Abstract
Nanoparticles are attractive carriers for vaccines. We have previously shown that a short peptide (Hp91) activates dendritic cells (DCs), which are critical for initiation of immune responses. In an effort to develop Hp91 as vaccine adjuvant with nanoparticle carriers, we evaluated its activity when packaged inside or conjugated to the outside of PLGA nanoparticles (PLGA-NPs). We found that Hp91 when packaged inside or conjugated to the outside of PLGA-NPs not only activates both human and mouse DCs, but was in fact more potent as compared to free Hp91. Hp91 packaged within NPs was ∼5-fold more potent than free peptide and Hp91 conjugated to the outside of NPs was ∼20-fold more potent as compared to free Hp91 peptide. Because of their capacity to activate DCs, such nanoparticle-Hp91 delivery systems are promising for subunit vaccines for infectious disease or cancer.
Keywords: PLGA-nanoparticles, peptide conjugation, immune-stimulation, dendritic cells, HMGB1
Introduction
Nanoparticles (NPs) are being evaluated as carriers for vaccine antigens. Vaccination remains the most successful prophylaxis for infectious disease and in the past decades it has also been explored as an approach to prevent or cure cancer [1, 2]. Since peptides tend to be unstable in vivo, NPs can protect them from degradation and potentially increase the immune response to peptide and possibly protein vaccines. Encapsulation of antigen peptides into biodegradable spheres has been shown to increase MHC-class-I presentation [3]. Delivery of OVA protein as antigen in Poly-γ-Glutamic acid nanoparticles (γPGA-NPs) lead to increased immune responses in comparison to vaccinations using the same amount of free OVA protein [4]. Additionally, enhanced immune responses were observed when the cancer associated antigen MUC-1 [5, 6] as well as Tetanus Toxoid [7] was delivered using Poly-lactic-glycolic acid nanoparticles (PLGA-NPs).
Dendritic cells (DCs) are the most potent antigen-presenting cells and central for the initiation of adaptive immune responses [8]. DCs need to receive a maturation signal in order to present antigen, upregulate co-stimulatory and adhesion molecules, and become potent activators of T cells. Antigen-displaying mature DCs can then activate T cells to act as CTLs. We have previously identified several immunostimulatory peptides (ISPs), derived from the endogenous protein HMGB1, that can activate both mouse and human DCs. We have shown that the 18 aa long ISP named Hp91, when used to activate DCs, can induce potent antigen-specific CTL responses [9]. Due to their immuno-activating properties, ISPs are attractive candidates for vaccine adjuvants. In an effort to develop ISPs for vaccine adjuvant usage we evaluated whether Hp91 could be incorporated into PLGA (poly-DL-lactic-co-glycolic acid) nanoparticles (PLGA-NPs) and still maintain its activity. PLGA was chosen as material for our NPs, since it is a biodegradable and biocompatible polymer [10–13] which has been employed for numerous in vivo applications [14–16]. In addition, polymeric NPs are more stable on the gastrointestinal tract as compared to other carriers like liposomes and can be used for oral vaccine development [17]. Different composition polymers allow for controlled and prolonged release of cargo, allowing for antigen depot formation at the injection site, again another major advantage for vaccine development. We found that delivery of Hp91 inside as well as conjugated to the outside of PLGA-NPs not only preserved their DC stimulatory capacity, but was more potent as compared to free Hp91 peptide. Thus, delivery of ISPs inside or outside of PLGA-NPs is a promising delivery platform for subunit vaccines of infectious disease and cancer.
Materials and Methods
Peptides
Peptides were purchased with an N-terminal biotin at 97% purity from Genscript Corporation, Piscatway, NJ, USA; Web:www.genscript.com. The peptides contain the following sequences: Hp91: DPNAPKRPPSAFFLFCSE and Hp121: SIGDVAKKLGEMWNNTAA. When used as free peptide, the peptides were dissolved at 2 mg/ml in 5% DMSO/RPMI.
Synthesis of PLGA nanoparticles (NPs)
PLGA (poly-DL-lactic-co-glycolic acid) nanoparticles (NPs) that carried the immunostimulatory peptide Hp91 peptide inside or on the outside were synthesized. Empty NPs were synthesized to serve as “carrier” control.
Loading of Hp91 inside of PLGA-NPs
The particles were made using the water-in-oil-in-water (w-o-w) double emulsion method. The PLGA co-polymer (polymer ratio 50:50) was dissolved at 25 mg/ml in chloroform and vortexed for 1h. To make a 30 mg batch of PLGA-NP loaded with Hp91, Hp91 was dissolved in DMSO at 20 mg/ml and 30 µl was added to 270 µl of an aqueous BSA solution (10 mg/ml). As a control, 300 µl BSA solution was used to make empty NPs. The Hp91/BSA solution was added to the PLGA dissolved in chloroform, and then vortexed and sonicated. This created the first emulsion (water in oil). Subsequently this mixture was slowly added to about 10× the volume of 2% (w/v) polyvinyl alcohol and the emulsion was vortexed and sonicated to create the double emulsion. The double emulsion was stirred over night at RT to evaporate off the chloroform, solidifying the particles. The next day the emulsion was stirred under a dessicator in vacuum to remove the remaining chloroform. The particles were washed 3 times with LPS-free water (Thermo Scientific, HyClone Laboratories, Inc., Logan, UT) by ultracentrifugation for 30 min at 30,000 rpm (Beckman Coulter Optima L-90K Ultracentrifuge) and the PLGA-NP were lyophilized for storage at −20°C. PLGA-NPs were dissolved in PBS at 1mg/ml before addition to the cells.
Conjugation of Hp91 to the surface of PLGA-NPs
Hp91 was conjugated to the surface of empty NPs using the linker molecule Denacol EX-521 (Figure.1A). NPs were suspended in borate buffer (pH5) containing Denacol EX-521 (linker) and zinctetrafluroborate hydrate (catalyst). The sample was mixed by vortexing and sonication. For activation of the NPs, the sample was stirred for 30 min at 37°C. To remove un-reacted Denacol, the activated NPs were washed with water by ultracentrifugation and resuspended in borate buffer. To couple the Hp91 peptide, the peptide was dissolved in DMSO and borate buffer and added to the activated NPs. The reaction was carried out for 2h at 37°C under constant stirring. Excess unbound peptide was removed by ultracentrifugation and the samples were lyophilized for storage. The amount of Hp91 on the surface of NPs was quantified by HPLC. PLGA-NPs were dissolved in PBS at 1mg/ml before addition to the cells.
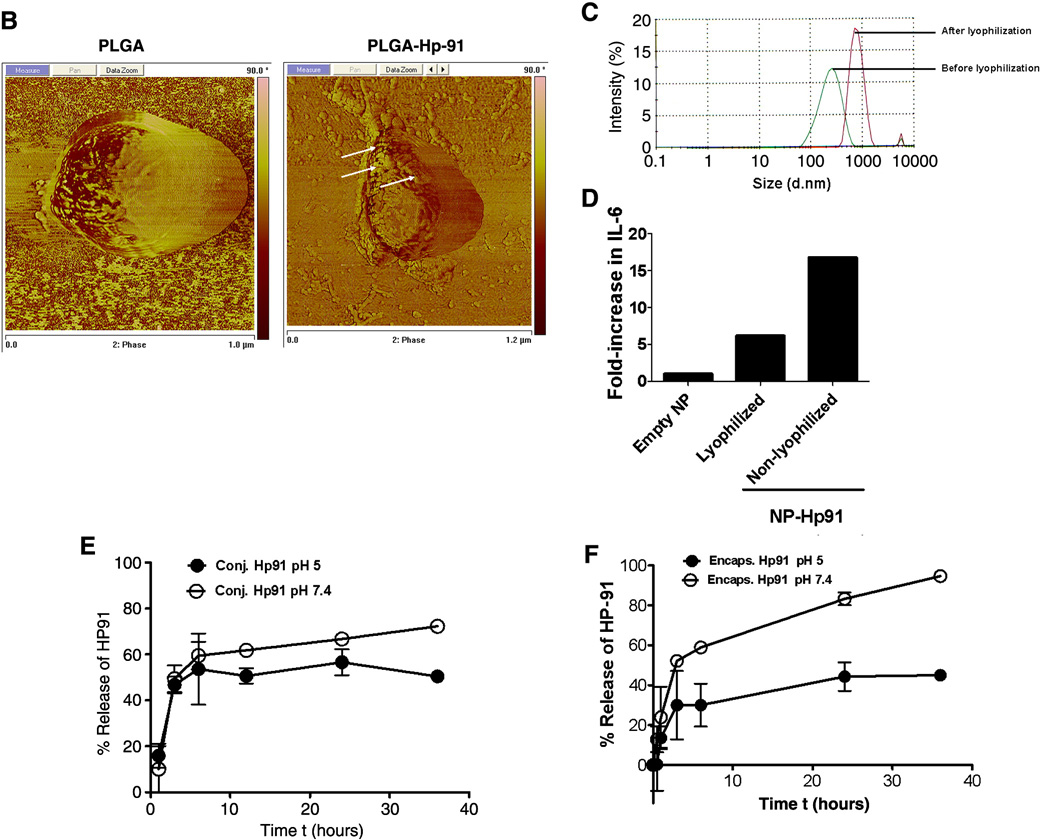
Figure 1. PLGA-NP characterization.
A) Synthesize scheme for conjugation of Hp91 to the surface of PLGA-NPs. B) Atomic force microscopy phase images of empty NPs and NPs conjugated with Hp91. Arrows point to the areas where peptide was detected on the NP surface. C) The size distribution of PLGA-NPs was analyzed before and after lyophilization by dynamic light scattering. The x-axis represents the size in nm. D) Human immature DCs were exposed to media, empty NPs (300 µg/ml), lyophilized PLGA-NPs containing Hp91 (300 µg/ml), and non-lyophilized PLGA-NPs containing Hp91 (240 µg/ml) for 48h. Cell culture supernatants were collected and analyzed for IL-6 by ELISA. Depicted is a representative result showing fold-increase in IL-6 secretion as compared to the empty NP control, which was set as 1. Normalized peptide release profile from PLGA-NPs. E) Hp91 conjugated to the surface of PLGA-NPs. F) PLGA-NPs with Hp91 encapsulated inside the NPs. The graphs show release profiles of Hp91 peptide from PLGA -NPs at pH 7.4 and pH 5 over 36 h. The peptide input at time 0 is set as 100%. Samples were taken at the indicated time points and the amount of peptide in PLGA-NPs was quantified by HPLC. Data shown are mean +/−SEM of triplicate measurements.
Characterization of PLGA-NPs
The NP formation was analyzed for particle size by dynamic light scattering (DLS) using a zetasizer (Zetasizer Nano ZS, Worcestershire, UK). A spectral scan of the Hp91 peptide was performed and the peptide but not the polymer was detected at 211 nm. To quantify the amount of Hp91 present inside or on the surface of the PLGA-NPs, per mg NPs, the NPs were weighted and then dissolved in acetonitrile for 30 min (for NPs loaded with Hp91 inside) or over night (for NPs with Hp91 conjugated to the outside) under constant shaking at room temperature and peptide content was quantified by HPLC (column: WATERS DELTA PAKC18 5 microns, Waters Corporation, Milford, MA) at 211 nm in comparison to a Hp91 standard curve.
To measure stability of the peptide-NPs, 100 µl of particle solution was added to multiple micro dialysis cassettes with a cutoff of 10,000 MW and dialyzed against 1 L of PBS buffer at pH 7.4 or potassium hydrogen phthalate buffer at pH 5. At each time point, two samples were recovered from the micro dialysis cassettes for each buffer condition and the volumes waere brought up to 125 µl to keep all volumes constant. To each sample, 125 µl of acetonitrile was added to dissolve the PLGA-NPs and release the remaining Hp91 peptide. The samples were shaken for 1 hour and then the total amount of Hp91 in each sample was quantified using HPLC. The amounts were normalized against the starting concentration of Hp91 before dialysis, which was set at 100% to calculate the percent release.
In order demonstrate that the peptide was covalently linked to the PLGA particles and not only adsorbed to the surface, 100 µl of Hp91 conjugated PLGA-NPs were sonicated for 3 min and the supernatant was removed using a 10k Microcon Amicon spin column (Millipore, Billerica, MA). As control, 10 µl of Hp91 conjugated PLGA particles were filtered using the spin column without the sonication step. Both the filtrate and the retentate were analyzed for peptide content by HPLC.
Atomic force microscopy
AFM experiments were performed with a Multimode V SPM system (Veeco Instruments Inc.). Height, amplitude, and phase images were obtained in tapping mode in ambient environment with Tapping Mode Etched Silicon Probes (TESP, Veeco Instruments Inc.). Only phase images are shown unless specified. The scan rate is 0.5Hz. Here, AFM phase imaging is used to provide nanometer-scale information about surface structure. During the topographic tapping mode scan, the AFM phase lag of the cantilever oscillation is simultaneously monitored, which is very sensitive to variations in material properties.
Animals
FVB.N\neu-tg mice were derived from in house breeding stocks at the Moores UCSD Cancer Center animal facility. All animal studies were performed with human care of animals and approved by the Institutional Animal Care and Use Committee of UCSD and were performed in accordance with the institutional guidelines.
Generation of mouse BM- DCs
Bone marrow-derived dendritic cells (BM-DC) were prepared from HER-2/neu transgenic mice (H-2q) as described by Inaba et al. [18] with minor modifications. Briefly, single bone marrow cell suspensions were obtained from femurs and tibias, depleted of lymphocytes, granulocytes, and Ia+ cells using a mixture of monoclonal antibodies (anti-CD4, anti-CD8, anti-B220/CD45R, and anti-Ia) for 45 min on ice, followed by incubation with low-toxicity rabbit complement for 30 min at 37°C. Cells were resuspended at a concentration of 106 cells/ml in medium supplemented with recombinant murine GM-CSF (10ng/ml). Fresh media (RPMI/5%FCS) containing GM-CSF was added on days 2 and 4 of culture. On day 6 cells were collected for the experiments.
Generation of human monocyte-derived DCs
Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of normal volunteers over a Ficoll-Hypaque (Amersham Biosciences, Uppsala, Sweden) density gradient. Anonymous blood was purchased from the San Diego Blood Bank, thus no IRB approvals are needed. To generate DCs, PBMCs were allowed to adhere to culture plates for 1h. The non-adherent cells were washed off and the adherent cells were cultured in RPMI 1640 medium supplemented with 2 mM L-glutamine (GIBCO-BRL Life Technologies; Grand Island, NY), 50 mM 2-mercaptoethanol (Sigma, St. Louis, MO), 10 mM HEPES (GIBCO-BRL), penicillin (100 U/ml)-streptomycin (100 µg/ml) (GIBCO-BRL), and 5% human AB serum (Human AB serum, Gemini Bio Products West Sacramento, CA), supplemented with 1000 U/ml GM-CSF (Bayer HealthCare Pharmaceuticals (Wayne, NJ) and 200 U/ml IL-4 (R&D Systems, Minneapolis, MN) at days 0, 2, and 4. Immature DCs were harvested on days 5–7.
Stimulation of DCs
105 immature DCs were incubated in 100 µl culture media with the indicated amounts of empty or peptide carrying NPs, free Hp91 peptide, free Hp121 peptide, a cocktail of inflammatory cytokines (CyC) consisting of IL-1β at 10 ng/ml, TNF-α at 10 ng/ml (R&D Systems), and PGE2 at 1 µg/ml (Sigma), or 10 ng/ml LPS (Sigma). 48h after activation the cell culture supernatants were collected and analyzed for cytokines by ELISA (eBioscience, Inc. San Diego, CA).
Analysis of DC phenotype
DCs were incubated for 20 min at 4°C in 100 µl of PBS/5% FCS/0.1% sodium azide (staining buffer) with Phycoerythrin-conjugated IgG mAb specific for CD80 and CD40, and APC-conjugated IgG mAb specific for CD11c (eBioscience). Cells were washed four times with staining buffer, fixed in 3.7% formaldehyde in PBS (pH 7.2–7.4), and examined by flow cytometry using the FACSCalibur (Beckon Dickinson). In all experiments, isotype controls were included using irrelevant mAb of the same Ig class conjugated to the same fluorophor. Data was analyzed using the FlowJo 7.2.2 software. Data are shown as mean fluorescence intensity gated on CD11c+ cells.
Statistical analysis
Data are represented as mean +/− SD if not otherwise indicated. Data were analyzed for statistical significance using Student’s t-test. p-values < 0.05 were considered statistically significant.
Results
Characterization of PLGA-NPs
To demonstrate the presence of Hp91 on the surface of the PLGA-NPs, we applied atomic force microscopy comparing empty PLGA-NPs to PLGA-NPs that have been conjugated with Hp91 (NP-Hp91). PLGA-NPs with peptide show a rough surface, note arrows (Figure 1 B right image) compared to empty PLGA-NPs generated in the absence of peptide (Figure 1B left image). Although AFM only indicates the presence of protein on the PLGA-NP surface, since the difference in synthesis was presence or absence of Hp91, we conclude that the detected protein on the surface is Hp91. The presence of Hp91 on the PLGA-NP surface was confirmed and quantified by HPLC at 211 nm, which detected peptide in the preparation with Hp91 on the PLGA-NP surface, but not on empty NPs. As measured by HPLC 112 µg Hp91 were conjugated per mg PLGA-NP. These data indicate that we successfully conjugated Hp91 to the outside of the PLGA-NPs.
In order to test whether or not the peptide was covalently linked to the PLGA particles or only adsorbed to the surface, the peptide-conjugated PLGA-NPs were sonicated to release loosely bound peptide, spun down using a using a 10k Microcon Amicon spin column, and the supernatants were analyzed for the presence of peptide by HPLC. After sonication for 3 min, approximately 4.4% of the total Hp91 was measured in the supernatant, while the majority of the peptide, 95.6% was still associated with the particles. Peptide-conjugated PLGA-NPs that were not sonicated, but simply filtered in the same manner, only 0.06% of the Hp91 was measured in the filtrate and 99.9% of the Hp91 remained associated with the particles.
We noted that the PLGA-NPs showed some level of aggregation when observed under the light microscope. In order to improve on the synthesis scheme we investigated at what stage of the synthesis process the aggregation occurred. The size distribution of the PLGA-NPs was measured by dynamic light scattering (see Materials and Methods) after each of the three ultracentrifugation spins which were used to remove un-incorporated peptide from the NPs. Only a slight increase in size was observed. The average size of the PLGA-NPs was 201, 235, and 243 nm after the first to third wash respectively (Table 1). Since the NPs were lyophilized for long-term storage, the NP size was measured before lyophilization and after resuspension of the lyophilized NPs. After resuspension of the lyophilized NPs their average size was 1365 nm (Figure 1C), which indicates formation of small aggregates/quadruplets
Table 1.
PLGA nanoparticles size distribution during preparation.
| PLGA-NP | Number of washes | a size of NP |
|---|---|---|
| one | 201 | |
| two | 235 | |
| three | 243 |
The size distribution of PLGA nanoparticles was measured by zetasizer after each of the three washes of ultracentrifugation.
Next, we tested whether sonication for prolonged periods for time would dissolve the aggregates. NPs were measured after different sonication times (1 to 30 min). After 30 min of sonication their size was reduced from 1365+/− 906 to 648+/− 254 nm (Table 2). This decrease in size after sonication indicates that although the big aggregates were disrupted, some small aggregates, most likely duplets, were still present. Since the aggregation occurred in the lyophilization step, for all subsequent experiments, the NPs were not lyophilized, but instead stored in a mixture of water/10% sucrose at −20°C to avoid aggregation.
Table 2.
PLGA nanoparticles size distribution after sonication.
PLGA nanoparticles were lyophilized after the final wash and resuspended in PBS. The suspension was then sonicated for 1, 15, or 30 min at 40 kHz in an iced sonicator waterbath (Brandon; Model 2510).
The NP size distribution (+/− SD) was measured by dynamic light scattering using three different batches of NPs after the different sonication times: 1, 15, and 30 min.
We hypothesized that non-aggregated PLGA nanoparticles are more potent than aggregated particles due to the increased surface area. To test this hypothesis lyophilized NPs that were conjugated with Hp91 on the surface were resuspended in PBS and compared to NPs that had never been lyophilized, but were instead frozen in PBS/10%sucrose solution immediately after synthesis. Immature human DCs were generated as we have previously described [19] and exposed to empty PLGA-NPs, previously lyophilized PLGA-NPs containing Hp91, and non-lyophilized PLGA-NPs containing Hp91 (Figure 1D). As expected the particles that were stored in solution (non-lyophilized) and had very few aggregates if any, caused increased activation of DCs even at the lower amount tested.
We next measured the stability of the Hp91 conjugated to the surface of PLGA-NPs (Figure 1E). After approximately 3 h, 50% of the conjugated peptide is released from the PLGA-NPs at pH 7.4 and pH5. Although the lower pH results in a slower release of the conjugated peptide over prolonger periods of time than at neutral pH, the difference is not as significant as that for the encapsulated peptide at different pH (Figure 1F). After 3 hours, the release rate begins to flatten out for both pH conditions. After 36 h, PLGA-NPs at pH 7.4 have released approximately 72% of the conjugated peptide, while at pH 5 approximately 50% of the peptide is released.
A similar trend was observed with Hp91 loaded PLGA-NPs with stronger peptide release at higher pH (Figure 1F). After approximately 3h 50% of the peptide is released from within the PLGA-NPs at pH7.4 and after 36 h, 95% of the peptide is released from the PLGA particles in pH 7.4, while only 45% is released from the PLGA-NPs at pH 5. Both conditions produce an initial burst release with a large proportion of the released peptide being released within the first 3 hours. After 3h, both curves flatten out and the release rate is reduced; however, the PLGA-NPs at pH 7.4 continue to release at a higher rate than at pH 5 throughout the time course (Figure 1F).
Hp91 conjugated to the surface of PLGA-NPs induces cytokine secretion in human DCs
DC activation is characterized by the secretion of inflammatory cytokines such as IL-6 [20]. We have previously shown that an 18-amino acid long peptide Hp91, whose sequence corresponds to a part of HMGB1-Bx induced IL-6 secretion in human monocyte-derived DCs [9]. To test whether Hp91 when conjugated to the surface of PLGA-NPs maintains its DC stimulatory capacity, immature human monocyte-derived DCs were exposed to empty PLGA-NPs (NP), PLGA-NPs with Hp91 conjugated to the surface (NP-Hp91), free Hp91 peptide, or a cocktail of inflammatory cytokines (CyC) known to activate DCs. Two days later the cell culture supernatants were collected and analyzed for the presence of IL-6 by ELISA. A highly significant increased in IL-6 secretion was observed when NP-Hp91 with a final Hp91 concentration of 11.2 µg/ml was added to DCs as compared to the same amount of empty PLHA-NPs (p<0.0001) and as compared to 200µg/ml of free Hp91 peptide (p<0.0001) (Figure.2). At 11.2 µg/ml Hp91 when conjugated to the surface of PLGA-NPs induced 3,923 (+/− 410) pg/ml IL-6, whereas 200 µg/ml free Hp91 induced only 1,485 (+/− 376) pg/ml IL-6. This is a 47-fold increased in IL-6 secretion when normalized for the same amount of Hp91. In addition Hp91 conjugated to the PLGA-NP surface induced higher IL-6 secretion as compared to a cocktail of inflammatory cytokines CyC known to mature DCs [21]. When lower amounts of Hp91 (1.12 µg/ml) conjugated to the PLGA-NP surface were used, although an increase in IL-6 was observed, the differences as compared to the controls were not significant (Figure 2).
Figure 2. Hp91 conjugated to the outside of PLGA-NPs causes stronger activation of human DCs as compared to free peptide.
105 immature human DCs were exposed to media, PLGA-NPs carrying Hp91 on the surface (Np-Hp91) with 2 concentrations of Hp91: 11.2 and 1.12 µg/ml, empty NPs matching the amount of PLGA-NPs used with peptides (as NP control), 200 µg/ml of free Hp91 peptide, or a cytokine cocktail CyC (see methods for composition). Cell culture supernatants were collected after 48h and analyzed for IL-6 by ELISA. Data shown is mean +/− SD of three independent experiments using DCs from different donors. * indicates a statistically significant increase.
Hp91 conjugated to the surface of PLGA-NPs causes cytokine secretion in mouse BM-DCs
To determine whether PLGA-NPs carrying Hp91 on the surface also elicit cytokine secretion in mouse DCs, immature mouse bone marrow-derived DCs (BM-DCs), were generated as previously described [9], and exposed to media only, indicated doses of empty PLGA-NPs (NP) (as particle control; matching the amount of PLGA-NP in peptide carrying PLGA-NP), 3 dilutions of PLGA-NPs that have been conjugated with Hp91 on the surface (NP-Hp91), free Hp91 peptide (200 µg/ml), or LPS (10 ng/ml) as positive control. Two days later the cell culture supernatants were collected and analyzed for the presence of IL-6 by ELISA. As observed with human DC, we found that the peptide Hp91 when conjugated to the surface of the PLGA-NPs induced high levels of IL-6 secretion. NP-Hp91 with a final peptide concentration of 34 µg/ml Hp91 induced an average of 5050 +/− 277 pg/ml IL-6, which was significantly higher than empty NPs at the same dose (p=0.008) and than 200 µg/ml free Hp91, which induced an average of 1446 +/− 559 pg/ml IL-6 (p=0.01) (Figure 3A). When normalized to the amount of Hp91, Hp91 is ∼20-fold more potent when delivered on the surface of PLGA-NPs as compared to free Hp91. NP-Hp91 with a final peptide concentration of 3.4 µg/ml Hp91 elicited significant IL-6 expression as compared to empty NPs at the same dose (p=0.03), but it was not significantly different from 200 µg/ml of free Hp91 (Figure 3A).
Figure 3. Hp91 conjugated to the outside of PLGA-NPs causes stronger activation of mouse DCs as compared to free peptide.
105 immature mouse BM-DCs were exposed to media only, PLGA-NPs carrying Hp91 on the surface (Np-Hp91) with 3 concentrations of Hp91: 34, 3.4, and 0.34 µg/ml, empty NPs matching the amount of PLGA-NPs used with peptides (as NP control), 200 µg/ml of free Hp91 peptide, or LPS (10 ng/ml). A) Cell culture supernatants were collected after 48h and analyzed for the presence of IL-6 by ELISA. Data shown is mean +/−SD of two independent experiments using DCs from different donors. B, C) Cells were collected 48h after exposure to the different NP conditions and analyzed for the expression of CD40 and CD80 by surface membrane immunofluorescence techniques using fluorophor conjugated mAbs. DCs were gated on CD11c+ cells and analyzed for expression of the indicated markers. B) depicts percent positive cells and C0 depicts mean fluorescence intensity (MFI). Data shown is mean +/−SD of three independent experiments using DCs from different donors. * indicates a statistically significant increase.
Hp91 conjugated to the surface of PLGA-NPs induces phenotypic maturation of mouse BM-DCs
To determine whether Hp91 when conjugated to the surface of PLGA-NPs can induce phenotypic maturation of mouse BM-DCs, immature BM-DCs were exposed to empty PLGA-NPs (NP), PLGA-NPs with Hp91 conjugated to the surface (NP-Hp91), free Hp91 peptide, or bacterial lipopolysaccharide (LPS), which served as positive control. After 48h cells were harvested and analyzed for surface expression of CD40, CD80, and MHC class II by flow cytometry (Figure. 3 B,C). Empty PLGA-NPs did not cause any changes in CD40 and CD80 expression, but they increased MHC II expression in immature DCs by 2-fold as compared to media control. However, no additional changes in MHC II expression were observed with peptide loaded NPs (data not shown). Interestingly, although free HP91 peptide did not increase expression of CD40 or CD80, Hp91 conjugated to the surface of PLGA-NPs caused a significant increase in CD40 and CD80 expression levels comparable to those induced by LPS (Figure. 3 B,C). During the process of DC maturation some surface molecules are weakly expressed on immature DCs and are upregulated upon activation. This manifest itself as an increase in mean fluorescence intensity (MFI) when measuring expression levels by flow cytometry. Other surface molecules are not or barely expressed on immature DCs and the percentage of cells positive for those molecules can increase upon activation.
On immature DCs (=media control) only a small percentage of DCs in the population expressed CD40 (Figure 3B) and on those the expression was very low (Figure 3C). After exposure to NP-Hp91 with a final Hp91 concentration of 34 µg/ml, the number of CD40 positive DCs significantly increased in comparison to media control (p=0.0009) and to empty NPs at the same concentration (p=0.004) (Figure 3B). Furthermore, NP-Hp91 with 34 µg/ml of Hp91 elicited a highly significant increase in the percentage of CD40 positive cells as compared to 200 µg/ml of free Hp91 (p=0.0008). The same was true for the lower concentration of NP-Hp91 with 3.4 µg/ml of Hp91. Even the low dose of peptide (3.4 µg/ml), NP-Hp91 elicited a significant increase in the percentage of CD40 positive DCs as compared to media control (p=0.04), the same dose of empty NPs (p=0.001), and 200 µg/ml of free Hp91 (p<0.0001) (Figure 3B).
In addition to the increase in the number of CD40-positive DCs, NP-Hp91 with 34 µg/ml of Hp91 elicited a significant increase in CD40 cell surface expression levels as compared to media (p=0.01), empty NPs at the same concentration (p=0.02), and as compared to 200 µg/ml of free Hp91 (p=0.005) (Figure 3C). Although lower amounts NP-Hp91 with a final concentration of 3.4 µg/ml of Hp91 significantly increasing the number of CD40 positive DCs (Figure 3B), no significant increase in CD40 expression were observe additionally (Figure 3C).
In the case of CD80, the majority of the immature DCs (∼80%) within the population were already positive for CD80, although the expression levels were very low (Figure 3C), there was no further increase in the number of positive cells (data not shown). However, NP-Hp91 with 34 µg/ml of Hp91 elicited a highly significant increase in CD80 cell surface expression levels as compared to media (p=0.0002), empty PLGA-NPs at the same concentration (p=0.002), and as compared to 200 µg/ml of free Hp91 (p<0.0001) (Figure 3C). Lower amounts NP-Hp91 with a final concentration of 3.4 µg/ml of Hp91 did not significantly increase the CD80 expression levels as compared to empty PLGA-NPs at the same concentration, but did cause a significant increase as compared to 200 µg/ml of free Hp91 (p=0.04) (Figure 3C). Hence Hp91 when delivered on the surface of PLGA-NPs not only maintains its ability to activate DCs, but the peptide is much more potent as a result and gained additional functions like induction of surface molecule expression on DCs, which is very favorable for vaccine adjuvants.
Delivery of Hp91 inside of PLGA-NPs leads to increased activation of human DCs
We next tested whether Hp91 when packaged inside PLGA-NPs would maintain its ability to activate human DCs. Immature human DCs were exposed to media only, empty-NPs, PLGA-NPs that contained Hp91 inside the nanoparticles NP-(Hp91), and the same amount of free Hp91 peptide as present in the PLGA-NPs. Two days later the cell culture supernatants were collected and analyzed for the presence of IL-6 by ELISA (Figure 4A). Hp91 loaded inside of PLGA-NPs at 9 µg/ml (p=0.01) and 18 µg/ml (p=0.03) significantly increased secretion of IL-6 by DC as compared to media control. In contrast, free Hp91 peptide at 9 or 18 µg/ml did not induce significant increase in IL-6 as compared to media control, indicating that PLGA-NP delivered Hp91 is more potent. Empty NPs did not induce significant changes in IL-6 expression. At 9 µg/ ml Hp91 loaded inside of PLGA-NPs a significant increase (5-fold; (p=0.02) in IL-6 secretion was observed as compared to free Hp91 peptide (Figure 4A). Since Hp91 was dissolved in DMSO for the synthesis of the PLGA-NPs and empty PLGA-NPs carry BSA, we also tested the effect of BSA/DMSO added at the same dose as present in the added PLGA-NPs to DCs. No increase in IL-6 was observed under these control conditions (Figure 4B). To further test whether the observed effects are due to Hp91 or simply caused by the presence of any peptide packaged inside of PLGA-NPs, we incorporated Hp121 as control peptide into PLGA-NPs. Hp121 is derived from the same molecule as Hp91, HMGB1, and we have previously shown has no activity on DCs as free peptide [9]. Here we show that the control peptide Hp121, neither as free peptide, nor packages inside of PLGA-NPs induces secretion of IL-6 by human DCs (Figure 4B).
Figure 4. Packaging of the immunostimulatory peptide Hp91 inside of PLGA-NPs increases their potency to activate human DCs.
A) Immature human DCs were exposed to media, empty PLGA-NPs, PLGA-NPs containing Hp91 encapsulated (Np-Hp91) at 2 doses of Hp91L 9 and 18 µg/ml, or the same amount of free Hp91 peptide was added to the cells. Cell culture supernatants were collected after 48h and analyzed for IL-6 by ELISA. Data shown is mean +/−SD of three independent experiments using DCs from different donors. * indicates a statistically significant increase. B) Immature human DCs were exposed to media only, BSA/DMSO control matching the amount present in the NP preparations, PLGA-NPs that have been filled with Hp91 (NP-Hp91) added at a final concentration of 9 µg/ml of Hp91, PLGA-NPs that have been filled with Hp121 (NP-Hp121) added at a final concentration of 4µg/ml of Hp121, free Hp91 peptide (200 µg/ml), free Hp121 peptide (200 µg/ml), or LPS (10ng/ml). 48h later the cell culture supernatants were collected and analyzed for the presence of IL-6 by ELISA. Data shown is mean +/−SD of three independent experiments using DCs from different donors. * indicates a statistically significant increase.
We also evaluated whether PLGA-NPs exert toxic effects on DCs by comparing empty and Hp91 loaded PLGA-NPs. We did not observe any toxicity with either empty or peptide loaded PLGA-NPs in comparison to media control at doses used in these experiments even after 4 days of culture (data not shown).
Hp91 packaged inside of PLGA-NPs activate mouse BM-DCs
Since increased biological activity using Hp91 loaded PLGA-NPs as compared to free peptide was observed in human DCs, we next evaluated whether the same was true for mouse BM-DCs. Immature mouse BM-DCs were exposed to media only, empty PLGA-NPs (NP), PLGA-NPs that have been filled with Hp91 (NP-(Hp91)) with the final peptide concentration being (13 µg/ml), or 200 µg/ml free Hp91 peptide. Two days later the cell culture supernatants were collected and analyzed for the presence of IL-6 by ELISA (Figure 5). As observed with human DCs, when Hp91 was packaged inside the PLGA-NPs the peptide maintained its ability to induce IL-6 secretion. Hp91 was delivered inside of the PLGA-NPs cause a significant increased in IL-6 expression as compared to media control (p=0.03) and as compared to empty NPs (p=0.04). At only 13 µg/ml when packaged inside the PLGA-NPs Hp91 induced in average 707 pg/ml IL-6 whereas free Hp91 at 200 µg/ml induced similar levels of 545 pg/ml of IL-6. Thus a 15-fold lower amount of Hp91 was sufficient to elicit a similar level of IL-6.
Figure 5. Packaging of the immunostimulatory peptide Hp91 inside of PLGA-NPs increases their potency to activate mouse DCs.
Immature mouse BM-DCs were exposed to medium only, empty NPs, PLGA-NPs that filled with 13 µg/ml of Hp91 (Np-Hp91), or, free Hp91 peptide (200 µg/ml). 48h later the cell culture supernatants were collected and analyzed for the presence of IL-6 by ELISA. Data shown is mean +/−SD of three independent experiments using DCs from different donors.
Discussion
Nano- and microparticles are being evaluated as vaccine carriers. They are very attractive platforms for vaccine delivery since antigen and adjuvant can be co-delivered to antigen presenting cells such as DCs. It has been shown that the generation of CD4 T cell epitopes requires antigen and TLR agonist (adjuvant) to co-translocate to the endosomes [22] and co-delivery of CpG-ODN together with antigen in PLGA microparticles lead to effective cross-presentation and antigen-specific CD8 T cell responses in vivo [23]. Another advantage of using micro- or nanoparticles (NPs) is that the cargo can be protected from degradation by serum or tissue proteases and the co-delivery of antigen and adjuvant ensures that a DC will always encounter both. This is very critical as the uptake of antigen in the absence of adjuvant can lead to generation of tolerance [24], which has to be avoided/minimized to achieve activation of effective immune responses. This is particularly important when trying to break tolerance in the context of a self antigen for cancer immunotherapy.
Although some materials carry intrinsic adjuvant properties like pluronic-stabilized polypropylene sulfide which activates the immune system via the complement cascade [25] or poly(γ-glutamic acid) [4, 26], addition of adjuvants into NPs should further increase immune responses. In some situations inert materials might be preferable as one can control the type of immune response by selecting appropriate adjuvants for incorporation or attachement. Adjuvants like monophosphoryl lipid A [6], as well TLR agonists: CpG ODN [7, 23, 27], and pI:C [28, 29] have been packaged inside micro- and nanoparticles. Although microparticles show efficacy, depending on the route of application nanoparticles could be of further advantage. Different size NPs are preferably taken up by different cell types. For example 40–50 nm NPs are predominantly taken up by DCs, 20 nm NPs by B cells, and 1 µm NPs by monocytes/macrophages [30]. Furthermore, it has been shown that 40 nm, but not 750 nm or larger NPs enter epidermal CD1a+ cells after transcutaneous application on human skin [31]. We have previously shown that the 18 aa long ISP Hp91 is a potent stimulus for mouse and human DCs [9]. In an effort to further develop Hp91 as vaccine adjuvant we tested whether it could be incorporated into or conjugated to the surface of PLGA NPs. PLGA was chosen as material for our NPs, since it is a biodegradable and biocompatible polymer [10–13] that has been employed for numerous in vivo applications [14–16].
We synthesized PLGA-NPs that were loaded with Hp91 peptide or that carried it on the surface via conjugation. We measured the amount of peptide loosely attached to the surface in the process of synthesized PLGA-NPs with peptide conjugated to the surface and found that PLGA-NPs approximately 4.4% of the Hp91 peptide dissociated from the PLGA particles after sonication, suggesting that it was not conjugated but loosely attached. Although sonication increased the amount of peptide released from the particles, the overall amount was still very low, indicating that the Hp91 is in fact covalently attached to the PLGA particles and not just loosely adsorbed to the surface. In addition to releasing any peptide loosely associated with the PLGA particles, sonication of the particles is likely to break up some particles, thus releasing a small amount of peptide. Therefore, we cannot firmly conclude that the 4.4% are truly loosely attached. However, the percentage released is small compared to the amount retained.
We found that Hp91, when packaged inside or outside of PLGA-NPs activates both mouse and human DCs. In both cases the DC stimulatory capacity was higher when the peptide was delivered via NPs as compared to free peptide. One possible explanation is that the delivery is more efficient, since the NPs are readily taken up by DCs and each NP will deliver many peptides, whereas free peptide will diffuse around the cells and the uptake is much less effective. In addition packaging of Hp91 inside of PLGA-NPs or conjugation to the surface most likely protects the peptide from degradation. The increase in Hp91 potency when delivered via NPs could be due to enhanced delivery into intracellular compartments that contain the receptor or other interacting molecules.
The peptide release characteristics show that at pH 5 the peptide is released slower than at pH 7.4. One possible reason for this is due to the protonation of carboxyl groups on the peptide at lower pH values, which would make the peptide less polar and therefore more likely to remain encapsulated in the hydrophobic polymer core. The slow release at pH 5, which is present in endosomes could also contribute to the increase effect of the peptide, as a DC might carry a reservoir that is slowly released and can exert the effect on DCs over a prolonged period of time within the cell. The peptide conjugated to the surface of the PLGA spheres is released slower than the peptide only encapsulated by the PLGA. This is expected as the peptide is covalently linked to the PLGA and must either be cleaved off the PLGA or the PLGA must be sufficiently degraded to be released from the particle.
Furthermore, Hp91 conjugated to the surface of NPs can potentially crosslink the receptor leading to stronger DC activation. The receptor for Hp91 on DCs remains to be identified. However, the fact that Hp91 packaged inside of NPs not only activates the DCs but shows increased potency as compared to free peptide suggests that the receptor is not on the cell surface, but most likely in an intracellular compartment, possibly endosomes, like other adjuvant binding receptors including TLR 3, 7/8 and 9 [32]. In conclusion, we show that the ISP Hp91 when delivered via PLGA-NPs not only maintains its ability to activate DCs, but PLGA-NPs carrying Hp91 are stronger in activating DCs than is free peptide. This warrants further exploration in vaccine settings.
Acknowledgements
We would like to thank Ila Bharati for breeding of the mouse colony.
Conflict of Interest: This work is supported by the US Army Medical Research and Materiel Command under Agreement No. W81XWH-07-1-0412 (to DM), 5 U54 CA119335 from the National Institutes of Health/NCI (to S.E.), the Swedish Research Council AI52731 and the Swedish International Development Cooperation Agency; SIDA and VINNMER (Vinnova) to (ML). Corporate affiliations: Call/Recall Inc., Nanogen, Genoptix, OMM, Orimedix, and Ziva Corp. (S.E.). D.M. holds the following patents: European Application: PTC/US2005/021691, US application: 11/570,695. Title: Immunogenic compositions comprising HMGB1 polypeptides.
Abbreviations
- DC
dendritic cells
- NP
nanoparticles
- PLGA
poly (D, L-lactic-co-glycolic acid)
- CTL
cytotoxic T lymphocyte
- ISP
immunostimulatory peptides
- TLR
Toll-like receptors
- aa
Amino acids
- IL-6
interleukin 6
- LPS
lipopolysaccharide
- BM-DC
Bone marrow-derived dendritic cells
- HMGB1
High mobility group box protein 1
- PBMC
peripheral blood mononuclear cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fournier P, Schirrmacher V. Randomized clinical studies of anti-tumor vaccination: state of the art in 2008. Expert Rev Vaccines. 2009 Jan;8(1):51–66. doi: 10.1586/14760584.8.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006 May;6(5):383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Audran R, Peter K, Dannull J, Men Y, Scandella E, Groettrup M, et al. Encapsulation of peptides in biodegradable microspheres prolongs their MHC class-I presentation by dendritic cells and macrophages in vitro. Vaccine. 2003 Mar 7;21(11–12):1250–1255. doi: 10.1016/s0264-410x(02)00521-2. [DOI] [PubMed] [Google Scholar]
- 4.Uto T, Wang X, Sato K, Haraguchi M, Akagi T, Akashi M, et al. Targeting of antigen to dendritic cells with poly(gamma-glutamic acid) nanoparticles induces antigen-specific humoral and cellular immunity. J Immunol. 2007 Mar 1;178(5):2979–2986. doi: 10.4049/jimmunol.178.5.2979. [DOI] [PubMed] [Google Scholar]
- 5.Diwan M, Elamanchili P, Lane H, Gainer A, Samuel J. Biodegradable nanoparticle mediated antigen delivery to human cord blood derived dendritic cells for induction of primary T cell responses. J Drug Target. 2003;11(8–10):495–507. doi: 10.1080/10611860410001670026. [DOI] [PubMed] [Google Scholar]
- 6.Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly(D,L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004 Jun 23;22(19):2406–2412. doi: 10.1016/j.vaccine.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Diwan M, Tafaghodi M, Samuel J. Enhancement of immune responses by co-delivery of a CpG oligodeoxynucleotide and tetanus toxoid in biodegradable nanospheres. J Control Release. 2002 Dec 13;85(1–3):247–262. doi: 10.1016/s0168-3659(02)00275-4. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998 Mar 19;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 9.Telusma G, Datta S, Mihajlov I, Ma W, Li J, Yang H, et al. Dendritic cell activating peptides induce distinct cytokine profiles. Int Immunol. 2006 Nov;18(11):1563–1573. doi: 10.1093/intimm/dxl089. [DOI] [PubMed] [Google Scholar]
- 10.Dillen K, Vandervoort J, Van den Mooter G, Verheyden L, Ludwig A. Factorial design, physicochemical characterisation and activity of ciprofloxacin-PLGA nanoparticles. Int J Pharm. 2004 May 4;275(1–2):171–187. doi: 10.1016/j.ijpharm.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca C, Simoes S, Gaspar R. Paclitaxel-loaded PLGA nanoparticles: preparation, physicochemical characterization and in vitro anti-tumoral activity. J Control Release. 2002 Oct 4;83(2):273–286. doi: 10.1016/s0168-3659(02)00212-2. [DOI] [PubMed] [Google Scholar]
- 12.Govender T, Stolnik S, Garnett MC, Illum L, Davis SS. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J Control Release. 1999 Feb 1;57(2):171–185. doi: 10.1016/s0168-3659(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 13.Lemoine D, Preat V. Polymeric nanoparticles as delivery system for influenza virus glycoproteins. J Control Release. 1998 Jun;54(1):15–27. doi: 10.1016/s0168-3659(97)00241-1. [DOI] [PubMed] [Google Scholar]
- 14.Ayalasomayajula SP, Kompella UB. Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur J Pharmacol. 2005 Mar 28;511(2–3):191–198. doi: 10.1016/j.ejphar.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Lee SY, Oh JH, Kim JC, Kim YH, Kim SH, Choi JW. In vivo conjunctival reconstruction using modified PLGA grafts for decreased scar formation and contraction. Biomaterials. 2003 Dec;24(27):5049–5059. doi: 10.1016/s0142-9612(03)00411-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhou T, Lewis H, Foster RE, Schwendeman SP. Development of a multiple-drug delivery implant for intraocular management of proliferative vitreoretinopathy. J Control Release. 1998 Nov 13;55(2–3):281–295. doi: 10.1016/s0168-3659(98)00061-3. [DOI] [PubMed] [Google Scholar]
- 17.des Rieux A, Fievez V, Garinot M, Schneider YJ, Preat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006 Nov;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. The Journal of Experimental Medicine. 1992 Dec;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004 Jul 1;173(1):307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 20.Morelli AE, Zahorchak AF, Larregina AT, Colvin BL, Logar AJ, Takayama T, et al. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 2001;98(5):1512–1523. doi: 10.1182/blood.v98.5.1512. [DOI] [PubMed] [Google Scholar]
- 21.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997 Dec;27(12):3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 22.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006 Apr 6;440(7085):808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 23.Heit A, Schmitz F, Haas T, Busch DH, Wagner H. Antigen co-encapsulated with adjuvants efficiently drive protective T cell immunity. Eur J Immunol. 2007 Aug;37(8):2063–2074. doi: 10.1002/eji.200737169. [DOI] [PubMed] [Google Scholar]
- 24.Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 2003 Mar;51(2):59–60. doi: 10.1016/s0369-8114(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 25.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007 Oct;25(10):1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 26.Yoshikawa T, Okada N, Oda A, Matsuo K, Matsuo K, Mukai Y, et al. Development of amphiphilic gamma-PGA-nanoparticle based tumor vaccine: potential of the nanoparticulate cytosolic protein delivery carrier. Biochem Biophys Res Commun. 2008 Feb 8;366(2):408–413. doi: 10.1016/j.bbrc.2007.11.153. [DOI] [PubMed] [Google Scholar]
- 27.de Jong S, Chikh G, Sekirov L, Raney S, Semple S, Klimuk S, et al. Encapsulation in liposomal nanoparticles enhances the immunostimulatory, adjuvant and anti-tumor activity of subcutaneously administered CpG ODN. Cancer Immunol Immunother. 2007 Aug;56(8):1251–1264. doi: 10.1007/s00262-006-0276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimura T, Nakagawa S, Ohtani T, Ito Y, Aiba S. Inhibitory effect of the polyinosinic-polycytidylic acid/cationic liposome on the progression of murine B16F10 melanoma. Eur J Immunol. 2006 Dec;36(12):3371–3380. doi: 10.1002/eji.200636053. [DOI] [PubMed] [Google Scholar]
- 29.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006 Jun 15;176(12):7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 30.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004 Sep 1;173(5):3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 31.Vogt A, Combadiere B, Hadam S, Stieler KM, Lademann J, Schaefer H, et al. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J Invest Dermatol. 2006 Jun;126(6):1316–1322. doi: 10.1038/sj.jid.5700226. [DOI] [PubMed] [Google Scholar]
- 32.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006 May;117(5):979–987. doi: 10.1016/j.jaci.2006.02.023. quiz 88. [DOI] [PubMed] [Google Scholar]