Abstract
β-Catenin plays important roles in liver physiology and hepatocarcinogenesis. While studying the role of β-catenin in diet-induced steatohepatitis, we recently found that liver-specific β-catenin knockout (KO) mice exhibit intrahepatic cholestasis. This study was undertaken to further characterize the role of β-catenin in biliary physiology. KO mice and wild type (WT) littermates were fed standard chow or 0.5% cholic acid supplemented diet for two weeks. Chow-fed KO mice had higher serum and hepatic total bile acid levels and lower bile flow rate than WT mice. Expression levels of bile acid biosynthetic genes were lower and levels of major bile acid exporters similar and, therefore, could not explain the KO phenotype. Despite loss of the tight junction protein claudin-2, KO mice had preserved functional integrity of tight junctions. KO mice had bile canalicular morphologic abnormalities as evidenced by staining for F-actin and zona occludens 1. Electron microscopy revealed dilated and tortuous bile canaliculi in KO livers along with decreased canalicular and sinusoidal microvilli. KO mice on cholic acid diet had higher hepatic and serum bile acid levels, bile ductular reaction, increased pericellular fibrosis, and dilated, misshapen bile canaliculi. Compensatory changes in expression levels of several bile acid transporters and regulatory genes were found in KO livers. In conclusion, liver-specific loss of β-catenin leads to defective bile canalicular morphology, bile secretory defect, and intrahepatic cholestasis. Thus, our results establish a critical role for β-catenin in biliary physiology.
Keywords: Wnt pathway, bile acids, tight junctions, claudin, bile canaliculi
Introduction
β-catenin, the primary effector of the canonical Wnt signaling pathway, plays critical roles in hepatocarcinogenesis and liver development. (1-6) However, its role in adult liver physiology is not well understood. Cytoplasmic levels and localization of β-catenin are tightly regulated (reviewed in MacDonald et al (7)). In the absence of Wnt signaling, β-catenin is bound in the cytoplasm by a multiprotein complex. Phosphorylation via the action of glycogen synthase kinase 3β and casein kinase 1 targets β-catenin for proteasomal degradation. In the presence of Wnt ligands, β-catenin remains unphosphorylated, translocates to the nucleus and activates transcription of its target genes by binding to T cell factor/Lymphoid-enhancing factor family of transcriptional activators. Through its association with E-cadherin at the cell membrane, where it links cadherins to the actin cytoskeleton, β-catenin also plays an important role in the formation of adherens junctions (reviewed in Hartsock and Nelson (8)).
We recently showed that liver-specific β-catenin KO mice have increased susceptibility to developing steatohepatitis on the experimental methionine choline deficient (MCD) diet.(9) Surprisingly, KO mice were found to have higher hepatic total bile acid levels on both MCD and MCD-control diets, suggesting a defect in bile acid metabolism in addition to lipid metabolic abnormalities. Audard et al reported recently that hepatocellular carcinomas containing β-catenin mutations exhibited striking cholestasis.(10) These findings raised the intriguing possibility that β-catenin plays an important role in bile acid homeostasis. Therefore, this study was undertaken to further characterize the role of β-catenin in bile acid physiology in the liver.
Materials and Methods
Animals and diets
Liver-specific β-catenin KO mice (Ctnnb1loxp/loxp;Albumin-Cre) in a C56BL/6 background were generated as previously described.(11) Age- and sex-matched littermates of KO mice with wild type Ctnnb1 alleles were used as WT controls. Genotypes of all mice were confirmed by PCR using primers specific for β-catenin. Mice were given ad libitum access to food and water and were maintained under a 12-hour light/dark cycle. For experiments on chow-diet, 2-3 month female mice were used. Mice were fasted overnight before sacrifice and collection of serum and liver tissue samples. For experiments on cholic acid diet, 2-5 month old male mice were fed either chow diet (Teklad Global Diet 2018; Harlan Teklad, Madison, WI) or chow supplemented with 0.5% cholic acid (Catalog no. TD.06026; Harlan Teklad). Mice were fasted for 4 hours before sacrifice. All animal experiments were performed in accordance with the University of Pittsburgh Animal Use and Care Committee and National Institutes of Health guidelines.
Histological analysis
Formalin-fixed, paraffin-embedded liver sections were stained with hematoxylin and eosin (H&E) or Reticulin by the University of Pittsburgh Research Histology services. An experienced pathologist (ES) evaluated liver histology while blinded to the treatment groups.
Sections were stained for F-actin with TRITC-phalloidin (Sigma-Aldrich, St. Louis, MO) or claudin-2 antibody. Fluorescence (Eclipse E600, Nikon) or confocal (LSM-510) microscopes were used to capture digital images.
Electron microscopy
Liver tissue was fixed with 2.5% glutaraldehyde in PBS by perfusion via the inferior vena cava. Transmission and scanning electron microscopy were performed as described previously by Wack et al. (12)
Bile flow rate and functional analysis of hepatic tight junction integrity
The common bile duct was ligated and the gallbladder cannulated after a midline laparotomy with a polyethylene (PE-10) tube. Bile was collected for 20 min to obtain the flow rate and for analysis of bile composition. Excretion rates of bile components were calculated by measuring the concentration of each component by biochemical assay as described below and adjusting for bile flow rate and body weight.
Functional integrity of hepatic tight junctions was measured as described by Han et al using fluorescein isothiocyanate (FITC)-conjugated dextran, molecular weight 40 kDa (FD-40).(13)
Serum, bile, and tissue biochemical assays
Glutathione, phospholipids, total bile acids, and total cholesterol levels in bile were determined using commercially available kits from Biovision (Mountain View, CA), Wako Chemicals (Richmond, VA), Diazyme (Poway, CA), and Stanbio (Boerne, TX), respectively, according to the manufacturer's instructions. Serum bilirubin, alkaline phosphatase, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels were determined using automated methods in the University of Pittsburgh clinical chemistry laboratory.
Hepatic total bile acid levels was determined by homogenizing 100 mg minced frozen liver tissue in 0.5 ml ice-cold PBS with Halt™ protease and phosphatase inhibitor cocktail (Pierce, Rockford, IL). Cellular debris was removed by centrifuging at 600 × g for 10 min at 4 °C, and the supernatant centrifuged at 105,000 × g at 4 °C to recover purified cytosol. Cytosolic total bile acid levels were measured using a total bile acids kit from Diazyme and normalized to protein concentration for each sample.
RNA extraction and real time PCR (RTPCR)
RNA extraction and RTPCR were performed as previously described using proprietary Taqman® primers from Applied Biosystems (Foster City, CA).(14) The list of primers is provided in Supplementary Table 1. The results are expressed as relative to that in WT mice on chow diet (set at 1).
Protein extraction and Western blots
Crude liver membrane extracts were prepared by homogenizing minced tissue in 100 volumes of ice-cold homogenizing buffer (0.25M sucrose, 10mM Tris-HCl [pH]) containing protease and phosphatase inhibitor cocktail (Pierce). The homogenate was filtered through a gauze sponge and centrifuged at 100,000 × g for 1 h at 4 °C. The resulting pellet was resuspended in resuspension buffer (0.25 M sucrose, 10 mM HEPES [pH 7.5]) containing a protease and phosphatase inhibitor cocktail. Protein concentration was determined by bicinchoninic acid protein assay. Western blot analysis was performed as previously described.(15) Antibodies used in this study are listed in Supplementary Table 2.
Statistical analysis
Results were analyzed using one-way analysis of variance followed by pairwise comparisons with the two-tailed Student's T-test. Values of p < 0.05 were considered significant.
Results
Higher serum and hepatic bile acid levels in KO mice
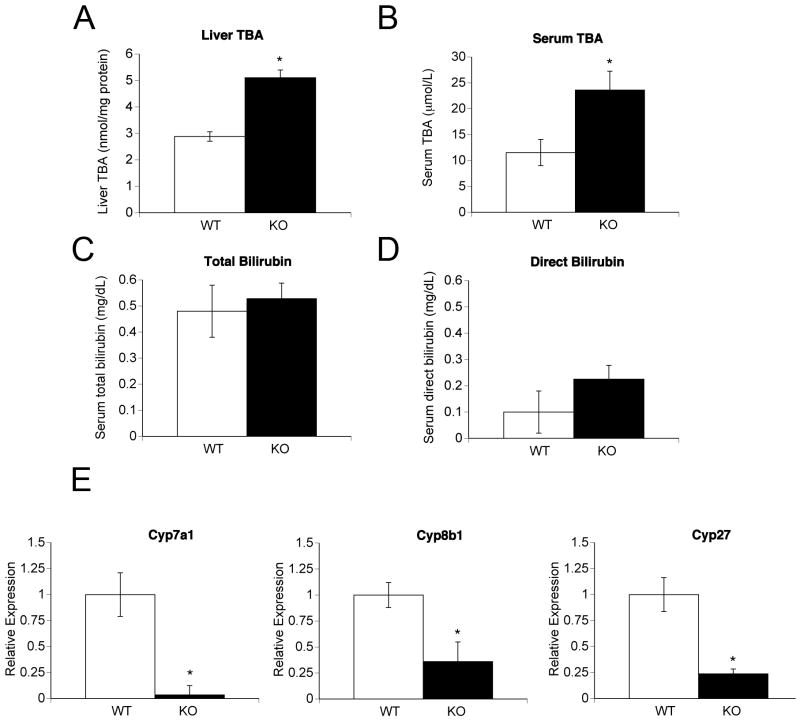
Hepatic total bile acid levels were measured in fasted chow-fed mice. Higher liver bile acid levels were found in both female (Figure 1A) and male (Figure 5D) KO mice. Serum bile acid levels were two-fold higher in KO mice of both sexes (Figures 1B and 5C). Serum total bilirubin levels were similar in the two strains (Figure 1C). Serum direct bilirubin (Figure 1D) and alkaline phosphatase (data not shown) levels were not significantly different (p = .07 for both).
Figure 1.
Liver bile acid levels, serum biochemistry, and expression of bile acid biosynthetic genes in chow-fed female WT and KO mice. (A) Hepatic total bile acid levels (TBA). (B) Serum total bile acid levels. (C) Serum total bilirubin levels. (D) Serum direct bilirubin levels. (E) Expression levels of bile acid biosynthetic genes by real time PCR. All results are expressed as means +/- standard error of mean (SEM) for each group (5-7 mice per group). *p < 0.05.
Figure 5.
Electron micrographs showing bile canalicular and sinusoidal morphology. (A) Low-power scanning electron micrograph from WT liver showing uniform and narrow bile canaliculi (arrows). (B) Low power view from KO liver showing dilated canaliculi (arrows). (C) High power view from WT liver showing numerous microvilli within the canaliculus (arrowheads). Also note the abundant microvilli at the sinusoidal surfaces of hepatocytes (asterisks). (D) High power scanning electron micrographs from KO liver showing dilated and tortuous canaliculi. Note the decreased microvilli within the canaliculus (black arrowhead) and areas devoid of microvilli within the lumen (white arrowheads). Also note the relative paucity of microvilli at the sinusoidal surface of the hepatocytes at the bottom left (asterisk). (E) Transmission electron micrograph from WT liver showing abundant microvilli (arrowheads) at the sinusoidal surface. (F) KO liver showing decreased microvilli at the sinusoidal surface (arrowheads). S, sinusoid; Ec, endothelial cell; RBC, red blood cell.
Lower expression levels of bile acid biosynthetic enzymes in KO mice
Higher bile acid levels in KO mice may result from increased bile acid synthesis. Therefore, we measured expression levels of bile acid biosynthetic genes by RTPCR (Figure 1E). Cytochrome P450 7a1 (Cyp7a1; cholesterol 7α-hydroxylase), the rate limiting enzyme in the classic pathway of bile acid synthesis, and cytochrome P450 27 (Cyp27; cholesterol 27-hydroxylase), the rate limiting enzyme of the alternate pathway of bile acid, were significantly down-regulated in KO livers of both sexes. Expression of cytochrome P450 8b1 (Cyp8b1; sterol 12a-hydroxylase), important in the classic pathway, was lower in female KO mice (Figure 1E) but not in male KO mice (data not shown). We conclude from these data that higher bile acid levels in KO mice did not result from increased bile acid biosynthesis.
Lower bile flow rate in KO mice
To determine whether KO mice had a bile secretory defect, we measured bile flow rate after bile duct cannulation. KO mice had bile flow rate less than 50% of WT levels (p < 0.001) after adjusting for either body weight (Figure 2A), or liver weight (data not shown). Excretion rates of all four major components of bile, namely total bile acids, total cholesterol, phospholipids, and total glutathione, were significantly lower in KO mice (Figure 2B).
Figure 2.
Bile flow rates and expression of canalicular bile acid transporters. (A) Bile flow rate in WT and KO mice (n = 5 per group). (B) Excretion rates of major components of bile as indicated. Excretion rates of bile constituents were calculated by measuring concentration in bile and correcting for the bile flow rate (5 mice per group). (C) Expression levels of Bsep (Abcb11) and Mrp2 (Abcc2) by real time PCR (8 samples per group). Results are expressed as means +/- SEM for each group. *p < 0.05. (D) Western blot analysis using crude membrane extracts for BSEP and MRP2. Actin is shown as the internal loading control.
Expression levels of bile salt export pump (BSEP or ABCB11), responsible for bile acid-dependent bile flow, and ATP-binding cassette sub-family C member 2 (ABCC2 or MRP2), responsible for bile acid-independent bile flow, were similar in WT and KO by RTPCR (Figure 2C). Western blot analysis showed no difference in BSEP and modestly higher MRP2 levels in KO mice (Figure 2D).
Downregulation of claudin-2 expression in KO mice
Liver tumors with activating β-catenin mutations exhibit cholestasis and increased expression of the hepatic tight junction protein claudin-2.(9, 10) We measured hepatic levels of claudin proteins by western blot analysis. While no differences were found in claudin 1 and 3 levels, claudin-2 was nearly undetectable in KO livers (Figure 3A). Bands for claudin-4, which is not expressed in the liver, and claudin-5, which is expressed only in endothelial junctions,(16) were not detected (Figure 3A and data not shown). RTPCR analysis showed that expression of the claudin-2 gene was ten-fold lower in KO mice, suggesting its regulation at the transcriptional level by β-catenin (Figure 3B). Immunostaining for claudin-2 showed prominent zone 3 staining in WT but not in KO livers (Supplementary Figure 2).
Figure 3.
Expression of claudins and evaluation of tight junction integrity. (A) Western blot analysis for claudin 1-4 in crude membrane fractions from livers of WT and KO mice. Actin was used as an internal loading control. (B) Expression levels of claudin-2 by real time PCR (5 samples per group). Results are expressed as means +/- SEM for each group. *p < 0.05. (C) Evaluation of hepatic tight junction integrity. Bile samples were collected in 2.5 min fractions after intravenous injection of FD-40 and analyzed for immunofluorescence. Results are expressed in arbitrary fluorescence units. Results are expressed as means +/- SEM for each group (5 mice per group). *p < 0.05. (D) Transmission electron micrographs showing bile canaliculi (BC), tight junctions (arrowheads), and hepatocytes (H). Scale bar = 500 nm.
Hepatic tight junctions form the blood-bile barrier that keeps sinusoidal blood spatially separated from canalicular bile. We asked whether disruption of hepatic tight junction integrity from loss of claudin-2 could account for the bile secretory defect in KO mice. We tested the functional integrity of hepatic tight junctions in KO mice by assaying for biliary FD-40 excretion after intravenous injection (Figure 3C). Disruption of tight junctions causes an early peak in bile fluorescence.(13) No such early peak in fluorescence was detected in KO mice to suggest defective tight junction integrity. Instead, KO mice had a significant delay in FD-40 excretion in bile, likely resulting from their lower bile flow rate. Evaluation by transmission electron microscopy showed normal appearing tight junctions in KO mice (Figure 3D).
Bile canalicular abnormalities in KO mice
To further evaluate the cholestatic phenotype in KO mice, we stained liver sections with TRITC-phalloidin for F-actin, which exhibits pericanalicular localization in hepatocytes. WT livers exhibited interconnected, evenly spaced “train-track” bile canaliculi (Figure 4, panels A and B). In contrast, KO livers showed distorted bile canaliculi (Figure 4, panels C and D) with occasional grossly misshapen “corkscrew” shaped canaliculi (Figure 4, panels D, E and F). We confirmed that these abnormal structures seen in KO livers on F-actin staining were bile canaliculi by double-labeling liver sections with TRITC-phalloidin and anti-ZO-1 antibody (Supplementary figure 2).
Figure 4.
Bile canalicular morphology in chow-fed WT and KO mice. Liver sections were stained for F-actin with TRITC-phalloidin. (A) WT liver showing an interconnected lattice of bile canaliculi (1000× magnification). A magnified view of a bile canaliculus within the white square is shown in panel B. (B) Magnified view of a bile canaliculus from a WT liver. Note the parallel walls and “train-track” appearance of the canaliculus. (C) KO liver showing distorted bile canaliculi and areas of dilatation interspersed with short segments of normal appearing canaliculi (1000× magnification). A magnified view of the area within the white square is shown in panel D. (D) Magnified view of a canaliculus from a KO liver showing an example of an abnormal “corkscrew”-shaped canaliculus. (E and F) Two more examples of abnormal bile canalicular structures seen in KO livers.
Bile canalicular morphology was also evaluated by scanning electron microscopy (Figure 5, panels A-D). Canaliculi in KO livers were dilated, with an average diameter of approximately 1 μM, which was 25-40% greater than in WT mice. Canaliculi in KO livers were tortuous and showed frequent blind loops. Strikingly, there was a marked paucity of microvilli within canaliculi in KO mice with corresponding bare areas within the canalicular lumina (Figure 5, panels C and D). The subsinusoidal surface of KO hepatocytes also showed a relative decrease in microvilli by both scanning (Figure 5D) and transmission electron microscopy (Figure 5F). The ultrastructure of bile ducts within portal triads appeared normal in KO mice (data not shown).
Effect of cholic acid-containing diet on liver histology in KO mice
Cholic acid feeding has been used to study bile acid-mediated liver toxicity.(17) To determine the effect of cholic acid feeding, mice were fed either chow or chow supplemented with 0.5% cholic acid for 2 weeks. Both strains of mice had body weight loss on the cholic acid diet (Figure 6A) but increase in liver weight and liver-to-body weight ratio (Figure 6B). The latter finding is consistent with the results of Huang et al showing bile acid-dependent liver growth and regeneration.(18)
Figure 6.
Effect of cholic acid feeding. (A) Growth curves on chow or cholic acid diet for male WT and KO mice. *p < 0.05 for body weight difference between WT and KO mice on chow diet. (B). Liver weight-body weight ratio. (C) Hepatic total bile acid (TBA) levels. (D) Serum total bile acid levels. Results are expressed as means +/- SEM for each group. *p < 0.05 for WT v. KO mice on the same diet; #p < 0.05 for chow and CA-fed mice of same genotype (N = 5-7 mice per group for all experiments).
Serum ALT and AST levels were higher in both cholic acid-fed groups, but not different between WT and KO mice (data not shown). However, serum total bile acid levels in cholic acid-fed KO mice were approximately 5-fold higher and hepatic bile acid levels 3-fold higher than in WT mice, suggesting defective ability to regulate serum and liver bile acid levels (Figure 6C and 6D).
Gene expression changes on cholic acid diet
Expression of the sinsusoidal bile acid uptake transporters Ntcp, Slco1a1, and Slco1b2 were lower in both cholic acid-fed groups (Figure 7A). Expression of the latter two genes was also lower in chow-fed KO mice. Expression of the basolateral efflux transporters Abcc3 (Mrp3) and Abcc4 (Mrp4) was not different, although there was a trend towards higher Abcc4 levels in KO mice. Amongst the canalicular transporters, expression of Bsep (Abcb11) was lower in cholic acid-fed KO mice, while Abcb4 (Mdr2) was upregulated in both cholic acid-fed groups (Figure 7B). Expression levels of other sinusoidal transporters were similar between the cholic acid-fed groups.
Figure 7.
Analysis of expression of hepatic bile acid transporters and bile acid regulatory genes in chow and cholic acid-fed WT and KO mice by real time PCR. (A) Basolateral transporters. (B) Canalicular transporters. (C) Genes involved in regulation of bile acid biosynthesis. #p < 0.05 between chow and cholic acid groups; *p < 0.05 between WT and KO groups (4-5 mice per group).
Of the bile acid regulatory genes, expression of farsenoid X receptor (FXR) was lower in both cholic acid-fed groups. However, Shp-1 expression, a downstream target of FXR, was upregulated in both WT and KO mice on cholic acid diet (Figure 7C). Fgf4r levels were similar in all four groups. Expression of constitutive androstane receptor (CAR) was lower in both cholic acid-fed groups and in chow-fed KO mice. However, despite lower expression levels of CAR, KO mice had higher expression of the downstream target of CAR, Cyp2b10. This result suggests that activation of CAR, possibly by a post-transcriptional mechanism, occurs in KO mice and may represent a protective mechanism to downregulate bile acid biosynthetic genes. PPARα expression was higher in both cholic acid groups while PXR expression was lower in cholic acid-fed KO mice.
Liver Histology and Bile canalicular morphology on cholic acid diet
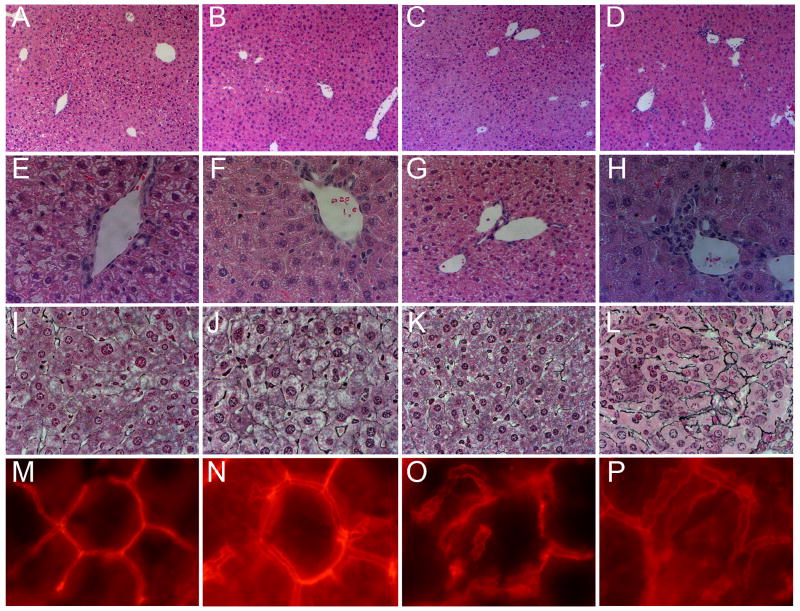
On H&E staining, WT mice showed mildly increased lobular inflammation on cholic acid diet (Figure 8). On the other hand, cholic acid-fed KO mice had increased ductular reaction and greater lobular inflammation on cholic acid diet along with increased pericellular fibrosis on reticulin staining.
Figure 8.
Liver histology in chow and cholic acid-fed WT and KO mice. A, E, I, and M: WT/chow fed; B, F, J, and N: WT/cholic acid fed; C, G, K, and O: KO/chow fed; D, H, L, and P: KO/cholic acid fed. Panels A-D, H&E stained liver sections showing increased lobular inflammation in both cholic acid fed groups. 100× magnification. Panels E-H, high power view of H&E stained sections showing portal triads. Note the bile ductular reaction in KO/cholic acid group in panel H. 400× magnification. Panels I-L, reticulin staining for fibrosis. Note the increased pericellular fibrosis in the KO/cholic acid group in panel L. 400× magnification. Panels M-P, TRITC-phalloidin staining for F-actin showing bile canalicular morphology. Note the dilated but uniformly contoured “train-track” canaliculi in cholic acid fed WT mice (panel N), areas of irregular F-actin staining and focal dilation in the KO/chow group (panel O) and the dilated, tortuous bile canaliculi in the KO/cholic acid group (panel P). 1000× magnification.
Staining for F-actin showed that WT mice on cholic acid diet had dilated bile canaliculi, likely reflecting the choleretic effect of bile acids (Figure 8, bottom panel). However the interconnected lattice-like structure of canaliculi was preserved in WT livers on cholic acid diet. On the other hand, KO mice on cholic acid diet had canaliculi that were not only dilated but also tortuous and distorted (Figure 8, panel P).
Discussion
Formation and excretion of bile is one of the important functions of the liver. This task is achieved by the coordinated action of multiple proteins and cellular organelles. Disruption in canalicular transporter function, loss of nuclear receptors, or disruption of tight junction integrity can result in defective bile secretion and intrahepatic cholestasis.(19-22) Results presented in this paper establish that the Wnt/β-catenin pathway is another regulatory input in the complex process of bile secretion and an important mediator of normal bile canalicular morphogenesis.
The most significant finding of our study is that loss of β-catenin causes bile canalicular abnormalities characterized by dilatation, tortuosity, and loss of canalicular microvilli. It is noteworthy that two downstream targets of β-catenin, claudin-2 and senescence marker protein-30 (SMP30), have been independently shown to be important in bile canalicular formation in vitro and SMP30 has been implicated in the formation of microvilli in hepatoma cells. (23, 24) In further support of a role of β-catenin in the process of canalicular morphogenesis, Theard et al have shown that depletion of β-catenin-E-cadherin based adherens junctions leads to defective canalicular lumen remodeling, suggesting that β-catenin may be involved in bile canalicular morphogenesis via more than one mechanism. (25)
Since β-catenin plays an important role in anchoring the actin cytoskeleton to the cell membrane, it is plausible that KO mice have defective bile canalicular contractility that leads to cholestasis and canalicular dilatation. A similar mechanism has been suggested for connexin-32-deficient mice that exhibit canalicular dilatation and decrease in sympathetic nerve-stimulated bile secretion. (26) Furthermore, disruption of the actin-containing microfilament network by cytochalasin leads to decreased cytoplasmic contractile movements and dilation of bile canalicular lumina. (27) Further experiments to test this hypothesis in β-catenin KO mice are currently ongoing.
We propose the following model for the cholestatic phenotype observed in β-catenin KO mice: Loss of β-catenin, either via alteration of the actin cytoskeleton-adherens junction interactions or via loss of claudin-2 and SMP30, results in bile canalicular morphological abnormalities and bile secretory defect. Decreased bile flow contributes to the development of intrahepatic cholestasis. As a compensatory mechanism, expression of bile acid biosynthetic enzymes is downregulated, along with that of bile acid uptake transporters, Slco1a1 and Slco1b2. CAR may be functionally activated and play a protective role in KO mice since expression of its downstream target Cyp2b10 is significantly higher in KO mice. On being stressed with cholic acid, there is activation of additional protective mechanisms, including downregulation of the uptake transporter, Ntcp, upregulation of the efflux transporter Mdr2, and upregulation of Shp-1, an FXR-target gene that is a negative regulator of bile acid biosynthesis. CAR and FXR/Shp-1 have previously been shown to be activated by bile acids and negatively regulate bile acid biosynthesis.(28, 29)
In summary, β-catenin KO mice exhibit intrahepatic cholestasis, lower bile flow rates, and abnormalities in bile canalicular morphology. Thus, in addition to its multifaceted roles in liver biology, β-catenin plays an important role in biliary physiology in the adult mammalian liver.
Supplementary Material
Acknowledgments
Grant Support: Funded by University of Pittsburgh Department of Medicine Startup Funds and National Institutes of Health Grant 1K08AA017622-01 to JB.
Abbreviations
- KO
knockout
- WT
wild type
- MCD
methionine choline deficient
- MCD
methionine choline-deficient
- H&E
hematoxylin and eosin
- TRITC
tetramethylrhodamine isothiocyanate
- PBS
phosphate buffered saline
- PE
polyethylene
- FITC
fluorescein isothiocyanate
- FD-40
FITC-conjugated dextran
- RTPCR
real time PCR
- Cyp
cytochrome P450
- BSEP
bile salt export pump
- MRP
multidrug resistance protein
- ABC
ATP-binding cassette
- mdr
multiple drug resistance
- ZO-1
zona occludens 1
- Slco
solute carrier organic anion transporter
- FXR
farsenoid X receptor
- Shp
small heterodimer partner
- CAR
constitutive androstane receptor
- PPARα
peroxisome proliferators activated receptor alpha
- PXR
pregnane X receptor
Footnotes
Disclosure: No conflicts of interest exist.
References
- 1.Decaens T, Godard C, de Reynies A, Rickman DS, Tronche F, Couty JP, Perret C, et al. Stabilization of beta-catenin affects mouse embryonic liver growth and hepatoblast fate. Hepatology. 2008;47:247–258. doi: 10.1002/hep.21952. [DOI] [PubMed] [Google Scholar]
- 2.Colnot S, Decaens T, Niwa-Kawakita M, Godard C, Hamard G, Kahn A, Giovannini M, et al. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proc Natl Acad Sci U S A. 2004;101:17216–17221. doi: 10.1073/pnas.0404761101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22:3050–3063. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan X, Yuan Y, Zeng G, Apte U, Thompson MD, Cieply B, Stolz DB, et al. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. 2008;47:1667–1679. doi: 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranganathan S, Tan X, Monga SP. beta-Catenin and met deregulation in childhood Hepatoblastomas. Pediatr Dev Pathol. 2005;8:435–447. doi: 10.1007/s10024-005-0028-5. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behari J, Yeh TH, Krauland L, Otruba W, Cieply B, Hauth B, Apte U, et al. Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis. Am J Pathol. 2010;176:744–753. doi: 10.2353/ajpath.2010.090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Audard V, Grimber G, Elie C, Radenen B, Audebourg A, Letourneur F, Soubrane O, et al. Cholestasis is a marker for hepatocellular carcinomas displaying beta-catenin mutations. J Pathol. 2007;212:345–352. doi: 10.1002/path.2169. [DOI] [PubMed] [Google Scholar]
- 11.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 12.Wack KE, Ross MA, Zegarra V, Sysko LR, Watkins SC, Stolz DB. Sinusoidal ultrastructure evaluated during the revascularization of regenerating rat liver. Hepatology. 2001;33:363–378. doi: 10.1053/jhep.2001.21998. [DOI] [PubMed] [Google Scholar]
- 13.Han X, Fink MP, Uchiyama T, Yang R, Delude RL. Increased iNOS activity is essential for hepatic epithelial tight junction dysfunction in endotoxemic mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G126–136. doi: 10.1152/ajpgi.00231.2003. [DOI] [PubMed] [Google Scholar]
- 14.Komoroski BJ, Zhang S, Cai H, Hutzler JM, Frye R, Tracy TS, Strom SC, et al. Induction and inhibition of cytochromes P450 by the St. John's wort constituent hyperforin in human hepatocyte cultures. Drug Metab Dispos. 2004;32:512–518. doi: 10.1124/dmd.32.5.512. [DOI] [PubMed] [Google Scholar]
- 15.Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33:1098–1109. doi: 10.1053/jhep.2001.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 17.Bochkis IM, Rubins NE, White P, Furth EE, Friedman JR, Kaestner KH. Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat Med. 2008;14:828–836. doi: 10.1038/nm.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 19.Boyer JL. New perspectives for the treatment of cholestasis: lessons from basic science applied clinically. J Hepatol. 2007;46:365–371. doi: 10.1016/j.jhep.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 21.Wagner M, Zollner G, Trauner M. New molecular insights into the mechanisms of cholestasis. J Hepatol. 2009;51:565–580. doi: 10.1016/j.jhep.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Kojima T, Yamamoto T, Murata M, Chiba H, Kokai Y, Sawada N. Regulation of the blood-biliary barrier: interaction between gap and tight junctions in hepatocytes. Med Electron Microsc. 2003;36:157–164. doi: 10.1007/s00795-003-0220-5. [DOI] [PubMed] [Google Scholar]
- 23.Ishigami A, Fujita T, Inoue H, Handa S, Kubo S, Kondo Y, Maruyama N. Senescence marker protein-30 (SMP30) induces formation of microvilli and bile canaliculi in Hep G2 cells. Cell Tissue Res. 2005;320:243–249. doi: 10.1007/s00441-004-1073-5. [DOI] [PubMed] [Google Scholar]
- 24.Son S, Kojima T, Decaens C, Yamaguchi H, Ito T, Imamura M, Murata M, et al. Knockdown of tight junction protein claudin-2 prevents bile canalicular formation in WIF-B9 cells. Histochem Cell Biol. 2009;131:411–424. doi: 10.1007/s00418-008-0546-0. [DOI] [PubMed] [Google Scholar]
- 25.Theard D, Steiner M, Kalicharan D, Hoekstra D, van Ijzendoorn SC. Cell polarity development and protein trafficking in hepatocytes lacking E-cadherin/beta-catenin-based adherens junctions. Mol Biol Cell. 2007;18:2313–2321. doi: 10.1091/mbc.E06-11-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Temme A, Stumpel F, Sohl G, Rieber EP, Jungermann K, Willecke K, Ott T. Dilated bile canaliculi and attenuated decrease of nerve-dependent bile secretion in connexin32-deficient mouse liver. Pflugers Arch. 2001;442:961–966. doi: 10.1007/s004240100623. [DOI] [PubMed] [Google Scholar]
- 27.Phillips MJ, Oshio C, Miyairi M, Smith CR. Intrahepatic cholestasis as a canalicular motility disorder. Evidence using cytochalasin. Lab Invest. 1983;48:205–211. [PubMed] [Google Scholar]
- 28.Soroka CJ, Mennone A, Hagey LR, Ballatori N, Boyer JL. Mouse organic solute transporter alpha deficiency enhances renal excretion of bile acids and attenuates cholestasis. Hepatology. 2010;51:181–190. doi: 10.1002/hep.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.