Abstract
In this report, the possibility of pharmacologically altering the hepatitis B virus (HBV) epitopes presented by major histocompatibility complex (MHC) class I on infected cells is demonstrated. The HBV middle envelope glycoprotein MHBs maturation appears to require calnexin mediated folding. This interaction is dependent upon glucosidases in the endoplasmic reticulum. Prevention of HBV envelope protein maturation in cultured cells with glucosidase inhibitors, such as 6-O-butanoyl castanospermine and N-nonyl deoxynorjirmycin, resulted in MHBs degradation by proteasomes. The de-N-glycosylation associated with polypeptide degradation was predicted to result in conversion of asparagine residues into aspartic acid residues. This prediction was confirmed by showing that peptides corresponding to the N-glycosylation sequons of MHBs, but with aspartic acid replacing asparagine, (a) can prime human CTLs that recognize HBV producing cells and (b) that the presentation of these envelope motifs by MHC class I is enhanced by incubation with glucosidase inhibitors. Moreover, although peripheral blood mononuclear cells isolated from woodchucks chronically infected with woodchuck hepatitis virus (WHV) and vaccinated with WHV surface antigen could be induced to recognize the natural MHBs asparagine-containing peptides, only cells isolated from glucosidase inhibitor treated animals recognized the aspartic containing peptides. Conclusion: These data suggest that pharmacological intervention with glucosidase inhibitors can alter the MHBs epitopes presented. This editing of the amino acid sequence of the polypeptide results in a new epitope, or “editope”, with possible medical significance.
Keywords: envelope glycoprotein, imino sugars, 6-O-butanoyl castanospermine, HBV
Chronic infection with hepatitis B virus (HBV) is characterized by a lack of robust T cell responsiveness to viral antigens (1, 2). Indeed, an inadequate CD8+ T cell response is thought to be key to the establishment of chronicity. Typically, virus-specific CD8+ cytotoxic T lymphocytes (CTLs) are elicited by infected cells presenting virus-derived peptides by major histocompatibility complex (MHC) class I. However, poor CTL responses in chronic HBV infection are likely a consequence of multiple factors (1, 2), including viral interference with efficient processing and presentation of HBV epitopes (3). Thus, methods that can cause enhanced recognition or presentation of viral epitopes by MHC class I might be useful as therapeutic interventions and as research tools.
Viral glycoproteins represent important targets for any antiviral immune response. HBV is an enveloped virus with three glycoproteins: LHBs, MHBs and SHBs (4). In tissue culture, the HBV envelope proteins are very stable, and are degraded by proteasomes less efficiently than host proteins (5). Resistance to proteasomal degradation might contribute to HBV’s refractoriness to presentation by MHC class I and even to establishment of chronicity (6). However, compared to most cellular N-glycoproteins, and even the SHBs, the MHBs protein is unusually dependent upon calnexin mediated protein folding (7, 8). Calnexin is a cellular lectin chaperone that recognizes N-glycans on nascent proteins that have been trimmed to a mono-glucose residue (9, 10). This trimming is mediated by glucosidases in the endoplasmic reticulum (ER). Inhibition of glucosidases resulted in significant and selective degradation of MHBs under conditions where most cellular glycoproteins are spared (7, 11). The sensitivity of MHBs to glucosidase inhibition was correlated with antiviral activity in animals (11).
Degradation of MHBs in the presence of glucosidase inhibitors was mediated by cellular proteasomes (5, 12). Since proteasomal degradation products are substrates for MHC class I-mediated presentation to T cells, it was hypothesized that glucosidase inhibitors could selectively enhance presentation of MHBs epitopes. This prediction was confirmed in cell culture; glucosidase inhibitor treatment of target cells resulted in increased killing by peptide-specific CTLs (13). A logical extension of this observation is that degradation of MHBs following glucosidase inhibition would be accompanied by de-N-glycosylation. Hydrolysis of N-linked glycan from asparagines of glycoproteins is thought to occur in the cytoplasm by the enzyme peptide:N-glycanase (PNGase) (14), resulting in conversion to aspartic acid (15, 16). Thus, de-N-glycosylation of MHBs in glucosidase-inhibited cells should be accompanied by altered polypeptide amino acid composition. It is thus postulated that such edited epitopes, or “editopes”, could be created by pharmacological intervention with glucosidase inhibitors. Although presentation of peptides containing aspartic acid in place of asparagines has been reported (17-19), the pharmacological induction of this modification would be unprecedented and have profound implications for therapy and how neo-antigens might be created. We report the results of such an intervention in tissue culture and in woodchucks chronically infected with woodchuck hepatitis virus (WHV), which mimics many of the immunologic features of chronic HBV infection in humans (20).
MATERIALS AND METHODS
Drugs used
6-O-butanoyl castanospermine (BuCas, or Celgosivir) was provided by Migenix, Inc. (Vancouver, BC). 1-(2-fluoro-5-methyl-beta-L-arabinofuranosyl)-uracil (L-FMAU; Clevudine) was provided by Pharmasett, Inc. (Princeton, NJ).
In vitro generation of peptide specific cytotoxic T lymphocytes (CTLs)
Heparinized blood from healthy HLA-A2 donors was purchased from Research Blood Components, LLC, (Brighton, MA). Peripheral blood mononuclear cells were purified and cultured as described (13, 21). After initial stimulation with synthetic peptide, T cells were re-stimulated with CD4/CD8 T cell depleted autologous monocytes pulsed with synthetic peptide at 10μg/ml for 5 days. IL-2 treatment and in vitro re-stimulation were repeated thrice prior to use of in vitro expanded T cells in ELISpot assays. Our previous work has demonstrated that T cells expanded in this manner secrete granzyme B and have surface CD8, hallmarks of the cytolytic potential of CD8+ T cells, so we refer to these cells as CTLs (21).
ELISpot assays
In vitro expanded CTLs were used as effectors in ELISpot assays to assess antigen stimulated interferon-γ release according to the manufacturer’s instructions (BD-Pharmingen, San Jose, CA). Target cells were HepG2 human hepatoma cells (HBV negative; American Type Culture Collection) or HBV-containing HepG2.2.15 cells (22). Cells were treated with glucosidase inhibitor BuCas (1 mg/ml) twice at an interval of 3 days prior to use as targets in ELISpot assays, and washed before incubation with T cells. Typically, 1×105 effectors (T cells) and 5×103 targets were used (20:1). Results are presented as number of interferon-γ producing cells per 106 CD8+ T cells.
Animals and treatments
All experimental procedures involving woodchucks were performed under protocols approved by the Cornell University Institutional Animal Care and Use Committee. Woodchucks were born to WHV-negative females in environmentally controlled laboratory animal facilities and inoculated at 3 days of age with 5 million infectious doses of a standardized WHV inoculum (23). Woodchucks were selected as chronic WHV carriers based on persistent detection of WHV surface antigen (WHsAg) and WHV DNA in serum prior to treatments. All animals were free of HCC at the beginning of the study as determined by hepatic ultrasound examination and normal serum activity of γ-glutamyl-transferase (GGT).
Twenty adult chronically infected woodchucks were stratified by age, sex, body weight, serum viral load, and serum GGT activity into four treatment groups of five animals each. Drug was administered orally at 100 mg/kg (in sterile water), chosen after an initial dose finding trial. Following a single oral dose of 100 mg/kg, the average observed Cmax was 7.7 μg/ml (range 5.0-12.1). The subunit vaccine consisted of 22-nm WHsAg particles, purified by zonal ultracentrifugation from serum of WHV7P1-infected WHV carriers (24), inactivated with formalin, and adsorbed onto alum. Prior to alum adsorption, vaccine was tested in naïve, WHV-susceptible animals and no residual virus was detected. Purified WHsAg was not pretreated with enzymes that remove preS sequences. Blood samples were obtained for WHV DNA analysis and serological testing while animals were under general anesthesia (ketamine 50 mg/kg and xylazine 5 mg/kg intramuscularly). Samples were taken prior to drug administration on the first day of treatment and at the indicated time points. Animals were weighed at bi-weekly intervals, and observed daily; no evidence of drug-related toxicity was seen.
Serologic assays
Serum WHV DNA was measured quantitatively by dot blot hybridization (assay sensitivity, ≥ 1.0 × 107 WHV genome equivalents per ml [WHVge/ml]) (25). Serum WHsAg, antibodies to WHV core antigen (anti-WHc), and WHV surface antigen (anti-WHs) were determined with WHV-specific enzyme immunoassays (26). Serum biochemical measurements included serum GGT, alkaline phosphatase (ALP), and marker of hepatocellular injury alanine aminotransferase (ALT), aspartate aminotransferase (AST), and sorbitol dehydrogenase (SDH) (25).
Glycan analysis
Sample preparation for glycan analysis was performed essentially as described (27). HPLC separation was performed using the Waters Alliance HPLC system with a Waters fluorescence detector, and quantified using the Millenium Chromatography Manager (Waters Corporation, Milford, MA). Tri-glucosylated structures were identified by comparison to known standards (27, 28).
PBMC proliferation assay
T cell responses against WHV were determined using in vitro stimulators at concentrations optimal for cultures of woodchuck PBMCs (29, 30). Stimulators consisted of native 22-nm WHsAg (2 μg/ml), recombinant WHcAg (2 μg/ml), or synthetic peptides (10 μg/ml) corresponding to either native viral sequences or predicted N-de-glycosylated sequences (Table 1). The in vitro proliferation assay using woodchuck PBMCs labeled dividing cells with [2-3H]adenine (Amersham Pharmacia Biotech, Inc., Arlington Heights, IL). Woodchuck PBMCs were isolated from whole blood and stimulated as described (30, 31). Counts per minute of triplicate PBMC cultures were averaged and expressed as a stimulation index (SI) by dividing the average sample counts per minute in the presence of the stimulator by that observed in the absence of stimulator (six replicates). A SI value of ≥ 3.1 was considered to represent a positive, specific T-cell response.
TABLE 1.
Peptides used in PBMC proliferation assay
| Previously used peptides | |
| S1: | MGNNIKVTFNPDKIA; |
| S7/8: | GRKPTPPTPPLRDTHPHLTM |
| S11: | DPALSPEMSPSSLLGLLAGLQVV |
| S12/13: | YFLWTKILTIAQNLDWWCTS |
| S18: | YCCCLKPTAGNCTCWPIPSS |
| S21: | LSILPPFIPIFVLFFLIWVYI. |
| New peptides used in this study: | |
| PreS2-N: | LTMKNQTFHLQGFVDGLR |
| PreS2-D: | LTMKDQTFHLQGFVDGLR |
| S-N: | CLKPTAGNCTCWPIPSSW |
| S-D: | CLKPTAGDCTCWPIPSSW. |
RESULTS
CTLs raised against aspartic acid-containing envelope peptides recognize HBV-producing cells
The ER chaperone calnexin (CNX) binds to nascent glycoproteins that are mono-glucosylated due to trimming of terminal glucoses by glucosidases (Fig. 1). We reasoned that inhibition of glucosidases would prevent HBV MHBs interaction with CNX and cause accumulation of misfolded MHBs. Misfolded protein might be retrotranslocated from the ER to the cytoplasm, and degraded by proteasomes. Accumulation of unglycosylated MHBs when cells were treated simultaneously with proteasome inhibitors and glucosidase inhibitor suggested that de-N-glycosylation occured (5). Cellular PNGase cleaves the N-glycosidic linkage between the core N-acetylglucosamine and asparagine (N), with deamidation to aspartic acid (D). Thus, formerly N-glycosylated peptides that emerge from the proteasome will differ from peptides that were never glycosylated. Since “D” containing epitopes are not specified by the viral genome and presumably result from posttranslational editing, we call them “editopes”.
Figure 1. Schematic representation of the consequences of endoplasmic reticulum associated degradation-linked de-N-glycosylation.
The figure depicts interference of the interaction of MHBs with calnexin (CNX) in the ER by glucosidase inhibitor (GluI), with subsequent retrotranslocation to the cytoplasm. Both de-N-glycosylation by PNGase and degradation by the proteasome result in the production of a novel D-peptide in place of the original N-peptide. These peptides are now available for re-import into the ER and loading into empty MHC class I complexes. Inverted triangle, tri-glucosylated N-glycan chain.
Peptides presented on the surface of a cell in the context of the MHC class I complex should be recognized with high sensitivity upon incubation with cognate peptide-primed CTLs, with specific killing of the target cells. Previously, we reported preparation of CTLs by stimulation with a known HLA-A2 restricted antigenic peptide, 183-FLLTRILTI (13). This peptide represents amino acids 183-191 of LHBs (32). Such CTLs recognized HepG2.2.15 target cells expressing viral antigens. HepG2.2.15, and the parental, HBV-negative, hepatoblastoma cell line HepG2, express HLA-A2 class I molecules, but not HLA class II (33). Thus, we tested whether a de-N-glycosylated HBs peptide could elicit CTLs from human peripheral blood mononuclear cells (PBMCs) that recognize peptides presented by HepG2.2.15 cells. PBMCs from healthy HLA-A2 positive donors were isolated and stimulated in vitro with either amino acids 304-312 KPSDGNCTC (N-peptide, Fig. 1), or the corresponding de-N-glycosylated KPSDGDCTC (D-peptide). Peptides conformed to the consensus for HLA-A2 binding according to the SYFPEITHI prediction algorithm (34). In vitro stimulated CTLs were incubated with either uninfected HepG2 cells or HBV-producing HepG2.2.15 cells. Target cell recognition was quantified by interferon-γ ELISpot assay.
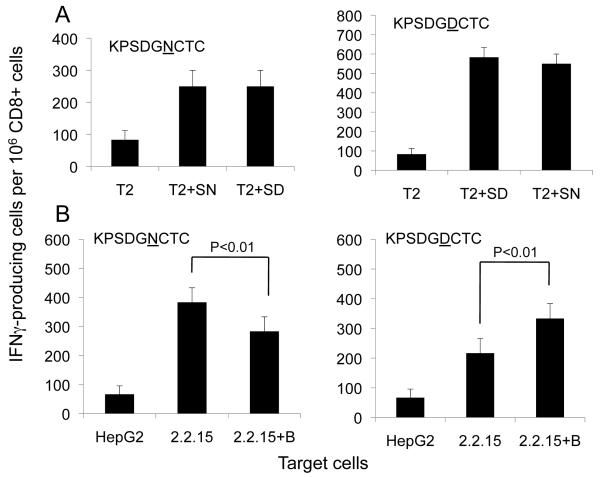
Both the natural N-peptide and the non-templated D-peptide were effective elicitors of specific CTLs that recognize HLA-A2 expressing T2 target cells, with significant cross-reactivity (Fig 2). Presentation of the D-peptide epitope by target cells was increased significantly by 6-O-butanoyl-castanospermine (BuCas) treatment, presumably because de-N-glycosylated epitope production was enhanced by glucosidase inhibition. Presentation of the N-peptide epitope was reduced in cells treated with the BuCas, consistent with increased protein turnover. Similar results were obtained in an independent experiment with another donor (data not shown). BuCas-induced changes were specific for the viral envelope glycoprotein, and not seen with CTLs primed with an epitope from HBV core antigen (13). These results show that (1) D-peptides are stimulatory and (2) glucosidase inhibition increases the degree to which HepG2.2.15 cells are recognized by CTLs primed with D-peptide but not N-peptide.
Figure 2. CTLs raised against aspartic containing envelope protein epitopes recognize HBV producing cells.
PBMCs isolated from healthy HLA-A2+ human donor blood were stimulated in vitro with peptides corresponding to the HLA-A2 restricted CTL epitope from HBs (KPSDGNCTC) or the ‘D’ substituted peptide (KPSDGDCTC). The ability of in vitro generated CTLs to recognize and secrete interferon-γ was evaluated by ELISpot assay.
A. CTLs generated against ‘N’ containing peptide and the corresponding ‘D’ containing peptide were incubated with T2 cells pulsed with either ‘N’ or ‘D’ containing peptide to assess T cell cross-reactivity.
B. HBV negative HepG2 cells or HBV positive HepG2.2.15 cells, either left untreated or treated with BuCas (1mg/ml) twice for three day intervals were used as targets. Target cells (5000 cells per well) were washed once before they were co-incubated with CTLs (100,000 cells/well) in an ELISpot plate. Error bars represent SEM of experimental replicates. P value was calculated from a Student’s t-Test analysis of experimental results.
Treatment of chronic WHV carrier woodchucks with antiviral and immunostimulatory agents
Next, we determined whether D-peptide-specific responses could be observed in vivo following glucosidase inhibition. Woodchuck hepatitis virus (WHV) shares DNA sequence homology and pathobiological features with human HBV. WHV establishes chronic infection in outbred woodchucks and is considered to be a model for the human virus (20). We showed that WHV MHBs is sensitive to glucosidase inhibition in vivo (11). Thus, antigen-specific proliferative cell responses of PMBCs were examined from woodchucks chronically infected with WHV as a function of treatment with BuCas.
Woodchucks chronically infected with WHV experienced significant immunological responses to envelope proteins following immunization with WHsAg-containing vaccines, especially in the context of low viral and antigen loads following treatment with an effective antiviral agent, 1-(2-fluoro-5-methyl-beta-L-arabinofuranosyl)-uracil (L-FMAU) (29, 35). Since BuCas treatment might be expected to reduce the amount of MHBs in the circulation and/or alter its immunological profile, we investigated the response to BuCas administration along with WHsAg vaccine. 25 woodchucks chronically infected with WHV were divided into five treatment groups: Placebo, Vaccine alone (V), BuCas alone (B), vaccine plus BuCas (V+B) and V+B plus L-FMAU (V+B+L). Four uninfected animals served as controls. Vaccine interventions were as shown in Fig. 3A.
Figure 3. Vaccination schedule for woodchucks, and proliferation of PBMCs in response to viral antigens.
A, Scheduled treatment of woodchucks. Arrows indicate vaccination with complexes of alum and surface antigen for selected groups of animals. Circle, vaccination of animals with D-peptide. See text for details on animal groupings. B, BuCas; V, vaccine; L, L-FMAU; P, placebo.
B, PBMCs were isolated at the indicated time points, and cultured as described in Materials and Methods. Peptide antigens are shown in Table 1; in addition, full length WHV core and HBs were used as antigens. Animals were scored as positive if cells proliferated above the cut-off value of ≥ 3.1. Treatment groups are designated as P, placebo; B, BuCas; V, vaccine; B+V, BuCa plus vaccine. Percentage of animals with a positive response to one or more WHsAg-related peptides is shown.
C, As for B, with percentage of animals with a positive response to the entire WHsAg and/or WHcAg shown.
Viremia and antigenemia remained relatively stable in all placebo animals (Table 2 and data not shown). These parameters were not altered significantly by treatment with either BuCas alone or the combination of BuCas and vaccine, at all times tested; representative data are shown from week 0 (baseline) and week 10 (4 weeks after the first vaccination). Markers of liver injury such as ALT, AST and GGT were also fairly stable (Table 2), excepting an animal in group V+B that succumbed to hepatocellular carcinoma ca. week 20. The triple combination V+B+L resulted in marked reduction of viremia, consistent with a previous trial (29, 35). Thus, BuCas treatment was not incompatible with reduction in viral load.
TABLE 2.
Summary of key woodchuck serum parameters at weeks 0 and 10
| WHV DNA^ | Av(range) | WHsAg* | Av(range) | ALT# | Av(range) | Glu3, % | |
|---|---|---|---|---|---|---|---|
| Group | Week 0 | Week 10 | Week 0 | Week 10 | Week 0 | Week 10 | Week 10 |
| U | UD+ | UD | 0 | 0 | 4.8 (4-7) | 5.5 (4-8) | NT+ |
| P | 5.3(.5-15) | 4.1(.7-16) | .39(.26-.55) | .41(.26-.6) | 6.0(5-7) | 9.6(8-11) | NT |
| V | 8.8(1.6-19) | 8.5(.22-16) | .40(.3-.47) | .44(.32-.56) | 6.2(4-11) | 9.8(5-18) | 0 |
| B | 2.7(.4-7.0) | 1.9(.9-4.8) | .38(.28-.43) | .42(.33-.52) | 7.4(4-9) | 15.6(10-22) | .49 (0-.90) |
| V+B | 13(1.6-50) | 13(2.1-51) | .40(.31-.55) | .44(.34-.55) | 8(4-20) | 12.6(9-18) | .33 (0-.55) |
| V+B+L | 19(12-29) | UD+ | .43(.27-.53) | .24(0-.47) | 7.2(4-16) | 9.4(6-13) | .53 (.41-.64) |
, DNA level is expressed as genome equivalents (X 1010)
, WHsAg level is indicated in optical density units
, ALT is indicated in Units/L
, UD, <E07 GE, the detection limit of dot blot hybridization; NT, not tested
In vivo, levels of circulating glycoproteins with N-glycans bearing three terminal glucose residues reflect the extent of glucosidase inhibition (11). Animals treated with BuCas were determined to have microgram per milliliter levels of the drug (Materials and Methods), which impaired glycan processing, seen as tri-glucosylated glycans in the sera of BuCas-treated animals (Table 2). Note that BuCas-treated animals that were negative for tri-glucosylated glycans at the 10-week time point were positive at one or more other time points (data not shown). No tri-glucosylated glycans were detected in any drug-naïve animals (Table 2).
Immunoblotting analysis of sera revealed visible drops in circulating MHBs in all of the animals in V+B+L group (Supplemental data), consistent with reductions in total surface antigen (Table 2). However, treatment with either vaccine or BuCas, alone or in combination, did not decrease MHBs levels at any time points (Supplemental data).
Proliferation of PBMCs from woodchucks chronically infected with WHV in response to viral antigens and pharmacologically induced neo-antigens
Although reagents to dissect the immune response of woodchucks are limited, assays to measure lymphocyte recognition of specific epitopes have been implemented. PMBCs are isolated from the animals and incubated with antigen in vitro; proliferation is assumed to be evidence of antigen recognition and stimulation. Therefore, PMBCs were isolated from animals at the indicated times (Fig. 3) and incubated with a panel of viral antigens, including intact WHsAg and various peptides of WHsAg (Table 1). Most the of the peptides were shown previously to induce strong proliferation of PBMCs from woodchucks with resolved WHV infections or vaccinated with WHsAg (29, 30, 35); these cells have been shown to be CD3+ T cells. The panel also included both D- and N-containing peptides spanning the two N-glycosylation sites of WHV MHBs. There was no recognition of naturally specified WHV HBs epitopes incubated with PMBCs from chronically infected woodchucks that were left untreated with either drug or vaccine at any time point (Fig. 3, group P). This is as expected, since chronically infected animals are considered tolerant and are unresponsive to HBV antigens (20).
Some vaccinated animals (Group V) produced PMBCs that recognized WHV epitopes (Fig. 3). The two responding animals at week 12 differ from those positive at week 8 (not shown), suggesting possible sampling variation, or variation in kinetics with respect to development of antibody and T cell responses. Strikingly, BuCas treatment alone resulted in proliferation in response to WHV HBs antigens (group B). BuCas plus vaccine also was potent at stimulating cellular responses (group B+V). Thus, despite the absence of detectable changes in antigenemia induced by the drug, virus-specific immune responses apparently occurred.
From the data in Fig. 2, we expected a cellular immune response to D-peptide antigens. Responses to the paired N/D peptides (glycosylation sequons at amino acids 4 and 146) were evaluated (Fig. 4). In untreated animals, none of the peptides elicited a response. For group V, responses was restricted to N-peptides. Since the D-peptides are not specified by WHV, the lack of response is not entirely surprising. In contrast, animals in groups B and B+V responded more strongly to D-peptides versus N-peptides. In some cases, both peptides were recognized (Fig. 4A). This response was observed as early as 8 weeks of treatment and persisted throughout (Fig. 4B).
Figure 4. Proliferation of PBMCs induced by viral neo-antigen in response to drug treatment.
A, Detailed responses of individual animals at a single time point to N-peptides versus D-peptides. Positive response is as defined in Fig. 4. Treatment groups are designated as Un, uninfected controls; P, placebo; B, BuCas; V, vaccine; B+V, BuCa plus vaccine.
B, Summary of responses of groups to N-peptides and D-peptides over time.
Lack of reactivity to D-peptides might be due to some animals being incapable of responding to these epitopes. To test this possibility, all animals in groups V, B, and B+V were inoculated with D-peptides in alum at week 28 (Fig. 3A). PBMCs were harvested at weeks 28 and 32, and analyzed for antigen-dependent proliferation (Fig. 5). Cellular responses to D-peptides were evident in all three groups at week 32 (3/5 animals positive), indicating that most animals were capable of responding to these epitopes. These data strongly suggest that D-peptides were produced and presented in animals treated with BuCas, and that these epitopes, which we refer to as “editopes” are not abundantly produced in the absence of pharmacological intervention.
Figure 5. Proliferation of PBMCs in response to neo-antigen vaccination.
Detailed responses of individual animals either pre-inoculation (week 28) or 4 weeks post-inoculation with D-peptides. Treatment groups are designated as Un, uninfected controls; P, placebo; B, BuCas; V, vaccine; B+V, BuCa plus vaccine. Woodchuck 7092 died following week 20 of the study, and thus is unscored.
DISCUSSION
Normally, wild-type MHBs is very stable in cultured cells (5). However, pharmacologic inhibition of ER glucosidases that trim N-glycans on nascent proteins results destabilization of MHBs. Such treatment leads to proteasome-mediated degradation, which in turn results in increased presentation of proteasome-derived peptides by MHC class I (13). De-N-glycosylation is expected to produce peptides in which asparagines are converted to aspartic acids (Fig. 1). The detection of D-peptides derived from MHBs presented by MHC class I on the surface of HepG2.2.15 cells treated with BuCas supported this hypothesis (Fig. 2). Thus, woodchucks chronically infected with WHV were treated with BuCas, and the effect of the drug on both viral replication and immune response to therapeutic vaccination were evaluated.
We were surprised that there was no detectable antiviral response in the drug treated woodchucks (Table 2), despite apparent efficacy in cell culture (13). Indeed, had previously observed we antiviral activity in woodchucks with a different iminocyclitol, N-nonyl deoxynojirimycin (11). There are several possible reasons for this discrepancy. First, the dose obtained with BuCas may have been insufficient to produce an antiviral effect, despite biochemical efficacy (tri-glucosylated proteins in the circulation, Table 2). Second, the two compounds do not act identically. Formation of the mono-glucosylated substrate for CNX requires sequential action of glucosidases I and II (10). Castanospermine and its derivative BuCas are more potent inhibitors of glucosidase I than deoxynojirimycin, but the latter may have more activity against glucosidase II (36-38). Thus, more tri-glucosylated MHBs should accumulate with BuCas. All three glucosylated species should be substrates for endomannosidase and escape from the ER (39). Finally, deoxynojirimycin prevents oligosaccharide addition some fraction of the time, but castanospermine does not (36). Secretion of MHBs is highly dependent upon the presence of N-glycan within the pre-S2 region (7).
A desirable therapeutic vaccine against chronic HBV would stimulate antiviral CTLs, which, combined with a reduction in viremia achieved by other treatments, should eliminate infected cells. Unfortunately, the response of chronically infected patients to such a vaccine was weak (40). Despite the absence of antiviral activity in the WHV infected animals, BuCas stimulated cellular immunity to viral antigen; only infected woodchucks treated with BuCas possessed PMBCs that could recognize and be primed by the D-peptides derived from MHBs. Based on the results presented, we propose that (a) D-peptide versions of the MHBs peptides can be presented by MHC class I and can activate CD8+ T cells and (b) the de-N-glycosylation can occur in vitro and in vivo following pharmacological intervention. The relatively weak response in the BuCas-treated animals to the natural N-peptides implies that there is little, if any, spontaneous generation of N-specific and that there may be limited cross recognition between cells that recognize the N- and D-epitopes.
Although we feel that we have some insight into the mechanism by which BuCas is stimulating cellular immunity, the actual in vivo situation is likely to be more complicated than the simplified model in Fig. 1. For instance, the limited cross recognition detected in animals is distinct from the tissue culture analysis of CD8+ CTLs from people (Fig. 2). It is unclear why non-BuCas treated HepG2.2.15 cells were recognized by D-peptide-primed CTLs. We believe the levels of spontaneously generated MHBs D-peptides are likely to be low, and that instead cross recognition of the N-peptide epitope by the CTLs primed with D-peptides is occurring, as was shown with exogenous peptide for the T2 cells. Some degree of cross recognition also was observed for a pair of tyrosinase peptides (41). The reason for this discrepancy is not known, and may reflect differences between the human or woodchuck processing machinery, or be intrinsic to the peptides themselves.
It also should be noted that the proliferative response in the woodchucks likely involves other immune cells as well as CD8+ T cells. The proliferating PBMCs include CD3+ T cells, although their CD8 status can not be determined due to lack of specific antibody. Drug treatment might affect components of the antigen processing and presentation apparatus; unoccupied MHC class I molecules are destabilized by glucosidase inhibition (42). The WHV MHBs protein itself has been reported to suppress MHC class I presentation levels (43). Although BuCas treatment does not detectably reduce circulating MHBs, it is possible that intracellular levels are decreased, influencing formation of MHC class I complexes.
There is evidence that “editope” production can occur naturally in the absence of glucosidase inhibition. For example, CTLs isolated from a chimpanzee chronically infected with hepatitis C virus showed strong preferential reactivity to a de-N-glycosylated, aspartic acid-containing version of an viral E1 glycoprotein epitope, with only weak reactivity against the templated asparagine-containing sequence (19). Similarly, D-epitopes derived from tyrosinase and from the Lymphocytic Choriomeningitis virus glycoprotein have been reported (17, 18).
Although the human genome is estimated to contain 25,000 or fewer protein-coding genes, post-translational modifications expand protein diversity. Posttranslational editing refers to the alteration of a polypeptide sequence such that it differs from the gene from which it was specified. The enzymatic hydrolysis of N-linked glycan from the asparagines of glycoproteins by the action of the mammalian PNGase results in the conversion of the asparagines to aspartic acids. We suggest that this is a form of posttranslational editing, and where it results in new epitopes, not specified by the genome, call it “editoping”.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Bertha Conyers for technical support. We are grateful to Migenix, Inc., for the gift of Celgosivir and to Pharmasett, Inc., for the gift of L-FMAU. We also thank Migenix for communicating woodchuck pharmacokinetic data.
Financial support: This work was supported by grants from NIAID, U01-AI053884 (T.M.B) and N01-AI-05399 (B.C.T), as well as by the Commonwealth of Pennsylvania and the Hepatitis B Foundation.
Abbreviations
- HBV
hepatitis B virus
- MHC
major histocompatibility complex
- MHBs
HBV middle envelope glycoprotein
- ER
endoplasmic reticulum
- PNGase
peptide:N-glycanase
- BuCas
6-O-butanoyl castanospermine
- L-FMAU
1-(2-fluoro-5-methyl-beta-L-arabinofuranosyl)-uracil
- HLA
human leukocyte antigen
- WHV
woodchuck hepatitis virus
- CTLs
cytotoxic T lymphocytes
- WHsAg
WHV surface antigen
- WHcAg
WHV core antigen
- GGT
γ-glutamyl-transferase
- Ge
genome equivalents
- PBMC
peripheral blood mononuclear cells
REFERENCES
- 1.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. Mech. Dis. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 2.Rehermann B. Chronic infections with hepatotropic viruses: mechanisms of impairment of cellular immune responses. Sem. Liver Dis. 2007;27:152–160. doi: 10.1055/s-2007-979468. [DOI] [PubMed] [Google Scholar]
- 3.Yewdell JW, Bennink JR. Mechanisms of viral interference with MHC class I antigen processing and presentation. Annu Rev Cell Dev Biol. 1999;15:579–606. doi: 10.1146/annurev.cellbio.15.1.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruss V. Envelopment of the hepatitis B virus nucleocapsid. Virus Res. 2004;106:199–209. doi: 10.1016/j.virusres.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Simsek E, Mehta A, Zhou T, Dwek RA, Block T. Hepatitis B Virus Large and Middle glycoproteins are degraded by a proteasome pathway in glucosidase-inhibited cells but not in cells with functional glucosidase enzyme. J Virol. 2005;79:12914–12920. doi: 10.1128/JVI.79.20.12914-12920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Block TM, Mehta AS, Blumberg BS, Dwek RA. Does rapid oligomerization of hepatitis B envelope proteins play a role in resistance to proteasome degradation and enhance chronicity? DNA Cell Biol. 2006;25:165–170. doi: 10.1089/dna.2006.25.165. [DOI] [PubMed] [Google Scholar]
- 7.Mehta A, Lu X, Block TM, Blumberg BS, Dwek RA. Hepatitis B virus (HBV) envelope glycoproteins vary drastically in their sensitivity to glycan processing: evidence that alteration of a single N-linked glycosylation site can regulate HBV secretion. Proc Natl Acad Sci USA. 1997;94:1822–1827. doi: 10.1073/pnas.94.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werr M, Prange R. Role for calnexin and N-linked glycosylation in the assembly and secretion of hepatitis B virus middle envelope protein particles. J Virol. 1998;72:778–782. doi: 10.1128/jvi.72.1.778-782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergeron JJ, Brenner MB, Thomas DY, Williams DB. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994;19:124–128. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 10.Parodi AJ. Protein glucosylation and its role in protein folding. Ann. Rev. Biochem. 2000;69:69–93. doi: 10.1146/annurev.biochem.69.1.69. [DOI] [PubMed] [Google Scholar]
- 11.Block TM, Lu X, Mehta A, Blumberg BS, Tennant B, Ebling M, Korba B, et al. Treatment of chronic hepadnavirus infection in a woodchuck animal model with an inhibitor of protein folding and trafficking. Nature Med. 1998;4:610–614. doi: 10.1038/nm0598-610. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Simsek E, Norton P, Sinnathamby G, Philip R, Block T, Zhou T, et al. The role of the downstream signal sequences in the maturation of the HBV middle surface glycoprotein: development of a novel therapeutic vaccine candidate. Virology. 2007;365:10–19. doi: 10.1016/j.virol.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 13.Simsek E, Sinnathamby G, Block TM, Liu Y, Philip R, Mehta AS, Norton PA. Inhibition of cellular alpha-glucosidases results in increased presentation of hepatitis B virus glycoprotein-derived peptides by MHC class I. Virology. 2009;384:12–15. doi: 10.1016/j.virol.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki T, Seko A, Kitajima K, Inoue Y, Inoue S. Purification and enzymatic properties of peptide:N-glycanase from C3H mouse-derived L-929 fibroblast cells. Possible widespread occurrence of post-translational remodification of proteins by N-deglycosylation. J Biol Chem. 1994;269:17611–17618. [PubMed] [Google Scholar]
- 15.Suzuki T, Lennarz W. Hypothesis: a glycoprotein-degradation complex formed by protein-protein interaction involves cytosolic peptide:N-glycanase. Biochem Biophys Res Comm. 2003;302:1–5. doi: 10.1016/s0006-291x(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 16.Wiertz E, Jones T, Sun L, Bogyo M, Geuze H, Ploegh H. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 17.Altrich-VanLith M, Ostankovitch M, Polefrone J, Mosse C, Shabanowitz J, Hunt D, Engelhard V. Processing of a class I-restricted epitope from tyrosinase requires peptide N-glycanse and the cooperative action of endoplasmic reticulum aminopeptidase 1 and clytosolic proteases. J Immunol. 2006;177:5440–5450. doi: 10.4049/jimmunol.177.8.5440. [DOI] [PubMed] [Google Scholar]
- 18.Hudrisier D, Riondi J, Mazarguil H, Gairin JE. Pleiotropic effects of post-translational modifications on the fate of viral glycoproteins a cytotoxic T cell epitopes. J Biol Chem. 2001;276:38255–38260. doi: 10.1074/jbc.M105974200. [DOI] [PubMed] [Google Scholar]
- 19.Selby M, Erickson A, Dong C, Cooper S, Parham P, Houghton M, Walker C. Hepatitis C virus envelope glycoprotein E1 originates in the endoplasmic reticulum and requires cytoplasmic processing for presentation by class I MHC molecules. J Immunol. 1999;162:669–676. [PubMed] [Google Scholar]
- 20.Menne S, Cote P. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J Gastroenterol. 2007;13:104–124. doi: 10.3748/wjg.v13.i1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinnathamby G, Lauer P, Zerfass J, Hanson B, Karabudak A, Krakover J, Secord AA, et al. Priming and activation of human ovarian and breast cancer-specific CD8+ T cells by polyvalent Listeria monocytogenes-based vaccines. J Immunother. 2009;32:856–869. doi: 10.1097/CJI.0b013e3181b0b125. [DOI] [PubMed] [Google Scholar]
- 22.Sells MA, Chen ML, Acs G. Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc. Natl. Acad. Sci. USA. 1987;84:1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cote PJ, Korba BE, Miller RH, Jacob JR, Baldwin BH, Hornbuckle WE, Purcell RH, et al. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatol. 2000;31:190–200. doi: 10.1002/hep.510310128. [DOI] [PubMed] [Google Scholar]
- 24.Gerin J, Faust R, Holland P. Biophysical characterization of the adr subtype of hepatitis B antigen and preparation of anti-r sera in rabbits. J Immunol. 1975;115:100–105. [PubMed] [Google Scholar]
- 25.Menne S, Cote PJ, Butler SD, George AL, Tochkov IA, Zhu Y, Xiong S, et al. Antiviral effect of orally administered lamivudine, emtricitabine, adefovir dipivoxil, and tenofovir disoproxil fumarate, alone and in combination in woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob Agents Chemother. 2008;52:3617–3632. doi: 10.1128/AAC.00654-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cote PJ, Roneker C, Cass K, Schodel F, Peterson D, Tennant BC, De Noronha F, et al. New enzyme immunoassays for the serologic detection of woodchuck hepatitis virus infection. Viral Immunol. 1993;6:161–169. doi: 10.1089/vim.1993.6.161. [DOI] [PubMed] [Google Scholar]
- 27.Comunale MA, Lowman M, Long RE, Krakover J, Philip R, Seeholzer S, Evans AA, Hann HWL, Block TM, Mehta AS. Proteomic analysis of serum associated fucosylated glycoproteins in the development of primary hepatocellular carcinoma. J Proteome Res. 2006;6:308–315. doi: 10.1021/pr050328x. [DOI] [PubMed] [Google Scholar]
- 28.Royle L, Mattu TS, Hart E, Langridge JI, Merry AH, Murphy N, Harvey DJ, et al. An analytical and structural database provides a strategy for sequencing O-glycans from microgram quantities of glycoproteins. Anal Biochem. 2002;304:70–90. doi: 10.1006/abio.2002.5619. [DOI] [PubMed] [Google Scholar]
- 29.Menne S, Roneker CA, Tennant BC, Korba BE, Gerin JL, Cote PJ. Immunization with surface antigen vaccine alone and after treatment with 1-(2-fluoro-5-methyl-beta-L-arabinofuranosyl)-uracil (L-FMAU) breaks humoral and cell-mediated immune tolerance in chronic woodchuck hepatitis virus infection. J Virol. 2002;76:5305–5314. doi: 10.1128/JVI.76.11.5305-5314.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menne S, Tennant BC, Gerin JL, Cote PJ. Chemoimmunotherapy of chronic hepatitis B virus infection in the woodchuck model overcomes immunologic tolerance and restores T-cell responses to pre-S and S regions of the viral envelope protein. J Virol. 2007;81:10614–10624. doi: 10.1128/JVI.00691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menne S, Roneker CA, Roggendorf M, Gerin JL, Cote PJ, Tennant BC. Deficiencies in the acute-phase cell-mediated immune response to viral antigens are associated with development of chronic woodchuck hepatitis virus infection following neonatal inoculation. J Virol. 2002;76:1769–1780. doi: 10.1128/JVI.76.4.1769-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 33.Ito Y, Kakumu S, Yoshioka K, Wakita T, Ishikawa T, Koike K. Cytotoxic T lymphocyte activity to hepatitis B virus DNA-transfected HepG2 cells in patients with chronic hepatitis B. Gastroenterol Jpn. 1993;28:657–665. doi: 10.1007/BF02806346. [DOI] [PubMed] [Google Scholar]
- 34.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanović S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 35.Menne S, Roneker CA, Tennant BC, Korba BE, Gerin JL, Cote PJ. Immunogenic effects of woodchuck hepatitis virus surface antigen vaccine in combination with antiviral therapy: breaking of humoral and cellular immune tolerance in chronic woodchuck hepatitis virus infection. Intervirology. 2002;45:237–250. doi: 10.1159/000067914. [DOI] [PubMed] [Google Scholar]
- 36.Gross V, Tran-Thi TA, Schwarz RT, Elbein AD, Decker K, Heinrich PC. Different effects of the glucosidase inhibitors 1-deoxynojirimycin, N-methyl-1-deoxynojirimycin and castanospermine on the glycosylation of rat alpha 1-proteinase inhibitor and alpha 1-acid glycoprotein. Biochem J. 1986;236:853–860. doi: 10.1042/bj2360853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaushal GP, Pan YT, Tropea JE, Mitchell M, Liu P, Elbein AD. Selective inhibition of glycoprotein-processing enzymes. Differential inhibition of glucosidases I and II in cell culture. J Biol Chem. 1988;263:17278–17283. [PubMed] [Google Scholar]
- 38.Taylor DL, Kang MS, Brennan TM, Bridges CG, Sunkara PS, Tyms AS. Inhibition of alpha-glucosidase I of the glycoprotein-processing enzymes by 6-O-butanoyl castanospermine (MDL 28,574) and its consequences in human immunodeficiency virus-infected T cells. Antimicrob Agents Chemother. 1994;38:1780–1787. doi: 10.1128/aac.38.8.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore SEH, Spiro RG. Demonstration that golgi endo-a-D-mannosidase provides a glucosidase-independent pathway for the formation of complex N-linked oligosaccharides of glycoproteins. J Biol Chem. 1990;265:13104–13112. [PubMed] [Google Scholar]
- 40.Heathcote J, McHutchinson J, Lee S, Tong M, Benner K, Minuk G, Wright T, et al. A pilot study of the CY-1899 T-cell vaccine in subjects chronically infected with hepatitis B virus. The CY1899 T Cell Vaccine Study Group. Hepatol. 1999;30:531–536. doi: 10.1002/hep.510300208. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell MS. Phase I trial of adoptive immunotherapy with cytolytic T lymphocytes immunized against a tyrosinase epitope - In Reply. J Clin Oncol. 2002;20:3176–3184. doi: 10.1200/JCO.2002.20.4.1075. [DOI] [PubMed] [Google Scholar]
- 42.Moore SE, Spiro RG. Inhibition of glucose trimming by castanospermine results in rapid degradation of unassembled major histocompatibilty complex class I molecules. J Biol Chem. 1993;268:3809–3812. [PubMed] [Google Scholar]
- 43.Wang J, Michalak TI. Inhibition by woodchuck hepatitis virus of class I major histocompatibility complex presentation on hepatocytes is mediated by virus envelope pre-S2 protein and can be reversed by treatment with gamma interferon. J Virol. 2006;80:8541–8553. doi: 10.1128/JVI.00830-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.