Abstract
Programmed Death-1 (PD-1)/B7-H1 co-stimulation acts as negative regulator of host allo-immune responses. Although CD4 T cells mediate innate immunity-dominated ischemia/reperfusion injury (IRI) in the liver, the underlying mechanisms remain to be elucidated. This study focuses on the role of PD-1/B7-H1 negative signaling in liver IRI. We used established mouse model of partial liver warm ischemia (90min) followed by reperfusion (6h). Although disruption of PD-1 signaling after anti-B7-H1 mAb treatment augmented hepatocellular damage, its stimulation following B7-H1Ig fusion protected livers from IRI, as evidenced by low sALT levels and well-preserved liver architecture. The therapeutic potential of B7-H1 engagement was evident by diminished intrahepatic T lymphocyte, neutrophil and macrophage infiltration/activation; reduced cell necrosis/apoptosis, yet enhanced anti-necrosis/apoptotic Bcl-2/Bcl-xl; decreased pro-inflammatory chemokine/cytokine gene expression, in parallel with selectively increased IL-10. Neutralization of IL-10 recreated liver IRI and rendered B7-H1Ig treated hosts susceptible to IRI. These findings were confirmed in T cell-macrophage in vitro co-culture in which B7-H1Ig diminished TNF-α/IL-6 levels in IL-10 dependent manner. Our novel findings document the essential role of PD-1/B7-H1 pathway in liver IRI. This study is the first to demonstrate that stimulating PD-1 signals ameliorated liver IRI by inhibiting T cell activation and Kupffer cell/macrophage function. Harnessing mechanisms of negative co-stimulation by PD-1 upon T cell-Kupffer cell cross-talk may be instrumental in the maintenance of hepatic homeostasis by minimizing organ damage and promoting IL-10 dependent cytoprotection.
Introduction
Liver ischemia and reperfusion injury (IRI), an exogenous Ag-independent inflammatory event, occurs in multiple clinical settings, including partial hepatectomy, trauma, and transplantation. Indeed, IRI remains one of the most critical problemsin liver transplant recipients causing up to 10% of early graft failure, leading to a higher incidence of acute and chronic rejection, and contributing to acute donor liver shortage (1,2). Although its mechanism has not been fully elucidated, IR-triggered generation of reactive oxygen species (ROS) inflicts tissue damage, which initiates circulatory disturbances, local inflammation, cell death, and ultimate organ failure. Back in 2003, we proposed that liver damage due to reperfusion following prolonged ischemia should be considered as an innate immunity-dominated inflammation response (2). Our group was among the first to document that activation of TLR4 was required for the induction of IR-triggered hepatic inflammation/damage (3). By releasing inflammatory mediators downstream of TLR4 signaling, such as TNF-α, IL-6 and CXCL-10, we have identified Kupffer cells as critical players in the mechanism of IRI (4,5).
In agreement with others (6), we have reported that T lymphocytes, particularly of CD4 phenotype, represent the key mediators in IR-triggered liver IRI (7). We have highlighted the role of CD154 co-stimulation, and documented the benefit of disrupting CD154-CD40 pathway (8–10). Our studies have shown that CD4 T cells can function via CD154 without de novo Ag-specific activation, and innate immunity-induced CD40 may trigger CD154-CD40 engagement to facilitate tissue inflammation/injury (11). Our recent study has focused at distinctive features of newly identified TIM-1–TIM-4 signaling in liver IRI (12). Collectively, these studies have documented previously unrecognized mechanism by which CD4 T cell-generated “positive” co-stimulation signal can amplify Kupffer cell activity/function and cross-talk to facilitate IR-triggered immune cascade.
Programmed death 1 (PD-1; CD279), is the CD28 homologue expressed selectively by activated T, B and myeloid cells (13). When cross-linked with PD-L1 (B7-H1; CD274) on hemopoetic and many nonhemopoetic tissues, the PD-1/B7-H-1 interaction delivers potent “negative” signal that inhibits T and B cell activation and may promote immune tolerance. This study is the first to examine putative role of PD-1/B7-H-1 in the pathophysiology of liver IRI. Our results demonstrate that stimulating PD-1 negative signals ameliorates local inflammation and liver damage, and suggest that engaging PD-1/B7-H1 co-stimulation is required for maintaining liver homeostasis during IR-induced insult.
Materials and Methods
Animals
Male C57BL/6 wild-type (WT) mice (8–12 weeks old) were used (Jackson Laboratory, Bar Harbor, ME). Animals were housed in the University of California Los Angeles animal facility under specific pathogen-free conditions and received humane care according to the criteria outlined in Guide for the Care and Use of Laboratory Animals (prepared by the National Academy of Sciences; National Institutes of Health publication 86-23, revised 1985).
Mouse model of liver IRI
We have used a mouse model of warm partial hepatic IRI (3–5,7–12). Animals were anesthetized, injected with heparin (100U/kg i.p.), and the arterial and portal venous blood supply to the cephalad lobes was interrupted by an atraumatic clip. After 90min of local ischemia, the clip was removed. Animals were sacrificed after 6h or 24h of reperfusion. Sham-operated mice underwent the same procedure, but without vascular occlusion.
Protein and mAb therapy
Rat anti-B7-H1 mAb (10F.9G2; Bio X Cell, West Lebanon, NH), recombinant B7-H1Ig, a dimeric B7-H1 immunoglobulin fusion protein (14) (courtesy of Dr. Xian C. Li, Harvard Medical School, Boston, MA), or control Ig (Bio X Cell) was given i.v. prior to the onset of ischemia (0.25mg at day -1 and 0.5mg at day 0). Rat anti-mouse IL-10 mAb (JES5-2A5; Bio X Cell) was given (0.5mg at day -1 and 0.5mg at day 0) with or without B7-H1Ig.
The hepatocellular damage
Serum alanine aminotransferase (sALT) levels, an indicator of liver injury, were measured with an autoanalyzer (ANTECH Diagnostics, Los Angeles, CA).
Histology
Liver specimens (4μm), stained with hematoxylin and eosin (H&E), were analyzed blindly by modified Suzuki’s criteria, as described (7–10).
Immunohistochemistry
Primary mAb against mouse T cells CD3 (17A2; BD Biosciences, San Jose, CA), neutrophils Ly-6G (1A8; BD Biosciences) and macrophages F4/80 (FA-11; AbD Serotec, Raleigh, NC) were used, as described (12). Liver sections were evaluated blindly by counting labeled cells in 10 high-power fields (HPF).
Myeloperoxidase activity assay
The presence of myeloperoxidase (MPO) was used as an index of neutrophil accumulation in the liver (7–10). One absorbance unit (U) of MPO activity was defined as the quantity of enzyme degrading 1 mol peroxide/min at 25°C/gram of tissue.
Quantitative RT-PCR
Quantitative PCR was performed with platinum SYBR green quantitative PCR kit (Invitrogen, Carlsbad, CA) by the Chromo 4 detector (MJ Research, Waltham, MA). Primers used to amplify specific gene fragments are listed in Supplementary Table 1. Target gene expressions were calculated by their ratios to the housekeeping gene hypoxanthine-guanine phosphoribosyl transferase (HPRT).
Western blots
Western blots were performed with liver proteins (30μg/sample) and rabbit anti-mouse cleaved caspase-3, Bcl-2, Bcl-xl, and β-actin mAbs (Cell Signaling Technology, Danvers, MA), as described (8–10,12). Relative quantities of protein were determined by densitometer and expressed in absorbance units (AU).
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay
DNA fragments in liver sections, resulting from oncotic necrosis and apoptosis were detected by TUNEL method (In Situ Cell Death Detection Kit, Roche, Indianapolis, IN), as described (7–9,12). TUNEL positive cells were counted in 10 HPF/section under light microscopy (x400).
Cell cultures
Spleen T cells from C57BL/6 mice wereincubated for 24h by addition of anti-CD3 (145-2C11, BD Biosciences; 0.5μg/ml) with B7-H1 or control Ig (20μg/ml). Supernatants were evaluated for IFN-γ/IL-10 levels by ELISA (eBioscience, San Diego, CA). Bone marrow-derived macrophages (BMM), separated from femurs/tibias of C57BL/6 mice were cultured (5×106/well) with 10% L929 conditioned medium for 6 days. The cell purity was assayed to be 94–99% CD11b+. In some experiments, BMM were co-cultured with spleen T cells at responder to stimulator ratios of 1:5 (12), incubated for 24h by using anti-CD3 (0.5μg/ml) with B7-H1Ig or control Ig +/− anti-IL-10 mAb (20μg/ml). Cell-free supernatants were assayed for TNF-α/IL-6 levels by ELISA (eBioscience).
Statistical analysis
All values are expressed as the mean ± standard deviation (SD). Data were analyzed with an unpaired, two-tailed Student’s t test. P < 0.05 was considered to be statistically significant.
Results
PD-1/B7-H1 signaling prevents hepatocellular damage and ameliorates liver IRI
We analyzed the hepatocellular damage in our model of 90min partial liver warm ischemia followed by reperfusion. As shown in Figure 1a, sALT levels increased as early at 1h of reperfusion, peaked at 6h, and decreased thereafter. To determine the function of PD-1/B7-H1 negative signaling in the acute phase of liver IRI in this model, WT mice treated with B7-H1Ig were subjected to 90min of partial liver warm ischemia followed by 6h or 24h of reperfusion. Unlike WT mice given control Ig, those conditioned with B7-H1Ig were resistant against IR-mediated hepatocellular damage, evidenced by reduced sALT levels (U/L; Fig. 1b: 163±30 vs. 845±166 [6h], p<0.01; 65±2 vs. 216±113 [24h], p<0.05). These data correlated with histological criteria of liver damage at the peak of 6h post-reperfusion (Fig. 1c). Control livers revealed severe lobular edema, congestion, ballooning/hepatocellular necrosis (Suzuki’s score=2.33±0.29 [6h]). In contrast, B7-H1Ig group showed well-preserved liver architecture and histological detail without edema, vacuolization or necrosis (score=0.33±0.58 [6h]; p<0.01). To document this effect was dependent on stimulation of PD-1–B7-H1, a separate group of WT mice was treated with anti-B7-H1 mAb. Indeed, PD-1/B7-H1 blockade recreated IR-triggered hepatocellular injury and augmented liver damage, compared with controls, and evidenced by increased sALT levels (U/L; Fig. 1b: 1497±164 [6h], 737±264 [24h]; p<0.05), and deterioration of liver histology (Fig. 1c), reflected by the Suzuki’s score (3.83±0.29 [6h]; p<0.01).
Figure 1.
Distinct effects of blockade vs. activation of PD-1/B7-H1 negative co-stimulation pathway in liver IRI (after 90min of warm ischemia). (a) The liver damage, assessed by sALT levels (U/L), peaks at 6h of reperfusion. (b) The hepatocellular function (sALT; U/L) was impaired after anti-B7-H1 mAb (1497±164 [6h], 737±264 [24h]; ***p<0.05), but improved after B7-H1Ig (163±30 [6h], 65±2 [24h]; **p<0.01, ***p<0.05) treatment, compared with controls (845±166 [6h], 216±113 [24h]; n=6–8/group). These data correlated with (c) Liver histology (H&E staining; magnification x100 and x400) of ischemic liver lobes [6h].
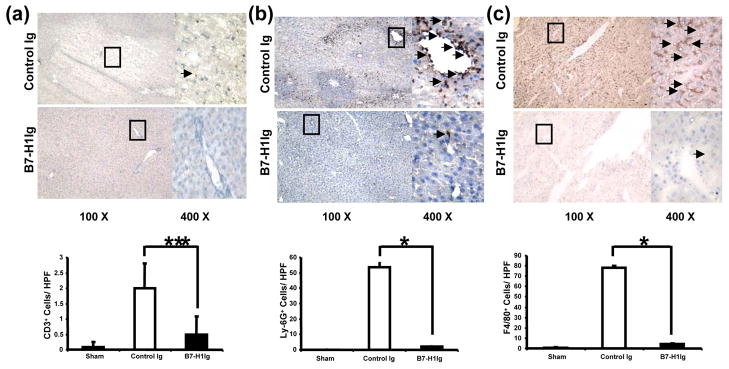
B7-H1Ig engagement attenuates T cell, neutrophil and macrophage infiltration
We performed immunohistochemical staining of liver infiltrating cells at 6h of reperfusion following 90min of warm ischemia. Treatment with B7-H1Ig diminished numbers/HPF of T cells in the ischemic liver lobe (Fig. 2a: 0.5±0.58 vs. 2.0±0.82, p<0.05), neutrophils (Fig. 2b: 2.0±1.0 vs. 53.7±3.2, p<0.001) and macrophages (Fig. 2c: 4.3±1.0 vs. 78.0±2.0, p<0.001), compared with controls. MPO assay, reflecting liver neutrophil activity, was also depressed in the treatment group, as compared with controls (0.03±0.2 vs 1.13±0.3 U/g; p<0.05; Supplementary Figure 1).
Figure 2.
T lymphocytes, neutrophils and macrophages in IR livers following activation of PD-1/B7-H1 pathway. Diminished infiltration of (a) CD3+ T lymphocytes (0.5±0.6 vs. 2.0±0.8, ***p<0.05); (b) Ly-6G+ neutrophils (2.0±1.0 vs. 53.7±3.2, *p<0.001); and (c) F4/80+ macrophages (4.3±1.0 vs. 78.0±2.0, *p<0.001) in B7-H1Ig treated liver lobes, compared with controls. Magnification x100 and x400.
B7-H1Ig treatment prevents IR-triggered liver cytokine/chemokine programs
To assess the immunoregulatory mechanism of PD-1/B7-H1 activation, we contrasted intrahepatic T lymphocyte-related cytokine expression patterns in our model. B7-H1Ig significantly (p<0.05) suppressed gene induction of Th1-type IFN-γ/granzyme B and largely abolished otherwise enhanced gene transcript levels of neutrophil/monocyte-derived pro-inflammatory chemokines (CXCL-1, CXCL-5, CCL-2, CXCL-10) and cytokines (TNF-α, IL-1β, IFN-β, IL-6) (Fig. 3a,b, p<0.01). In contrast, Th2-type IL-10 but not IL-4 expression significantly (p<0.01) increased after B7-H1Ig engagement (Fig. 3c).
Figure 3.
Quantitative RT-PCR-assisted detection of: (a) chemokines (CXCL-10, CXCL-1, CXCL-5, and CCL-2); (b) cytokines (IFN-γ, Granzyme B, TNF-α, IL-1β, IFN-β, IL-6); and (c) IL-4 and IL-10. Selectively increased IL-10 expression after activation of PD-1/B7-H1 signaling. Data were normalized to HPRT gene expression (**p<0.01, ***p<0.05, n=6–8/group).
B7-H1Ig inhibits IR-induced liver necrosis/apoptosis
We performed TUNEL assay to detect IR-triggered oncotic necrosis/apoptosis. B7-H1Ig treatment diminished otherwise abundant hepatocellular necrosis/apoptosis in IR-injured livers (Fig. 4a,b: 2.3±0.6 vs. 38.0±2.0; p<0.001). In parallel, Western blot analysis has revealed selectively decreased expression (AU) of cleaved caspase-3, and increased anti-necrosis/apoptotic Bcl-2/Bcl-xl proteins in B7-H1Ig group (Fig. 4c: Control Ig vs. B7-H1Ig: 1.84±0.041 vs. 0.07±0.020 [cleaved caspase-3], 0.20±0.081 vs. 2.12±0.086 [Bcl-2], 0.29±0.064 vs. 2.08±0.120 [Bcl-xl].
Figure 4.
Necrotic/apoptotic and anti-necrotic/apoptotic pathways in IR livers following activation of PD-1/B7-H1. (a) TUNEL-assisted detection of hepatic necrosis/apoptosis (arrows) in ischemic lobes (magnification x400); (b) Lower frequency of hepatic TUNEL+ cells in B7-H1Ig-treated group, compared with controls (2.3±0.6 vs. 38.0±2.0, *p<0.001); (c) Western blot-assisted detection of cleaved caspased-3, Bcl-2/Bcl-xl after B7-H1Ig treatment, compared with controls (0.07±0.020 vs. 1.84±0.041 AU [cleaved caspase-3], 2.12±0.086 vs. 0.20±0.081 AU [Bcl-2], 2.08±0.120 vs. 0.29±0.064 AU [Bcl-xl], **p<0.01). Representative of n=4.
IL-10 neutralization restores liver IRI in B7-H1Ig treated mice
As liver inflammation response to IR in B7-H1Ig-treated mice was characterized by selectively increased IL-10 (Fig. 3c), the question of whether IL-10 played a cytoprotective function was addressed by neutralizing IL-10. Indeed, significant increase in liver injury was observed after infusion of B7-H1Ig treated mice with anti-IL-10 mAb, as shown by sALT levels (Fig. 5a: 1656.7±358 vs. 163±30 U/L after B7-H1Ig monotherapy, p<0.001), and liver histology (Fig. 5b). Livers in B7-H1Ig treated mice in which IL-10 has been neutralized were characterized by zonal/pan-lobular parenchyma necrosis (Suzuki’s score=3.88±0.25), comparable with controls (Fig. 1b). Infusion of anti-IL-10 mAb triggered significant (p<0.01) increase in the inflammatory gene expression programs (CXCL-10, TNF-α, and IL-6). Thus, IL-10 neutralization recreated liver IRI, rendered B7-H1Ig treated host susceptible to IR, and confirmed the pivotal cytoprotective role of IL-10 produced by B7-H1Ig engagement.
Figure 5.
Functional significance of IL-10 for liver cytoprotection after activation of PD-1/B7-H1 pathway. The neutralization of IL-10 re-stored liver injury in B7-H1Ig-treated mice, as evidenced by (a) sALT levels (U/L; 1656.7±358 vs. 163±30 in B7-H1Ig monotherapy group; **p<0.01, n=6/group), (b) liver histology (representative H&E staining; magnification x100 and x400, Suzuki’s score=3.88±0.25 vs. 0.33±0.58 B7-H1Ig group in Figure 1b, **p<0.01), and (c) increased CXCL-10, TNF-α, and IL-6 expression.
PD-1/B7-H1 pathway regulates T lymphocyte - macrophage cross-talk
We analyzed the immunomodulatory function of PD-1/B7-H1 signaling in well-controlled cell culture system, designed to mimic liver IRI. First, we screened anti-CD3 mAb-mediated activation of T cells with control Ig/B7-H1Ig by ELISA (Fig. 6a). Addition of B7-H1Ig decreased IFN-γ (88.3±21 vs. 1267.8±30 pg/ml, p<0.001) yet increased IL-10 (641.8±42 vs. 302.1±72 pg/ml, p<0.05) levels, as compared with control Ig cultures. This data confirms our in vivo findings (Fig. 3) that activation of PD-1/B7-H1 pathway preferentially induces T cell-derived IL-10.
Figure 6.
The effects of B7-H1Ig upon T cell/macrophage activation in vitro. (a) IFN-γ depression (88.3±21 vs. 1267.8±30 pg/ml) and IL-10 increase (641.8±42 vs. 302.1±72 pg/ml) after treatment of anti-CD3 stimulated T cells with B7-H1Ig (*p<0.001, n=3/group). (b) Upregulated TNF-α/IL-6 production in anti-CD3-stimulated T cell + BMM co-cultures. Addition of B7-H1Ig suppressed TNF-α (62.0±6 vs. 174.6±11 pg/ml) and IL-6 (129.2±8 vs. 653.4±7 pg/ml) levels (**p<0.01, n=3/group). Concomitant neutralization of IL-10 recreated TNF-α (123.0±3 pg/ml) and IL-6 (356.5±9 pg/ml) levels (**p<0.01, n=3/group).
The cross-talk between T lymphocytes and macrophages is essential for progression of liver injury in the early phase of IRI (6,15). To address mechanism by which B7-H1 engagement may affect macrophage priming, we cultured mouse BMM plus anti-CD3 mAb-stimulated T cells with control Ig, B7-H1Ig or B7-H1Ig plus anti-IL-10 mAb (Fig. 6b). Anti-CD3 activated T lymphocytes primed BMM in this co-culture system, as evidenced by increased TNF-α/IL-6 elaboration (p<0.01). Interestingly, B7-H1Ig suppressed macrophage-induced TNF-α and IL-6 levels (62.0±6 vs. 174.6±11 pg/ml [TNF-α], and 129.2±8 vs. 653.4±7 pg/ml [IL-6]; p<0.01). However, concomitant anti-IL-10 mAb recreated BMM activation, as evidenced by augmented TNF-α (123.0±3 pg/ml) and IL-6 (356.5±9 pg/ml) expression. These results document the key regulatory function of PD-1/B7-H1 signaling, and suggest that the defect of B7-H1Ig activated T cells to activate macrophages was IL-10 dependent.
Discussion
In this study we demonstrate, for the first time, that PD-1 negative T cell co-stimulation regulates local innate immunity-driven inflammation response leading to liver IRI. Indeed, although disruption of PD-1 signaling augmented the hepatocellular damage, its deliberate stimulation following B7-H1 engagement protected livers from fulminant IRI by local IL-10 mediated mechanism. These data suggests that engaging negative PD-1/B7-H1 signal is required for maintaining liver homeostasis during IR-mediated hepatocellular insult.
Triggering negative signals through PD-1/B7-H1 in mice has been shown to promote corneal, skin and cardiac allograft survival (16–18), and peripheral transplantation tolerance (19–22). In addition, PD-1/B7-H1 interaction is essential for the spontaneous acceptance of mouse liver allografts (23). The necessity for PD-1/B7-H1 co-stimulation in hepatic defense against IR insult became evident after treatment of WT mice with anti-B7-H1 mAb. PD-1 blockade increased sALT levels and histological Suzuki’s grading of liver injury. We have reported similar findings in mice deficient of anti-oxidant HO-1, in which decreased basal HO-1 levels exacerbated IR-mediated liver damage (24). In analogy with cytoprotection facilitated by HO-1 (25), we asked whether stimulating PD-1/B7-H1 signals might improve liver function. We have chosen an approach of Freeman et al. (26) by engaging the negative receptor PD-1 with a dimeric recombinant fusion protein consisting of the extracellular domain of B7-H1 and the Fc portion of the IgG. This construct has been used in mouse islet (14) and cardiac (18) allograft models. In our series, stimulating PD-1 signaling decreased sALT levels and ameliorated the cardinal histological features of liver injury. The therapeutic potential of PD-1 stimulation was also evident by diminished local T lymphocyte, neutrophil and macrophage infiltration/activation; reduced parenchyma cell necrosis/apoptosis, yet enhanced anti-necrosis/apoptotic Bcl-2/Bcl-xl protein levels; decreased inflammatory chemokine/cytokine gene programs, in parallel with increased IL-10. Strikingly, neutralization of IL-10 recreated liver IRI and rendered IR-resistant B7-H1Ig pre-treated hosts fully susceptible to the panoply of hepatic pro-inflammatory cascade.
In addition to Kupffer and epithelial cells, liver sinusoidal endothelial cells constitutively express B7-H1 (27–30). Hence, PD-1/B7-H1 negative signaling might act as a local traffic regulator to suspend the pathological cell sequestration in the target tissue. Indeed, B7-H1 fusion protein has been shown to determine the accumulation of intrahepatic CD8+ T cells (31). As in our previous studies (12), relatively few CD3+ and CD4+ cells could be found in IR livers, consistent with activation/recruitment of CD4+ T cells within the first hour of reperfusion. Of note, B7-H1 ligation further diminished inflammatory T lymphocyte infiltration/activation, as evidenced by immunohistology and mRNA coding for IFN-γ, granzyme B, and CXCL-10. Interestingly, the levels of anti-inflammatory IL-10 (but not IL-4) were selectively heightened, in agreement with the ability of B7-H1Ig treated T cells to preferentially secrete IL-10 (32), and increased PD-1 and IL-10 levels found in liver transplant patients at high risk for CMV disease (33). Moreover, PD-1 induced IL-10 may impair CD4+ T cell activation during HIV infection (34). Such an altered local inflammation was responsible for liver protection, because IL-10 neutralization restored inflammation and the hepatocellular damage. In support of this notion, we have reported that IL-10 was required for liver protection in mice deficient of CXCL-10 (4), and that vIL-10 gene transfer in WT recipients prevented hepatic IR insult in association with depressed Th1 cytokine/chemokine programs (35). It is plausible that by virtue of selective IL-10 expression, B7-H1Ig might raise the defensive threshold to inflammatory response in IR-exposed livers.
Our results suggest that PD-1/B7-H1 interaction mediates local inflammatory cell infiltration and activation. In the first phase of IR-mediated inflammation response, activation of macrophages/Kupffer cells results in the release of TNF-α, IL-1β, IL-6, CXCL-10, and CCL-2 (MCP-1), the “signature” markers of liver IRI (1–5). These cytokines/chemokines also influence T cell/macrophage trafficking patterns, as evidenced by increased numbers of infiltrating CD3+ cells and F4/80+ cells. However, stimulating PD-1 signals blunted the number of macrophages sequestered in the liver and their inflammation/chemotactic expression programs. In the second phase of IRI, activated neutrophils dominate local damage cascade (1,2). We observed a marked increase in Ly-6G+ neutrophil infiltration and MPO activity in control livers, compared to sham controls. Unlike in control group, livers in B7-H1Ig-treated mice were characterized by decreased neutrophil sequestration, along with diminished CXCL-1 (KC) and CXCL-5 (ENA-78), the key chemoattractants facilitating neutrophil recruitment in the hepatic IR inflammation. As Th1-derived IFN-γ acts directly on neutrophils to enhance their sequestration in the liver, B7-H1 crosslinking can regulate neutrophil function through cytokine/chemokine networks.
One of the principal mechanisms by which PD-1/B7-H1 ligation affects host alloimmunity is through modulation of T cell apoptosis (12). B7-H1 but not PD-1 blockade inhibited apoptosis of allo-Ag specific T cells in transplant recipients (20), and B7-H1 was identified as a key protein controlling deletion of hepatic CD8+ T cells (31). During liver IRI, the death receptor activation, mitochondrial Ca2+ loading, and ROS promote mitochondrial permeability transition, which leads to hepatocellular swelling, rupture of the plasma membrane, and release of cytochrome C and other enzymes, resulting in adenosine triphosphate depletion-dependent oncotic necrosis and caspase-dependent apoptosis (1,36). Both oncotic necrosis and apoptosis proceed via DNA degradation, which can be detected by TUNEL assay (36). In addition to nonparenchymal liver cells, hepatocytes constitutively express low levels of PD-L1, which is strongly enhanced by activated T cells or viral infection, and augmented by stimulation with type I or type II IFNs (28). B7-H1Ig engagement did inhibit necrosis/apoptosis in IR livers, evidenced by decreased frequency of TUNEL+ cells, and consistent with diminished cleaved caspase-3 expression. Simultaneously, we have detected increased Bcl-2/Bcl-xl levels, which are known to exert anti-necrotic/apoptotic functions (37). Hence, it is plausible that cellular and physiological mechanism by which B7-H1 ligation exerts cytoprotection accompanied by enhanced local expression of Bcl-2/Bcl-xl. Consistent with our findings, increased Bcl-2/Bcl-xl levels prevented cell apoptosis in mouse liver IRI (38).
We attempted to mimic in vivo liver damage scenario by employing B7-H1Ig in anti-CD3 mAb-activated murine T cell cultures. Consistent with published data (32), B7-H1Ig treated T lymphocytes failed to elaborate IFN-γ, yet their IL-10 levels increased over 2-fold. This is in agreement with our in vivo findings where PD-1 signals attenuated IFN-γ and promoted IL-10 production. We used BMM and anti-CD3 mAb-activated T cell co-cultures to analyze direct cellular interactions. Although B7-H1Ig failed to affect TNF-α/IL-6 in macrophages, it diminished cytokine elaboration profiles in IL-10 dependent fashion in the co-culture system. Thus, PD-1 ligation by B7-H1 regulates T cell-macrophage cross-talk; IL-10 exerts pivotal cytoprotective function in innate–adaptive cytoprotective feedback mechanism. However, other complementary IL-10 protective mechanisms may be at work. Indeed, IL-10 producing conventional DCs requiring TLR9 can provide protection in a sterile inflammation model of liver IRI (39).
Our results document the essential role of PD-1/B7-H1 pathway in liver inflammation leading to organ damage due to warm IR (Fig. 7). This study is the first to demonstrate that stimulating PD-1 negative signals ameliorated liver IRI by inhibiting T cell activation and Kupffer cell/macrophage functions. Our results provide evidence that harnessing physiological mechanisms of negative co-stimulation by PD-1 upon T cell–Kupffer cell cross-talk may be instrumental in the maintenance of hepatic homeostasis by minimizing organ damage and promoting IL-10 dependent cytoprotection. Targeting PD-1 represents a novel means to improve liver function, expand organ donor pool, and improve the overall success of liver transplantation.
Figure 7.
Cross-talk interactions between liver IR and PD-1 signaling. IR-triggered liver damage leads to CD4 T cell activation, which can transmit the signal to macrophage and neutrophil via IFN-γ. PD-1 is being induced on activated CD4 T cells. After B7-H1Ig engagement, preferential IL-10 secretion attenuates activated macrophage and neutrophil function, and ultimately prevents IR-induced hepatic injury.
Supplementary Material
The MPO activity was significantly suppressed in B7-H1Ig treated group, as compared with controls (0.03±0.2 vs 1.13±0.3 U/g; ***p<0.05, n=6–8/group).
The effects of PD-1/B7-H1 activation (B7-H1Ig) vs. blockade (anti-B7-H1 mAb) as analyzed by the hepatocellular function (sALT; U/L) in liver IRI (6h of reperfusion after 90min of ischemia). A single dose at day 0 exerted comparable effects as compared with 2-dose (day –1 and day 0) regimens (248±38 vs. 163±30 [B7-H1Ig]; 1257±278 vs. 1497±164 [anti-B7-H1 mAb]). In contrast, the efficacy of a 1-dose treatment applied at day –1 was diminished as compared with 2-dose regimen (347±158, **p<0.01 [B7-H1Ig]; 955±92, ***p<0.05 [anti-B7-H1 mAb], n=4–5/group).
Primers used for Quantitative RT-PCR
Footnotes
Supported by: NIH Grants RO1 DK062357, AI23847 (JWKW), and The Dumont Research Foundation. HJ is a recipient of American Society of Transplantation Fellowship Grant.
References
- 1.Farmer DG, Amersi F, Kupiec-Weglinski JW, Busuttil RW. Current status of ischemia and reperfusion injury in the liver. Transplant Rev. 2000;14:106. [Google Scholar]
- 2.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 3.Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173(12):7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 4.Zhai Y, Shen XD, Gao F, Zhao A, Freitas MC, Lassman C, et al. CXCL-10 regulates liver innate immune response against ischemia and reperfusion injury. Hepatology. 2008;47(1):207–214. doi: 10.1002/hep.21986. [DOI] [PubMed] [Google Scholar]
- 5.Zhai Y, Qiao B, Gao F, Shen X, Vardanian A, Busuttil RW, et al. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology. 2008;47(1):199–206. doi: 10.1002/hep.21970. [DOI] [PubMed] [Google Scholar]
- 6.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen XD, Ke B, Zhai Y, Gao F, Anselmo D, Lassman CR, et al. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology. 2003;37(2):296–303. doi: 10.1053/jhep.2003.50066. [DOI] [PubMed] [Google Scholar]
- 8.Shen XD, Ke B, Zhai Y, Amersi F, Gao F, Anselmo DM, et al. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation. 2002;74(3):315–319. doi: 10.1097/00007890-200208150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Ke B, Shen XD, Gao F, Busuttil RW, Löwenstein PR, Castro MG, et al. Gene therapy for liver transplantation using adenoviral vectors: CD40-CD154 blockade by gene transfer of CD40Ig protects rat livers from cold ischemia and reperfusion injury. Mol Ther. 2004;9(1):38–45. doi: 10.1016/j.ymthe.2003.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ke B, Shen XD, Gao F, Tsuchihashi S, Farmer DG, Briscoe D, et al. The CD154-CD40 T-cell co-stimulation pathway in liver ischemia and reperfusion inflammatory responses. Transplantation. 2005;79(9):1078–1083. doi: 10.1097/01.tp.0000161248.43481.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen X, Wang Y, Gao F, Ren F, Busuttil RW, Kupiec-Weglinski JW, et al. CD4 T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury. Hepatology. 2009;50(5):1537–1546. doi: 10.1002/hep.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchida Y, Ke B, Freitas MC, Ji H, Zhao D, Benjamin ER, et al. The emerging role of T cell immunoglobulin mucin-1 in the mechanism of liver ischemia and reperfusion injury in the mouse. Hepatology. 2010;51:1363–1372. doi: 10.1002/hep.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao W, Demirci G, Strom TB, Li XC. Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival. Transplantation. 2003;76(6):994–999. doi: 10.1097/01.TP.0000085010.39567.FB. [DOI] [PubMed] [Google Scholar]
- 15.Hanschen M, Zahler S, Krombach F, Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation. 2008;86:710–718. doi: 10.1097/TP.0b013e3181821aa7. [DOI] [PubMed] [Google Scholar]
- 16.Hori J, Wang M, Miyashita M, Tanemoto K, Takahashi H, Takemori T, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177(9):5928–5935. doi: 10.4049/jimmunol.177.9.5928. [DOI] [PubMed] [Google Scholar]
- 17.Dai H, Zhu H, Lei P, Yagita H, Liu J, Wen X, et al. Programmed death-1 signaling is essential for the skin allograft protection by alternatively activated dendritic cell infusion in mice. Transplantation. 2009;88(7):864–873. doi: 10.1097/TP.0b013e3181b6ea74. [DOI] [PubMed] [Google Scholar]
- 18.Ozkaynak E, Wang L, Goodearl A, McDonald K, Qin S, O’Keefe T, et al. Programmed death-1 targeting can promote allograft survival. J Immunol. 2002;169(11):6546–6553. doi: 10.4049/jimmunol.169.11.6546. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Han R, Hancock WW. Programmed cell death 1 (PD-1) and its ligand PD-L1 are required for allograft tolerance. Eur J Immunol. 2007;37(10):2983–2990. doi: 10.1002/eji.200737583. [DOI] [PubMed] [Google Scholar]
- 20.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204(2):299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179(8):5204–5210. doi: 10.4049/jimmunol.179.8.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morita M, Fujino M, Jiang G, Kitazawa Y, Xie L, Azuma M, et al. PD-1 – B7-H1 Interaction Contribute to the Spontaneous Acceptance of Mouse Liver Allograft. Am J Transplant. 2010;10(1):40–46. doi: 10.1111/j.1600-6143.2009.02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuchihashi S, Livhits M, Zhai Y, Busuttil RW, Araujo JA, Kupiec-Weglinski JW. Basal rather than induced Heme Oxygenase-1 levels are crucial in the antioxidant cytoprotection. J Immunol. 2006;177:4749–4757. doi: 10.4049/jimmunol.177.7.4749. [DOI] [PubMed] [Google Scholar]
- 25.Katori M, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase 1 system in organ transplantation. Transplantation. 2002;74:905–912. doi: 10.1097/00007890-200210150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahl C, Bochtler P, Chen L, Schirmbeck R, Reimann J. B7-H1 on hepatocytes facilitates priming of specific CD8 T cells but limits the specific recall of primed responses. Gastroenterology. 2008;135(3):980–988. doi: 10.1053/j.gastro.2008.05.076. [DOI] [PubMed] [Google Scholar]
- 28.Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C, et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45(4):520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Chen CH, Kuo LM, Chang Y, Wu W, Goldbach C, Ross MA, et al. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology. 2006;44(5):1171–1181. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]
- 30.Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69(20):8067–8075. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20(3):327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 32.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan A, Zhou W, Lacey SF, Limaye AP, Diamond DJ, La Rosa C. Programmed death-1 receptor and interleukin-10 in liver transplant recipients at high risk for late cytomegalovirus disease. Transpl Infect Dis. 2010 doi: 10.1111/j.1399-3062.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4(+) T cell activation during HIV infection. Nat Med. 2010 doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ke B, Shen XD, Tsuchihashi S, Gao F, Araujo JA, Busuttil RW, et al. Viral interleukin-10 gene transfer prevents liver ischemia-reperfusion injury: Toll-like receptor-4 and heme oxygenase-1 signaling in innate and adaptive immunity. Hum Gene Ther. 2007;18(4):355–366. doi: 10.1089/hum.2007.181. [DOI] [PubMed] [Google Scholar]
- 36.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125(4):1246–1257. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- 37.Tsujimoto Y, Shimizu S, Eguchi Y, Kamiike W, Matsuda H. Bcl-2 and Bcl-xL block apoptosis as well as necrosis: possible involvement of common mediators in apoptotic and necrotic signal transduction pathways. Leukemia. 1997;11 (Suppl 3):380–382. [PubMed] [Google Scholar]
- 38.Bilbao G, Contreras JL, Eckhoff DE, Mikheeva G, Krasnykh V, Douglas JT, et al. Reduction of ischemia-reperfusion injury of the liver by in vivo adenovirus-mediated gene transfer of the antiapoptotic Bcl-2 gene. Ann Surg. 1999;230(2):185–193. doi: 10.1097/00000658-199908000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest. 2010;120(2):559–569. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The MPO activity was significantly suppressed in B7-H1Ig treated group, as compared with controls (0.03±0.2 vs 1.13±0.3 U/g; ***p<0.05, n=6–8/group).
The effects of PD-1/B7-H1 activation (B7-H1Ig) vs. blockade (anti-B7-H1 mAb) as analyzed by the hepatocellular function (sALT; U/L) in liver IRI (6h of reperfusion after 90min of ischemia). A single dose at day 0 exerted comparable effects as compared with 2-dose (day –1 and day 0) regimens (248±38 vs. 163±30 [B7-H1Ig]; 1257±278 vs. 1497±164 [anti-B7-H1 mAb]). In contrast, the efficacy of a 1-dose treatment applied at day –1 was diminished as compared with 2-dose regimen (347±158, **p<0.01 [B7-H1Ig]; 955±92, ***p<0.05 [anti-B7-H1 mAb], n=4–5/group).
Primers used for Quantitative RT-PCR