Abstract
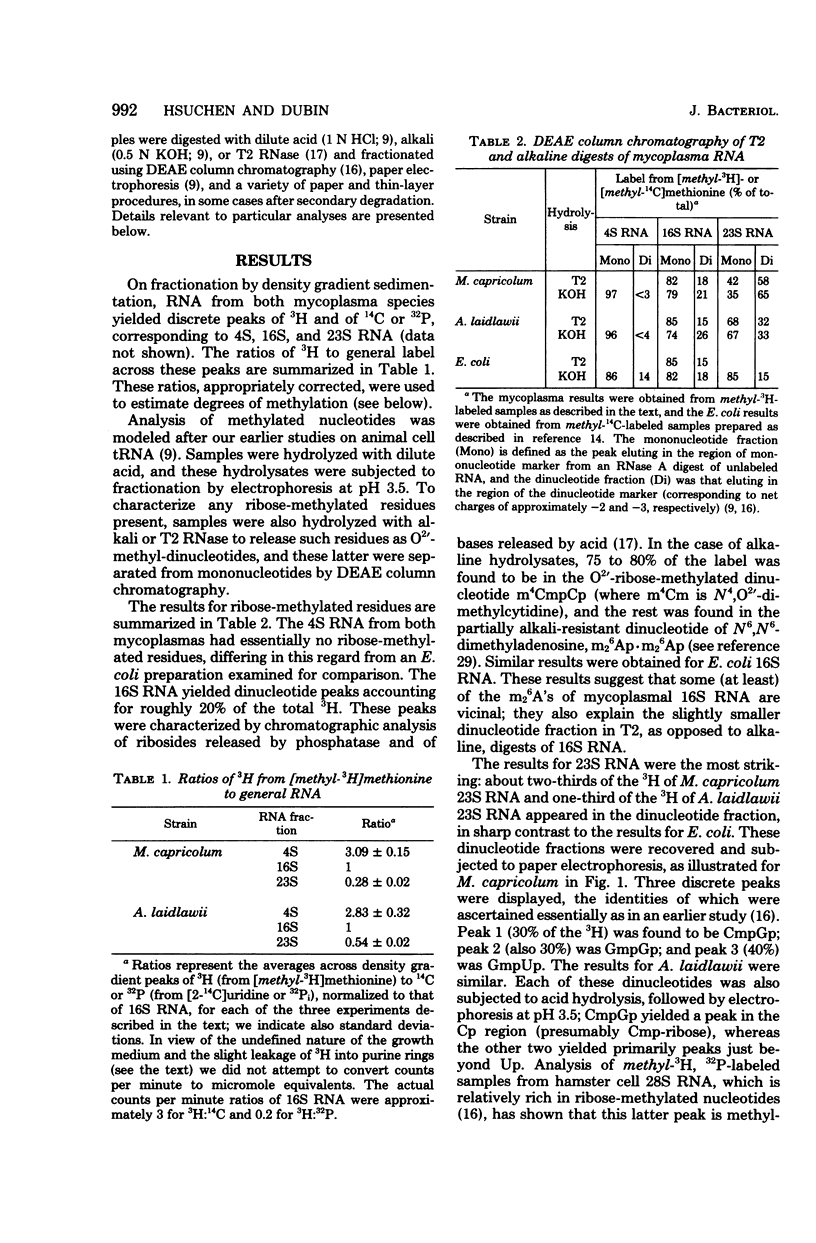
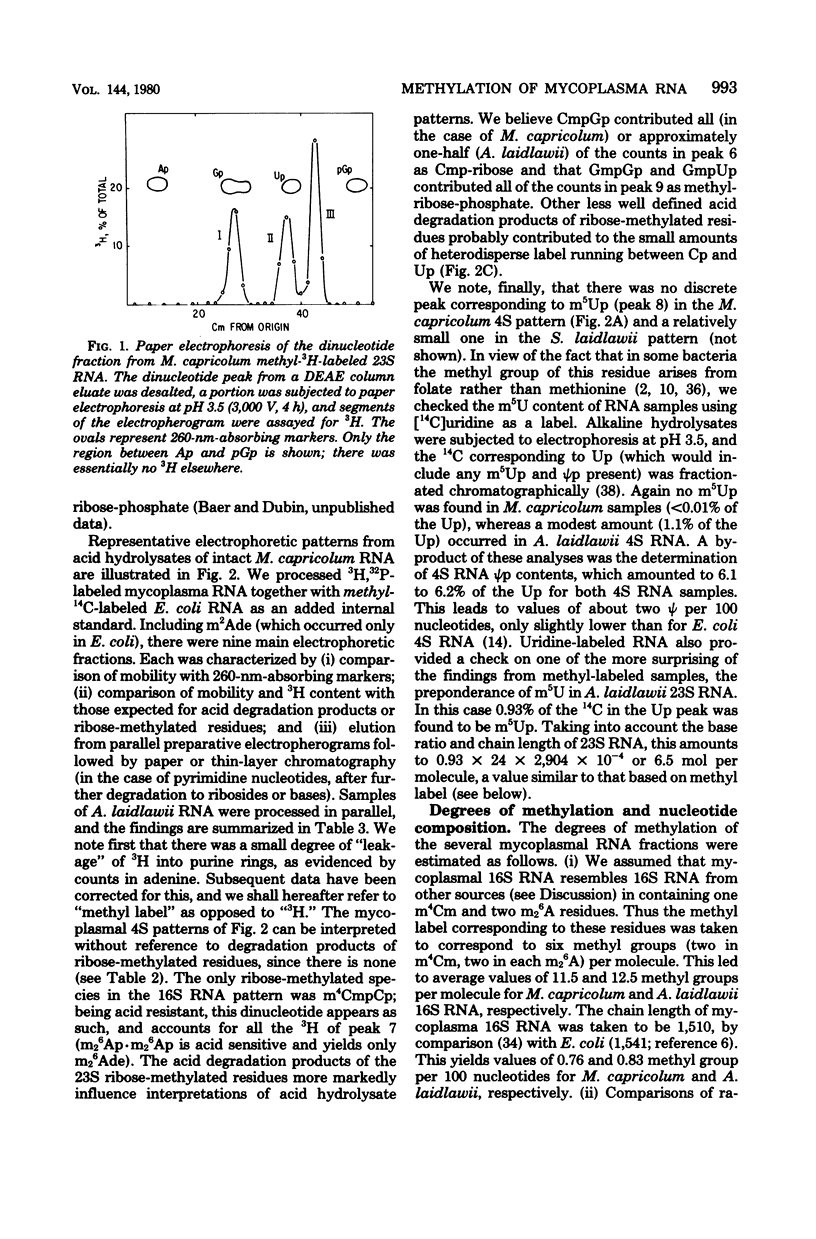
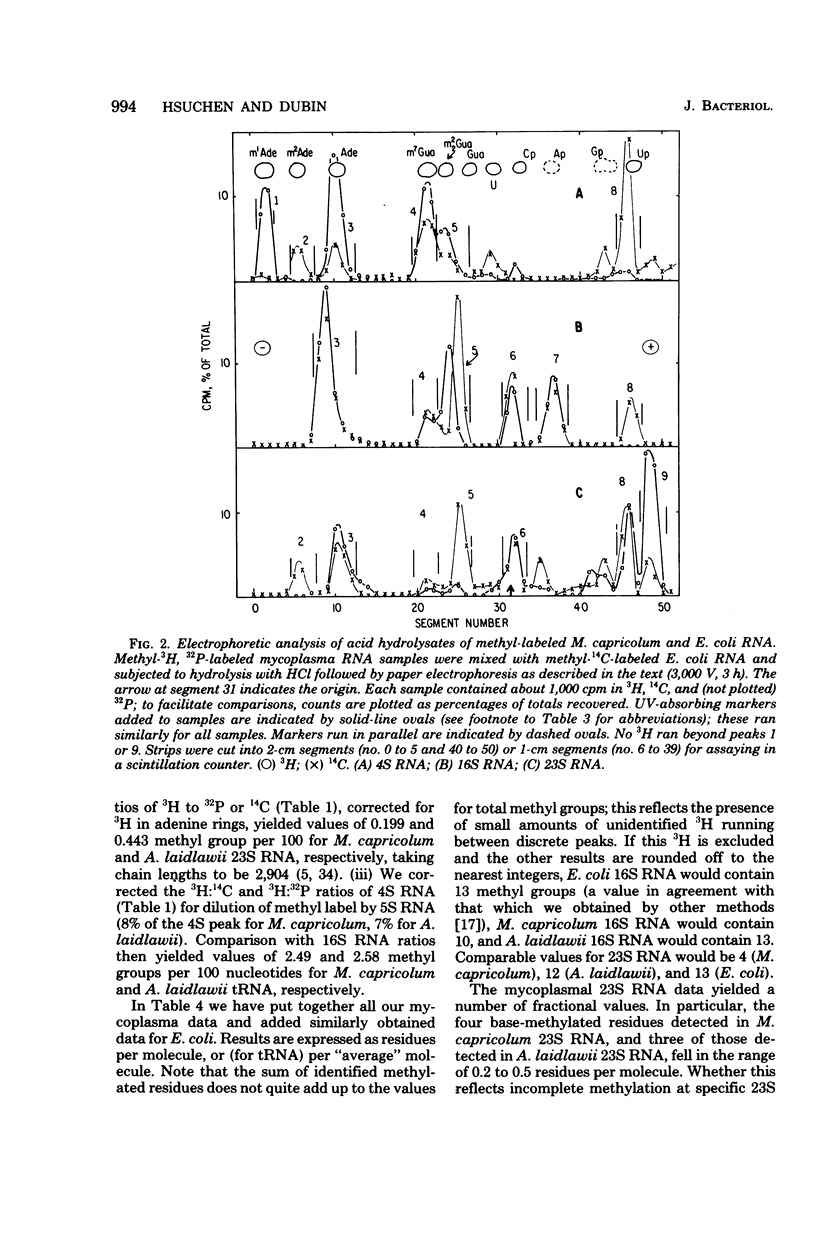
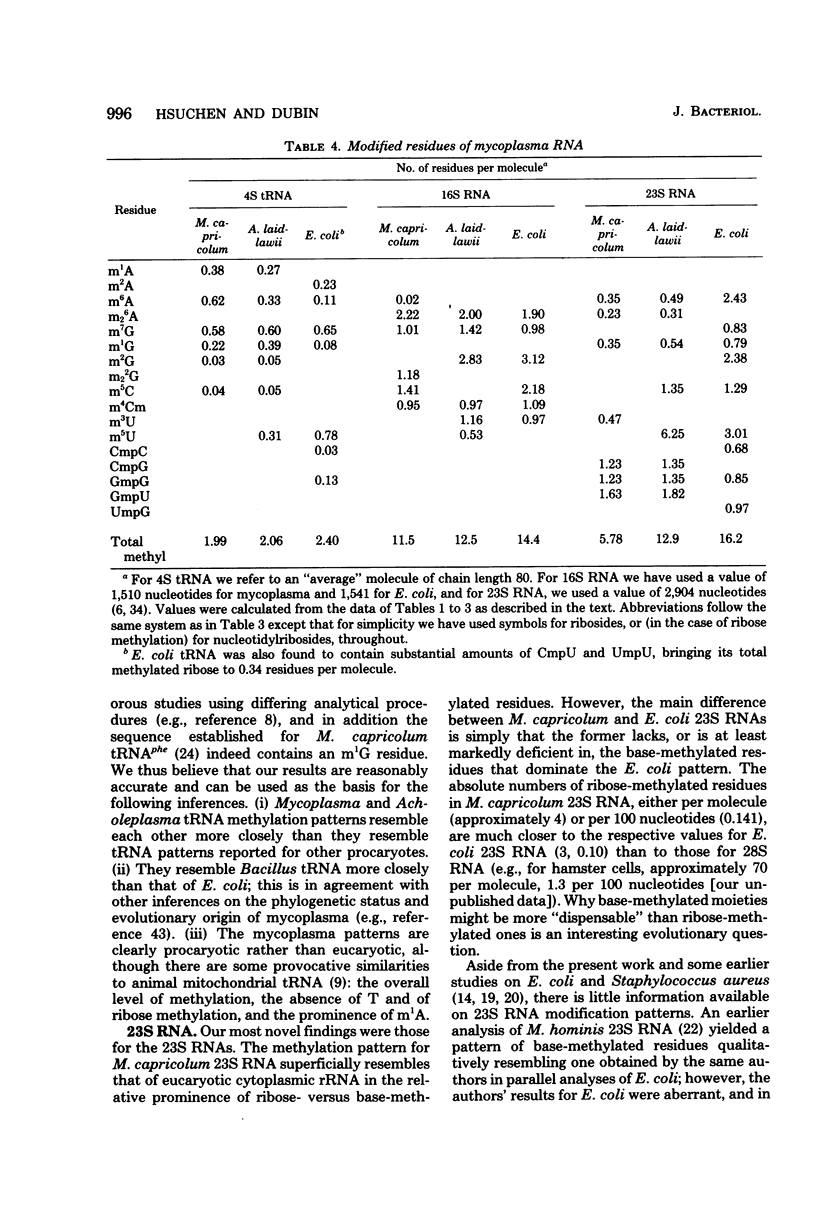
The methylation patterns of transfer and ribosomal ribonucleic acid (RNA) from two mycoplasmas, Mycoplasma capricolum and Acholeplasma laidlawii, have been examined. The transfer RNA from the two mycoplasmas resembled that of other procaryotes in degree of methylation and general diversity of methylated nucleotides, and bore particular resemblance to Bacillus subtilis transfer RNA. The only unusual feature was the absence of m5U from M. capricolum transfer RNA. The methylation patterns of the mycoplasma 16S RNAs were also typically procaryotic, retaining the methylated residues previously shown to be highly conserved among eubacterial 16S RNAs. The mycoplasma 23S RNA methylation patterns were, on the other hand, quite unusual. M. capricolum 23S RNA contained only four methylated residues in stoichiometric amounts, all of which were ribose methylated. A. laidlawii 23S RNA contained the same ribose-methylated residues, plus in addition approximately six m5U residues. These findings are discussed in relation to the phylogenetic status of mycoplasma, as well as the possible role of RNA methylation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold H. H., Schmidt W., Kersten H. Occurrence and biosynthesis of ribothymidine in tRNAs of B. subtilis. FEBS Lett. 1975 Mar 15;52(1):62–65. doi: 10.1016/0014-5793(75)80638-7. [DOI] [PubMed] [Google Scholar]
- Arnold H., Kersten H. The occurrence of ribothymidine, 1-methyladenosine, methylated guanosines and the corresponding methyltransferases in E. coli and Bacillus subtilis. FEBS Lett. 1973 Oct 1;36(1):34–38. doi: 10.1016/0014-5793(73)80331-x. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best A. N. Composition and Characterization of tRNA from Methanococcus vannielii. J Bacteriol. 1978 Jan;133(1):240–250. doi: 10.1128/jb.133.1.240-250.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon P., Ehresmann C., Ehresmann B., Ebel J. P. The complete nucleotide sequence of the ribosomal 16-S RNA from Excherichia coli. Experimental details and cistron heterogeneities. Eur J Biochem. 1979 Oct 15;100(2):399–410. doi: 10.1111/j.1432-1033.1979.tb04183.x. [DOI] [PubMed] [Google Scholar]
- Cecchini J. P., Miassod R. Studies on the methylation of cytoplasmic ribosomal RNA from cultured higher plant cells. Eur J Biochem. 1979 Jul;98(1):203–214. doi: 10.1111/j.1432-1033.1979.tb13178.x. [DOI] [PubMed] [Google Scholar]
- Chia L. L., Morris H. P., Randerath K., Randerath E. Base composition studies on mitochondrial 4 S RNA from rat liver and Morris hepatomas 5123D and 7777. Biochim Biophys Acta. 1976 Feb 18;425(1):49–62. doi: 10.1016/0005-2787(76)90215-x. [DOI] [PubMed] [Google Scholar]
- Davenport L., Taylor R. H., Dubin D. T. Comparison of human and hamster mitochondrial transfer RNA. Physical properties and methylation status. Biochim Biophys Acta. 1976 Oct 18;447(3):285–293. doi: 10.1016/0005-2787(76)90051-4. [DOI] [PubMed] [Google Scholar]
- Delk A. S., Romeo J. M., Nagle D. P., Jr, Rabinowitz J. C. Biosynthesis of ribothymidine in the transfer RNA of Streptococcus faecalis and Bacillus subtilis. A methylation of RNA involving 5,10-methylenetetrahydrofolate. J Biol Chem. 1976 Dec 10;251(23):7649–7656. [PubMed] [Google Scholar]
- Dubin D. T., Friend D. A. Comparison of cytoplasmic and mitochondrial 4 S RNA from cultured hamster cells: physical and metabolic properties. J Mol Biol. 1972 Nov 14;71(2):163–175. doi: 10.1016/0022-2836(72)90344-0. [DOI] [PubMed] [Google Scholar]
- Dubin D. T. Methylated nucleotide content of mitochondrial ribosomal RNA from hamster cells. J Mol Biol. 1974 Apr 5;84(2):257–273. doi: 10.1016/0022-2836(74)90584-1. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., Taylor R. H., Davenport L. W. Methylation status of 13S ribosomal RNA from hamster mitochondria: the presence of a novel riboside, N4-methylcytidine. Nucleic Acids Res. 1978 Nov;5(11):4385–4397. doi: 10.1093/nar/5.11.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin D. T., Taylor R. H. Modification of mitochondrial ribosomal RNA from hamster cells: the presence of GmG and late-methylated UmGmU in the large subunit (17S) RNA. J Mol Biol. 1978 Jun 5;121(4):523–540. doi: 10.1016/0022-2836(78)90398-4. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., Taylor R. H. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 1975 Oct;2(10):1653–1668. doi: 10.1093/nar/2.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H., Falter H. Transfer ribonucleic acid from Mycoplasma laidlawii A. Eur J Biochem. 1971 Feb;18(4):573–581. doi: 10.1111/j.1432-1033.1971.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Fisher H., Söll D. Transfer ribonucleic acid from Mycoplasma. Biochemistry. 1969 Sep;8(9):3680–3686. doi: 10.1021/bi00837a028. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Horowitz J. Characterization of ribosomes and RNAs from Mycoplasma hominis. Biochim Biophys Acta. 1971 Oct 14;247(2):262–279. doi: 10.1016/0005-2787(71)90675-7. [DOI] [PubMed] [Google Scholar]
- Khan M. S., Salim M., Maden B. E. Extensive homologies between the methylated nucleotide sequences in several vertebrate ribosomal ribonucleic acids. Biochem J. 1978 Mar 1;169(3):531–542. doi: 10.1042/bj1690531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball M. E., Szeto K. S., Soll D. The nucleotide sequence of phenylalanine tRNA from Mycoplasma sp. (Kid). Nucleic Acids Res. 1974 Dec;1(12):1721–1732. [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M. An evolutionary study of the methylation of transfer and ribosomal ribonucleic acid in prokaryote and eukaryote organisms. J Biol Chem. 1973 Apr 10;248(7):2612–2620. [PubMed] [Google Scholar]
- LITTLEFIELD J. W., DUNN D. B. The occurrence and distribution of thymine and three methylated-adenine bases in ribonucleic acids from several sources. Biochem J. 1958 Dec;70(4):642–651. doi: 10.1042/bj0700642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKHAM R., SMITH J. D. The structure of ribonucleic acid. I. Cyclic nucleotides produced by ribonuclease and by alkaline hydrolysis. Biochem J. 1952 Dec;52(4):552–557. doi: 10.1042/bj0520552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J. L., Lane B. G. N-4-methyl-2'-O-methyl cytidine and other methyl-substituted nucleoside constituents of Escherichia coli ribosomal and soluble RNA. Biochim Biophys Acta. 1966 Jun 22;119(3):649–651. doi: 10.1016/0005-2787(66)90147-x. [DOI] [PubMed] [Google Scholar]
- Razin S., Morowitz H. J., Terry T. M. Membrane subunits of Mycoplasma laidlawii and their assembly to membranelike structures. Proc Natl Acad Sci U S A. 1965 Jul;54(1):219–225. doi: 10.1073/pnas.54.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. Physiology of mycoplasmas. Adv Microb Physiol. 1973;10:1–80. doi: 10.1016/s0065-2911(08)60086-7. [DOI] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogg H., Brambilla R., Keith G., Staehelin M. An improved method for the separation and quantitation of the modified nucleosides of transfer RNA. Nucleic Acids Res. 1976 Jan;3(1):285–295. doi: 10.1093/nar/3.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W., Arnold H. H., Kersten H. Tetrahydrofolate-dependent biosynthesis of ribothymidine in transfer ribonucleic acids of Gram-positive bacteria. J Bacteriol. 1977 Jan;129(1):15–21. doi: 10.1128/jb.129.1.15-21.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Grueter F., Spelzhaus A., Gauss D. H. Compilation of tRNA sequences. Nucleic Acids Res. 1980 Jan 11;8(1):r1–r22. [PMC free article] [PubMed] [Google Scholar]
- Taylor R. H., Varricchio F., Dubin D. T. Hamster mitochondrial transfer RNA lacks T and the universal GUUCG sequence. Biochim Biophys Acta. 1980 May 30;607(3):521–526. doi: 10.1016/0005-2787(80)90162-8. [DOI] [PubMed] [Google Scholar]
- Vani B. R., Ramakrishnan T., Taya Y., Noguchi S., Yamaizumi Z., Nishimura S. Occurrence of 1-methyladenosine and absence of ribothymidine in transfer ribonucleic acid of Mycobacterium smegmatis. J Bacteriol. 1979 Mar;137(3):1084–1087. doi: 10.1128/jb.137.3.1084-1087.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. Modified nucleosides of Bacillus subtilis transfer ribonucleic acids. J Bacteriol. 1976 Jul;127(1):258–267. doi: 10.1128/jb.127.1.258-267.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Oshima T., Saneyoshi M., Nishimura S. Replacement of ribothymidine by 5-methyl-2-thiouridine in sequence GT psi C in tRNA of an extreme thermophile. FEBS Lett. 1974 Jul 1;43(1):59–63. doi: 10.1016/0014-5793(74)81105-1. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E., Zablen L., Uchida T., Bonen L., Pechman K., Lewis B. J., Stahl D. Conservation of primary structure in 16S ribosomal RNA. Nature. 1975 Mar 6;254(5495):83–86. doi: 10.1038/254083a0. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Maniloff J., Zablen L. B. Phylogenetic analysis of the mycoplasmas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):494–498. doi: 10.1073/pnas.77.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]


