Abstract
Background
Induced hypothermia is a promising neuroprotective therapy. We studied the feasibility and safety of hypothermia and thrombolysis after acute ischemic stroke.
Methods
ICTuS-L was a randomized, multi-center trial of hypothermia and intravenous t-PA in patients treated within 6 hours after ischemic stroke. Enrollment was stratified to the treatment time windows 0–3 and 3–6 hours. Patients presenting within 3 hours of symptom onset received standard dose intravenous alteplase (IV tPA) and were randomized to undergo 24 hours of endovascular cooling to 33°C followed by 12 hours of controlled re-warming or normothermia treatment. Patients presenting between 3 and 6 hours were randomized twice: to receive t-PA or not and to receive hypothermia or not.
Results
In total, 59 patients were enrolled. One patient was enrolled but not treated when pneumonia was discovered just prior to treatment. All 44 patients enrolled within 3 hours and 4 of 14 patients enrolled between 3–6 hours received t-PA. Overall, 28 patients randomized to receive hypothermia (HY) and 30 to normothermia (NT). Baseline demographics and risk factors were similar between groups. Mean age was 65.5±12.1 years and baseline NIHSS was 14.0±5.0; 32 (55%) were male. Cooling was achieved in all patients except 2 in whom there were technical difficulties. The median time to target temperature after catheter placement was 67min (Q1 57.3 –Q3 99.4). At 3 months, 18% of patients treated with HY had a modified Rankin Scale (mRS) of 0 or 1, versus 24% in the NT groups (NS). Symptomatic intracranial hemorrhage occurred in 4 patients (68), all were treated with tPA less than 3 hours (1 received HY). Six patients in the HY and 5 in the NT groups died within 90 days (NS). Pneumonia occurred in 14 patients in the HY and in 3 of the NT groups (p=0.001). The pneumonia rate did not significantly adversely affect 3 month mRS (p=0.32).
Conclusion
This study demonstrates the feasibility and preliminary safety of combining endovascular hypothermia after stroke with intravenous thrombolysis. Pneumonia was more frequent after hypothermia, but further studies are needed to determine its effect on patient outcome and whether it can be prevented. A definitive efficacy trial is necessary to evaluate the efficacy of therapeutic hypothermia for acute stroke.
Keywords: Ischemic stroke, Neuroprotection, Thrombolysis, Hypothermia
INTRODUCTION
Based on experimental data and early human experience, hypothermia is one of the most active modes of neuroprotection.(1, 2) Hypothermia improves survival and neurological outcome after cardiac arrest(3, 4) and its use in this setting is recommended by the International Liaison Committee on Resuscitation(ILCOR).(5) In infants with hypoxic-ischemic encephalopathy, hypothermia at 33.5°C for 72 hours is safe, reduces fatality and improves neurodevelopmental outcome.(6) Other researchers have used surface cooling methods to reduce brain edema and treat increased intracranial pressure after stroke.(7)
The COOL-AID study group completed two clinical trials of hypothermia in acute ischemic stroke. The first study used surface cooling(8), and the second endovascular cooling.(9) Both trials demonstrated feasibility but were not powered to answer questions regarding safety and efficacy. Side effects of endovascular hypothermia have been pneumonia, cardiac arrhythmia and deep vein thrombosis (DVT).(9)
Only few patients have been treated with hypothermia below 35°C while awake.(10, 11) Other than small uncontrolled case series, no prior study has confirmed the safety of combining intravascular cooling catheters with thrombolytic therapy for acute stroke.
We sought to determine feasibility and safety of endovascular hypothermia in patients receiving thrombolytic therapy in a randomized, controlled study of endovascular cooling in awake patients after stroke, The Intravascular Cooling in the Treatment of Stroke – Longer tPA window ( ICTuS-L) study.(12)
MATERIALS AND METHODS
The ICTuS-L trial was a controlled, prospective, randomized trial designed to investigate the feasibility and safety of induced endovascular hypothermia with thrombolysis in patients presenting with acute ischemic stroke less than six hours from onset, age 18–80, an National Institutes of Health Stroke Scale (NIHSS) ≥ 7, and a score of 0 or 1 on NIHSS Item 1a (arousal) at the time of cooling catheter placement. The institutional review boards of the participating centers approved this protocol. All patients or their surrogates gave written informed consent. Enrollment was stratified to the tPA treatment time windows 0–3 and 3–6 hours. Patients presenting within 3 hours received 0.9mg/kg (maximum 90mg) intravenous tPA and were randomized to undergo 24 hours of intravascular cooling to 33°C using the Celsius Control system (Innercool, San Diego) followed by 12 hours of controlled re-warming at a rate of 0.3°C per hour versus no cooling (2 Groups). Patients presenting between 3 and 6 hours were randomized twice: tPA vs. no tPA and hypothermia (HY) versus normothermia (NT) (4 Groups) (Table 1). Participants were randomized using a randomization list generated and maintained by the UCSD SPOTRIAS Data Core.
Table 1.
Patient group randomization by time of tPA treatment from stroke onset.
| hours from stroke | Group | Patients(n) | tPA | HY |
|---|---|---|---|---|
| 0–3 | 1 | 22 | + | − |
| 2 | 22 | + | + | |
| 3–6 | 3 | 6 | − | − |
| 4 | 2 | + | − | |
| 5 | 4 | − | + | |
| 6 | 2 | + | + | |
| total | 58 |
We used a previously published anti-shivering protocol combining meperidine, buspirone and warming blankets.(10) In tPA treated patients the catheter insertion was initiated between 30 and 180 minutes after completion of the tPA infusion. Patients received a head CT before treatment, at 36 (±12) hours and at, 30 days, and a clinical evaluation before treatment; and at 24 hours, 7, 30 and 90 days after stroke. A full description of the clinical trial methods has been published.(12)The primary safety outcome was the incidence of a serious adverse event at 3 months. The primary feasibility outcome was achievement of cooling defined as reaching a target temperature as close to 33°C as possible.
Secondary safety outcomes of interest included (a) the incidence and volume of hemorrhages on head CT 36 hours after stroke onset, (b) the incidence of adverse events (AE), and (c) 90-day mortality. Efficacy outcomes of interest were the NIHSS at 24 hours, 30 and 90 days; and the mRS at 90 days.
Statistical analysis
Safety, feasibility and efficacy analyses were conducted using the intent-to-treat principle. Since this was primarily a feasibility study, no adjustments were made for multiple comparisons. A p value less than 0.05 was considered to be statistically significant. Baseline comparisons between the HY and NT groups were done using a Wilcoxon Rank Sum test for continuous outcomes and a Fisher’s Exact Test for categorical outcomes. Fisher’s Exact Test was used to compare the rates of serious adverse events, adverse events, hemorrhages, deaths and 90-day mRS between the HY and NT groups. The Wilcoxon Rank Sum Test was used for the NIHSS comparisons. All statistical analyses were conducted using the statistical software R version 2.10.1 (http://www.r-project.org/)
RESULTS
Fifty-nine patients were randomized but one patient was found to have pneumonia prior to receiving any study procedures and was excluded, leaving 58 patients for the intention to treat (ITT) analysis. Cooling was performed in 28 of 58 patients (48.2%); in 22 of the 28 (78.6%), tPA was begun within 3 hours from stroke onset and in 2 patients between 3 and 6 hours (Groups 5 and 6). Four patients in the 3–6 hour window underwent cooling alone. (Table 1)
The mean (±SE) age was 65±14 years with a range from 21 to 81 years. Patients treated with HY were older, 68.93±7.87 years, compared to NT patients, 62.30±14.48 years. (NS) 55% were men. Several risk factors were reported by at least 50% of the patients, including hypertension and hyperlipidemia. Between 25% and 50% of the patients reported coronary disease, atrial fibrillation, or myocardial infarction. The pre-stroke mRS was >1 in four HY treated and one NT treated patient. The baseline (mean±SE) NIHSS was 14.3 (±5) in the HY groups and 13.7 (±5) in the NT groups. (Table 2)
Table 2.
Baseline demographics and risk factors between the hypothermia and normothermia groups. (* peripheral vascular disease)
| Hypothermia (Group 2, 5, 6) n=28 | Normothermia (Group 1, 3,4) n=30 | Fisher’s Exact Test p-value: | |
|---|---|---|---|
| Male gender | 56.7% | 53.8% | 0.999 |
| Race (white) | 89.3% | 80.0% | 0.707 |
| Age in years (±SD) | 68.9 (7.9) | 62.3 (14.5) | 0.109 |
| Known atrial fibrillation | 21.4% | 13.3% | 0.499 |
| Diabetes | 21.4% | 30.0% | 0.554 |
| Arterial hypertension | 71.4% | 63.3% | 0.583 |
| Hyperlipidemia | 53.7% | 50.0% | 0.793 |
| Present smoking | 10.7% | 26.7% | 0.102 |
| Congestive heart failure | 3.6% | 16.7% | 0.109 |
| PVD* | 3.6% | 6.7% | 0.411 |
| Myocardial infarction | 14.3% | 16.7% | 0.426 |
| Coronary artery disease | 39.4% | 26.7% | 0.695 |
| Prior stroke or TIA | 17.9% | 20.0% | 0.869 |
| Liver disease | 3.6% | 0 | 0.229 |
| Lung disease | 10.7% | 13.3% | 0.848 |
| Renal disease | 7.1% | 13.3% | 0.671 |
| Gastrointestinal disease | 21.4% | 33.3% | 0.298 |
| Pre-stroke Modified Rankin >1 | 14.3% | 3.3% | 0.214 |
| Pre-treatment NIHSS (±SD) | 14.3(5.0) | 13.7(5.1) | 0.651 |
Peripheral Vascular Disease
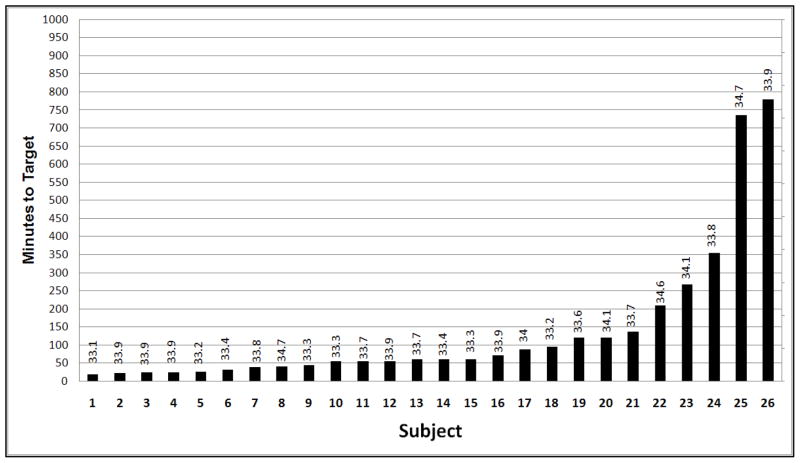
The mean (±SE) meperidine dose used in HY patients was 14.5±6.9mg/kg. Target temperature was reached in 20/28 patients (71.4%). In two patients the hypothermia console failed, 4 had poorly controlled shivering that lead to an increase in target temperature and 2 were maintained at 34°C and 34.1°C. The mean (±SE) temperature that was achieved in these patients was 33.4°C (±0.6). The median time to target temperature after catheter placement was 67min (Q1 57.3 – Q3 99.4), the mean (± SD) was 138.3±198.9 min. (Figure 1)
Figure 1.
Time to target temperature (lowest temperature achieved OR first 33°C) in 26 patients in whom cooling was attempted. In 4 patients effective anti-shivering was not accomplished, resulting in very long times to target.
In the under 3 hour patients (Group 2), the median time from stroke onset to cooling start was 355 min (Q1 269 – Q3 399) and to target temperature was 421 min (Q1 331 – Q3 594). The respiratory rate (mean±SD) in the HY groups was lower throughout the cooling period (at 1 hour 14.15±2.85 vs 18.65±4.18). There was a trend towards lower respiratory rate during rewarming, but by hour 36 the respiratory rate was similar between groups. There were no significant differences in blood pressure, oxygen saturation or heart rate between groups.
There were no differences in outcome or occurrence of adverse events comparing patients who were treated with t-PA and those who were not. Forty serious adverse events (SAE) occurred in 21 of the 28 patients treated with HY versus 21 SAEs in 13 of the 30 NT patients. Pneumonia occurred more frequently in the HY patients than in the NT patients (7/28 versus 2/30, p<0.05, Fishers Exact Test). The rate of any intracerebral hemorrhage (ICH) at 48 hours including both asymptomatic and symptomatic ICH was similar in both groups, at 30% (33% HY, 25% NT.) Symptomatic ICH occurred in 4 patients, all treated with t-PA within 3 hours from stroke onset, one with HY, three in the NT groups.
The risk of Deep vein thrombosis (DVT), urinary tract infection, pancreatitis, renal failure or cardiac arrhythmia was not significantly increased in patients treated with HY compared to NT. DVT occurred in 4 HY and 1 NT patient. It was possibly related to the hypothermia catheter in 2 patients. One received an inferior vena cava filter.
There were no differences in baseline laboratory values between groups. Mild oliguria occurred in almost all patients while undergoing HY, but was not associated with renal failure and reversed during re-warming. There was a transient increase in Blood Urea Nitrogen (BUN) (mean±SD) at day 2 in the HY groups to 23.3 mg/dL (±9.9) vs 12.9 mg/dL (±5.93) in the NT group. No significant change in creatinine was observed and the BUN upon follow up at day 7 was 16.3 mg/dL (±6.9) in the HT vs 15.1 mg/dL (±8.3) in the NT group. Amylase was increased in patients with HY at day 2 (243.3 U/L ± 260.9 vs 62.5 U/L ± 25.6) and remained elevated (99.7 U/L ± 63.0 vs 49.7 U/L ±28.4) at Day 7, but no patient was diagnosed with pancreatitis.
Due to sedation with meperidine, the NIHSS at 24 hours was 17.0 (±8.9) in the HY and 11.1(±8.1) in the NT groups (p=0.02). The NIHSS was equivalent in both groups at 30 days. It was 8.0 (±6.5) in the HY and 5.0 (±4.1) in the NT groups (NS). At 90 days the NIHSS was 6.3 (±6.6) vs 3.8 (±3.0) (NS).
At 3 months 18% of patients in the HY groups had a mRS of 0 or 1, versus 24% in the NT groups. The difference was not statistically significant p<0.77 (Fishers Exact Test). Six patients treated with HY died, five died in the NT groups. Of the patients suffering pneumonia 11.8% had a mRS of 0 or 1 at 90 days, versus 25% of those without pneumonia (NS). (Table 3)
Table 3.
Outcome measures between Hypothermia and normothermia patients.
| Hypothermia (Group 2, 5, 6) n=28 | Normothermia (Group 1, 3,4) n=30 | Fisher’s Exact Test p-value: | |
|---|---|---|---|
| mRS 0–1 at 90 days | 5 | 7 | 0.747 |
| NIHSS at 90 day (mean ±SD) | 6.3 (±6.6) | 3.8 (±3.0) | 0.355 |
| At least one SAE (%) | 75 | 43.3 | 0.018 |
| Pneumonia (%) | 50 | 10 | 0.001 |
| All ICH (%) | 28.6 | 20 | 0.752 |
| Symptomatic ICH (%) | 3.6 | 10 | 0.609 |
| Mortality by 90 days (%) | 21.4% | 16.7 | 0.744 |
DISCUSSION
This study is the largest randomized, controlled study of awake stroke patients who received hypothermia targeted at 33°C. Past studies used surface cooling methods with aggressive anti-shivering regimens that cause skin irritation and respiratory suppression.(8) We used an anti-shivering treatment with intravenous meperidine, oral buspirone and surface skin warming, without severe respiratory suppression and were able to combine endovascular cooling with thrombolysis in awake patients after acute ischemic stroke.
Our protocol was designed as a safety study and the aim was to avoid bleeding complications when hypothermia was combined with t-PA. The median time to target temperature was over 7 hours (421 min) and was mainly driven by the delay in catheter placement. For safety concerns, endovascular hypothermia was not begun until 30 – 180 min after completion of the t-PA infusion. While we did not observe groin hematomas or other bleeding complications attributable to the femoral venous catheterization in patients who had received thrombolytic therapy, cooling was significantly delayed due to this precaution; however, our calculation of the times to cooling may have been an overestimate as we did not record temperature for 20 minutes after cooling catheter placement (Figure 1). The delayed cooling may have reduced the potential for neuroprotective benefit in our patients.
Hypothermia induction with non-invasive methods such as intravenous cold saline or external cooling may have further increased the time to target temperature.
Preclinical studies have shown that hypothermia is more effective the sooner it is implemented after stroke, suggesting that future studies should contain provisions for more prompt cooling initiation.(13–16)
In addition to the time window, cooling duration may affect patient outcome.(17) Preclinical studies have shown that longer cooling increase neuroprotective effects.(18) The patients in our study were cooled for 24 hours. Future research should focus on the optimal cooling duration after ischemic stroke in man.
While the re-warming paradigm used in the present study was the same in all patients, Schwab et al have shown that hypothermia can reduce edema after cerebral ischemia.(7) It may be possible that adjusting the re-warming paradigm to physical exam findings, intracranial pressure monitoring or other surrogate markers we could benefit patient outcome. In our own earlier experience we found that hypothermia reduced brain edema.(19) The aim of ICTuS-L, however, was acute neuroprotection and edema therapy after completed ischemia was not targeted.
In 8/28 patients target temperature was not reached. In two patients this was due to failure of the cooling console, in four patients shivering was poorly controlled and in two the temperature was maintained at 34.0 and 34.1°C by the investigator. The cooling failure was, in part, attributed to the subjects being obese and the use of the 10.7 F catheter. Midway through the trial a larger and more powerful 14F catheter became available, and it was noted that obese patients were easier to cool. Future studies that examine the efficacy of hypothermic neuroprotection will need to use more reliable cooling devices and use sufficiently powerful cooling catheters in patients with a high body mass index.
One initial hypothesis of our study was that cooling extends the time window for t-PA. We were not able to test this hypothesis because enrolment into the treatment groups between 3 and 6 hours was low. After our trial was initiated, survey data confirmed that few stroke patients arrive between 3 and 6 hours.(20) Patients with higher NIHSS are more likely to present within 3 hours.(21–23) Since our protocol required an NIHSS ≥ 7 very few patients arriving between 3 and 6 hours qualified for study enrolment. We were, therefore, unable to show that extending the treatment window for IV tPA is possible with the addition of hypothermia. In the limited number of patients studied between 3 and 6 hours we did not observe an excess in safety concerns. This finding is consistent with the more recent ECASS III study that demonstrated the safety and efficacy of IV t-PA between 3 and 4.5 hours.(24)
We did not find a difference in mortality and mRS in patients treated with or without HY in this small safety and feasibility study. The increased NIHSS at 24 hours in the HY groups may have been due to mild sedation caused by the anti-shivering protocol as we found no significant difference in NIHSS at follow-up.
SAEs were more common in the HY groups and the most common SAE was pneumonia. The occurrence of pneumonia did not significantly affect outcome at 90 days. Stroke causes a transient immune-depression, which leads to increased pneumonia risk.(25, 26) Data regarding the effect of pneumonia on stroke outcome is conflicting. The GAIN investigators found an increased risk of poor outcome after stroke associated with pneumonia and urinary tract infection.(25) The PANTHERIS study showed a reduction of pneumonia after stroke when prophylactic antibiotics were used.(27) The reduction was associated with a trend towards better outcome. This benefit was not confirmed, however, in a similar study by Chamorra et al.(28)
The increase in pneumonia rate in the HY treated patients may be affected in part by an ascertainment bias as patients undergoing HY were more closely monitored for side effects they were all hospitalized in intensive care units for a minimum of 36 hours, while NT patients may have been admitted to lower acuity level units. Hypothermia, in addition to stroke, however, is known to suppress the immune system(29) and patients who suffer sepsis with hypothermia or postoperative hypothermia suffer increased risks of infections.(30) Furthermore, the use of meperidine reduces respiratory frequency and may contribute to aspiration. Compared to other anti-shivering treatments, meperidine carries a lower risk of respiratory depression.(31–33) Our data do not allow us to attribute the pneumonia risk to hypothermic immune suppression, elevated aspiration risk due to the meperidine, or other unknown factors, and further studies are needed to establish the effect of hypothermia on immune suppression. The increased pneumonia rate in hypothermia patients may have obscured any possible beneficial effect on 3 month outcomes.
Conclusions
Endovascular hypothermia can be combined with thrombolytic therapy. The anti-shivering protocol is feasible in awake stroke patients. An increased rate of pneumonia in the cooled patients was observed, but was not associated with poor outcome. Further studies with larger patient samples and earlier cooling are needed to evaluate the efficacy of hypothermia after ischemic stroke.
Acknowledgments
Funding:
The ICTuS-L study was funded by a National Institutes of Health Grant, SPOTRIAS, P50N5044148. Innercool Therapies, Inc supplied cooling catheters and devices for this clinical trial but no other financial or other support.
We thank the patients and families who participated in this clinical trial.
Appendix
The Intravenous Thrombolysis plus Hypothermia for Acute Treatment of Ischemic Stroke (ICTuS-L) Investigators are as follows: University of California San Diego, CA and Scripps Mercy Hospital, San Diego, CA: TM Hemmen, T Rzesiewicz, KZ Guluma, BC Meyer; University of Texas Medical School, Houston, TX: J Grotta, MJ Hess, S Martin-Schild, A Barreto, H Hallevi, N Gonzales, S Savitz; University of Connecticut, Hartford, CT: J Gomes, J Blum; University of Pittsburgh, Pittsburgh, PA: M Hammer, E Gruendler; Stanford University Medical Center, Palo Alto, CA: CAC Wijman, GW Albers, MS Buckwalter, JS Castle, A Finley Caulfield, M Garcia, S Kemp, MA Kumar, MG Lansberg, NE Schwartz, C Venkatasubramanian; Saint Louis University, St. Louis, MO: S Cruz-Flores, E Holzemer, UCSD Stroke Clinical Trials Coordinating Center, San Diego, CA: PD Lyden, KS Rapp.
Footnotes
Disclosures:
None
References
- 1.O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59(3):467–77. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 2.Popovic R, Liniger R, Bickler PE. Anesthetics and mild hypothermia similarly prevent hippocampal neuron death in an in vitro model of cerebral ischemia. Anesthesiology. 2000;92(5):1343–9. doi: 10.1097/00000542-200005000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 4.The Hypothermia After Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 5.Nolan JP, Morley PT, Vanden Hoek TL, Hickey RW, Kloeck WG, Billi J, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108(1):118–21. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 6.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 7.Schwab S, Schwarz S, Spranger M, Keller E, Bertram M, Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. 1998;29(12):2461–6. doi: 10.1161/01.str.29.12.2461. [DOI] [PubMed] [Google Scholar]
- 8.Krieger DW, De Georgia MA, Abou-Chebl A, Andrefsky JC, Sila CA, Katzan IL, et al. Cooling for acute ischemic brain damage (cool aid): an open pilot study of induced hypothermia in acute ischemic stroke. Stroke. 2001;32(8):1847–54. doi: 10.1161/01.str.32.8.1847. [DOI] [PubMed] [Google Scholar]
- 9.De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, et al. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology. 2004;63(2):312–7. doi: 10.1212/01.wnl.0000129840.66938.75. [DOI] [PubMed] [Google Scholar]
- 10.Lyden PD, Allgren RL, Ng K, Akins P, Meyer B, Al-Sanani F, et al. Intravascular Cooling in the Treatment of Stroke (ICTuS): early clinical experience. J Stroke Cerebrovasc Dis. 2005;14(3):107–14. doi: 10.1016/j.jstrokecerebrovasdis.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Zweifler RM, Voorhees ME, Mahmood MA, Alday DD. Induction and maintenance of mild hypothermia by surface cooling in non-intubated subjects. J Stroke Cerebrovasc Dis. 2003;12(5):237–43. doi: 10.1016/j.jstrokecerebrovasdis.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Guluma KZ, Hemmen TM, Olsen SE, Rapp KS, Lyden PD. A trial of therapeutic hypothermia via endovascular approach in awake patients with acute ischemic stroke: methodology. Acad Emerg Med. 2006;13(8):820–7. doi: 10.1197/j.aem.2006.03.559. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich WD, Busto R, Alonso O, Globus MY, Ginsberg MD. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993;13(4):541–9. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- 14.Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15(11):7250–60. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Chopp M, Welch KM. Effect of mild hyperthermia on the ischemic infarct volume after middle cerebral artery occlusion in the rat. Neurology. 1991;41(7):1133–5. doi: 10.1212/wnl.41.7.1133. [DOI] [PubMed] [Google Scholar]
- 16.Zhang RL, Chopp M, Chen H, Garcia JH, Zhang ZG. Postischemic (1 hour) hypothermia significantly reduces ischemic cell damage in rats subjected to 2 hours of middle cerebral artery occlusion. Stroke. 1993;24(8):1235–40. doi: 10.1161/01.str.24.8.1235. [DOI] [PubMed] [Google Scholar]
- 17.Colbourne F, Corbett D, Zhao Z, Yang J, Buchan AM. Prolonged but delayed postischemic hypothermia: a long-term outcome study in the rat middle cerebral artery occlusion model. J Cereb Blood Flow Metab. 2000;20(12):1702–8. doi: 10.1097/00004647-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Clark DL, Penner M, Wowk S, Orellana-Jordan I, Colbourne F. Treatments (12 and 48 h) with systemic and brain-selective hypothermia techniques after permanent focal cerebral ischemia in rat. Exp Neurol. 2009;220(2):391–9. doi: 10.1016/j.expneurol.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Guluma KZ, Oh H, Yu SW, Meyer BC, Rapp K, Lyden PD. Effect of endovascular hypothermia on acute ischemic edema: morphometric analysis of the ICTuS trial. Neurocrit Care. 2008;8(1):42–7. doi: 10.1007/s12028-007-9009-z. [DOI] [PubMed] [Google Scholar]
- 20.Investigators CASPRC. Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology. 2005;64(4):654–9. doi: 10.1212/01.WNL.0000151850.39648.51. [DOI] [PubMed] [Google Scholar]
- 21.Chang KC, Tseng MC, Tan TY. Prehospital delay after acute stroke in Kaohsiung, Taiwan. Stroke. 2004;35(3):700–4. doi: 10.1161/01.STR.0000117236.90827.17. [DOI] [PubMed] [Google Scholar]
- 22.Agyeman O, Nedeltchev K, Arnold M, Fischer U, Remonda L, Isenegger J, et al. Time to admission in acute ischemic stroke and transient ischemic attack. Stroke. 2006;37(4):963–6. doi: 10.1161/01.STR.0000206546.76860.6b. [DOI] [PubMed] [Google Scholar]
- 23.Mandelzweig L, Goldbourt U, Boyko V, Tanne D. Perceptual, social, and behavioral factors associated with delays in seeking medical care in patients with symptoms of acute stroke. Stroke. 2006;37(5):1248–53. doi: 10.1161/01.STR.0000217200.61167.39. [DOI] [PubMed] [Google Scholar]
- 24.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 25.Aslanyan S, Weir CJ, Diener HC, Kaste M, Lees KR. Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. Eur J Neurol. 2004;11(1):49–53. doi: 10.1046/j.1468-1331.2003.00749.x. [DOI] [PubMed] [Google Scholar]
- 26.Hilker R, Poetter C, Findeisen N, Sobesky J, Jacobs A, Neveling M, et al. Nosocomial pneumonia after acute stroke: implications for neurological intensive care medicine. Stroke. 2003;34(4):975–81. doi: 10.1161/01.STR.0000063373.70993.CD. [DOI] [PubMed] [Google Scholar]
- 27.Harms H, Prass K, Meisel C, Klehmet J, Rogge W, Drenckhahn C, et al. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS ONE. 2008;3(5):e2158. doi: 10.1371/journal.pone.0002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chamorro A, Horcajada JP, Obach V, Vargas M, Revilla M, Torres F, et al. The Early Systemic Prophylaxis of Infection After Stroke study: a randomized clinical trial. Stroke. 2005;36(7):1495–500. doi: 10.1161/01.STR.0000170644.15504.49. [DOI] [PubMed] [Google Scholar]
- 29.Lee SL, Battistella FD, Go K. Hypothermia induces T-cell production of immunosuppressive cytokines. J Surg Res. 2001;100(2):150–3. doi: 10.1006/jsre.2001.6230. [DOI] [PubMed] [Google Scholar]
- 30.Remick DG, Xioa H. Hypothermia and sepsis. Front Biosci. 2006;11:1006–13. doi: 10.2741/1858. [DOI] [PubMed] [Google Scholar]
- 31.Kurz A, Ikeda T, Sessler DI, Larson MD, Bjorksten AR, Dechert M, et al. Meperidine decreases the shivering threshold twice as much as the vasoconstriction threshold. Anesthesiology. 1997;86(5):1046–54. doi: 10.1097/00000542-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Mokhtarani M, Mahgoub AN, Morioka N, Doufas AG, Dae M, Shaughnessy TE, et al. Buspirone and meperidine synergistically reduce the shivering threshold. Anesth Analg. 2001;93(5):1233–9. doi: 10.1097/00000539-200111000-00038. [DOI] [PubMed] [Google Scholar]
- 33.Doufas AG, Lin CM, Suleman MI, Liem EB, Lenhardt R, Morioka N, et al. Dexmedetomidine and meperidine additively reduce the shivering threshold in humans. Stroke. 2003;34(5):1218–23. doi: 10.1161/01.STR.0000068787.76670.A4. [DOI] [PubMed] [Google Scholar]