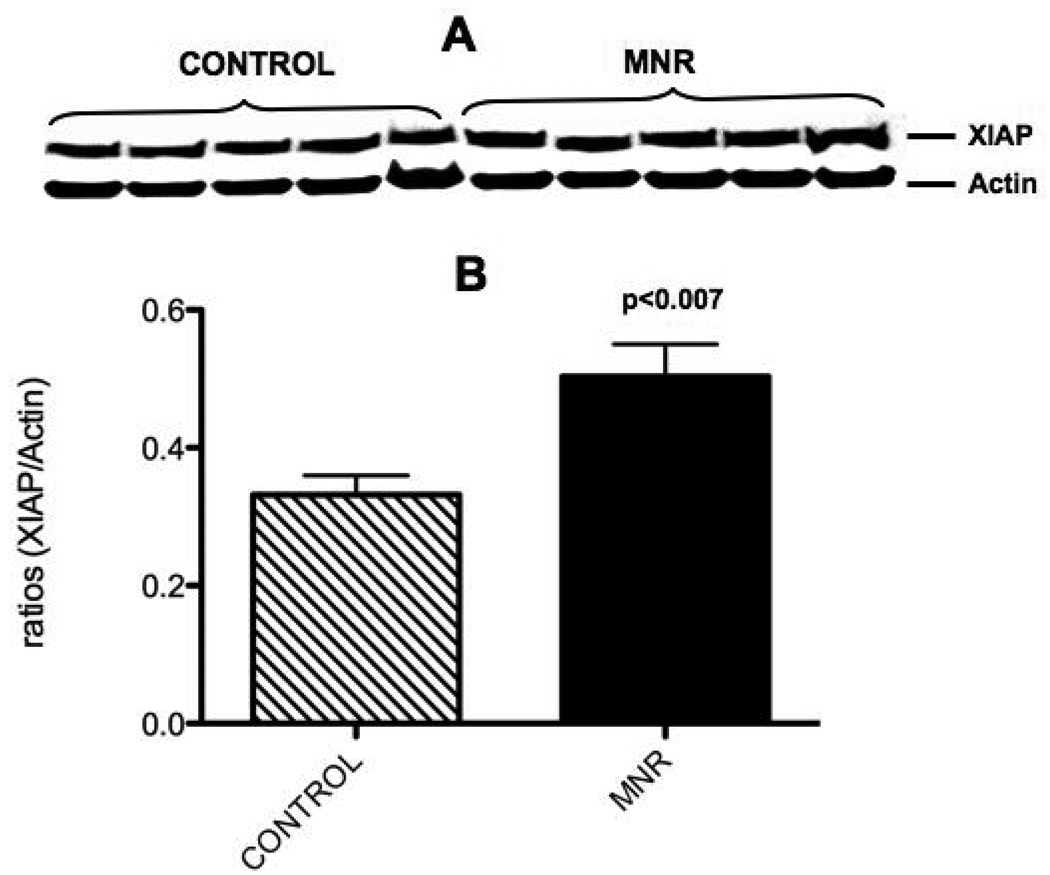
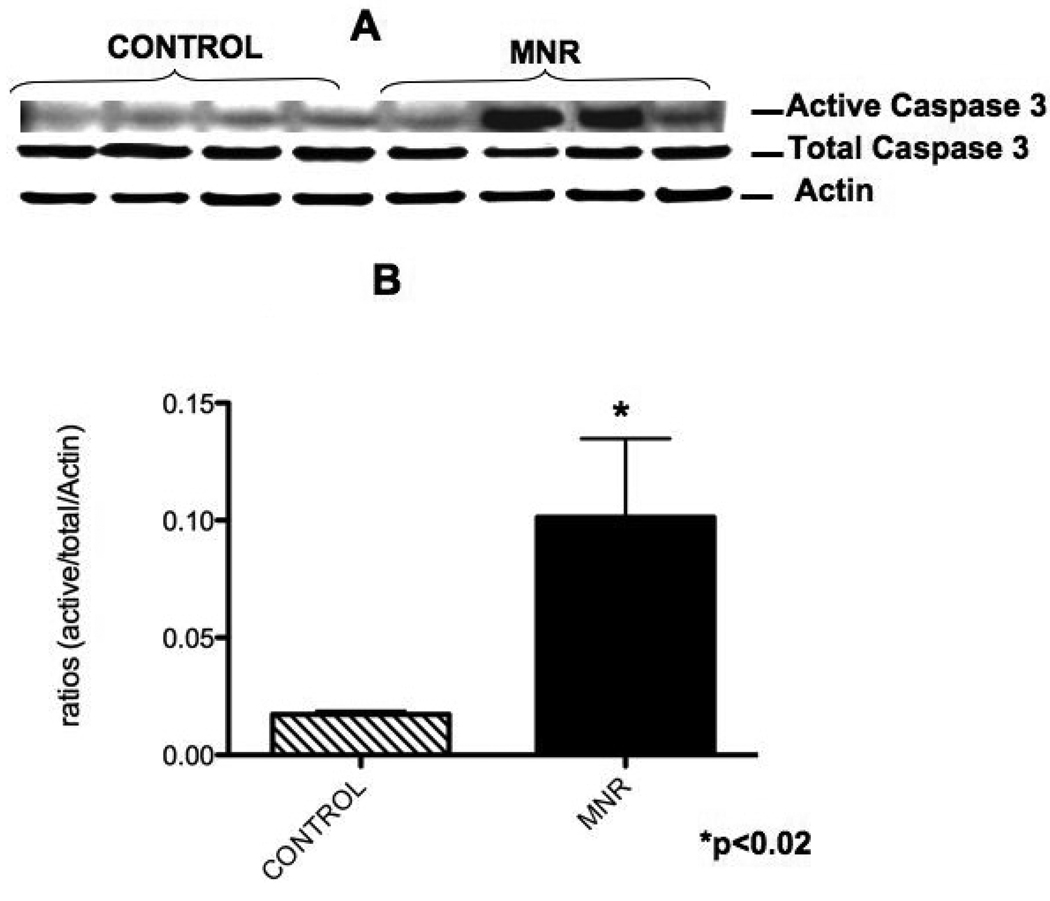
XIAP protein was significantly increased at 165 dG (1.5-fold) in the placenta of mothers in the nutrient reduction group (Figure 4). Similarly, active caspase 3 protein (5.8-fold) was also increased in the placenta of mothers in the nutrient reduction group (Figure 5).
Figure 4.
Placental villi XIAP protein from the control ad libitum fed and maternal nutrient reduction groups. Characteristic western blot of CIAP (A) and the corresponding densitometry quantification (B)
Figure 5.
Placental villi active caspase 3 protein from control ad libitum fed and maternal nutrient reduction groups. Panel A shows a characteristic western blot, and panel B reflects the corresponding densitometry quantification.
ERK AKT and JNK
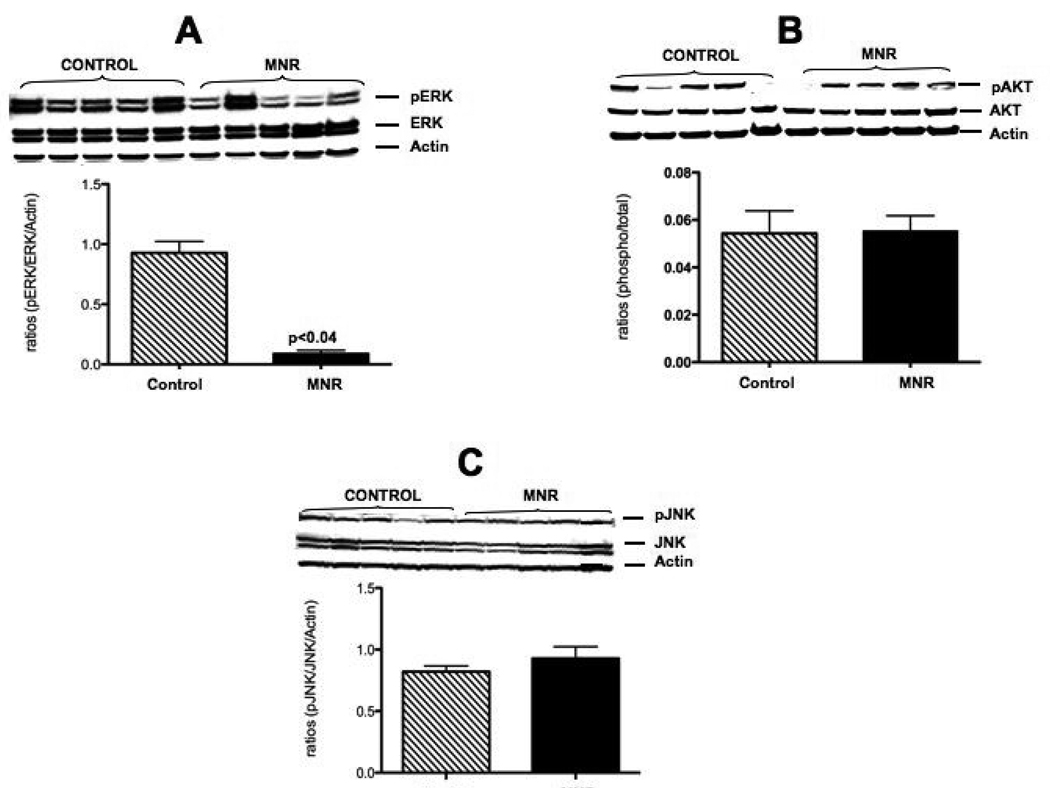
pERK protein increased 10.0-fold (p<0.04; Figure 6 A) while neither pAKT(Figure 6 B) nor pJNK (Figure 6 C) show differences in the placentas of the two groups.
Figure 6.
Characteristic western blots and protein quantification for ERK (A), AKT (B) and JNK (C) in placental villi from control ad libitum fed and maternal nutrient reduction groups.
Comments
The baboon model of MNR is characterized by a decrease in villous number and volume and a decrease in overall placental size near-term. 1, 16 We found that the smaller placentas of the MNR baboon group were characterized by an increase in apoptosis (TUNEL studies), which was further validated, by a qualitative increase in M30 from the IHC studies. To further elucidate the underlying mechanisms of apoptosis, XIAP and caspase 3 protein levels were determined in the placenta of MNR and controls animals. XIAP protein was significantly increased in MNR treated animals as compared to controls. Similarly, active caspase 3 was also increased in the placenta of MNR animals. Suggesting that the increase in XIAP protein could be a result of the increased apoptosis found at this point.
Cell death or apoptosis is normally present during cell growth and in the development of tissues, including the placenta. 21, 22 Increased trophoblast apoptosis is present in clinical obstetrics pathology such as preeclampsia and intrauterine growth restriction. 2, 7, 12, 23, 24 Trophoblast are epithelial cells that facilitate the exchange of nutrients and wastes between maternal and fetal compartments. Proper functioning of these cells is critical for placental development leading to a successful pregnancy. Results from the current study, including the TUNEL assay, and cleavage of cytokeratin 18, indicate that apoptosis is present in the placenta in this baboon model of MNR and that is greater than in the matched controls. This may contribute to the decreased placental weight observed in our model. Interestingly, the decrease in weight seen in the placenta was not seen in the fetus between groups near-term. Although unexpected, this finding has been described in other animal models of MNR. Ozaki et al showed that there was no significant decrease in fetal weight by day 20 of gestation, but growth restriction was present at birth in the rat dietary restriction model. 25 Collectively, these results suggest that decreased placental weight and apoptosis does not affect fetal size near-term in this baboon model of MNR although it remains to be seen if fetal organ systems are impacted by these placental changes. 25
In addition, experiments were performed to determine signaling molecules associated with the above findings during MNR. More specifically, the protein activation (phosphorylation) of ERK, AKT and JNK was studied. There was a significant decrease in ERK activation in the MNR placenta as compared to controls. In contrast, no significant differences were observed in the activation of placental AKT or JNK proteins during MNR in the baboon. The association of decreased ERK with the decreased in placental size and increased apoptosis is not entirely surprising as ERK is commonly associated with cell proliferation. While the above findings seem logical and consistent with published literature in apoptosis, some findings were not as easily interpreted. We did not observed any changes in activation of AKT or JNK proteins. This was unexpected because these proteins are usually involved in apoptosis and cell stress. At the same time we observed an increase in the anti apoptotic protein XIAP and an increase in caspase 3 activation. Given that XIAP normally inhibits caspase activation and subsequent apoptosis, this increase may represent a compensatory mechanism for the trophoblast for survival in the placenta of these MNR animals. We speculate that the XIAP increase could be involved in protecting the trophoblast from stress resulting in the lack of activation of AKT or JNK leading to improved trophoblast survival and function during the maternal food restriction in this baboon model. The fact that there is an increase in activation of caspase 3 and M30 staining, suggests that other mechanism independent of XIAP cold be a factor inducing this activation and leading to the apoptosis that we see in the MNR placenta. While there is no direct clinical application of the findings in this study, it remains relevant to normal and abnormal human placental nutrient transport for several reasons. The mechanisms that underlie the effect of diminished maternal nutrition on fetal-placental development as well as its role in fetal origins of adult disease has received considerable over the last few decades and has received worldwide interest. 26–27 The Barker Hypothesis 28 is based on end organ changes in function and structure during fetal life that subsequently leads to disease as adults. Several studies have shown that moderate degrees of nutrient restriction can impact organ structure and function including the kidney, heart, liver and placenta. 29–30 The finding of increased placental apoptosis in this baboon model of MNR at term is a finding that has been described the human IUGR placenta. 31 Why the finding of an increase in XIAP, which is known to be protective against apoptosis, did not prevent apoptosis compared to controls is not known. It may be that without XIAP, apoptosis is not controlled, a question that may only be answered with knock-out models.
Finally we would note that both groups of baboons were of mixed age and parity. The extent to which these factors affect the changes we report remains to be determined. We have recently shown that placental efficiency calculated as fetal divided by placental weight is higher in mature adult multiparous than primiparous baboons of the same weight (unpublished observations). The degree to which that may be due to a lesser degree of apoptosis in multiparity remains to be evaluated.
In summary, apoptosis is increased in the baboon placenta leading to a reduction in placental weight in this MNR baboon model. In addition, XIAP protein expression is increased in the placenta near-term in this model of MNR. We speculate that a possible mechanism of protection for the increased apoptosis observed in the placenta of treated animals is by increasing XIAP expression in the placenta of MNR animals as compared to controls. Also, we speculate that although XIAP was increased, this increase is not sufficient to prevent the decrease placental weight in this model of MNR. To our knowledge this is the first report to show an increase in XIAP protein associated with an increase in placental apoptosis during MNR in animal or human studies. Further studies are needed to determine the role of XIAP and trophoblast apoptosis earlier in gestation in this baboon model of MNR.
Acknowledgments
This study was supported by the HD21350-17, HDO34837-08 and HL071990
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schlabritz-Loutsevitch N, Ballesteros B, Dudley C, et al. Moderate maternal nutrient restriction, but not glucocorticoid administration, leads to placental morphological changes in the baboon (Papio sp.) Placenta. 2007;28:783–793. doi: 10.1016/j.placenta.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hetts SW. To die or not to die: an overview of apoptosis and its role in disease. JAMA. 1998;279:300–307. doi: 10.1001/jama.279.4.300. [DOI] [PubMed] [Google Scholar]
- 3.Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxiareoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90:1274–1281. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- 4.Huppertz B, Frank HG, Reister F, Kingdom J, Korr H, Kaufmann P. Apoptosis cascade progresses during turnover of human trophoblast: analysis of villous cytotrophoblast and syncytial fragments in vitro. Lab Invest. 1999;79:1687–1702. [PubMed] [Google Scholar]
- 5.Leung DN, Smith SC, To KF, Sahota DS, Baker PN. Increased placental apoptosis in pregnancies complicated by preeclampsia. Am.J.Obstet.Gynecol. 2001;184:1249–1250. doi: 10.1067/mob.2001.112906. [DOI] [PubMed] [Google Scholar]
- 6.Levy R, Nelson DM. To be, or not to be, that is the question. Apoptosis in human trophoblast. Placenta. 2000;21:1–13. doi: 10.1053/plac.1999.0450. [DOI] [PubMed] [Google Scholar]
- 7.Ratts VS, Tao XJ, Webster CB, et al. Expression of BCL-2, BAX and BAK in the trophoblast layer of the term human placenta: a unique model of apoptosis within a syncytium. Placenta. 2000;21:361–366. doi: 10.1053/plac.1999.0486. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y, Nakabayashi Y, Nakata K, Reed JC, Takahashi R. X-linked inhibitor of apoptosis protein (XIAP) inhibits caspase-3 and -7 in distinct modes. J.Biol.Chem. 2001;276:27058–27063. doi: 10.1074/jbc.M102415200. [DOI] [PubMed] [Google Scholar]
- 9.Creagh EM, Martin SJ. Caspases: cellular demolition experts. Biochem.Soc.Trans. 2001;29:696–702. doi: 10.1042/0300-5127:0290696. [DOI] [PubMed] [Google Scholar]
- 10.Kadyrov M, Kaufmann P, Huppertz B. Expression of a cytokeratin 18 neoepitope is a specific marker for trophoblast apoptosis in human placenta. Placenta. 2001 Jan;22(1):44–48. doi: 10.1053/plac.2000.0616. [DOI] [PubMed] [Google Scholar]
- 11.Gruslin A, Qiu Q, Tsang BK. X-linked inhibitor of apoptosis protein expression and the regulation of apoptosis during human placental development. Biol.Reprod. 2001;64:1264–1272. doi: 10.1095/biolreprod64.4.1264. [DOI] [PubMed] [Google Scholar]
- 12.Arroyo JA, Anthony RV, Galan HL. Decreased placental X-linked inhibitor of apoptosis protein in an ovine model of intrauterine growth restriction. Am.J.Obstet.Gynecol. 2008;199:80–88. doi: 10.1016/j.ajog.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM. Serine/threonine protein kinases and apoptosis. Exp Cell Res. 2000;256(1):34–41. doi: 10.1006/excr.2000.4836. [DOI] [PubMed] [Google Scholar]
- 14.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000 Oct 13;103(2):239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 15.Ain R, Canham LN, Soares MJ. Dexamethasone-induced intrauterine growth restriction impacts the placental prolactin family, insulin-like growth factor-II and the Akt signaling pathway. J Endocrinol. 2005;185:253–263. doi: 10.1677/joe.1.06039. [DOI] [PubMed] [Google Scholar]
- 16.Gruppuso PA, Boylan JM, Anand P, Bienieki TC. Effects of maternal starvation on hepatocyte proliferation in the late gestation fetal rat. Pediatr.Res. 2005;57:185–191. doi: 10.1203/01.PDR.0000151646.55587.0F. [DOI] [PubMed] [Google Scholar]
- 17.Lea RG, Wooding P, Stewart I, et al. The expression of ovine placental lactogen, StAR and progesterone-associated steroidogenic enzymes in placentae of overnourished growing adolescent ewes. Reproduction. 2007;133:785–796. doi: 10.1530/REP-06-0294. [DOI] [PubMed] [Google Scholar]
- 18.McDonald TJ, Nijland MJ, Nathanielsz PW. The insulin-like growth factor system and the fetal brain: effects of poor maternal nutrition. Rev.Endocr.Metab Disord. 2007;8:71–84. doi: 10.1007/s11154-007-9044-2. [DOI] [PubMed] [Google Scholar]
- 19.Murdoch WJ, Van Kirk EA, Vonnahme KA, Ford SP. Ovarian responses to undernutrition in pregnant ewes, USA. Reprod.Biol.Endocrinol. 2003;1:6. doi: 10.1186/1477-7827-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlabritz-Loutsevitch NE, Dudley CJ, Gomez JJ, et al. Metabolic adjustments to moderate maternal nutrient restriction. Br.J.Nutr. 2007;98:276–284. doi: 10.1017/S0007114507700727. [DOI] [PubMed] [Google Scholar]
- 21.Smith SC, Baker PN, Symonds EM. Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol. 1997;177:1395–1401. doi: 10.1016/s0002-9378(97)70081-4. [DOI] [PubMed] [Google Scholar]
- 22.Smith SC, Price E, Hewitt MJ, Symonds EM, Baker PN. Cellular proliferation in the placenta in normal human pregnancy and pregnancy complicated by intrauterine growth restriction. J.Soc.Gynecol.Investig. 1998;5:317–323. doi: 10.1016/s1071-5576(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 23.Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet.Gynecol. 2000;96:271–276. doi: 10.1016/s0029-7844(00)00895-4. [DOI] [PubMed] [Google Scholar]
- 24.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am.J.Obstet.Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 25.Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J.Physiol. 2001;530:141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol. 2004 Dec 1;561(Pt 2):355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heasman L, Clarke L, Stephenson TJ, Symonds ME. The influence of maternal nutrient restriction in early to mid-pregnancy on placental and fetal development in sheep. Proc Nutr Soc. 1999;58(2):283–288. doi: 10.1017/s0029665199000397. [DOI] [PubMed] [Google Scholar]
- 28.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49(2):270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Cox LA, Nijland MJ, Gilbert JS, Schlabritz-Loutsevitch NE, Hubbard GB, McDonald TJ, Shade RE, Nathanielsz PW. Effect of 30 per cent maternal nutrient restriction from 0.16 to 0.5 gestation on fetal baboon kidney gene expression. J Physiol. 2006;572(Pt 1):67–85. doi: 10.1113/jphysiol.2006.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Schlabritz-Loutsevitch NE, Hubbard GB, Han V, Nygard K, Cox LA, McDonald TJ, Nathanielsz PW. Effects of maternal global nutrient restriction on fetal baboon hepatic insulin-like growth factor system genes and gene products. Endocrinology. 2009;150(10):4634–4642. doi: 10.1210/en.2008-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increasedapoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]